Published online Nov 26, 2022. doi: 10.12998/wjcc.v10.i33.12337
Peer-review started: July 15, 2022
First decision: September 25, 2022
Revised: October 5, 2022
Accepted: October 26, 2022
Article in press: October 26, 2022
Published online: November 26, 2022
Processing time: 131 Days and 5.4 Hours
The goal of periodontal disease treatment is to completely remove bacteria and promote wound healing. The erbium-doped yttrium aluminum garnet (Er:YAG) laser is commonly used to treat periodontal disease. Advanced platelet-rich fibrin+ (A-PRF+) secrets growth factors that accelerates soft- and hard-tissue regeneration and wound healing. Herein I present 2 cases of patients with oral diseases treated with a combination of Er:YAG laser and A-PRF+.
Case 1 was a female with pocket depth bone loss over 8 mm and infection of tooth 31 and 41, and severe advanced periodontitis with grade III mobility. Case 2 was a male with tooth 22 root end apical swelling and infection and alveolar bony defects. Clinical outcomes were recorded at 6 and 36 mo. In case 1, the Er:YAG laser was used to perform open flap debridement (100 mJ/pulse, 15 Hz) and remove calculus and granulation tissue (50 mJ/pulse, 30 Hz). In case 2 the laser was used to create a semilunar full thickness flap incision (80 mJ/pulse, 20 Hz) and eliminate the pathogen (100 mJ/pulse, 15 Hz). In both patients, A-PRF+ mixed with bone was used to fill bone defects, and A-PRF+ autologous membranes were used to cover tension-free primary flaps. There was no recurrent infection at 36 mo, and tissue regeneration and would healing occurred.
Debridement with an Er:YAG laser followed by treatment with A-PRF+ is effective for the treatment periodontal diseases with bone defects.
Core Tip: Combined treatment with an erbium-doped yttrium aluminum garnet laser and advanced platelet-rich fibrin+ is effective for the management of severe periodontal disease and infection and in alveolar bone defects.
- Citation: Tan KS. Erbium-doped yttrium aluminum garnet laser and advanced platelet-rich fibrin+ in periodontal diseases: Two case reports and review of the literature. World J Clin Cases 2022; 10(33): 12337-12344
- URL: https://www.wjgnet.com/2307-8960/full/v10/i33/12337.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i33.12337
The goal of periodontal disease treatment is to completely remove periodontal pathogens with surgical and/or non-surgical procedures. Conventional scaling and root planing is sufficient to remove pathogens on the teeth surface, but not in the root or cementum[1]. Thus, other methods are needed to eliminate pathogens in the root or cementum.
Phototherapy using lasers is one of the methods used to eliminate harmful substances. The erbium-doped yttrium aluminum garnet (Er:YAG) laser (wavelength 2940 nm) has a high absorption rate in water and thus a low penetration into biological tissues[2-4]. It can be used to create incisions and ablation of hard and soft tissue without thermal injury to surrounding healthy tissue[5,6]. Er:YAG lasers are used to remove periodontopathic bacteria, including Porphyromonas gingivalis (P. gingivalis) and Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans)[7,8], which can be used in periodontal pockets[9,10] and for intrabony socket debridement[11]. In addition, Er:YAG laser treatment induces blood cell attachment[12] and fibrin formation[13] to influence gingival fibroblast adhesion and proliferation of wound healing processes[14,15], and increases osteoblast proliferation to promote new bone formation[16,17].
Periodontal diseases are chronic inflammatory diseases, the tooth-support tissue damage, including atrophy or bone loss, is due to periodontal disease. The clinical measure of periodontal disease is based on bone level and clinical attachment level (CAL), and reduces probing depths (PD)[16,17]. Therefore, regeneration of damaged tooth-supporting tissue is important in periodontal disease treatment. Platelet-rich fibrin (PRF), an autologous platelet concentrates, consisted of 97% platelets and more than 50% leukocytes[18]. It secrets growth factors to promote angiogenesis, cell migration and proliferation of connective tissue[19-21], and increases the bone fill-in of bone defects area[22,23]. PRF can be modified by low speed centrifugation to form the advanced PRF and advanced PRF+ (A-PRF+)[24]. Compared with PRF, A-PRF+ releases greater amounts of growth factors that promote fibroblast migration that directly influences the wound healing process[25,26].
Based on the aforementioned findings, we hypothesized that treatment of severe periodontal disease with an Er:YAG laser to remove pathogens and dental calculus followed by application of A-PRF+ to improve tissue regeneration would provide superior results to other methods. Herein, we present 2 cases of severe periodontal disease with root infections treated with an Er:YAG laser and application of A-PRF+. After 36 mo of follow-up, there were no recurrent infections and tissue regeneration and bone formation were satisfactory.
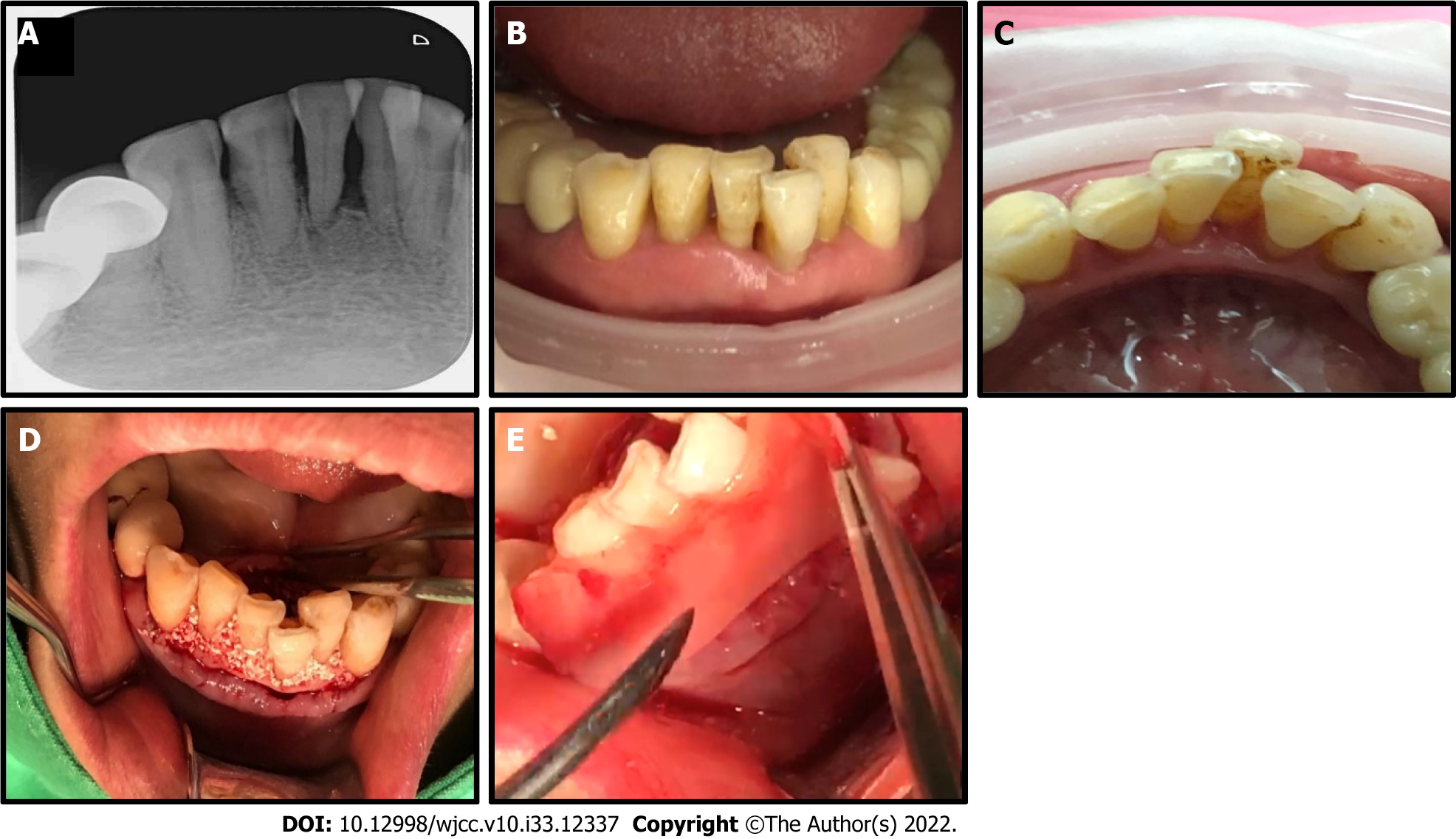
Case 1: A 54-year-old female presented with pocket depth bone loss over 8 mm and infection of tooth 31 and 41, and severe advanced periodontitis with grade III mobility (Figure 1A-C).
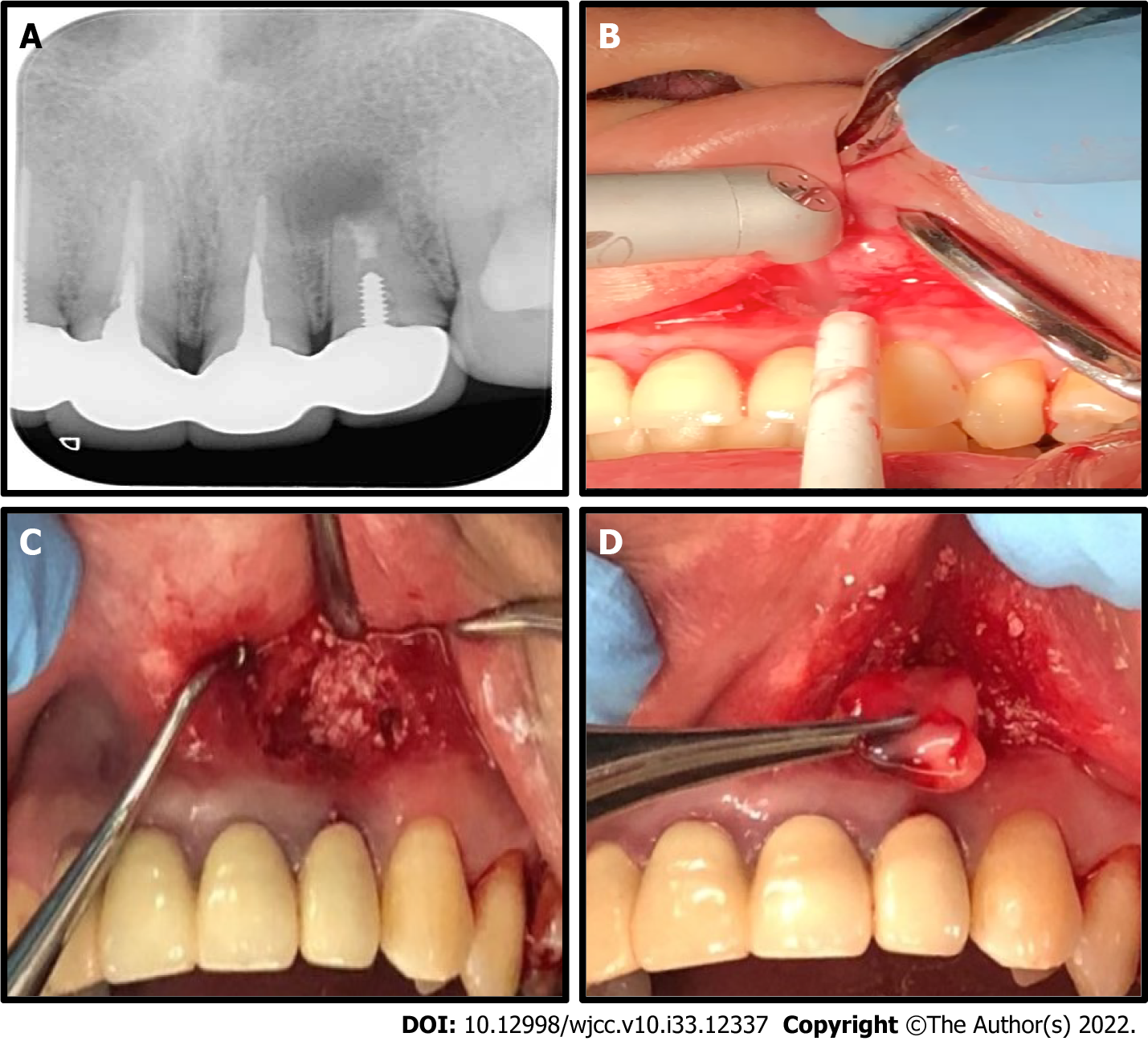
Case 2: A 43-year-old male patient presented tooth 22 root end apical swelling and purulent discharge (Figure 2A).
Case 1: The patient underwent full mouth scaling, and chlorhexidine 0.12% rinses for a week before treatment.
Case 2: The patient had received conventional apicoectomy surgery twice in a nearby general hospital 2 years prior, but swelling, pain, and other symptoms persisted. The patient received amoxicillin 500 mg and scanol 500 mg, 4 times a day, for 3 d before treatment.
The patient had no significant medical history.
The patient had no significant personal or family history.
None.
None.
None.
Periodontal Diseases.
Case 1: An Er:YAG laser (LiteTouch Syneron, Yokneam Elite, Israel) was used to create a full-thickness, tension-free flap with extension of the 2 adjacent teeth mesial and distally. A 17 mm chisel-shape fiber tip was used, and the laser parameters were an energy level of 100 mJ/pulse, repetition rate of 15 Hz (hard tissue/calculus removal mode). The calculus and the granulation tissue on the infected root was also removed with a 17 mm conical-shape fiber tip and the laser parameters were an energy level of 50 mJ/pulse, repetition rate of 30 Hz (soft tissue/periodontal pocket debridement mode). The granulation tissue from the healthy epithelium lining the mucosa in the periodontal pocket was vaporized, followed by decortication of the labial and lingual walls with the aid of 3 × magnification (LiteTouch Syneron, Yokneam Elite, Israel). The buccal and lingual flaps were further advanced using soft brush instruments in order to obtain a better tension-free primary closure.
A-PRF+ was prepared from autologous blood, and extraction was performed following a PRF Instrument kit protocol (Process for PRF, Nice, France). A-PRF+ liquid was mixed with particulate osseous graft material FDBA (allograft, Maxxeus, Kettering, OH, United States) to yield a moldable product, referred to as “sticky bone”. The sticky bone was harvested and compressed into intrabony defects. The labial and lingual root surfaces were covered with a double layer of an A-PRF+ membrane to promote tissue regeneration (Figure 1D and E). Tension-free primary closure was performed using an interrupted and single-sling suture techniques.
Case 2: To remove the apical purulent lesion, a semilunar full-thickness flap incision was made using the Er:YAG laser with a 17 mm chisel-shape fiber tip, set at energy level of 80 mJ/pulse, repetition rate of 20 Hz (soft tissue mode). Since an apicoectomy was done prior, to clean the pathogens the Er:YAG laser with a 17 mm conical-shape fiber tip was set at an energy level of 100 mJ/pulse, repetition rate of 15 Hz to generate a vortex shock in the cavity space via the laser photoacoustic effect (Figure 2B). The surgery was performed with the aid of 3 × magnification (LiteTouch Syneron, Yokneam Elite, Israel).
Sticky bone, consisting of A-PRF+ liquid and FDBA, was inserted and compressed the entire intrabony defect dead space and the periodontal wound was covered with a double layer of A-PRF+ membrane (Process for PRF, Nice, France), then the flap was sutured with simple interrupted sutures in a tension-free manner (Figure 2C and D).
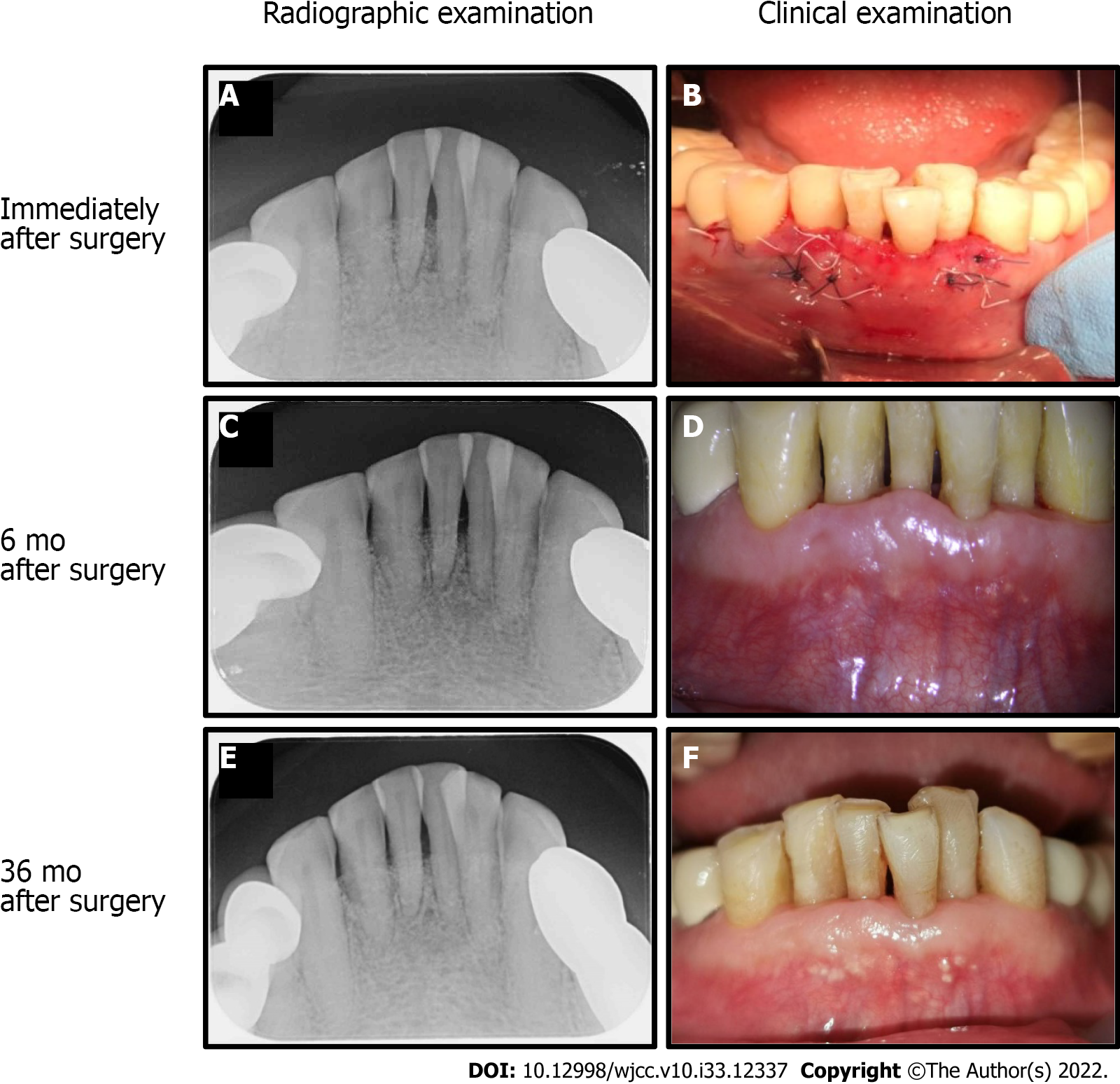
Case 1: Occlusal reduction and tooth splinting were not detected after surgery. Periapical intraoral radiographs were obtained immediately after surgery (Figure 3A and B). At the 6 mo followed, a reduction in PD, gain in CAL, and bone fill-in of the bone defect was observed (Figure 3C and D). At 36 mo, lamina dura appearance and periodontal regeneration were noted (Figure 3E and F).
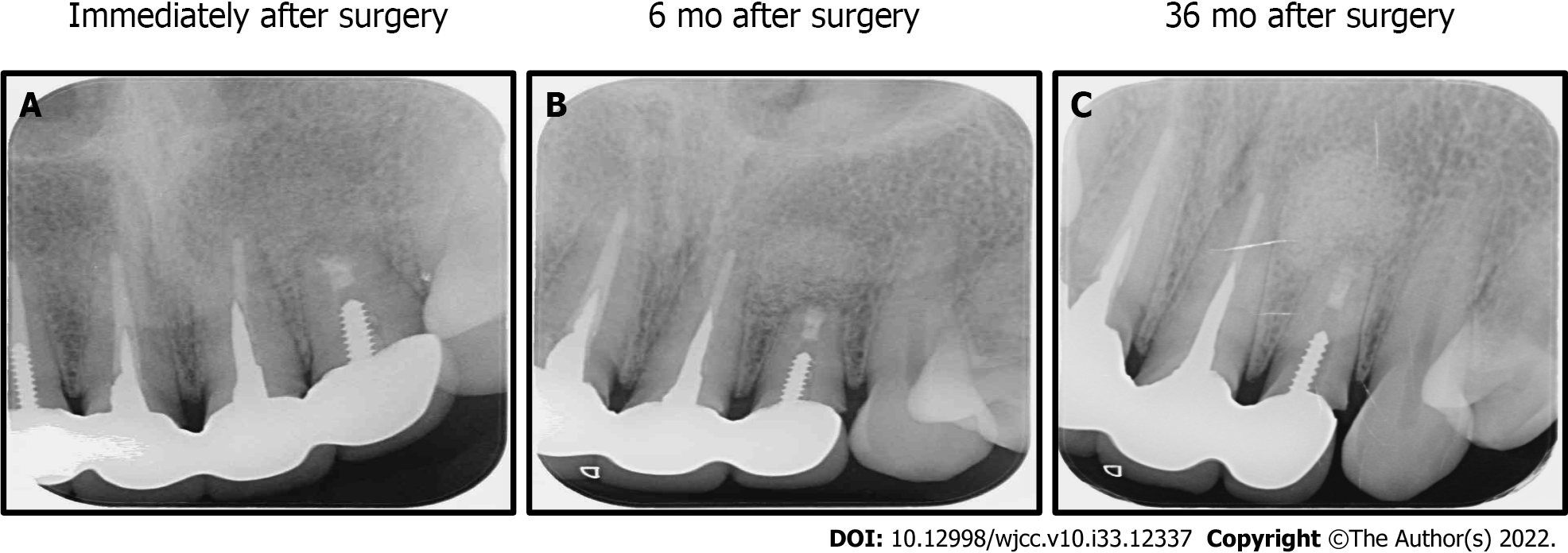
Case 2: Periapical intraoral radiographic were taken immediately after surgery (Figure 4A), and at 6 mo and 36 mo follow-up. At 6 mo there was no root end apical swelling or purulent discharge (Figure 4B). At 36 mo periodontal regeneration and fill-in of the bone defect were observed (Figure 4C).
The photoablative and bactericidal effects of the Er:YAG laser can eliminate the pathogens and the photobiomodulatory effects of low-level laser therapy using an Er:YAG laser promotes new bone formation[16,17]. Treatment with A-PRF+ increases tissue regeneration during the wound healing process[26]. In this study, we described the therapeutic effects of combined treatment using an Er:YAG laser and A-PRF+ for periodontitis and alveolar bony defects. The Er:YAG laser was used to remove pathogens and there was no recurrence in either patient. In case 1, a reduction in PD, gained in CAL, and defect bone fill-in were observed after 6 mo, and lamina dura appearance and periodontal regeneration were observed at 36 mo. In case 2, combined treatment resulted in tissue regeneration and no recurrence of the infection was noted at long-term follow-up. Our results suggest that combined treatment with an Er:YAG laser and A-PRF+ is effective for the management of severe periodontal disease and infection. Combined treatment is a relative “new regeneration” clinical treatment in modern dentistry.
Based on the previous studies and my clinical experience, successful Er:YAG laser therapy is based on the correct adjustment of 9 parameters[27-31]: (1) Energy delivered per pulse; (2) frequency of the pulse; (3) water control; (4) time of exposure; (5) contact or non-contact working distance; (6) angulation of the beam; (7) choice of tips; (8) fiber or non-fiber; and (9) reflected mirrors in Er:YAG laser. For the treatment of soft tissues, the general principle is low energy (mJ), high frequency (Hz), low water pressure, and relatively short time of exposure. Working distance is either contact or non-contact, and angulation of the beam at a 45-degree angle avoids excessive accumulation of energy transmission, scattering, and reflection which can cause surrounding healthy tissue damage. For treating hard tissue, a high energy (mJ), low frequency (Hz), and high water pressure are used. A greater exposure time is required, and angulation of the beam is the same as for treating soft tissues. Different tips can be used for tissue ablation or other purposes according to personal preferences. The fiber or reflected mirror surface inside the handpiece of Er:YAG laser reflects the laser energy, carbonization or damage of the reflected mirror will affect energy transmission which finally reduce the laser output efficiency.
Appropriate suture of the flap is also important for wound healing. After debridement, a precise buccal and lingual side flap should be designed and advanced to release tension in order to subsequently achieve tension-free primary closure. Adequate suture can prevent secondary infection and unexpected soft tissue ingrowth.
After open-flap debridement and treatment with Er:YAG laser, a bone-graft material needs to be applied to the intrabony defect and adjacent root surface to increase the bone level and CAL, and reduce PD[12,14,17,28]. Periodontitis and alveolar bony defects are mainly caused by anaerobic gram (-) bacteria, such as P. gingivalis and A. actinomycetemcomitans[7,8,30,32,33]. The water and air turbine effects of the Er:YAG laser during open flap surgery alter the anaerobic environment of the defect site. In addition, treatment with A-PRF+, enrich growth factors and leukocytes promote angiogenesis, provides oxygen to improve tissue regeneration, and prevention of recurrent infection[25,26].
The present clinical data show that combined treatment with an Er:YAG laser and A-PRF+ is effective for the management of severe periodontal disease and infection and alveolar bone defects. However, more clinical case evaluations are required before promoting further use of combined treatment with Er:YAG laser and A-PRF+.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Dentistry, oral surgery and medicine
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Feng J, China; Gupta A, Nepal; Heboyan A, Armenia S-Editor: Chang KL L-Editor: A P-Editor: Chang KL
| 1. | Adriaens PA, Edwards CA, De Boever JA, Loesche WJ. Ultrastructural observations on bacterial invasion in cementum and radicular dentin of periodontally diseased human teeth. J Periodontol. 1988;59:493-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 190] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 2. | Hale GM, Querry MR. Optical Constants of Water in the 200-nm to 200-microm Wavelength Region. Appl Opt. 1973;12:555-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3107] [Cited by in RCA: 1600] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 3. | Robertson CW, Williams D. Lambert Absorption Coefficients of Water in the Infrared. JOSA. 1971;61:1316-1320. [RCA] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 81] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Zolotarev VM, Mikhailov BA, Alperovich LI, Popov SI. Dispersion and Absorption of Liquid Water in the Infrared and Radio Regions of the Spectrum. Opt Spectosc. 1969;27:430-432. [DOI] [Full Text] |
| 5. | Ishikawa I, Aoki A, Takasaki AA, Mizutani K, Sasaki KM, Izumi Y. Application of lasers in periodontics: true innovation or myth? Periodontol 2000. 2009;50:90-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Visuri SR, Walsh JT Jr, Wigdor HA. Erbium laser ablation of dental hard tissue: effect of water cooling. Lasers Surg Med. 1996;18:294-300. [PubMed] [DOI] [Full Text] |
| 7. | Akiyama F, Aoki A, Miura-Uchiyama M, Sasaki KM, Ichinose S, Umeda M, Ishikawa I, Izumi Y. In vitro studies of the ablation mechanism of periodontopathic bacteria and decontamination effect on periodontally diseased root surfaces by erbium:yttrium-aluminum-garnet laser. Lasers Med Sci. 2011;26:193-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Ando Y, Aoki A, Watanabe H, Ishikawa I. Bactericidal effect of erbium YAG laser on periodontopathic bacteria. Lasers Surg Med. 1996;19:190-200. [PubMed] [DOI] [Full Text] |
| 9. | Folwaczny M, Aggstaller H, Mehl A, Hickel R. Removal of bacterial endotoxin from root surface with Er:YAG laser. Am J Dent. 2003;16:3-5. [PubMed] |
| 10. | Ishikawa I, Sasaki KM, Aoki A, Watanabe H. Effects of Er:YAG laser on periodontal therapy. J Int Acad Periodontol. 2003;5:23-28. [PubMed] |
| 11. | Aoki A, Mizutani K, Schwarz F, Sculean A, Yukna RA, Takasaki AA, Romanos GE, Taniguchi Y, Sasaki KM, Zeredo JL, Koshy G, Coluzzi DJ, White JM, Abiko Y, Ishikawa I, Izumi Y. Periodontal and peri-implant wound healing following laser therapy. Periodontol 2000. 2015;68:217-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 209] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 12. | Cekici A, Maden I, Yildiz S, San T, Isik G. Evaluation of blood cell attachment on Er: YAG laser applied root surface using scanning electron microscopy. Int J Med Sci. 2013;10:560-566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Bolortuya G, Ebihara A, Ichinose S, Watanabe S, Anjo T, Kokuzawa C, Saegusa H, Kawashima N, Suda H. Initial fibroblast attachment to Erbium:YAG laser-irradiated dentine. Int Endod J. 2011;44:1134-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Crespi R, Capparè P, Toscanelli I, Gherlone E, Romanos GE. Effects of Er:YAG laser compared to ultrasonic scaler in periodontal treatment: a 2-year follow-up split-mouth clinical study. J Periodontol. 2007;78:1195-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Feist IS, De Micheli G, Carneiro SR, Eduardo CP, Miyagi S, Marques MM. Adhesion and growth of cultured human gingival fibroblasts on periodontally involved root surfaces treated by Er:YAG laser. J Periodontol. 2003;74:1368-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Aleksic V, Aoki A, Iwasaki K, Takasaki AA, Wang CY, Abiko Y, Ishikawa I, Izumi Y. Low-level Er:YAG laser irradiation enhances osteoblast proliferation through activation of MAPK/ERK. Lasers Med Sci. 2010;25:559-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | Izumi Y, Aoki A, Yamada Y, Kobayashi H, Iwata T, Akizuki T, Suda T, Nakamura S, Wara-Aswapati N, Ueda M, Ishikawa I. Current and future periodontal tissue engineering. Periodontol 2000. 2011;56:166-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Dohan Ehrenfest DM, Del Corso M, Diss A, Mouhyi J, Charrier JB. Three-dimensional architecture and cell composition of a Choukroun's platelet-rich fibrin clot and membrane. J Periodontol. 2010;81:546-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 290] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 19. | Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e45-e50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 692] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 20. | Fujioka-Kobayashi M, J Miron R. Biological Components of Platelet Rich Fibrin: Growth Factor Release and Cellular Activity. In: Miron RJ, Choukroun J. Platelet Rich Fibrin in Regenerative Dentistry: Biological Background and Clinical Indications: Biological Background and Clinical Indications, One. New York: John Wiley & Sons, 2017: 15-31. [DOI] [Full Text] |
| 21. | Kornsuthisopon C, Pirarat N, Osathanon T, Kalpravidh C. Autologous platelet-rich fibrin stimulates canine periodontal regeneration. Sci Rep. 2020;10:1850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Li A, Yang H, Zhang J, Chen S, Wang H, Gao Y. Additive effectiveness of autologous platelet-rich fibrin in the treatment of intrabony defects: A PRISMA-compliant meta-analysis. Medicine (Baltimore). 2019;98:e14759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Panda S, Jayakumar ND, Sankari M, Varghese SS, Kumar DS. Platelet rich fibrin and xenograft in treatment of intrabony defect. Contemp Clin Dent. 2014;5:550-554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Miron RJ, Choukroun J. Future Research with Platelet Rich Fibrin. In: Miron RJ, Choukroun J. Platelet Rich Fibrin in Regenerative Dentistry: Biological Background and Clinical Indications: Biological Background and Clinical Indications, One. New York: John Wiley & Sons, 2017: 251-261. [DOI] [Full Text] |
| 25. | El Bagdadi K, Kubesch A, Yu X, Al-Maawi S, Orlowska A, Dias A, Booms P, Dohle E, Sader R, Kirkpatrick CJ, Choukroun J, Ghanaati S. Reduction of relative centrifugal forces increases growth factor release within solid platelet-rich-fibrin (PRF)-based matrices: a proof of concept of LSCC (low speed centrifugation concept). Eur J Trauma Emerg Surg. 2019;45:467-479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 26. | Fujioka-Kobayashi M, Miron RJ, Hernandez M, Kandalam U, Zhang Y, Choukroun J. Optimized Platelet-Rich Fibrin With the Low-Speed Concept: Growth Factor Release, Biocompatibility, and Cellular Response. J Periodontol. 2017;88:112-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 266] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 27. | Birang R, Yaghini J, Nasri N, Noordeh N, Iranmanesh P, Saeidi A, Naghsh N. Comparison of Er:YAG Laser and Ultrasonic Scaler in the Treatment of Moderate Chronic Periodontitis: A Randomized Clinical Trial. J Lasers Med Sci. 2017;8:51-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Cobb CM, Low SB, Coluzzi DJ. Lasers and the treatment of chronic periodontitis. Dent Clin North Am. 2010;54:35-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Ishikawa I, Aoki A, Takasaki AA. Potential applications of Erbium:YAG laser in periodontics. J Periodontal Res. 2004;39:275-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 122] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Vardhan PK, Paramashivaiah R, Prabhuji MLV, Bhavikatti SK, Basha S, Arora S, Basheer SN, Peeran SW, Aldowah O, Heboyan A. The Effect of Photodynamic Therapy on the Early Outcome of Implants Placed on Patients with Periodontitis. Photonics. 2022;9:480. [RCA] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 31. | Zhou X, Lin M, Zhang D, Song Y, Wang Z. Efficacy of Er:YAG laser on periodontitis as an adjunctive non-surgical treatment: A split-mouth randomized controlled study. J Clin Periodontol. 2019;46:539-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Heboyan A, Manrikyan M, Zafar MS, Rokaya D, Nushikyan R, Vardanyan I, Vardanyan A, Khurshid Z. Bacteriological Evaluation of Gingival Crevicular Fluid in Teeth Restored Using Fixed Dental Prostheses: An In Vivo Study. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 33. | Srimaneepong V, Heboyan A, Zafar MS, Khurshid Z, Marya A, Fernandes GVO, Rokaya D. Fixed Prosthetic Restorations and Periodontal Health: A Narrative Review. J Funct Biomater. 2022;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 63] [Article Influence: 21.0] [Reference Citation Analysis (0)] |