Published online Dec 16, 2022. doi: 10.12998/wjcc.v10.i35.13006
Peer-review started: August 27, 2022
First decision: November 4, 2022
Revised: November 17, 2022
Accepted: November 23, 2022
Article in press: November 23, 2022
Published online: December 16, 2022
Processing time: 108 Days and 16.7 Hours
Lung cancer is the leading cause of cancer-related death. Early diagnosis is critical to improving a patient’s chance of survival. However, lung cancer associated with cystic airspaces is often misdiagnosed or underdiagnosed due to the absence of clinical symptoms, poor imaging specificity, and high risk of biopsy-related complications.
We report an unusual case of cancer in a 55-year-old man, in which the lesion evolved from a small solitary thin-walled cyst to lung squamous cell carcinoma (SCC) with metastases in both lungs. The SCC manifested as rare clustered cystic lesions, detected on chest computed tomography. There were air-fluid levels, compartments, and bronchial arteries in the cystic lesions. Additionally, there was no clear extrathoracic metastasis. After chemotherapy, the patient achieved a partial response, type I respiratory failure was relieved, and the lung lesions became a clustered thin-walled cyst.
Pulmonary cystic lesions require regular imaging follow-up. Lung SCC should be a diagnostic consideration in cases of thin-walled cysts as well as multiple clustered cystic lesions.
Core Tip: Lung cancer associated with cystic airspaces is often delayed diagnosis. We report a case in which the lesion evolved from a thin-walled cyst to advanced lung squamous cell carcinoma (SCC). The SCC manifested as rare clustered cystic lesions in bilateral lungs. There are air-fluid levels, compartments and bronchial arteries in the cyst. Additionally, there was no obvious extrathoracic metastasis. After chemotherapy, the lesions became clustered thin-walled cysts. Our report highlights SCC should be a diagnostic consideration in thin-walled cyst as well as multiple clustered cystic lesions.
- Citation: Shen YY, Jiang J, Zhao J, Song J. Lung squamous cell carcinoma presenting as rare clustered cystic lesions: A case report and review of literature. World J Clin Cases 2022; 10(35): 13006-13014
- URL: https://www.wjgnet.com/2307-8960/full/v10/i35/13006.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i35.13006
Lung cancer is the leading cause of cancer-related death worldwide[1]. Early diagnosis is critical to improving a patient’s chance of survival. Low-dose computed tomography (CT) has become an important method for the early screening of lung cancer[2]. On imaging, it typically presents as a mass or nodule. Lung cancer associated with cystic airspaces (LCCA) was first described by Anderson and Pierce in 1954[3]. It is a special form of lung cancer, which is rare in clinical practice, with a reported overall prevalence of 0.46% in a surgical series and 3.7% in a lung cancer–screening cohort[4]. The most common histology was adenocarcinoma, followed by squamous cell carcinoma (9.1%)[5]. Its diagnosis is very challenging and it is increasingly recognized as a cause of delayed diagnosis[6].
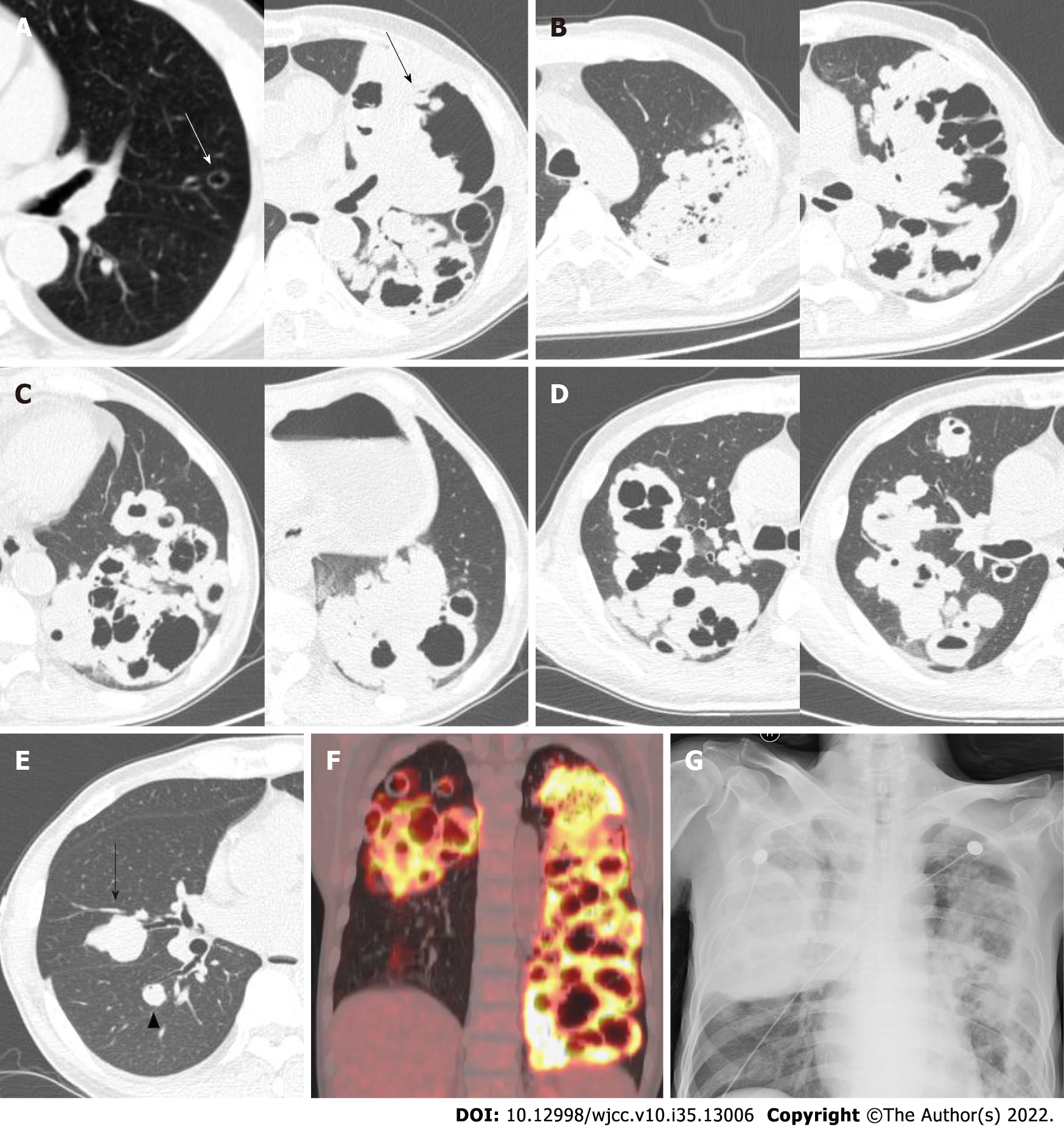
We report an unusual case of lung cancer in which the lesion evolved from a solitary thin-walled cyst to bilateral clustered cystic lesions in 4 years (Figure 1A-E). Additionally, there was no obvious extrathoracic metastasis. After chemotherapy, the lesions became clustered thin-walled cysts.
A 55-year-old man was transferred to our department with complaints of dry cough and dyspnea for 6 mo without fever, chills, or chest pain, accompanied by fatigue, anorexia, and weight loss.
The patient was admitted to a local hospital and chest enhanced CT revealed multiple cavities and masses in both lungs. CT-guided percutaneous lung biopsy was performed, but only necrotic tissue was found. He was referred to our hospital in May 2019.
The patient had hypertension, for which he was treated with telmisartan (40 mg once daily). He also suffered from diabetes, for which he was treated with gansulin30R (30/70 mixed recombinant insulin, 24 U before breakfast and 20 U before dinner) and acarbose (50 mg three times a day).
The patient had a 76-pack year history of smoking but had stopped smoking 6 mo prior to hospital admission. He also had a long history of alcohol abuse. His family history was unremarkable.
Vital signs on arrival were unremarkable, except for a mild decrease in blood pressure (to 109/59 mmHg). There was no enlargement of superficial lymph nodes and no rales on auscultation of the lungs.
The results of tumor biomarker tests for neuron-specific enolase and serum cytokeratin 19 fragments (CYFRA21-1) showed an abnormal increase to 32.06 ng/mL and 101.3 ng/mL, respectively. Levels of other relevant serum tumor markers (carcinoembryonic antigen, squamous cell carcinoma (SCC) antigen, carbohydrate antigen 19-9 (CA 19-9), cancer antigen 125, and pro-gastrin-releasing peptide) were normal. Arterial blood gas analysis demonstrated type I respiratory failure (PaO2 60 mmHg, PaCO2 32.1 mmHg, and pH 7.44, with oxygen 3 L/min via nasal catheter).
The other laboratory results were as follows: white blood cell count of 6.6 × 109/L; hemoglobin of 106 g/L; platelet count of 455 × 109/L; C-reactive protein of 70.5 mg/L; erythrocyte sedimentation rate of 34 mm/h; and procalcitonin of 0.108 ng/mL. Blood biochemical index results showed that albumin decreased to 31.9 g/L (reference range: 40.0–55.0 g/L), creatine kinase decreased to 15 U/L (reference range: 50-310 U/L), and blood urea nitrogen decreased to 2.59 mmol/L (reference range: 3.10-8.00 mmol/L). The result of the tuberculosis-specific enzyme-linked immunospot assay was positive. The 1,3-beta-D-glucan test (G test), galactomannan test (GM test), and cryptococcal capsular antigen test results were negative. Anti-DNA and anti-nuclear antibodies were not detected.
The patient underwent a bronchoscopy examination with bronchoalveolar lavage fluid (BALF) collection on day 3 of admission. BALF Gram stain, acid-fast stain, Mycobacterium tuberculosis DNA detection, and the G and GM tests were negative. In addition, BALF culturing for fungi and bacteria showed no organisms.
Positron emission tomography-CT revealed an elevated standardized uptake value (SUV) of 13.3 in multiple round nodules and irregular cavities in both lungs, as well as mildly enlarged lymph nodes in the mediastinum and left axilla but low SUV (Figure 1F).
Percutaneous lung biopsy was performed again, and the pathology showed poorly differentiated lung SCC (Figures 2A and B).
After diagnosis, six cycles of systemic chemotherapy were administered, consisting of carboplatin (area under the concentration time-curve 5) and paclitaxel 150 mg/m2 every 21 d.
Tumor assessment showed partial response (Response Evaluation Criteria in Solid Tumors 1.1) (Figure 3). The patient’s symptoms, including cough and dyspnea, were relieved. The patient discontinued maintenance treatment, immunotherapy, and regular imaging follow-up. He was hospitalized again at 8 mo after his first chemotherapy treatment and complained of dyspnea for 1 mo. The examination indicated type I respiratory failure, and the bedside chest radiograph showed a mass in the right lung and bilateral cavities (Figure 1G). He was treated with the best supportive care with clinical improvement but eventually died of hemoptysis 1 mo later.
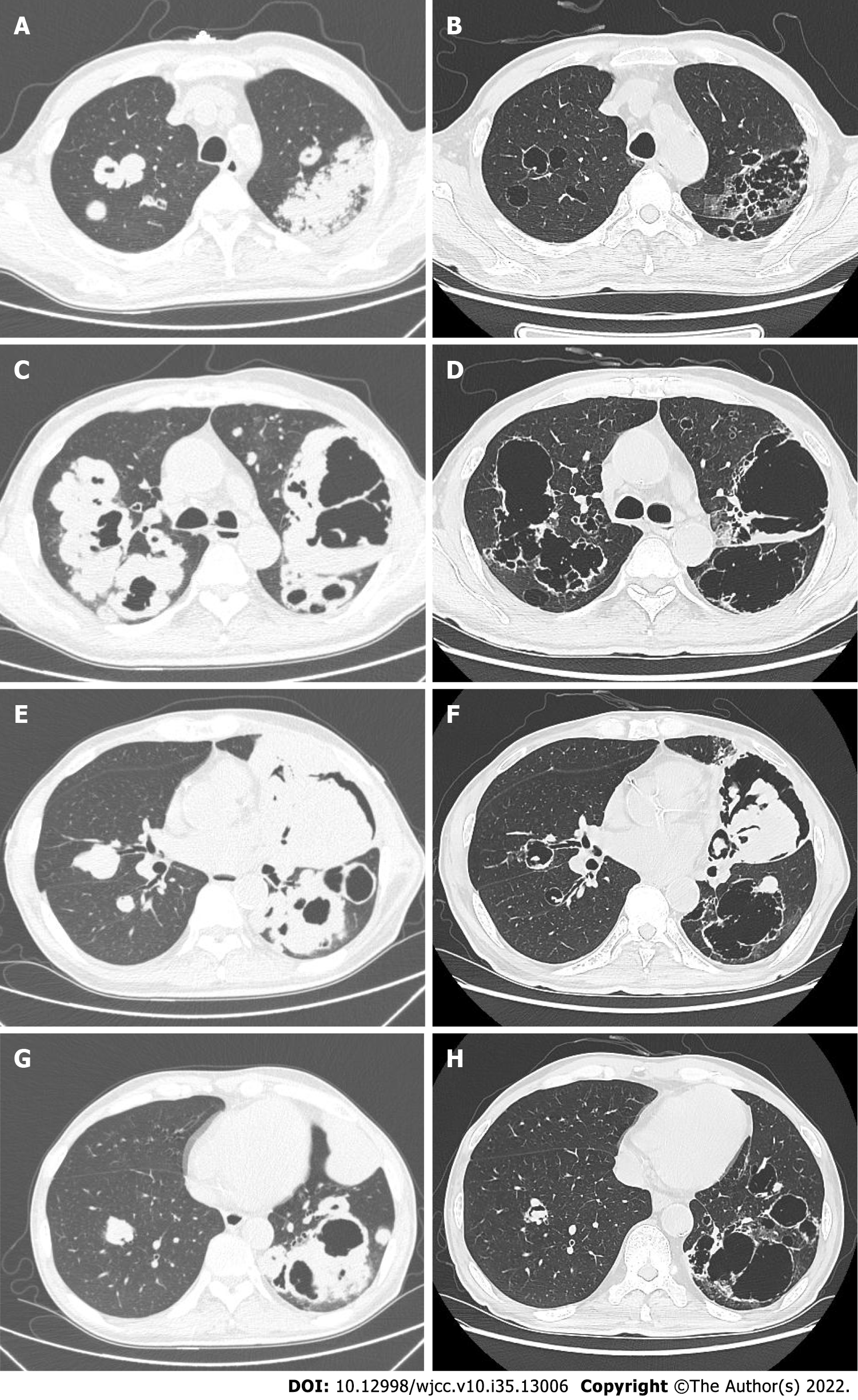
Upon review of the patient’s electronic medical records, it was found that a chest CT was performed in March 2015. Interestingly, no abnormality in the lungs or mediastinum had been observed, except a well-defined thin-walled cyst in the left lingual segment of about 10.5 mm × 7.5 mm in size. He was asymptomatic and did not have a follow-up CT. Four years later, a thick-walled cavity with a diameter of 125 mm × 80 mm and height of 189 mm had formed in the left upper lobe, and multiple clustered cystic lesions and masses had developed with bilateral lung involvement. The cystic lesions had air-fluid levels (Figures 1D and 3C), compartments (Figures 1 and 3), and bronchial arteries (Figure 2C-E).
We report a rare case in which a lung SCC changed over time from a small cyst to bilateral dissemination. Four years before onset, only a well-defined thin-walled cystic lesion in the left upper lobe was found on chest CT. To the best of our knowledge, a case of lung cancer presenting as clustered cystic lesions with such extensive bilateral dissemination has not been reported.
The definition of the cyst was first standardized by the Fleischner Society in 1996 and was updated in 2008[7]. It was defined as a round parenchymal lucency consisting of a well-defined interface with normal lungs. Cysts are commonly seen on CT scans, and their differential diagnosis is challenging. Previous research has indicated that LCCA is an important cause of missed diagnosis[8,9] due to the morphology being difficult to distinguish from benign diseases and the fact that morphology may be unchanged for prolonged periods[6]. Patients usually present with either no symptoms, with the cysts discovered on chest imaging for another reason, or with nonspecific symptoms such as cough and shortness of breath[10]. Moreover, surrounding cystic areas render patients at high risk for biopsy-related complications, such as pneumothorax, and also pose diagnostic challenges for lung cancer.
However, LCCA has been increasingly identified in lung cancer screening programs[11]. Guo et al[4] identified 15 cases of LCCA from 3268 surgical resections of primary lung cancer and 306 cases of benign cysts during a period of 5.5 years. The findings suggested a rate of malignancy in pulmonary cysts’ airspaces of 4.7% (15 cancers in 321 cysts). Mascalchi et a[12] classified LCCA into four types. Type I corresponds to the presence of a nodule extruding from the cystic airspace wall. Type II corresponds to a nodule confined within the lumen of the cystic airspace. Type III corresponds to a soft tissue density extending along the wall of the cystic airspace. And, type IV corresponds to solid or nonsolid tissue intermixed within a cluster of multiple cystic airspaces. Shen et al[13] classified LCCA as thin-walled (I), thick-walled (II), cystic lesion with a mural nodule (either endophytic or exophytic; III), or multiloculated with nodular components (IV). Woodring et al[14] reported that cystic airspaces with maximum wall thicknesses ≤ 4 mm were mostly benign, whereas those with maximum wall thicknesses > 15 mm were mostly malignant.
Several studies have shown that LCCA is more common in adenocarcinoma[13,15,16]. Mendoza et al[5] found that adenocarcinoma was the most common histological type of LCCA, with a frequency of 88.1% (289/328), followed by squamous cell carcinoma, with a frequency of 9.1% (30/328). The pathogenesis of cyst formation is considered to be due to two main mechanisms: Central necrosis within the nodule and check-valve obstruction at the terminal bronchiolar level[15,16].
Early identification of any focal lesion is crucial because cancer is typically curable when it is in the early stage[17]. Tumor biopsy is the gold standard for lung cancer diagnosis, but the early lesions with few solid components, LCCA lesions are prone to pneumothorax and other biopsy-related complications, which limit the application of biopsy. Based on the results of The National Lung Cancer Screening Trial, low-dose CT screening of heavy smokers has been recommended by the major American and European scientific societies[18-21]. Furthermore, the unpredictable growth rate of lung cancer, which ranges from indolent to aggressive cancers, necessitates attention to the wide spectrum of progression in lung cancer appearance on serial CT scans[22]. The role of 18F-FDG-PET to differentiate between benign and malignant cystic airspace lesions is very limited, since infectious (including fungal) diseases, inflammatory abnormalities, and granulomatous diseases can also show high uptake[23,24].
Several studies have suggested that cystic airspaces indicative of lung cancer usually develop wall thickening and/or mural nodularity during follow-up[5,11,12]. LCCA is usually slow growing[25]. Nevertheless, with tumor growth, airspace size can increase, decrease, or remain unchanged[5,12].
The drawback of low-dose computed tomography screening is the presence of uncertainties about high costs, risk of radiation exposure, and false positives observed in the screening population[26]. And late stage cancers still emerge between screening intervals[9]. Liquid biopsy has emerged as a promising tool for the early diagnosis and management of lung cancer due to its non-invasive sampling, easily repeatable, and economic[27]. Liquid biopsy biomarkers include cell-free DNA, circulating tumor DNA, microRNA, exosomes, and circulating tumor cells. The key issue is the sensitivity and specificity of detection for application to early diagnosis. Several studies underline the importance of integrating different molecular technologies with imaging, radionics, and artificial intelligence to improve the sensitivity and specificity of early diagnosis[28,29].
Unfortunately, in our study, the solitary cyst did not attract clinical suspicion, and the patient did not undergo regular imaging follow-up.
Despite advances in screening, detection, molecular classification, and therapy, a substantial proportion of individuals who initially present with localized or locoregional disease eventually succumb to recurrent malignancy[30]. Local recurrence is seen in 13%-24% of patients after curative resection[31,32] and distant recurrence is reported to be the most common type of the first recurrence[32]. The bone, lung, brain, adrenals, and liver are the most frequent sites of lung cancer metastasis. Occasionally, metastases of lung cancer can be found in the soft tissue, such as the shoulder[33], back muscles[34], and subcutaneous[35].
When looking up the recurrence of LCCA, there is no definite conclusion. Shen et al[36] evaluated the prognosis by using propensity score matching and found that the LCCA group exhibited a better three-year recurrence-free survival than the non-LCCA group. Shinohara et al[37] reported that patients with lung cancer adjoining pulmonary bullae (LC-AB) exhibit better overall survival than those with non-LC-AB. Kaneda et al[38] stated that LC-AB, even a small lesion, exhibits a poor prognosis. Hanaoka et al[39] reported that postoperative survival of patients with lung carcinoma arising from bullae is comparable to that of patients with lung carcinoma without bullae if the carcinoma is resected in the early stages. In conclusion, the prognosis of LCCA remains controversial because of the rarity of LCCA and inconsistencies in the definitions of LCCA in multiple studies. Interestingly, in the present study, there was widely bilateral lung involvement, while no obvious extrathoracic metastasis.
The lack of continuous imaging follow-up of the cyst in our case proved limiting to our ability to make further comment.
Due to the extremely poor prognosis of advanced lung cancer and the high 5-year survival rate of patients with early-stage surgically resectable lung cancer, early diagnosis is very important. SCC should be considered during the differential diagnosis of solitary cystic lesions as well as multiple clustered cystic lesions. Cystic lesions require long-term imaging follow-up to ensure stability and exclude malignancy if the lesions are not resected.
We thank the patient’s wife for allowing us to use his pictures and clinical data. We also thank the pathology technicians for their efforts in the clinical diagnosis of this patient.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Respiratory system
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hashimoto K, Japan;
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13300] [Cited by in RCA: 15468] [Article Influence: 2578.0] [Reference Citation Analysis (2)] |
| 2. | National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8099] [Cited by in RCA: 7659] [Article Influence: 547.1] [Reference Citation Analysis (0)] |
| 3. | Anderson HJ, Pierce JW. Carcinoma of the bronchus presenting as thin-walled cysts. Thorax. 1954;9:100-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Guo J, Liang C, Sun Y, Zhou N, Liu Y, Chu X. Lung cancer presenting as thin-walled cysts: An analysis of 15 cases and review of literature. Asia Pac J Clin Oncol. 2016;12:e105-e112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Mendoza DP, Heeger A, Mino-Kenudson M, Lanuti M, Shepard JO, Sequist LV, Digumarthy SR. Clinicopathologic and Longitudinal Imaging Features of Lung Cancer Associated With Cystic Airspaces: A Systematic Review and Meta-Analysis. AJR Am J Roentgenol. 2021;216:318-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Sheard S, Moser J, Sayer C, Stefanidis K, Devaraj A, Vlahos I. Lung Cancers Associated with Cystic Airspaces: Underrecognized Features of Early Disease. Radiographics. 2018;38:704-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2471] [Cited by in RCA: 2674] [Article Influence: 157.3] [Reference Citation Analysis (0)] |
| 8. | Horeweg N, Scholten ET, de Jong PA, van der Aalst CM, Weenink C, Lammers JW, Nackaerts K, Vliegenthart R, ten Haaf K, Yousaf-Khan UA, Heuvelmans MA, Thunnissen E, Oudkerk M, Mali W, de Koning HJ. Detection of lung cancer through low-dose CT screening (NELSON): a prespecified analysis of screening test performance and interval cancers. Lancet Oncol. 2014;15:1342-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 259] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 9. | Scholten ET, Horeweg N, de Koning HJ, Vliegenthart R, Oudkerk M, Mali WP, de Jong PA. Computed tomographic characteristics of interval and post screen carcinomas in lung cancer screening. Eur Radiol. 2015;25:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Raoof S, Bondalapati P, Vydyula R, Ryu JH, Gupta N, Raoof S, Galvin J, Rosen MJ, Lynch D, Travis W, Mehta S, Lazzaro R, Naidich D. Cystic Lung Diseases: Algorithmic Approach. Chest. 2016;150:945-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 11. | Farooqi AO, Cham M, Zhang L, Beasley MB, Austin JH, Miller A, Zulueta JJ, Roberts H, Enser C, Kao SJ, Thorsen MK, Smith JP, Libby DM, Yip R, Yankelevitz DF, Henschke CI; International Early Lung Cancer Action Program Investigators. Lung cancer associated with cystic airspaces. AJR Am J Roentgenol. 2012;199:781-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 12. | Mascalchi M, Attinà D, Bertelli E, Falchini M, Vella A, Pegna AL, Ambrosini V, Zompatori M. Lung cancer associated with cystic airspaces. J Comput Assist Tomogr. 2015;39:102-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Shen Y, Xu X, Zhang Y, Li W, Dai J, Jiang S, Wu T, Cai H, Sihoe A, Shi J, Jiang G. Lung cancers associated with cystic airspaces: CT features and pathologic correlation. Lung Cancer. 2019;135:110-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Woodring JH, Fried AM, Chuang VP. Solitary cavities of the lung: diagnostic implications of cavity wall thickness. AJR Am J Roentgenol. 1980;135:1269-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 153] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Weisbrod GL, Towers MJ, Chamberlain DW, Herman SJ, Matzinger FR. Thin-walled cystic lesions in bronchioalveolar carcinoma. Radiology. 1992;185:401-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Xue X, Wang P, Xue Q, Wang N, Zhang L, Sun J, Wang K, Yang B, Wang J. Comparative study of solitary thin-walled cavity lung cancer with computed tomography and pathological findings. Lung Cancer. 2012;78:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | International Early Lung Cancer Action Program Investigators, Henschke CI, Yankelevitz DF, Libby DM, Pasmantier MW, Smith JP, Miettinen OS. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med. 2006;355:1763-1771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1163] [Cited by in RCA: 1170] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 18. | Detterbeck FC, Mazzone PJ, Naidich DP, Bach PB. Screening for lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e78S-e92S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 332] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 19. | Jaklitsch MT, Jacobson FL, Austin JH, Field JK, Jett JR, Keshavjee S, MacMahon H, Mulshine JL, Munden RF, Salgia R, Strauss GM, Swanson SJ, Travis WD, Sugarbaker DJ. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J Thorac Cardiovasc Surg. 2012;144:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 463] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 20. | Wender R, Fontham ET, Barrera E Jr, Colditz GA, Church TR, Ettinger DS, Etzioni R, Flowers CR, Gazelle GS, Kelsey DK, LaMonte SJ, Michaelson JS, Oeffinger KC, Shih YC, Sullivan DC, Travis W, Walter L, Wolf AM, Brawley OW, Smith RA. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin. 2013;63:107-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 538] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 21. | Kauczor HU, Bonomo L, Gaga M, Nackaerts K, Peled N, Prokop M, Remy-Jardin M, von Stackelberg O, Sculier JP; European Society of Radiology (ESR); European Respiratory Society (ERS). ESR/ERS white paper on lung cancer screening. Eur Respir J. 2015;46:28-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 22. | Rampinelli C, Calloni SF, Minotti M, Bellomi M. Spectrum of early lung cancer presentation in low-dose screening CT: a pictorial review. Insights Imaging. 2016;7:449-459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Sharma P, Mukherjee A, Karunanithi S, Bal C, Kumar R. Potential role of 18F-FDG PET/CT in patients with fungal infections. AJR Am J Roentgenol. 2014;203:180-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Sathekge M, Maes A, Van de Wiele C. FDG-PET imaging in HIV infection and tuberculosis. Semin Nucl Med. 2013;43:349-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Naidich DP, Bankier AA, MacMahon H, Schaefer-Prokop CM, Pistolesi M, Goo JM, Macchiarini P, Crapo JD, Herold CJ, Austin JH, Travis WD. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology. 2013;266:304-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 661] [Cited by in RCA: 727] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 26. | Kinsinger LS, Anderson C, Kim J, Larson M, Chan SH, King HA, Rice KL, Slatore CG, Tanner NT, Pittman K, Monte RJ, McNeil RB, Grubber JM, Kelley MJ, Provenzale D, Datta SK, Sperber NS, Barnes LK, Abbott DH, Sims KJ, Whitley RL, Wu RR, Jackson GL. Implementation of Lung Cancer Screening in the Veterans Health Administration. JAMA Intern Med. 2017;177:399-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 271] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 27. | Li W, Liu JB, Hou LK, Yu F, Zhang J, Wu W, Tang XM, Sun F, Lu HM, Deng J, Bai J, Li J, Wu CY, Lin QL, Lv ZW, Wang GR, Jiang GX, Ma YS, Fu D. Liquid biopsy in lung cancer: significance in diagnostics, prediction, and treatment monitoring. Mol Cancer. 2022;21:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 238] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 28. | Seijo LM, Peled N, Ajona D, Boeri M, Field JK, Sozzi G, Pio R, Zulueta JJ, Spira A, Massion PP, Mazzone PJ, Montuenga LM. Biomarkers in Lung Cancer Screening: Achievements, Promises, and Challenges. J Thorac Oncol. 2019;14:343-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 347] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 29. | Dama E, Colangelo T, Fina E, Cremonesi M, Kallikourdis M, Veronesi G, Bianchi F. Biomarkers and Lung Cancer Early Detection: State of the Art. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 30. | Kay FU, Kandathil A, Batra K, Saboo SS, Abbara S, Rajiah P. Revisions to the Tumor, Node, Metastasis staging of lung cancer (8th edition): Rationale, radiologic findings and clinical implications. World J Radiol. 2017;9:269-279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (4)] |
| 31. | Fedor D, Johnson WR, Singhal S. Local recurrence following lung cancer surgery: incidence, risk factors, and outcomes. Surg Oncol. 2013;22:156-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 32. | Karacz CM, Yan J, Zhu H, Gerber DE. Timing, Sites, and Correlates of Lung Cancer Recurrence. Clin Lung Cancer. 2020;21:127-135.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 33. | Hashimoto K, Nishimura S, Akagi M. Lung Adenocarcinoma Presenting as a Soft Tissue Metastasis to the Shoulder: A Case Report. Medicina (Kaunas). 2021;57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Zhang P, Meng X, Xia L, Xie P, Sun X, Gao Y, Wang S, Zhao X, Yu J. Non-small cell lung cancer with concomitant intramuscular myxoma of the right psoas mimicking intramuscular metastasis: A case report and literature review. Oncol Lett. 2015;10:3059-3063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Sinha N, Niazi M, Diaz-Fuentes G, Duncalf R. An innocent appearing subcutaneous nodule diagnoses a small cell lung cancer in a never-smoker female. Case Rep Oncol Med. 2014;2014:268404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Shen Y, Zhang Y, Guo Y, Li W, Huang Y, Wu T, Jiang G, Dai J. Prognosis of lung cancer associated with cystic airspaces: A propensity score matching analysis. Lung Cancer. 2021;159:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Shinohara S, Sugaya M, Onitsuka T, Machida K, Matsuo M, Kato K, Tanaka F. Impact of the favorable prognosis of patients with lung cancer adjoining bullae. J Thorac Dis. 2018;10:3289-3297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 38. | Kaneda M, Tarukawa T, Watanabe F, Adachi K, Sakai T, Nakabayashi H. Clinical features of primary lung cancer adjoining pulmonary bulla. Interact Cardiovasc Thorac Surg. 2010;10:940-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Hanaoka N, Tanaka F, Otake Y, Yanagihara K, Nakagawa T, Kawano Y, Miyahara R, Li M, Wada H. Primary lung carcinoma arising from emphysematous bullae. Lung Cancer. 2002;38:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |