Published online Nov 16, 2022. doi: 10.12998/wjcc.v10.i32.11869
Peer-review started: May 16, 2022
First decision: June 27, 2022
Revised: July 25, 2022
Accepted: August 21, 2022
Article in press: August 21, 2022
Published online: November 16, 2022
Processing time: 175 Days and 10.2 Hours
Anti-glomerular basement membrane (GBM) disease is a rare rapidly progressive glomerulonephritis, frequently associated with alveolar hemorrhage in the lungs and involving the kidney by crescentic glomerulonephritis. It has been described in association with other glomerulonephritides [such as anti-neutrophilic antibody (ANCA)-glomerulonephritis, membranous nephropathy, and immunoglobulin (Ig)A nephropathy].
Herein we present an unusual case of concurrent anti-GBM disease, ANCA-associated crescentic glomerulonephritis and diffuse proliferative immune complex mediated glomerulonephritis with predominant staining for IgA and C3 by immunofluorescence. The patient is a 46-year-old Caucasian male who pre
Our case emphasizes the importance of a renal biopsy in anti-GBM disease, even in the presence of positive serum anti-GBM antibodies, to identify other potential causes of rapidly progressive glomerulonephritis. The challenge in treating such cases lies in the different therapy modalities.
Core Tip: Unusual constellation of morphologic findings in the kidney, that include anti-glomerular basement membrane disease, anti-neutrophilic antibody-associated crescentic and necrotizing glomerulonephritis and immune-complex mediated glomerulonephritis with unusual pathogenesis and grave outcome for the patient. Kidney biopsy is warranted in patients with rapidly progressive decline in kidney function and hematuria to reveal the diagnosis, pathogenesis and the optimal treatment course.
- Citation: Ibrahim D, Brodsky SV, Satoskar AA, Biederman L, Maroz N. Triple hit to the kidney-dual pathological crescentic glomerulonephritis and diffuse proliferative immune complex-mediated glomerulonephritis: A case report. World J Clin Cases 2022; 10(32): 11869-11876
- URL: https://www.wjgnet.com/2307-8960/full/v10/i32/11869.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i32.11869
The first depiction of anti-glomerular basement membrane (anti-GBM) disease was by Ernest Goodpasture in 1919. He described a fatal pulmonary–renal syndrome secondary to an atypical influenza during the Spanish flu pandemic[1]. Diagnosis of anti-GBM disease is done by a kidney biopsy and laboratory data. There is bright linear staining of the GBM for immunoglobulin (Ig)G by immunofluorescence and positive anti-GBM antibody serologist testing. Early diagnosis and treatment are essential determinants of the response and long-term prognosis[2]. Patients have acute renal failure, proteinuria (usually sub-nephrotic range) and a nephritic sediment with dysmorphic red blood cells (RBC), white blood cells, and RBC casts. Kidney biopsy helps exclude other causes of glomerulonephritis, in addition to providing key information about the activity and chronicity of renal involvement[3]. Indications of an unfavorable renal course include high serum levels of anti-GBM antibody, high serum creatinine, oligo/anuria, and a high percentage of global crescents[4].
Concurrent anti-GBM disease and anti-neutrophilic antibody (ANCA)-associated (pauci-immune) glomerulonephritis is well recognized. ANCA antibodies are detected in 21%-43% of anti-GBM disease patients[4,5]. Coexisting anti-GBM disease and immune complex-mediated glomerulonephritis (ICGN) is much less common. The most frequent form of combined anti-GBM and ICGN is membranous nephropathy[6,7].
Rare cases with anti-GBM glomerulonephritis and IgA nephropathy have been described[8]. Recently it has been recognized that IgA- and C3-containing immune complex mediated glomerulonephritis could be seen in patients with an underlying infection, especially Staphylococcal, and morphologic findings in kidney biopsy are similar to those in IgA nephropathy[9].
Here we describe a case of anti-GBM disease concurrent with ANCA-associated crescentic glomerulonephritis and ICGN with IgA and C3-containing immune complex deposits.
A 46-year-old Caucasian male presented to the emergency department with a presenting complaint of right flank pain for one day duration.
He has medical history of diabetes mellitus type II, hypertension, chronic lymphedema of lower extremities, and obesity. Five months earlier he developed gross hematuria and was diagnosed with non-obstructive nephrolithiasis. Cystoscopy was unremarkable, serum creatinine was normal, (0.8 mg/dL), no quantification of proteinuria was performed. He had several episodes of cellulitis of lower extremities, the latest being 6 mo prior to the current admission.
He was not in acute distress and there was no apparent neurological deficit. Heart sounds were unremarkable. Lungs were clear to auscultation but diminished in both bases. He had significant distention of the abdomen with a positive fluid shift. Skin exam was remarkable for hardening of the skin of lower extremities due to bilateral lymphedema. Chest X-ray showed no infiltrates. Computed tomography (CT) scan of the abdomen without contrast demonstrated cirrhosis and sequela of portal hypertension. There was no renal pathology by CT scan (repeated at day 4 with similar results). Patient had rapid decline of kidney function with anuria, and hemodialysis was initiated on day 2 of admission. Serological work-up was remarkable for high level of anti-GBM antibodies 151 units (normal < 20 units), high cytoplasmic anti-neutrophilic antibody (C-ANCA) titer 1:80 (normal < 1:20), normal perinuclear-ANCA, MPO, and PR3. Antinuclear antibodies were negative, serum and urine protein electrophoresis were negative for monoclonal protein. Complement levels were normal (C3-94 mg/dL and C4-22 mg/dL). Due to the high clinical suspicion of rapid progressive glomerulonephritis from anti-GBM disease, therapeutic plasmapheresis with fresh frozen plasma was initiated. Patient received 2 doses of Solu-Medrol (methylprednisolone) 1 g intravenously, one dose of 150 mg of oral cyclophosphamide and one session of plasmapheresis.
On the fourth day, the patient developed worsening dyspnea with hypoxia and lethargy. Within hours he became hypotensive and required initiation of vasopressors and mechanical ventilation. Continuous renal placement therapy was initiated. Blood cultures demonstrated extended spectrum beta-lactamase producing Escherichia coli, but urine culture showed no growth. Left lower extremity purplish discoloration of the calf associated with hemorrhagic bullae was noted. There was no evidence of wound or drainage to suspect a localized infection. Despite treatment with broad-spectrum antibiotics and multi-organ support, the patient was deteriorating rapidly and passed from asystole on day 6 of hospitalization.
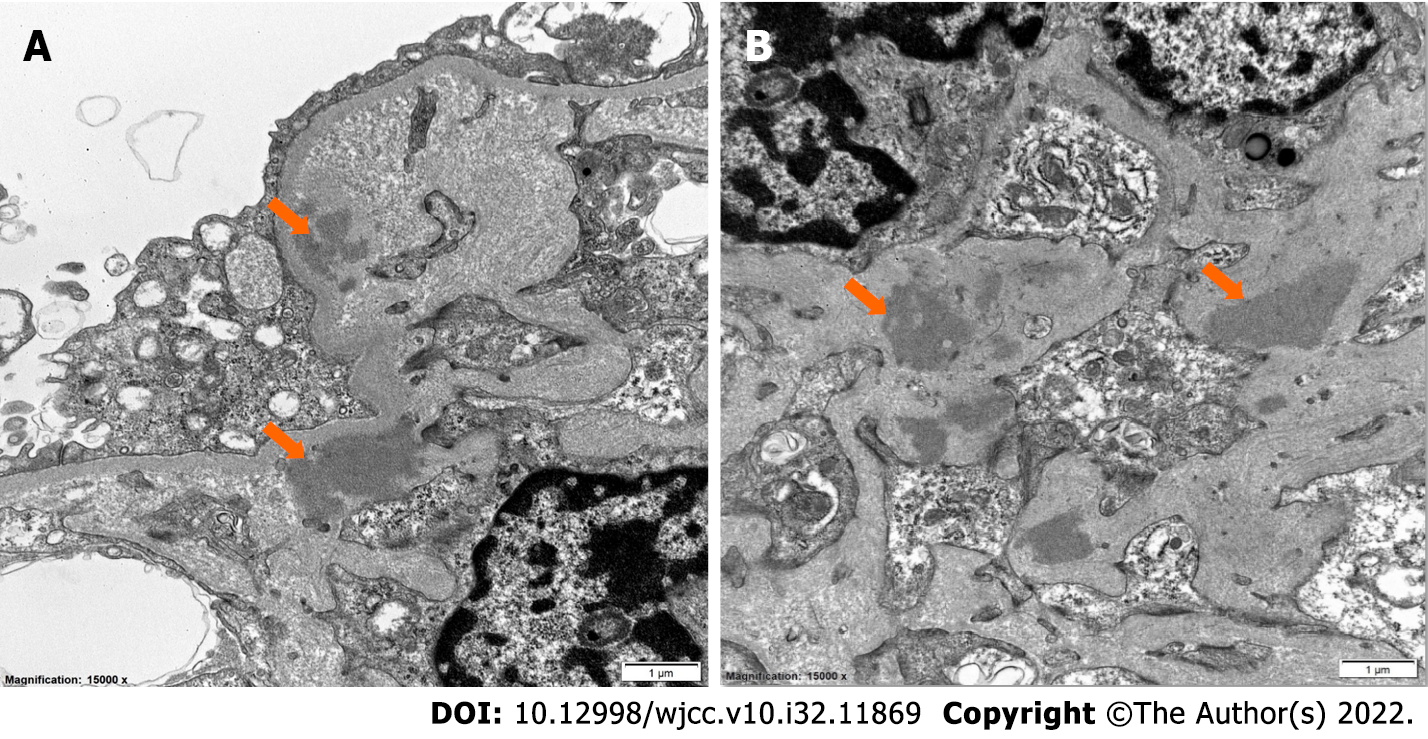
Renal biopsy was performed on the third day of hospitalization. Paraffin sections stained with Hematoxylin and Eosin, Periodic Acid-Schiff, Trichrome, and methenamine silver contained renal cortex with up to 20 glomeruli on different levels, two of which were globally sclerotic. Ten glomeruli had active cellular crescents with polymorphonuclear leukocytes, and segmental necrotizing lesions (Figure 1A). Four glomeruli had fibrocellular/fibrous crescents. Glomeruli with open capillary loops had mesangial hypercellularity (Figure 1B). Widespread active-appearing interstitial inflammation with interstitial edema and acute tubular epithelial cell injury were noted. A few tubules had RBC casts. Immunofluorescence displayed bright linear IgG staining along the glomerular capillary loops as compared with albumin (Figure 2A and B), granular moderate to prominent staining for IgA in the mesangium (Figure 2C) and prominent C3 staining in the mesangium (Figure 2D). Ultrastructurally, there were discrete electron-dense immune-type deposits, predominantly in the mesangium and paramesangium (Figure 3). Rare densities were also noted in the GBM along the glomerular capillary loops. The GBM was of normal thickness and texture. There was prominent podocyte foot process effacement.
Laboratory data on admission are shown in Table 1.
| Laboratory parameter, units | Value | Normal range |
| Temperature, oF (oC) | 96.7 (35.9) | 95.3-98.4 (35.2-36.9) |
| Blood pressure, systolic/diastolic, mmHg | 171/85 | < 120/< 80 |
| Heart rate, min-1 | 67 | 60-120 |
| Respiratory rate, min-1 | 16 | 14-26 |
| Serum creatinine, mg/dL | 15.1 | 0.5-1.2 |
| Blood urea nitrogen, mg/dL | 96 | 7-25 |
| Serum albumin, g/dL | 2.7 | 3.5-5.0 |
| Total bilirubin, mg/dL | 0.7 | < 1.5 |
| White blood cells, K/uL | 8.4 | 3.99-11.19 |
| Hemoglobin, g/dL | 8.0 | 11.4-15.2 |
| Platelets, K/uL | 92 | 150-393 |
| Urinary spot protein/creatinine ratio, g/g | 6.9 | < 0.03 |
| Hematuria | Moderate | Absent |
| Anti-GBM antibodies, a.u. | 151 | < 20 |
| C-ANCA | 1:80 | Negative |
| P-ANCA | Negative | Negative |
| ANA | Negative | Negative |
| Complement C3, mg/dL | 94 | 87-200 |
| Complement C4, mg/dL | 22 | 18 × 52 |
Anti-GBM disease, ANCA-associated crescentic glomerulonephritis, and diffuse proliferative ICGN.
Patient received 2 doses of Solu-Medrol (methylprednisolone) 1 g intravenously, one dose of 150 mg of oral cyclophosphamide and one session of plasmapheresis.
Despite treatment with broad-spectrum antibiotics and multi-organ support, the patient was deteriorating rapidly and passed from asystole on day 6 of hospitalization.
Herein we present an unusual case with constellation of anti-GBM disease and ICGN. In addition to the linear IgG staining along the glomerular capillaries, there was granular IgA and C3 mesangial staining by immunofluorescence and electron dense deposits by electron microscopy.
Crescentic glomerulonephritis is an aggressive form of glomerulonephritis. According to the underlying pathophysiological mechanisms, it is classified into three types: Anti-GBM disease, ICGN, and pauci-immune crescentic and necrotizing glomerulonephritis, that often is associated with ANCA[10]. Among these, anti-GBM disease is the rarest and most severe form of glomerular injury[11].
The pathophysiology of anti-GBM disease is multifactorial including immune, genetic, and environmental causes[12,13]. An autoimmune response is directed against the non-collagenase domain of the alpha 3 chain of type 4 collagen [a3(IV)NC1] found in the glomerular and alveolar basement membrane[12]. Environmental factors as smoking, hydrocarbon exposure, lithotripsy, and infection, may trigger the disease in genetically susceptible individuals[2,12].
Analogous to our case, concurrence with other kidney diseases such as ANCA-associated glomerulonephritis and membranous nephropathy is at a higher frequency than would be expected by chance alone. Few cases have been described associated with IgA nephropathy[14]. It is thought that IgA-containing immune complex deposition causes disruption of glomerular architecture exposing hidden epitopes that allow initiation of anti-GBM disease[15]. Conversely, anti-GBM antibodies might alter the permeability of the GBM, allowing circulating immune complexes to access parts of the GBM[6].
Anti-GBM disease is characterized by the presence of cellular crescents on light microscopy, with glomeruli showing lesions of a similar stage. Segmental fibrinoid necrosis can also be present. Characteristically, uninvolved glomerular do not show endocapillary or mesangial hypercellularity. Immunofluorescence demonstrates the pathognomonic finding of bright linear staining for IgG along the glomerular capillary loops and ultrastructural examination does not show deposits[16]. Linear staining for IgG and kappa and/or lambda light chains may be seen in other diseases, such as monoclonal immunoglobulin deposition or diabetic nephropathy. In our case, there was no differences in the staining for kappa and lambda light chains by immunofluorescence and no evidence of finely granular, "powdery," electron-dense deposits on ultrastructural examination, which is a hallmark of monoclonal immunoglobulin deposition in the kidney. Linear IgG staining along the GBM may be seen in diabetic nephropathy. However, such linear staining along the glomerular capillary loops in diabetic patients is seen for both albumin and IgG. It is present in both GBM and tubular basement membranes and Bowman’s capsule. In our case, there was bright linear staining for IgG in the glomerular capillary loops only, and this staining was stronger than albumin. The glomeruli uninvolved by crescents showed mesangial hypercellularity. In addition to the linear IgG staining in the GBM, there was granular IgA and C3 mesangial staining by immunofluorescence and electron microscopy revealed electron dense deposits in the mesangium and paramesangium, indicating immune complex deposits in the glomeruli.
It is quite plausible that the patient had a form of IgA nephropathy with minimal clinical symptoms. Infection could exacerbate the underlying IgA nephropathy and it became symptomatic. On admission, the patient had no fever, elevated white blood cell or apparent clinical or radiographic finding on admission to suspect infection. Unfortunately, biopsy findings in IgA nephropathy and infection-associated glomerulonephritis are hard to differentiate; therefore, the possibility that these immune-complex mediated glomerulonephritis is associated with infection cannot be excluded. Mesangial IgA deposits may be seen in patients with liver cirrhosis[17]. Usually this is asymptomatic and rarely does it cause hematuria or proteinuria. Interestingly, bacterial infection can act as a “second-hit” and lead to acute glomerulonephritis with large immune complex deposits[18]. In addition, it has been speculated that infection may play a role in the pathogenesis of anti-GBM disease[19].
Moreover, it has been shown that a significant proportion of ANCA-associated crescentic GN show evidence of immune complex deposition in renal biopsies. In such cases, immune complex deposition was associated with a greater amount of proteinuria[20,21], a higher frequency of hypocomplementemia and greater glomerular hypercellularity[21]. In addition, it is believed that ANCA-mediated glomerular inflammation may contribute to development of anti-GBM disease through disrupting the structure of the GBM leading to exposure of normally sequestered epitopes. On the other hand, glomerular neutrophil recruitment and activation in anti-GBM disease can contribute to ANCA-associated GN via abnormal expression of myeloperoxidase triggering anti-MPO antibodies production[22]. In our case, X ray showed no evidence of infiltrates suggestive of vasculitis. Patient had no hemoptysis.
One of the unusual clinical symptoms in our patient was flank pain. CT scan of the abdomen was done without contrast was done twice-the day of admission and the date prior to the patient’s death: It demonstrated liver enlargement and hepatic steatosis. Spleen was enlarged (15.3 cm). Adrenals were normal. Retroperitoneal structure had no pathology. There were gastric varices. Bowel showed wall thickening involving the right colon and transverse colon. There were moderate volume ascites[23]. We believe that flank pain was a part of the glomerulonephritis presentation in our case.
Our case represents an unusual constellation of different glomerulonephritides in a single patient. The rapidly progressive glomerulonephritis in our patient is likely associated with the ongoing infection. C3 immunofluorescence was stronger than IgA, suggesting infectious or IgA vasculitis and not typical IgA nephropathy. IgA vasculitis may also be a consequence of Gram-negative infection. The infection could trigger an ANCA- or IgA-vasculitis, which can provoke the production of an anti-GBM antibody by damaging the GBM, thereby causing further kidney damage. However, the pathogenetic role of infection triggered C-ANCA and anti-GBM serological positivity is not unequivocal. There were no previous symptoms of extrarenal vasculitis, anti-MPO and anti-PR3 were negative. However, upon admission patient had no signs of infection on physical examination. He had stable hemodynamics, had normal white blood cells 8.4 K/uL and was afebrile. He remained hemodynamically stable until day 4 of hospitalization with persistently normal white blood cell count. Blood culture and urine culture were collected on day 4 due to cardiac arrest.
Clinical and laboratory presentation of our patient on admission was not consistent with infection but rather RPGN picture with very high anti-GBM antibodies. Recommended approach to the treatment of anti-GBM disease requires prompt initiation of immunosuppressive therapy. Our case supports the importance of the thorough workup of hidden infection prior to initiation of immunosuppression. Concomitant presence of the infection may put the treating providers in front of the challenging choice between two life-threatening conditions, treatment of which is mutually exclusive. Recommendations to the clinical management in similar cases need to be developed.
Our case underscores the importance of a kidney biopsy in anti-GBM disease, even if diagnosis is clinically based on the positive serum anti-GBM antibodies.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ghazanfar A, United Kingdom; Večerić-Haler Ž, Slovenia S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Collins RD. Dr Goodpasture: "I was not aware of such a connection between lung and kidney disease". Ann Diagn Pathol. 2010;14:194-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | McAdoo SP, Pusey CD. Anti-Glomerular Basement Membrane Disease. Clin J Am Soc Nephrol. 2017;12:1162-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 259] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 3. | Pusey CD. Anti-glomerular basement membrane disease. Kidney Int. 2003;64:1535-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 127] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | Alchi B, Griffiths M, Sivalingam M, Jayne D, Farrington K. Predictors of renal and patient outcomes in anti-GBM disease: clinicopathologic analysis of a two-centre cohort. Nephrol Dial Transplant. 2015;30:814-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Rutgers A, Slot M, van Paassen P, van Breda Vriesman P, Heeringa P, Tervaert JW. Coexistence of anti-glomerular basement membrane antibodies and myeloperoxidase-ANCAs in crescentic glomerulonephritis. Am J Kidney Dis. 2005;46:253-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 145] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Cui Z, Zhao MH, Wang SX, Liu G, Zou WZ, Wang HY. Concurrent antiglomerular basement membrane disease and immune complex glomerulonephritis. Ren Fail. 2006;28:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Nikolopoulou A, Huang-Doran I, McAdoo SP, Griffith ME, Cook HT, Pusey CD. Membranous Glomerulonephritis With Crescents. Kidney Int Rep. 2019;4:1577-1584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Yamaguchi H, Takizawa H, Ogawa Y, Takada T, Yamaji I, Ura N. A case report of the anti-glomerular basement membrane glomerulonephritis with mesangial IgA deposition. CEN Case Rep. 2013;2:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Satoskar AA, Nadasdy G, Plaza JA, Sedmak D, Shidham G, Hebert L, Nadasdy T. Staphylococcus infection-associated glomerulonephritis mimicking IgA nephropathy. Clin J Am Soc Nephrol. 2006;1:1179-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 107] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Jennette J, D'Agati JD, Olson JL Silva FG. Heptinstall's Pathology of the Kidney. Lippincott Williams and Wilkins; 2014: 978-1451144116. |
| 11. | Parmar MS, Bashir K. Crescentic Glomerulonephritis. 2022 May 15. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. [PubMed] |
| 12. | Ooi JD, Holdsworth SR, Kitching AR. Advances in the pathogenesis of Goodpasture's disease: from epitopes to autoantibodies to effector T cells. J Autoimmun. 2008;31:295-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Canney M, O'Hara PV, McEvoy CM, Medani S, Connaughton DM, Abdalla AA, Doyle R, Stack AG, O'Seaghdha CM, Clarkson MR, Griffin MD, Holian J, Dorman AM, Niland A, Keogan M, Wallace EM, Conlon NP, Walsh C, Kelly A, Little MA. Spatial and Temporal Clustering of Anti-Glomerular Basement Membrane Disease. Clin J Am Soc Nephrol. 2016;11:1392-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 14. | Kamimura H, Honda K, Nitta K, Horita S, Kobayashi H, Uchida K, Yamaguchi Y, Yumura W, Nihei H. Glomerular expression of alpha2(IV) and alpha5(IV) chains of type IV collagen in patients with IgA nephropathy. Nephron. 2002;91:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Kurts C, Panzer U, Anders HJ, Rees AJ. The immune system and kidney disease: basic concepts and clinical implications. Nat Rev Immunol. 2013;13:738-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 515] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 16. | Fischer EG, Lager DJ. Anti-glomerular basement membrane glomerulonephritis: a morphologic study of 80 cases. Am J Clin Pathol. 2006;125:445-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Sinniah R. Heterogeneous IgA glomerulonephropathy in liver cirrhosis. Histopathology. 1984;8:947-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Hemminger J, Arole V, Ayoub I, Brodsky SV, Nadasdy T, Satoskar AA. Acute glomerulonephritis with large confluent IgA-dominant deposits associated with liver cirrhosis. PLoS One. 2018;13:e0193274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Kashif W, Yaqub S, Mahmood SF, Patel J. Double-positive Goodpasture's syndrome with concomitant active pulmonary tuberculosis. Saudi J Kidney Dis Transpl. 2013;24:783-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Neumann I, Regele H, Kain R, Birck R, Meisl FT. Glomerular immune deposits are associated with increased proteinuria in patients with ANCA-associated crescentic nephritis. Nephrol Dial Transplant. 2003;18:524-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Yu F, Chen M, Wang SX, Zou WZ, Zhao MH, Wang HY. Clinical and pathological characteristics and outcomes of Chinese patients with primary anti-neutrophil cytoplasmic antibodies-associated systemic vasculitis with immune complex deposition in kidney. Nephrology (Carlton). 2007;12:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | McAdoo SP, Tanna A, Hrušková Z, Holm L, Weiner M, Arulkumaran N, Kang A, Satrapová V, Levy J, Ohlsson S, Tesar V, Segelmark M, Pusey CD. Patients double-seropositive for ANCA and anti-GBM antibodies have varied renal survival, frequency of relapse, and outcomes compared to single-seropositive patients. Kidney Int. 2017;92:693-702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 150] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 23. | Troxell ML, Saxena AB, Kambham N. Concurrent anti-glomerular basement membrane disease and membranous glomerulonephritis: a case report and literature review. Clin Nephrol. 2006;66:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |