Published online Dec 16, 2022. doi: 10.12998/wjcc.v10.i35.13064
Peer-review started: September 7, 2022
First decision: September 27, 2022
Revised: October 2, 2022
Accepted: November 17, 2022
Article in press: November 17, 2022
Published online: December 16, 2022
Processing time: 97 Days and 22.7 Hours
We report on a case of Vibrio vulnificus (V. vulnificus) detected by metagenomics next-generation sequencing (mNGS) in a 53-year-old male patient with polymicrobial gas gangrene and successful treatment by surgery. This report raises awareness among dermatologists that when a patient is clinically suspected of a special type of pathogenic infection, the mNGS method should be preferred to identify the patient’s pathogen infection as soon as possible and then take effective treatment in time to save patients’ lives.
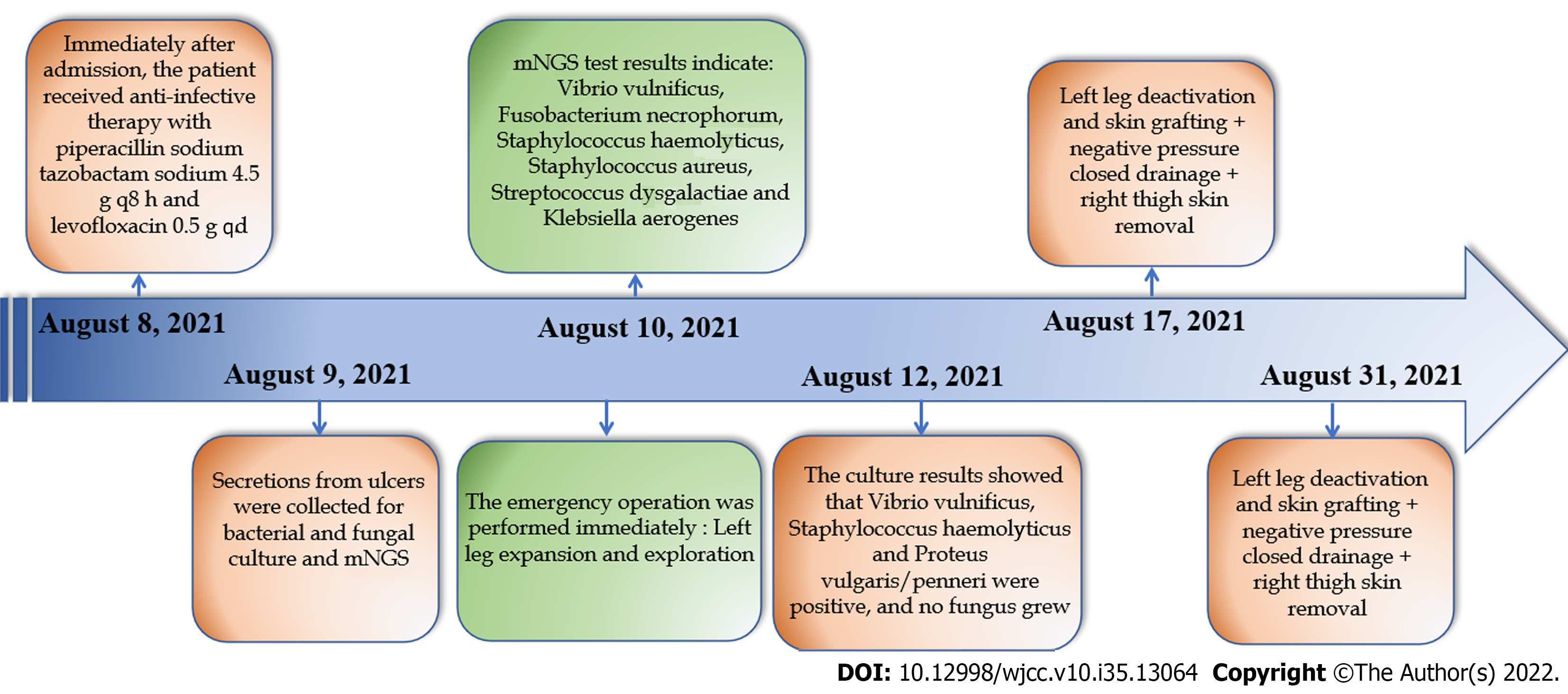
A 53-year-old male who worked in the aquatic market complained of redness and swelling of the lower limbs, blisters and ulcers with fever for 3 d. We used mNGS to test the pathogens in ulcer secretions. The results were returned in 24 h and indicated: V. vulnificus, Fusobacterium necrophorum, Staphylococcus haemolyticus, Staphylococcus aureus, Streptococcus dysgalactiae and Klebsiella aerogenes. This patient was diagnosed with V. vulnificus infection. The emergency operation was per
We could confirm the diagnosis of Vibrio vulnificus infection within 24 h through mNGS detection and then immediately performed emergency surgery.
Core Tip: We report on a case of Vibrio vulnificus detected by metagenomic next-generation sequencing (mNGS) in a 53-year-old male patient with polymicrobial gas gangrene and successful treatment by surgery. This report raises awareness among dermatologists that when a patient is clinically suspected of a special type of pathogenic infection, the mNGS method should be preferred to identify the patient’s pathogen infection as soon as possible, and then take effective treatment in time to save patients’ lives.
- Citation: Lu HY, Gao YB, Qiu XW, Wang Q, Liu CM, Huang XW, Chen HY, Zeng K, Li CX. Successful surgical treatment of polybacterial gas gangrene confirmed by metagenomic next-generation sequencing detection: A case report. World J Clin Cases 2022; 10(35): 13064-13073
- URL: https://www.wjgnet.com/2307-8960/full/v10/i35/13064.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i35.13064
Vibrio vulnificus (V. vulnificus) is a halophilic, motile, gram-negative bacterium that is an important opportunistic pathogen. V. vulnificus can cause septicemia and necrotizing infections[1]. V. vulnificus necrotizing skin and soft tissue infections are a serious, highly fatal and disabling disease that can cause fulminant sepsis with a mortality rate of over 50%[2-4].
V. vulnificus usually causes infection via exposure to seawater or through the consumption of seafood, and its pathophysiology can be divided into three types: (1) Primary sepsis; (2) Gastrointestinal diseases; and (3) Wound infections. Men (90% of cases) and older patients (85% > 40 years) are susceptible, especially those with liver disease, diabetes and underlying conditions such as immunodeficiency and hemochromatosis[5].
Primary V. vulnificus sepsis is a serious disease with a high mortality rate. Approximately 1/3 of patients with primary sepsis develop shock or hypotension within 12 h of admission. Bullous lesions are characteristic in three quarters of patients. Thrombocytopenia is common and patients often have evidence of diffuse intravascular coagulation[6].
The diagnosis of V. vulnificus infection is usually verified by a nonculture method or by traditional culture. V. vulnificus grows easily on standard media. Isolation of this bacterium from feces usually requires the use of a specific selective medium, namely, thiosulfate-citrate-bile salts-sucrose medium[7].
Here, we report the case of a 53-year-old male with polymicrobial gas gangrene complicated with V. vulnificus infection. The patient was diagnosed with V. vulnificus infection by metagenomic next-generation sequencing (mNGS) within 24 h and underwent immediate emergency debridement, followed by effective antibiotics and further surgical treatment. The patient recovered 1 mo later.
Redness and swelling of lower limbs, blisters and ulcers with fever for 3 d.
Three months prior, erythema and blisters had appeared in both lower extremities. He was diagnosed with a “skin infection” at a local hospital and improved after treatment. Three days prior, erythema, blisters, ulcers and fever had suddenly appeared in the left lower extremities. The local hospital administered cefoperazone for anti-infection, mannitol for dehydration, a magnesium sulfate wet compress, and other treatments without significant improvement.
The patient denied a history of medical illness. System physical examination showed no obvious abnormality.
The patient had no relevant personal or family history.
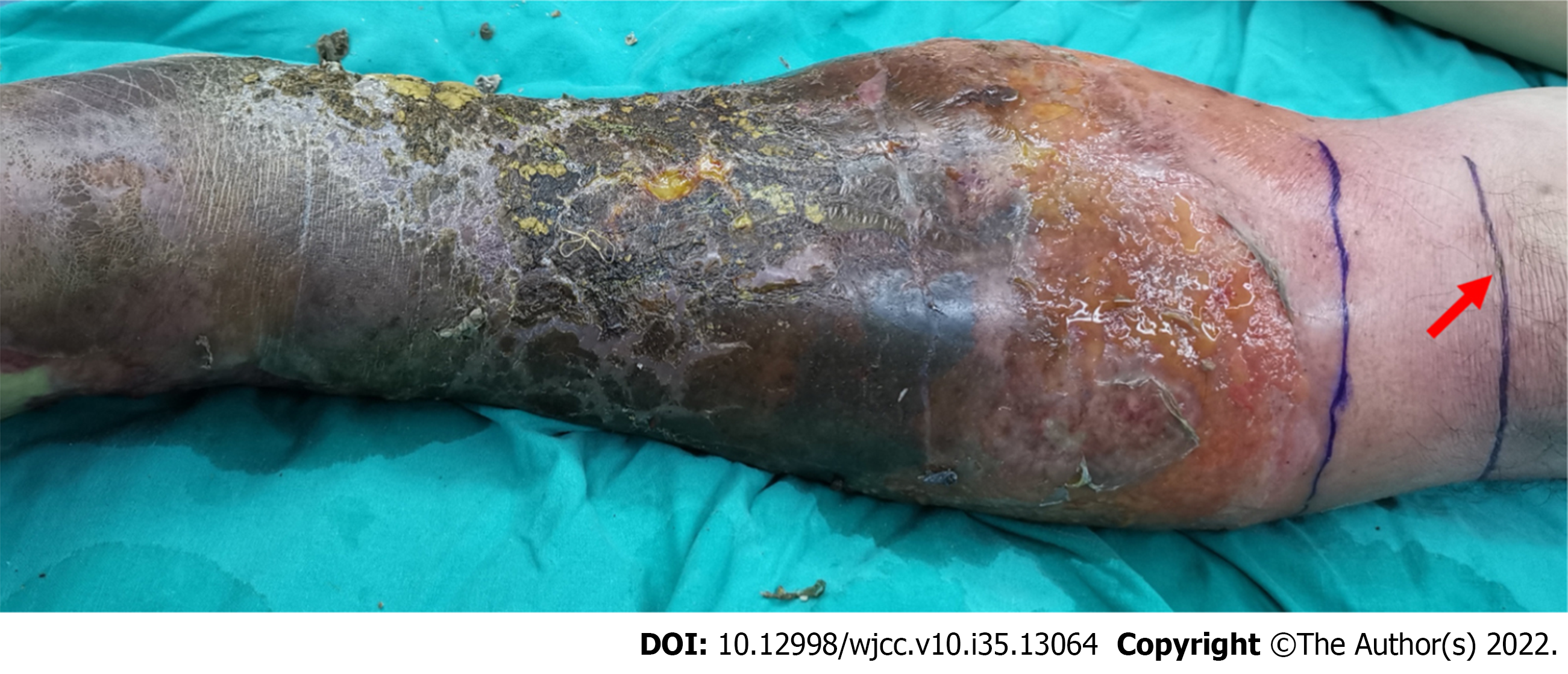
Obvious ulcerations and a large blister area were observed on the left calf; the blister walls were thin, and the blister fluid was pale yellow or bloody; the left inguinal lymph nodes were palpable and swollen (Figure 1); the patient also had a fever, with a maximum temperature of 38.9 ℃.
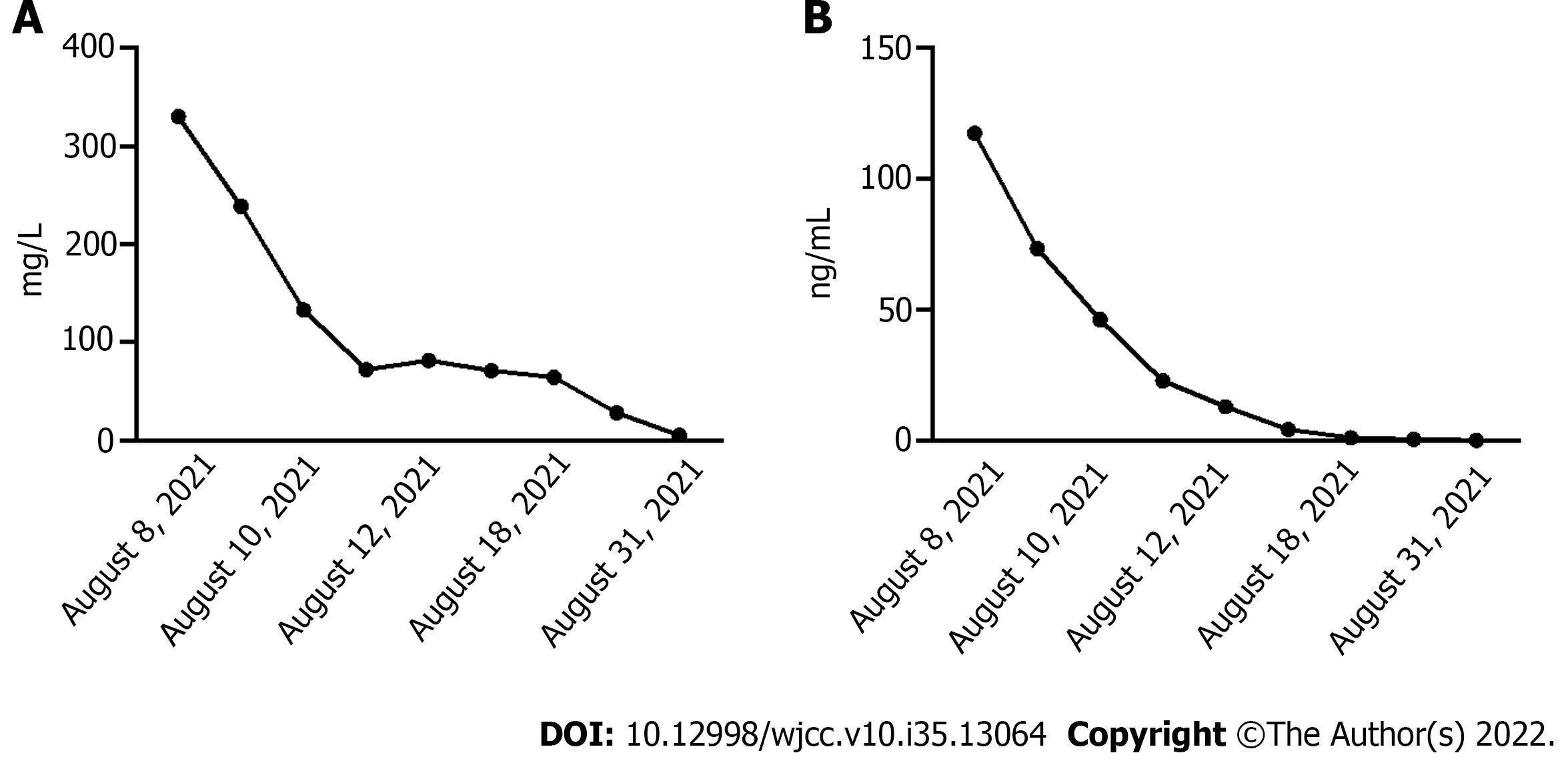
Infection index C-reactive protein (CRP) 134.30 mg/L (0.00-6.00 mg/L) (August 6, 2021), liver function: Alanine aminotransferase 47 U/L (9-50 U/L), Aspartate aminotransferase (AST) 40 U/L (15-40 U/L). The culture results showed that V. vulnificus, Staphylococcus haemolyticus and Proteus vulgaris/penneri were positive, and no fungus grew. The antimicrobial sensitivity of S. haemolyticus shows that it was sensitive to Linezolid, Nitrofurantoin, Rifampin, Trimethoprim-Sulfamethoxazole, Teicoplanin, Trimethoprim, and Vancomycin. The antimicrobial sensitivity of P. vulgaris/penneri shows that it was sensitive to Amoxicillin/Clavulanic, Amikacin, Aztreonam, Ceftazidime, Chloramphenicol, Cip
| Code | Antimicrobial | Result | MIC |
| AMC | Amoxicillin/Clavulanic | Sensitivity | ≤ 4/2 |
| AMK | Amikacin | Sensitivity | ≤ 8 |
| AMP | Ampicillin | Resistance | 8 |
| ATM | Aztreonam | Sensitivity | ≤ 2 |
| CAZ | Ceftazidime | Sensitivity | ≤ 1 |
| CDR | Cefdinir | - | - |
| CEC | Cefaclor | - | - |
| CEP | Cephalothin | - | - |
| CFM | Cefixime | - | - |
| CFP | Cefoperazone | - | - |
| CHL | Chloramphenicol | Sensitivity | ≤ 4 |
| CID | Cefonicid | - | - |
| CIN | Cinoxacin | - | - |
| CIP | Ciprofloxacin | Sensitivity | 0.25 |
| CL | Colistin | Resistance | > 2 |
| CMZ | Cefmetazole | - | - |
| CPR | Cefprozil | - | - |
| CPX | Cefpodoxime proxetil | - | - |
| CRB | Carbenicillin | - | - |
| CRO | Ceftriaxone | - | - |
| CSL | Cefoperazone/Sulbactam | - | - |
| CTT | Cefotetan | - | - |
| CTX | Cefotaxime | Sensitivity | ≤ 1 |
| CXM | Cefuroxime | - | - |
| CZO | Cefazolin | Resistance | > 16 |
| CZX | Ceftizoxime | - | - |
| DOX | Doxycycline | - | - |
| ETP | Ertapenem | - | - |
| FEP | Cefepime | Sensitivity | ≤ 2 |
| FLE | Fleroxacin | - | - |
| FOS | Fosfomycin | - | - |
| FOX | Cefoxitin | - | - |
| GAT | Gatifloxacin | - | - |
| GEN | Gentamicin | Sensitivity | ≤ 2 |
| IPM | Imipenem | Sensitivity | ≤ 1 |
| KAN | Kanamycin | - | - |
| LOM | Lomefloxaccin | - | - |
| LOR | Loracarbef | - | - |
| LVX | Levofloxacin | Sensitivity | 0.5 |
| MAN | Cefamandole | - | - |
| MEC | Mecillinam | - | - |
| MEM | Meropenem | Sensitivity | ≤ 1 |
| MEZ | Mezlocillin | - | - |
| MFX | Moxifloxacin | Mediation | 4 |
| MNO | Minocycline | - | - |
| MOX | Moxalactam | - | - |
| NET | Netilmicin | - | - |
| NIT | Nitrofurantoin | - | - |
| NOR | Norfloxacin | - | - |
| OFX | Ofloxacin | - | - |
| PIP | Piperacillin | Sensitivity | ≤ 4 |
| SAM | Ampicillin-Sulbactam | Sensitivity | ≤ 4/2 |
| SSS | Sulfonamides | - | - |
| STR | Streptomycin | - | - |
| SXT | Trimethoprim-Sulfamethoxazole | Sensitivity | ≤ 0.5/9.5 |
| TCC | Ticarcillin/Clavulanic | - | - |
| TCY | Tetracycline | Resistance | ≤ 2 |
| TIC | Ticarcillin | - | - |
| TMP | Trimethoprim | - | - |
| TOB | Tobramycin | - | - |
| TZP | Piperacillin-Tazobactam | Sensitivity | ≤ 4/4 |
| Code | Antimicrobial | Result | MIC |
| AMC | Amoxicillin/Clavulanic | Resistance | > 4/2 |
| AMP | Ampicillin | Resistance | > 8 |
| AZM | Azithromycin | - | - |
| CHL | Chloramphenicol | - | - |
| CIP | Ciprofloxacin | Resistance | > 4 |
| CLI | Clindamycin | - | - |
| CLR | Clarithromycin | - | - |
| DAP | Daptomycin | - | - |
| DOX | Doxycycline | - | - |
| ERY | Erythromycin | Resistance | > 4 |
| GEN | Gentamicin | Resistance | > 8 |
| LNZ | Linezolid | Sensitivity | 1 |
| LVX | Levofloxacin | - | - |
| MFX | Moxifloxacin | - | - |
| MUH | Mupirocin | Resistance | > 256 |
| NIT | Nitrofurantoin | Sensitivity | ≤ 16 |
| OFX | Ofloxacin | - | - |
| OXA | Oxacillin | Resistance | > 2 |
| PEN | Penicillin | Resistance | > 0.25 |
| QDA | Quinupristin-Dalfopristin | - | - |
| RA | Rifampin | Sensitivity | ≤ 0.5 |
| RIF | Rifampin | ||
| SXT | Trimethoprim-Sulfamethoxazole | Sensitivity | ≤ 1/19 |
| TCY | Tetracycline | Resistance | > 8 |
| TEC | Teicoplanin | Sensitivity | 4 |
| TLT | Telithromycin | - | - |
| TMP | Trimethoprim | Sensitivity | 2 |
| VAN | Vancomycin | Sensitivity | 2 |
Lung radiographs and computed tomography showed exudative changes in both lungs. The left lower limb ultrasound showed a dark fluid area. Abdominal ultrasonography revealed chronic liver disease.
The patient did not undergo a multidisciplinary consultation.
Polybacterial gas gangrene.
The emergency operation was performed immediately under combined lumbar and epidural anesthesia; a left leg expansion and exploration (August 10, 2021). After surgery, we continued to use piperacillin sodium tazobactam sodium 4.5 g every 8 h and levofloxacin 0.5 g for anti-infection treatment. The patient underwent further surgery under lumbar anesthesia on August 17, 2021, and again on August 31, 2021, including left leg deactivation and skin grafting, negative pressure closed drainage and right thigh skin removal.
After treatment, the transplanted flap survived (Figure 2). The patients’ infection indicators, CRP and Procalcitonin, decreased gradually and returned to normal on September 1, 2021 (Figure 3 and 4).
Although V. vulnificus infections are rare, V. vulnificus is responsible for the largest number of vibrio deaths[8,9]. Recent surveillance by the Centers for Disease Control and Prevention shows that the geographic area affected by V. vulnificus is expanding and that infection rates are rising worldwide due to global warming and rising ocean temperatures[10,11].
Infections caused by V. vulnificus were first reported by Hollis et al[12] in 1976. V. vulnificus has the highest case fatality rate among all foodborne pathogens. Infections spread extremely rapidly, with an average incubation period of only 16 h for wound infection and 26 h for septicemia[13]. A case series study from South Korea reported that the incubation period for sepsis ranged from 3 h to 6 d[14].
Primary V. vulnificus sepsis is a serious disease with a high mortality rate. V. vulnificus has the highest case fatality rate (39%) of all reported foodborne infections in the United States, with a fatality rate of more than 90% in cases with existing hypotension at the time of presentation[15,16]. In Japan, the majority of cases of V. vulnificus infection manifest as primary septicemia, with a mortality rate of up to 70%, and more than one-half of the patients die within 3 d[17]. Researchers have found that the longer the interval between the onset and the initiation of antimicrobial therapy, the higher the mortality rate of V. vulnificus sepsis and severe wound infection[18]. Although patients with V. vulnificus infection can fully recover, complications associated with multiple organ system failure may persist. Therefore, it is very important to recognize this disease and diagnose it correctly as soon as possible.
However, the identification of microorganisms by culturing methods usually takes 7-10 d or longer[19]. In addition, many microorganisms require specific growth conditions that are difficult to simulate in a laboratory environment, and microbial culture methods may not always detect pathogenic agents[20,21]. Using blood samples from patients with skin and soft tissue infections, C-polymerase chain reaction (PCR) and N-PCR were found to be 45% and 86% sensitive to V. vulnificus target gene toxicity R gene, respectively. Previous studies have reported that Q-PCR detection of V. vulnificus-specific genes is the most sensitive and specific technology and the fastest diagnostic method at present[5]. In this study, we propose that mNGS is a promising new diagnostic technique that can theoretically identify all known microbial genomes from clinical specimens. This analysis is usually performed in a short period of time (24-36 h)[22].
The traditional medical treatment for V. vulnificus infection is third-generation cephalosporins combined with tetracycline or fluoroquinolones. However, as a result of the excessive use of antibiotics in human, agriculture, and aquaculture systems, antibiotic resistance has emerged and evolved in many bacterial genera, including Vibrio, during the past few decades[23,24]. A case-series study of 121 Taiwanese patients presenting with necrotizing fasciitis found that surgical treatment within 12 h of admission resulted in significantly improved survival[25]. Of the 423 V. vulnificus wound infections reported in the United States, 10% required some type of amputation. However, some patients still die after undergoing surgical treatment[26]. In 1992, Chuang et al[27] reviewed 28 cases of V. vulnificus infection in 27 patients in Taiwan. They argued that clearing the infected area within the first 24 h was crucial because most patients died within 48 h of being hospitalized.
In this study, the patient developed a skin infection of the left lower limb 3 mo prior to admission, and a hemorrhagic blister with fever had suddenly appeared at the site of the original skin infection 3 d prior. The infection progressed rapidly with a tendency toward multiple organ failure. We found V. vulnificus, a highly lethal pathogen of skin infection, in time through the mNGS method. Meanwhile, Staphylococcus hemolyticus, Staphylococcus aureus, Streptococcus dysgalactiae, Fusobacterium necrophorum and Klebsiella were also detected through mNGS (Table 3). This result suggested that the patient had a mixed infection with multiple pathogenic bacteria. Therefore, we quickly chose antibiotic treatment covering V. vulnificus and other pathogens, followed by immediate surgical debridement and treatment resulting in effective control of the patient’s infection and avoiding amputation and even death.
| Type | Genera | Number of sequences | Species | Number of sequences |
| G+ | Staphylococcus | 7166 | Staphylococcus haemolyticus | 6493 |
| Staphylococcus aureus | 88 | |||
| G- | Vibrio | 7026 | Vibrio vulnificus | 6491 |
| G+ | Streptococcus | 973 | Streptococcus dysgalactiae | 664 |
| G- | Fusobacterium | 43 | Fusobacterium necrophorum | 40 |
| G- | Klebsiella | 11 | Klebsiella aerogenes | 10 |
Therefore, this report raises awareness among dermatologists that when a patient is clinically suspected of a special type of pathogenic infection, the mNGS method can be preferred to identify the patient’s pathogen infection as soon as possible, and then take effective treatment measures in time, which can benefit the patient to a greater extent and even save the patient’s life.
In conclusion, we reported a case of V. vulnificus detected by mNGS in a 53-year-old male patient with polymicrobial gas gangrene and successful treatment by surgery. This patient was successfully treated with surgery, while amputation or even death was avoided. The main benefit was that we were able to confirm the diagnosis of V. vulnificus infection within 24 h through mNGS detection. We immediately performed emergency surgery which helped gain precious time to save the patient’s life. Conversely, the traditional method of bacterial and fungal culture has a low positive rate, a small detection range and is time-consuming.
We would like to thank Precision Medicine Center of Nanfang Hospital for doing mNGS and analysis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chang CP, Taiwan; Paparoupa M, Germany S-Editor: Wei ZH L-Editor: Filipodia P-Editor: Cai YX
| 1. | Daniels NA. Vibrio vulnificus oysters: pearls and perils. Clin Infect Dis. 2011;52:788-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | Dickerson J Jr, Gooch-Moore J, Jacobs JM, Mott JB. Characteristics of Vibrio vulnificus isolates from clinical and environmental sources. Mol Cell Probes. 2021;56:101695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Huang KC, Weng HH, Yang TY, Chang TS, Huang TW, Lee MS. Distribution of Fatal Vibrio Vulnificus Necrotizing Skin and Soft-Tissue Infections: A Systematic Review and Meta-Analysis. Medicine (Baltimore). 2016;95:e2627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Chuang PY, Yang TY, Huang TW, Tsai YH, Huang KC, Weng HH. Hepatic disease and the risk of mortality of Vibrio vulnificus necrotizing skin and soft tissue infections: A systematic review and meta-analysis. PLoS One. 2019;14:e0223513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Hori M, Nakayama A, Kitagawa D, Fukushima H, Asai H, Kawai Y, Okuchi K. A case of Vibrio vulnificus infection complicated with fulminant purpura: gene and biotype analysis of the pathogen. JMM Case Rep. 2017;4:e005096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Kim K, Kim NJ, Kim SY, Kim IH, Kim KS, Lee GR. Cyclo(Phe-Pro) produced by the human pathogen Vibrio vulnificus inhibits host innate immune responses through the NF-κB pathway. Infect Immun. 2015;83:1150-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Bhat P, Bhaskar M, Sistla S, Kadhiravan T. Fatal case of necrotising fasciitis due to Vibrio vulnificus in a patient with alcoholic liver disease and diabetes mellitus. BMJ Case Rep. 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Kang SJ, Jung SI, Peck KR. Historical and Clinical Perspective of Vibrio vulnificus Infections in Korea. Infect Chemother. 2020;52:245-251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Jayakumar JM, Shapiro OH, Almagro-Moreno S. Improved Method for Transformation of Vibrio vulnificus by Electroporation. Curr Protoc Microbiol. 2020;58:e106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Heng SP, Letchumanan V, Deng CY, Ab Mutalib NS, Khan TM, Chuah LH, Chan KG, Goh BH, Pusparajah P, Lee LH. Vibrio vulnificus: An Environmental and Clinical Burden. Front Microbiol. 2017;8:997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 11. | Lee MT, Dinh AQ, Nguyen S, Krucke G, Tran TT. Late-onset Vibrio vulnificus septicemia without cirrhosis. Proc (Bayl Univ Med Cent). 2019;32:286-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Hollis DG, Weaver RE, Baker CN, Thornsberry C. Halophilic Vibrio species isolated from blood cultures. J Clin Microbiol. 1976;3:425-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 161] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Huang KC, Tsai YH, Huang KC, Lee MS. Model for end-stage liver disease (MELD) score as a predictor and monitor of mortality in patients with Vibrio vulnificus necrotizing skin and soft tissue infections. PLoS Negl Trop Dis. 2015;9:e0003720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Griffin PM, Tauxe RV. Food-related illness and death in the United States. Emerg Infect Dis. 1999;5:607-625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4755] [Cited by in RCA: 4079] [Article Influence: 156.9] [Reference Citation Analysis (0)] |
| 15. | Liu JW, Lee IK, Tang HJ, Ko WC, Lee HC, Liu YC, Hsueh PR, Chuang YC. Prognostic factors and antibiotics in Vibrio vulnificus septicemia. Arch Intern Med. 2006;166:2117-2123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Otomo S, Maekawa K, Goto T, Baba T, Yoshitake A. Pre-existing cerebral infarcts as a risk factor for delirium after coronary artery bypass graft surgery. Interact Cardiovasc Thorac Surg. 2013;17:799-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Kim HS, Kim DM, Neupane GP, Lee YM, Yang NW, Jang SJ, Jung SI, Park KH, Park HR, Lee CS, Lee SH. Comparison of conventional, nested, and real-time PCR assays for rapid and accurate detection of Vibrio vulnificus. J Clin Microbiol. 2008;46:2992-2998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Kuo Chou TN, Chao WN, Yang C, Wong RH, Ueng KC, Chen SC. Predictors of mortality in skin and soft-tissue infections caused by Vibrio vulnificus. World J Surg. 2010;34:1669-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Raghuram AC, Yu RP, Lo AY, Sung CJ, Bircan M, Thompson HJ, Wong AK. Role of stem cell therapies in treating chronic wounds: A systematic review. World J Stem Cells. 2020;12:659-675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (2)] |
| 20. | Sawaya AP, Jozic I, Stone RC, Pastar I, Egger AN, Stojadinovic O, Glinos GD, Kirsner RS, Tomic-Canic M. Mevastatin promotes healing by targeting caveolin-1 to restore EGFR signaling. JCI Insight. 2019;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Omar A, Wright JB, Schultz G, Burrell R, Nadworny P. Microbial Biofilms and Chronic Wounds. Microorganisms. 2017;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 234] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 22. | Rhoads DD, Cox SB, Rees EJ, Sun Y, Wolcott RD. Clinical identification of bacteria in human chronic wound infections: culturing vs. 16S ribosomal DNA sequencing. BMC Infect Dis. 2012;12:321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 23. | Kim SE, Shin SU, Oh TH, Kim UJ, Darboe KS, Kang SJ, Jang HC, Jung SI, Shin HY, Park KH. Outcomes of Third-Generation Cephalosporin Plus Ciprofloxacin or Doxycycline Therapy in Patients with Vibrio vulnificus Septicemia: A Propensity Score-Matched Analysis. PLoS Negl Trop Dis. 2019;13:e0007478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Tang HJ, Chen CC, Lai CC, Zhang CC, Weng TC, Chiu YH, Toh HS, Chiang SR, Yu WL, Ko WC, Chuang YC. In vitro and in vivo antibacterial activity of tigecycline against Vibrio vulnificus. J Microbiol Immunol Infect. 2018;51:76-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Chao WN, Tsai CF, Chang HR, Chan KS, Su CH, Lee YT, Ueng KC, Chen CC, Chen SC, Lee MC. Impact of timing of surgery on outcome of Vibrio vulnificus-related necrotizing fasciitis. Am J Surg. 2013;206:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 26. | Dechet AM, Yu PA, Koram N, Painter J. Nonfoodborne Vibrio infections: an important cause of morbidity and mortality in the United States, 1997-2006. Clin Infect Dis. 2008;46:970-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 27. | Chuang YC, Yuan CY, Liu CY, Lan CK, Huang AH. Vibrio vulnificus infection in Taiwan: report of 28 cases and review of clinical manifestations and treatment. Clin Infect Dis. 1992;15:271-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 144] [Article Influence: 4.4] [Reference Citation Analysis (0)] |