Published online Nov 16, 2022. doi: 10.12998/wjcc.v10.i32.11753
Peer-review started: May 30, 2022
First decision: July 13, 2022
Revised: July 26, 2022
Accepted: October 17, 2022
Article in press: October 17, 2022
Published online: November 16, 2022
Processing time: 161 Days and 20.9 Hours
Pulsed electromagnetic field (PEMF) therapy is widely used to treat myofascial pain syndrome (MPS). Damp-clearing and pain-reducing paste (DPP) comprises medical herbs and has been a traditional method of reducing myofascial pain in China for a long time, and it is usually administered with heating. However, the synergistic effect of PEMF therapy on heating-DPP in patients with MPS is unclear.
To investigate the synergistic effect of PEMF therapy plus heating-DPP in lumbar MPS.
This double-blind, randomized, placebo-controlled trial was conducted on 120 patients with lumbar MPS who were randomly divided into an experimental group (EG, n = 60) and a control group (CG, n = 60). Patients in both groups were treated with heating-DPP combined with PEMF therapy; however, the electromagnetic function of the therapeutic apparatus used in the CG was disabled. Each treatment lasted for 20 min and was applied five times a week for two weeks. The short-form McGill Pain Questionnaire was applied at five time points: pretest, end of the first and second weeks of treatment, and end of the first and fourth week after completing treatment. Visual analog scale (VAS), present pain intensity index (PPI), and pain rating index (PRI; total, affective pain, and sensory pain scores) scores were then analyzed.
Compared with the CG, the VAS, PPI and PRI scores (total, affective pain and sensory pain scores) in the EG were significantly lower after treatment and during follow-up.
PEMF therapy combined with heating-DPP showed better efficacy than heating-DPP alone in reducing the overall intensity of pain and sensory and affective pain.
Core Tip: The present study was a double-blind, randomized, placebo-controlled trial designed to compare the clinical efficacy of pulsed electromagnetic field (PEMF) therapy combined with heating-damp-clearing and pain-reducing paste (DPP) to that of heating-DPP alone for lower back myofascial pain syndromes. The main finding was that PEMF therapy combined with heating-DPP had better efficacy than heating-DPP alone in reducing the intensity of pain.
- Citation: Xiao J, Cao BY, Xie Z, Ji YX, Zhao XL, Yang HJ, Zhuang W, Sun HH, Liang WM. Clinical efficacy of electromagnetic field therapy combined with traditional Chinese pain-reducing paste in myofascial pain syndrome. World J Clin Cases 2022; 10(32): 11753-11765
- URL: https://www.wjgnet.com/2307-8960/full/v10/i32/11753.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i32.11753
Pain is defined as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage”[1]. Myofascial pain syndrome (MPS) is a musculoskeletal disorder in which tension (called the myofascial trigger point (MTrP)) is felt in local areas of muscle and fascia. Pain is felt when contact pressure is applied on MTrP, and snapping palpation or needle insertion causes a local twitching reaction[2]. MPS can result from muscle overuse and trauma; psychological stress; or ergonomic, structural, or systemic factors[3]. MPS often has a referred neuro
In addition to PEMF therapy, a traditional Chinese herbal formula - damp-clearing and pain-reducing paste (DPP) - has been used to treat MPS for decades at Xiyuan Hospital of the China Academy of Chinese Medical Sciences. The application of this traditional Chinese medicine topical paste has a long history. After use, the topical paste quickly exerts its effect at the site of administration but can also exert its effect systemically via the systemic circulation. The paste has a long-lasting effect and a wide treatment range[12]. Because the EMF alters ion channels and accelerates blood circulation[13,14], this therapy may increase the penetration of the DPP molecules into target tissues. To the best of our knowledge, this is the first study to investigate the synergistic effects of PEMF therapy and DPP in patients with MPS. We hypothesized that PEMF therapy combined with heating-DPP has better efficacy than heating-DPP alone in pain reduction.
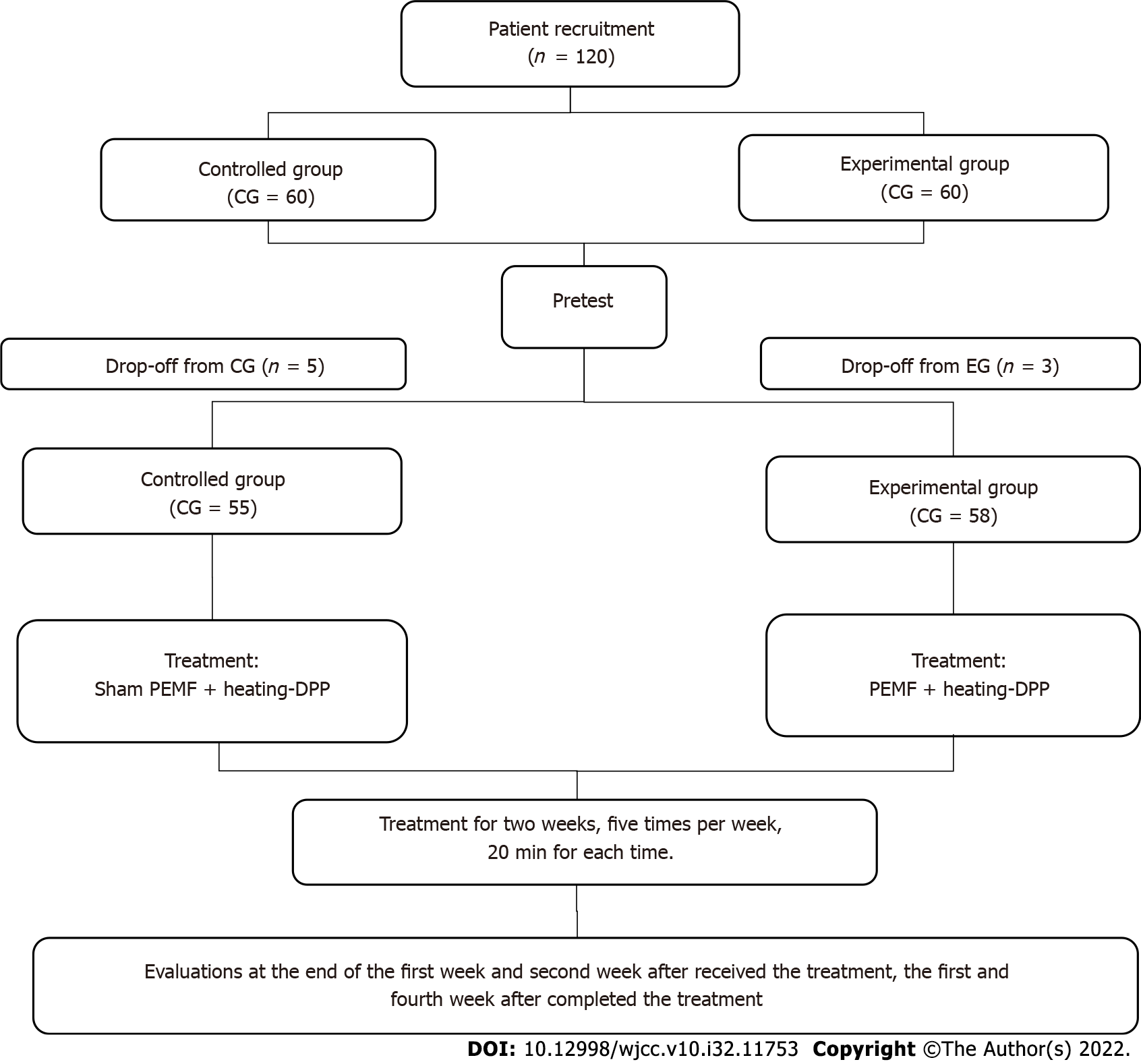
A total of 120 participants diagnosed with lower back MPS according to the China National Diagnostic Criteria were recruited and divided into the experimental (EG) and control (CG) groups using the block randomization method. Seven patients dropped out, while 113 (EG = 58, CG = 55) completed all the tests, as shown in Figure 1.
The inclusion criteria were as follows: Patients who (1) Were aged 18–75 years; (2) met the diagnostic criteria listed in the textbook of Musculoskeletal Rehabilitation (third edition, 2018)[15]; (3) underwent imaging (radiography, computed tomography, or magnetic resonance imaging) and biochemical examinations except in the case of myofascial pain caused by tumor, infection, fracture, or other reasons; and (4) provided written informed consent.
Exclusion criteria were as follows: (1) Hypertension, severe cardiovascular and cerebrovascular diseases, superficial open wounds on the skin, infectious diseases, or bleeding tendency; (2) suspected or confirmed vertebral or spinal tumors, tuberculosis, and severe osteoporosis; (3) history of lumbar surgery or severe congenital deformity of the lumbar spine; (4) administration of other treatment methods 48 h before seeing the doctor; (5) pregnant or lactating women; and (6) inability to comprehend the contents of the scale for judging degree of pain.
Drop-out criteria for patients included in this study were as follows: (1) Inability to attend meetings on time or receive treatment as required; (2) use of drug or treatment that influenced the efficacy of the study or performance of the behavior required for the index detection; (3) damage or discomfort during treatment; and (4) voluntary withdrawal.
This study was a randomized, double-blind, placebo-controlled clinical trial. The research protocol was approved by the ethics committee of Xiyuan Hospital of the China Academy of Chinese Medical Sciences (ethical approval No. 2018XLA049-7) and registered in the Chinese Clinical Trial Registry (registration no. ChiCTR2000033700) http://www.chictr.org.cn.
PEMF therapy: An EMF was generated using a PEMF therapeutic instrument (CZ-02B, Xingchen Wanyou Technology Co., Ltd., Beijing, China), which generates a 50 Hz and 3 mT PEMF. The instrument contains a host and belt that not only generates an EMF but also generates heat. DPP, a topical prescription produced at Xiyuan Hospital of the China Academy of Chinese Medical Sciences, is composed of Radix paeoniae rubra, Achyranthis bidentatae radix, Angelica sinensis radix, Cassia twig, Caulis spatholobi, Clubmoss herb, Semen persicae, Tougucao, Kusnezoff monkshood root, Radix aconiti, Frankincense, and Myrrh. All herbs were ground to a fine powder and mixed with vinegar to make a paste. According to traditional Chinese medical theory, this paste can invigorate circulation, eliminate stagnation, and reduce pain.
DDP was applied to the skin of the lower back, and the belt of the electromagnetic and thermal generator was fastened around the lower back. Each treatment lasted for 20 min and was applied five times a week for two weeks. The same procedure and dose were applied in both groups. However, the electromagnetic function of the therapeutic apparatus used in the CG was disabled by technicians.
Pain is a symptom and not a sign, and its evaluation is dependent on the report or demonstration[16]. Questionnaires represent an appropriate reporting option, and in this study, the short-form McGill Pain Questionnaire (SF-MPQ) was used to evaluate patients' pain, and it is reportedly reliable and valid[17-19]. The SF-MPQ is sensitive to the effectiveness of pain therapies in different population settings[20,21] and measures the sensory and affective components of pain. The questionnaire included the visual analog scale (VAS), present pain intensity index (PPI), and pain rating index (PRI), to detect the intensity of pain, sensory pain, and affective pain.
VAS was used to evaluate pain intensity. A 10 cm ruler was included on the form, with anchor statements on the left (no pain) and right (extreme pain). The patient was asked to mark their current pain level on the ruler. The PPI measures the magnitude of pain experienced by an individual and represents a numeric-verbal combination to assess overall pain intensity[22]. The PPI was scored from 1 to 5, with 1 = mild pain, 2 = discomfort, 3 = distressing pain, 4 = horrible pain, and 5 = excruciating pain. The PRI has 15 descriptors - descriptors 1-11 for sensory pain and 12-15 for affective pain. Each descriptor was ranked on an intensity scale (0 = none, 1 = mild, 2 = moderate, and 3 = severe)[23]. To clarify the effect of the interventions on sensory and affective pain, the scores from these two pain types were analyzed individually.
For all pain scores, a higher value means worse pain.
The main outcomes were changes in the overall pain intensity (VAS and PPI scores) and in sensory and affective pain (PRI scores) at the end of the first and second weeks of receiving treatment and at the end of the first and fourth weeks after completing treatment. The secondary outcome, which specified the effectiveness of the intervention on biological and psychological pain, was the change in sensory and affective pain scores.
The long power package was used to estimate the sample size for analysis by the generalized estimating equation (GEE)[24]. Based on Chang's estimation, α was set to 0.5 (two-sided) and β to 0.2, and a sample size of 43 was obtained for each group. Owing to the estimated 30% dropout rate, 56 participants were required for each group[25]. 60 participants were enrolled in each group.
The Good Clinical Practice Statistical Office at Xiyuan Hospital was entrusted with generating random envelopes using the block randomization method. 120 cases were numbered and split into 30-case groups: A1-A30, B1-B30, C1-C30, and D1-D30. Before joining the group, an instrument technician adjusted and numbered the instruments according to the envelopes. The number was reflected on the instruments, whereas the grouping result was not. During enrollment, the participants were assigned numbers from small to large according to the sequence of enrollment.
Blinding was executed by special personnel who took charge of blind editing and implementation. The number of envelopes was the same as that of the PEMF instruments and remained unchanged throughout the experiment. Patients and physicians were not informed of the differences in equipment and grouping. Blinding was removed by the time data collection was completed.
The difference in age between the EG and CG was calculated using an independent sample t-test, while that in sex was calculated using the χ2 test. The pretest pain scores were calculated using the Mann–Whitney U test. The pain scores from the pretest were then set as covariates to calculate the posttest outcomes using GEE. The ordinal logistic model was used in the GEE analysis to calculate the ordinal data - VAS and PPI scores. The linear with a link function of log was used to analyze the continuous data - PRI total, sensory, and affective scores. The main effects of time and group, interactions between time and group, and simple effects between the two groups at each time point were tested. All calculations were performed using SPSS version 20 (IBM Corp., Armonk, NY, USA).
A total of 120 patients with MPS were enrolled; however, seven of them were dropped. Finally, 113 patients were included for statistical analysis (EG: n = 58, men = 16, women = 42, mean age = 50.7 ± 13.3 years; CG: n = 55, men = 15, women = 40, mean age = 52.6 ± 13.6 years). The basic characteristics of the patients and the pretest results in each group are listed in Table 1. The chi-square test was used to test for sex equality between the groups, while an independent t-test was used to test for age; VAS; PPI, PRI, and RRI sensory pain scores; and PRI affective pain scores. All tests showed no significant differences between the EG and CG (Table 1).
| Group | Sex | Age | Pretest | |||||
| Men | Women | VAS | PPI | PRI | PRI sensory | PRI affective | ||
| CG (n = 55) | 15 | 40 | 52.62 ± 13.62 | 5 (4-7) | 3 (1-3) | 7 (4-11) | 4 (3-7) | 2 (1-4) |
| EG (n = 58) | 16 | 42 | 50.74 ± 13.33 | 5 (4-7) | 3 (1-3) | 7 (4.75-10.25) | 5.5 (3.75-8.25) | 2 (1-3) |
| Test statistic | χ2 = 0.001 | t = 0.740 | Z = 0.911 | Z = 0.800 | Z = 0.823 | Z = 1.199 | Z = 0.049 | |
| P value | 0.97 | 0.46 | 0.36 | 0.42 | 0.411 | 0.231 | 0.961 | |
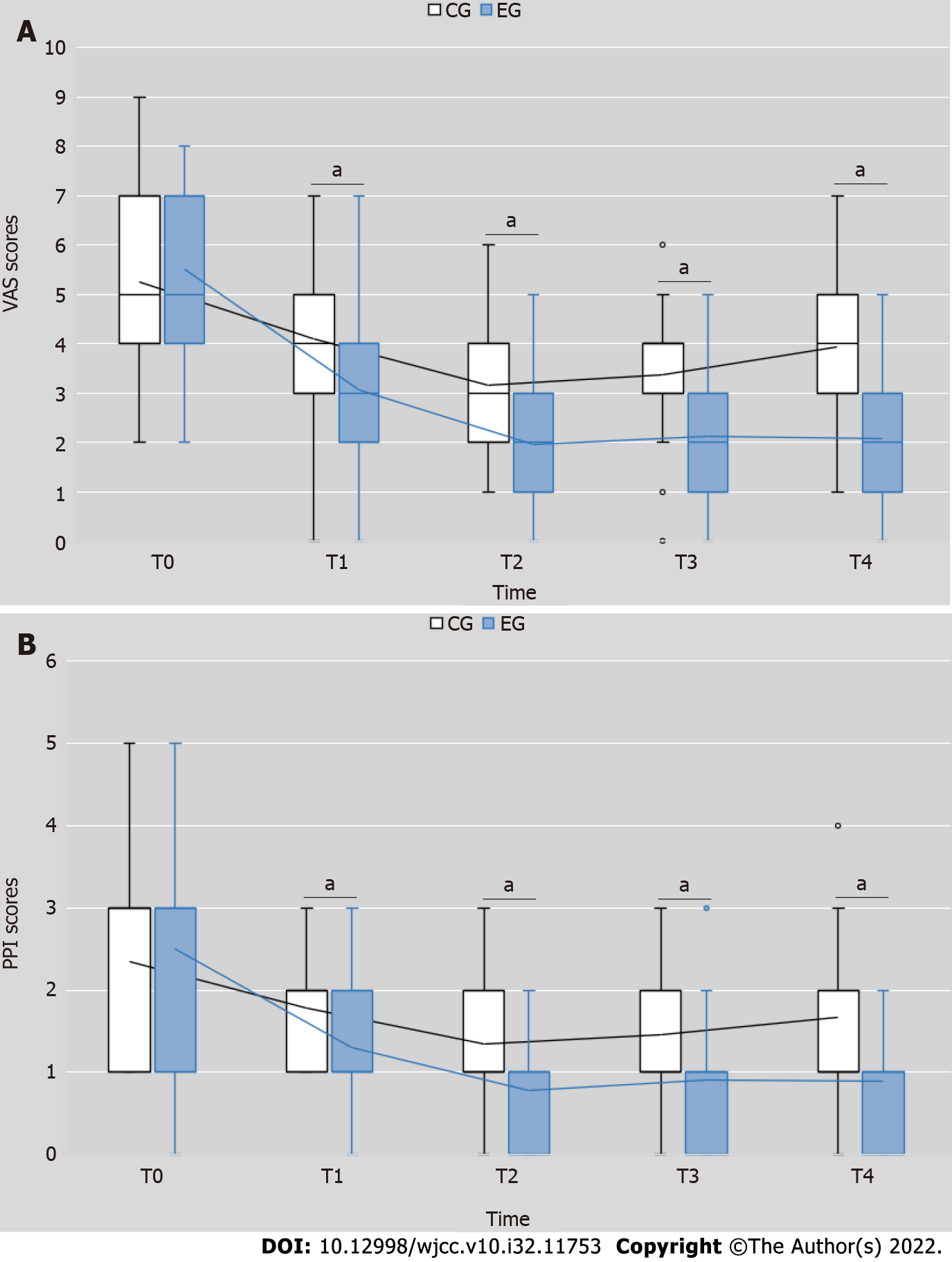
The VAS scores in the EG were significantly lesser than those in the CG after the intervention (Wald χ2 = 4.198, P = 0.040); the time effect (Wald χ2 = 3.806, P = 0.283) and interaction effect of time and interventions (Wald χ2 = 14.736, P = 0.002) were also significant (Figure 2A). At each testing point, the VAS scores of the EG were lesser than those of the CG (T1: Wald χ2 = 22.231, P = 0.000; T2: Wald χ2 = 33.434, P = 0.000; T3: Wald χ2 = 28.782, P = 0.000; T4: Wald χ2 = 60.137, P = 0.000) (Table 2). As shown in Table 2, the VAS scores of both groups were significantly reduced (EG: Wald χ2 = 109.567, P = 0.000; CG: Wald χ2 = 48.753, P = 0.000) (Table 2). These results indicated that overall pain in EG was reduced more than that in CG, and the difference in efficacy increased over time.
| Group | T1 | T2 | T3 | T4 | Wald χ2 | P value |
| CG (n = 55) | 4 (3-5) | 3 (2-4) | 4 (3-4) | 4 (3-5) | 48.753 | 0.000 |
| EG (n = 58) | 3 (2-4) | 2 (1-3) | 2 (1-3) | 2 (1-3) | 109.567 | 0.000 |
| Wald χ2 | 22.231 | 33.434 | 28.782 | 60.137 | ||
| P value | 0.000 | 0.000 | 0.000 | 0.000 |
Similar results were observed for the PPI scores, as its decrease in the EG was significantly larger than that in the CG after the intervention (Wald χ2 = 30.545, P = 0.000); the time effect (Wald χ2 = 39.539, P = 0.000). However, and interaction effect of time and interventions (Wald χ2 = 3.237, P = 0.356) was not statistically significant (Figure 2B). Compared with the CG, the PPI scores of the EG were lesser at each testing point (T1: Wald χ2 = 16.402, P = 0.000; T2: Wald χ2 = 25.200, P = 0.000; T3: Wald χ2 = 17.110, P = 0.002; T4: Wald χ2 = 16.577, P = 0.000) (Table 3). The reduction in PPI scores was also significant in both groups (EG: Wald χ2 = 93.582, P = 0.000; CG: Wald χ2 = 38.414, P = 0.000) (Table 3). These results suggest that the overall pain tested from PPI was also decreased more in EG relative to that in CG. However, the difference in efficacy between the two groups was not changed significantly over time.
| Group | T1 | T2 | T3 | T4 | Wald χ2 | P value |
| CG (n = 55) | 2 (1-2) | 1 (1-2) | 1 (1-2) | 1 (1-2) | 38.414 | 0.000 |
| EG (n = 58) | 1 (1-2) | 1 (0-1) | 1 (0-1) | 1 (0-1) | 93.582 | 0.000 |
| Wald χ2 | 16.402 | 25.200 | 17.110 | 16.577 | ||
| P value | 0.000 | 0.000 | 0.002 | 0.000 |
We analyzed the PRI scores in three ways: Total pain, sensory pain, and affective pain scores.
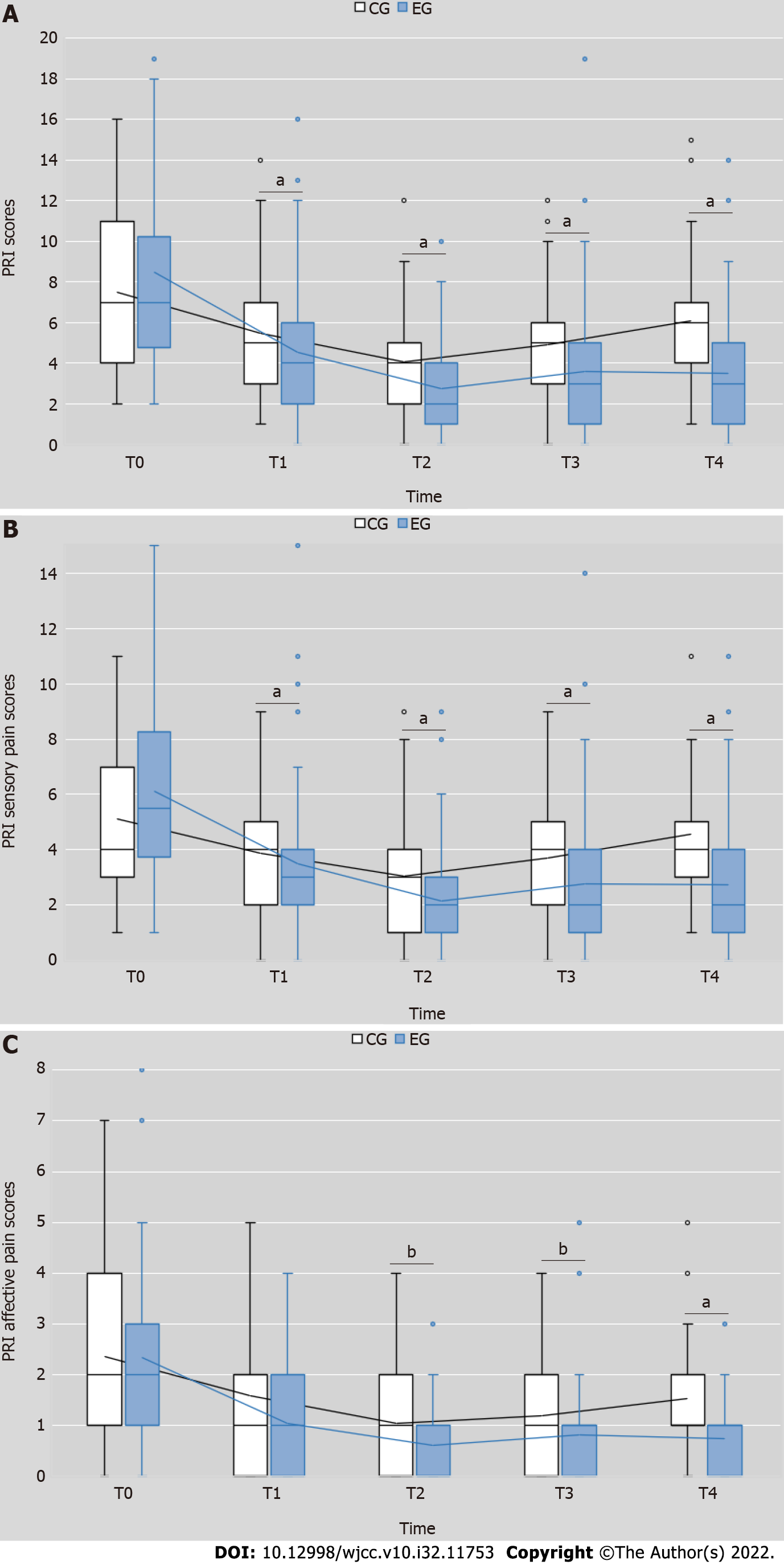
The total PRI scores of the EG were significantly lesser than those of the CG after the intervention (Wald χ2 = 47.934, P = 0.000). The time (Wald χ2 = 49.423, P = 0.000) and interaction effect of time and interventions (Wald χ2 = 11.180, P = 0.000) were also significant (Figure 3A). There was a statistically significant difference in total PRI scores between the EG and CG at each testing point (T1: Wald χ2 = 13.836, P = 0.001; T2: Wald χ2 = 15.177, P = 0.000; T3 (Wald χ2 = 26.756, P = 0.002; T4: Wald χ2 = 61.454, P = 0.000) (Table 4). A significant decrease in total PRI scores was also found in both groups (EG: Wald χ2 = 238.185, P = 0.000; CG: Wald χ2 = 65.551, P = 0.000) (Table 4).
| Group | T1 | T2 | T3 | T4 | Wald χ2 | P value |
| CG (n = 55) | 5 (3-7) | 4 (2-5) | 5 (3-6) | 6 (4-7) | 65.551 | 0.000 |
| EG (n = 58) | 4 (2-6) | 2 (1-4) | 3 (1-5) | 3 (1-5) | 238.185 | 0.000 |
| Wald χ2 | 13.836 | 15.177 | 26.756 | 61.454 | ||
| P value | 0.001 | 0.000 | 0.002 | 0.000 |
The PRI sensory pain scores of the EG were significantly lesser than those of the CG after the intervention (Wald χ2 = 46.196, P = 0.000). The time effect (Wald χ2 = 39.984, P = 0.000) and interaction effect of time and interventions (Wald χ2 = 12.986, P = 0.005) were also significant (Figure 3B). There was a statistically significant difference in PRI sensory pain scores between the EG and CG at each testing point (T1: Wald χ2 = 10.353, P = 0.001; T2: Wald χ2 = 13.305, P = 0.000; T3 (Wald χ2 = 28.271, P = 0.000; T4: Wald χ2 = 63.510, P = 0.000) (Table 5). A significant decrease in PRI sensory pain scores was found in both EG and CG (Wald χ2 = 197.864, P = 0.000; Wald χ2 = 45.488, P = 0.000) (Table 5).
| Group | T1 | T2 | T3 | T4 | Wald χ2 | P value |
| CG (n = 55) | 4 (2-5) | 3 (1-4) | 4 (2-5) | 4 (3-5) | 45.488 | 0.000 |
| EG (n = 58) | 3 (2-4) | 3 (2-4) | 2 (1-3) | 2 (1-4) | 197.864 | 0.000 |
| Wald χ2 | 10.353 | 13.305 | 28.271 | 63.510 | ||
| P value | 0.001 | 0.000 | 0.000 | 0.000 |
Similar to the PRI sensory pain scores, the PRI affective pain scores decrease in the EG was significantly larger than that in the CG after the intervention (Wald χ2 = 16.057, P = 0.000); the time effect (Wald χ2 = 23.546, P = 0.000) and interaction effect (Wald χ2 = 8.325, P = 0.040) were also significant (Figure 3C). Compared to the CG, the PRI affective pain scores of the EG were lesser at each testing point (T1: Wald χ2 = 3.227, P = 0.072; T2: Wald χ2 = 5.489, P = 0.019; T3: Wald χ2 = 5.588, P = 0.018; T4: Wald χ2 = 23.485, P = 0.000) (Table 6). The reduction in PRI affective pain scores was also significant in both groups (EG: Wald χ2 = 128.366, P = 0.000; CG: Wald χ2 = 78.635, P = 0.000) (Table 6).
| Group | T1 | T2 | T3 | T4 | Wald χ2 | P value |
| CG (n = 55) | 1 (0-2) | 1 (0-2) | 1 (0-2) | 1 (1-2) | 78.635 | 0.000 |
| EG (n = 58) | 1 (0-2) | 0 (0-1) | 1 (0-1) | 1 (0-1) | 128.366 | 0.000 |
| Wald χ2 | 3.227 | 5.489 | 5.588 | 23.485 | ||
| P value | 0.072 | 0.019 | 0.018 | 0.000 |
In general, total pain, sensory pain and affective pain from PRI scores were reduced in two groups, while the reduction in EG was more than that in CG. Interestingly, the significant difference in affective pain between the two groups was observed after two week’s treatment.
The present study was designed as a double-blind, randomized, placebo-controlled trial to control bias. The experiment was performed to compare the clinical efficacies of PEMF therapy combined with heating-DPP and heating-DPP alone for lower back MPS. The main finding of our study was that PEMF therapy combined with heating-DPP had better efficacy than heating-DPP alone in reducing the intensity of pain as well as sensory and affective pain. heating-DPP also had a positive effect on pain reduction.
The VAS is a commonly used visual scale for evaluating the general intensity of pain, whereas the PPI is a numeric-verbal scale[18]. Both VAS and PPI provide data on overall pain intensity[26]. Regarding the significant reduction in overall pain intensity observed after PEMF therapy, our findings were consistent with another study conducted by Elshiwi et al[8] that PEMF therapy combined with physiotherapy (Transcutaneous Electrical Nerve Stimulation(TENS) therapy and exercise) decreased overall pain intensity better than physiotherapy alone[26]. To explain these results and biological mechanisms, the MTrP should be discussed. The MTrP is a typical indicator and evaluation factor for MPS. The incidence of MPS with associated MTrPs varies between 30% and 85% in patients presenting to pain clinics[27]. Latent ischemia at MTrPs is believed to be a cause of pain. Ischemia creates an acidic environment that lowers acetylcholinesterase levels, enhances the effects of acetylcholine (ACH), and prolongs muscular contraction[5]. Following an acidified environment and prolonged muscle contraction, nociceptive substances (e.g., calcitonin gene-related peptide) are released, causing an increase in the number of ACH receptors and in ACH release[28]. This vicious cycle depletes adenosine triphosphate at trigger points, inhibiting the withdrawal of Ca2+ from muscle fibers. The accumulation of Ca2+ within myocytes is cytotoxic and stimulates inflammatory mediators, increases nociceptive sensitization, and results in severe pain[28,29]. Ion balance is maintained through the exchange of sodium, potassium, calcium, and chloride ions. By directing the movement of ions, the EMF can modify the charge distribution within cells and influence the opening and closing of some voltage-gated calcium channels[13]. Since the accumulation of Ca2+ in MTrPs causes MSP, the stimulation of calcium channels could lead to calcium withdrawal, relaxation of taut muscles, improved blood circulation, and consequently, reduced pain. Sun et al[14] found that PEMF could increase vein blood flow velocity in patients with diabetes, which supports the abovementioned theories. A review article summarized that low-frequency EMF downregulated pro-inflammatory proteins such as nuclear factor kappa B, tumor necrosis factor-α, interleukin (IL)-6, and interferon-γ and upregulated anti-inflammatory cytokines such as IL-4, IL-10, and IL-13, meaning it can eliminate inflammation in MPS[30]. The elimination of inflammation relieves pain[31].
The mechanism underlying the effect of DPP is difficult to explain since its pharmacology has not yet been fully studied. However, the functions of some of the herbs used in DPP have been tested. Radix paeoniae rubra can substantially improve the function of vascular endothelial cells and promote microcirculation[32]. Achyranthis bidentate radix has antioxidant and anti-inflammatory properties and promotes wound healing and angiogenesis[33]. Angelica sinensis radix contains ligustilide, which has a positive effect in chronic inflammatory pain. Many components of Caulis spatholobi exert an analgesic effect through multiple pathways with multiple targets[34], whereas its active ingredients mainly regulate inflammation-related pathways through targets such as PRKCA, IL6, and AKT1 to exert anti-inflammatory effects[35,36].
Since DPP is usually administered with heat to improve efficacy, the thermal effect was maintained in both groups, and symptom relief might be contributed to the thermal effect. When tissue temperature is higher than 41.5℃, heat causes localized blood flow, increasing the availability of nutrients and oxygen, improving tissue elasticity, normalizing tissue pH value, and exerting an analgesic effect[37].
Pain has a sensory and affective dimension[38]; it persists despite blockade of peripheral nociceptive inputs, which represents an effective motivational aspect of pain[39,40]. An example of this is the phenomenon of phantom limb pain. The PRI consists of a sensory and an affective subscale and can discriminate among various pain syndromes[26,41] and evaluate the level of pain after treatment. As summarized by Main[16], quantitative sensory testing offers reliability and precision, which may help identify those likely to develop central pain and may inform on the relationship between psychological factors and pain perception. The PRI results indicated that PEMF therapy combined with heating-DPP treatment was superior to heating-DPP treatment alone in decreasing sensory and affective pain.
However, concluding on whether PEMF therapy and heating-DPP treatment reduce psychological pain (affective pain) based on the PRI score is difficult. Therefore, we analyzed the sensory and affective pain scores separately. As shown in Table 5 and Table 6, PEMF therapy combined with heating-DPP had better results than heating-DPP treatment alone in the treatment of affective and sensory pain. This could be because overall pain intensity, sensory pain, and affective pain interact with each other; therefore, once the overall pain intensity was reduced, sensory and affective pain decreased simultaneously.
PEMF therapy combined with heating-DPP showed better efficacy than heating-DPP alone in reducing overall pain intensity, sensory pain, and affective pain. Distinctively, for affective pain, two weeks of treatment were required for superior clinical efficacy to manifest.
Myofascial pain syndrome (MPS) is a common musculoskeletal disorder. Pulsed electromagnetic field (PEMF) therapy is a modern treatment for MPS, while damp-clearing and pain-reducing paste (DPP) combined with a heating effect has been used as an herbal ointment in China for a very long time.
Both heating-DPP and PEMF are effective in the treatment of MPS. However, their synergistic effects remain unclear.
This manuscript aimed to study whether PEMF therapy combined with heating-DPP is better than heating-DPP alone in the treatment of lumbar MPS.
In total, 120 patients with MPS were randomly assigned to two groups: the experimental (EG) and control (CG) groups. Both groups were treated with heating-DPP combined with PEMF therapy; however, the electromagnetic function was disabled in the therapeutic apparatus used for patients in the CG. All patients received a two-week intervention, and the short-form McGill Pain Questionnaire, which comprises a visual analog scale (VAS), present pain intensity index (PPI), and pain rating index (PRI), was completed by participants at five time points: pre-test, end of the first and second week of receiving treatment, and end of the first and fourth week after completing treatment.
The VAS, PPI, and PRI (total, affective pain, and sensory pain scores) scores of the EG were significantly lesser than those of the CG after treatment and follow-up tests.
PEMF therapy combined with heating-DPP had better efficacy than heating-DPP alone in reducing overall pain intensity, sensory pain, and affective pain.
PEMF therapy plus heating-DPP, with their tested synergistic effects, may be a better option for treating lumbar MPS than heating-DPP alone. Further research on the pharmacology of DPP is needed.
The authors would like to thank all the participants for their cooperation and support.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Rehabilitation
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Akiyama Y, Japan; Nowlan NC, United Kingdom S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Pain terms: a list with definitions and notes on usage. Recommended by the IASP Subcommittee on Taxonomy. Pain. 1979;6:249. [PubMed] |
| 2. | Wheeler AH. Myofascial pain disorders: theory to therapy. Drugs. 2004;64:45-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Galasso A, Urits I, An D, Nguyen D, Borchart M, Yazdi C, Manchikanti L, Kaye RJ, Kaye AD, Mancuso KF, Viswanath O. A Comprehensive Review of the Treatment and Management of Myofascial Pain Syndrome. Curr Pain Headache Rep. 2020;24:43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 4. | Weller JL, Comeau D, Otis JAD. Myofascial Pain. Semin Neurol. 2018;38:640-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Bordoni B, Sugumar K, Varacallo M. Myofascial Pain. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2022. [PubMed] |
| 6. | Mattsson MO, Simkó M. Emerging medical applications based on non-ionizing electromagnetic fields from 0 Hz to 10 THz. Med Devices (Auckl). 2019;12:347-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Cichon N, Synowiec E, Miller E, Sliwinski T, Ceremuga M, Saluk-Bijak J, Bijak M. Effect of Rehabilitation with Extremely Low Frequency Electromagnetic Field on Molecular Mechanism of Apoptosis in Post-Stroke Patients. Brain Sci. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Elshiwi AM, Hamada HA, Mosaad D, Ragab IMA, Koura GM, Alrawaili SM. Effect of pulsed electromagnetic field on nonspecific low back pain patients: a randomized controlled trial. Braz J Phys Ther. 2019;23:244-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Sutbeyaz ST, Sezer N, Koseoglu F, Kibar S. Low-frequency pulsed electromagnetic field therapy in fibromyalgia: a randomized, double-blind, sham-controlled clinical study. Clin J Pain. 2009;25:722-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Cao QW, Peng BG, Wang L, Huang YQ, Jia DL, Jiang H, Lv Y, Liu XG, Liu RG, Li Y, Song T, Shen W, Yu LZ, Zheng YJ, Liu YQ, Huang D. Expert consensus on the diagnosis and treatment of myofascial pain syndrome. World J Clin Cases. 2021;9:2077-2089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (5)] |
| 11. | Paolucci T, Pezzi L, Centra AM, Giannandrea N, Bellomo RG, Saggini R. Electromagnetic Field Therapy: A Rehabilitative Perspective in the Management of Musculoskeletal Pain - A Systematic Review. J Pain Res. 2020;13:1385-1400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Li GS. Study on in iitro Transdernal Absorption and Quality Standard Improvement of Compound Nanxing Acesodyne Plster. 2019. Available from: https://kns-cnki-net-443.webvpn.ecut.edu.cn/kcms/detail/detail.aspx?dbcode=CMFD&dbname=CMFD201902&filename=1019064023.nh&uniplatform=NZKPT&v=ivCMb-ZL1GfdCNe1JdFao9iJOrdWsVbqZ8-QXyj-N6jtwB2u2b4qi3KpMBiif3b8. |
| 13. | Isaković J, Gorup D, Mitrečić D. Molecular mechanisms of microglia- and astrocyte-driven neurorestoration triggered by application of electromagnetic fields. Croat Med J. 2019;60:127-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Sun J, Kwan RL, Zheng Y, Cheing GL. Effects of pulsed electromagnetic fields on peripheral blood circulation in people with diabetes: A randomized controlled trial. Bioelectromagnetics. 2016;37:290-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Yue SW. Musculoskeletal Rehabilitation. 3rd ed. Beijing: People’s Medical Publishing House, 2018: 310-311. |
| 16. | Main CJ. Pain assessment in context: a state of the science review of the McGill pain questionnaire 40 years on. Pain. 2016;157:1387-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 17. | Burckhardt CS, Bjelle A. A Swedish version of the short-form McGill Pain Questionnaire. Scand J Rheumatol. 1994;23:77-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken). 2011;63 Suppl 11:S240-S252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2308] [Cited by in RCA: 2974] [Article Influence: 228.8] [Reference Citation Analysis (0)] |
| 19. | Strand LI, Ljunggren AE, Bogen B, Ask T, Johnsen TB. The Short-Form McGill Pain Questionnaire as an outcome measure: test-retest reliability and responsiveness to change. Eur J Pain. 2008;12:917-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Zhai J, Mu W, Si J, Li Y, Zhao C, Shang H, Li H, Tian G. Acupuncture for constipation in patients with stroke: protocol of a systematic review and meta-analysis. BMJ Open. 2018;8:e020400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Ruoff GE, Rosenthal N, Jordan D, Karim R, Kamin M; Protocol CAPSS-112 Study Group. Tramadol/acetaminophen combination tablets for the treatment of chronic lower back pain: a multicenter, randomized, double-blind, placebo-controlled outpatient study. Clin Ther. 2003;25:1123-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 124] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1:277-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4782] [Cited by in RCA: 4425] [Article Influence: 88.5] [Reference Citation Analysis (0)] |
| 23. | Alharbi HA, Albabtain MA, Alobiad N, Aba Alhasan J, Alruhaimi M, Alnefisah M, Alateeq S, Alghosoon H, Alarfaj SJ, Arafat AA, Algarni KD. Pain perception assessment using the short-form McGill pain questionnaire after cardiac surgery. Saudi J Anaesth. 2020;14:343-348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Peter D, Patrick H, Liang KY, Scott Z. Analysis of longitudinal data. 2nd ed. USA: Oxford University Press, 2013. |
| 25. | Chang MY, Yeh SC, Chu MC, Wu TM, Huang TH. Associations between Tai Chi Chung program, anxiety, and cardiovascular risk factors. Am J Health Promot. 2013;28:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2935] [Cited by in RCA: 3046] [Article Influence: 80.2] [Reference Citation Analysis (0)] |
| 27. | Han SC, Harrison P. Myofascial pain syndrome and trigger-point management. Reg Anesth. 1997;22:89-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 145] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Bron C, Dommerholt JD. Etiology of myofascial trigger points. Curr Pain Headache Rep. 2012;16:439-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 29. | Simons DG. New views of myofascial trigger points: etiology and diagnosis. Arch Phys Med Rehabil. 2008;89:157-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 30. | Ross CL, Syed I, Smith TL, Harrison BS. The regenerative effects of electromagnetic field on spinal cord injury. Electromagn Biol Med. 2017;36:74-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Bruehl S. Complex regional pain syndrome. BMJ. 2015;351:h2730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 236] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 32. | Tan YQ, Chen HW, Li J, Wu QJ. Efficacy, Chemical Constituents, and Pharmacological Actions of Radix Paeoniae Rubra and Radix Paeoniae Alba. Front Pharmacol. 2020;11:1054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 33. | Wu L, Hao Y, Dai C, Zhang Z, Ijaz M, Ibrahim SM, Murtaza G, Yao Z. Network Pharmacological Study of Achyranthis bidentatae Radix Effect on Bone Trauma. Biomed Res Int. 2021;2021:5692039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Zhao LX, Jiang BC, Wu XB, Cao DL, Gao YJ. Ligustilide attenuates inflammatory pain via inhibition of NFκB-mediated chemokines production in spinal astrocytes. Eur J Neurosci. 2014;39:1391-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 35. | Deng ZJ, Li AR, Lou YJ, Li jh, Guo JW, Huang CF, Zhong JF, Liu RX. [Based on network pharmacology technology to study the mechanism of Ji Xueteng in treating pain]. Re Dai Yi Xue. 2021;21:835-840. |
| 36. | Zheng SC, Wang Q, He XY, Wang JN, Li H, Dang BY, Liu Y, Zhang F, Zhang Z, Yang GL. Mechanism of Caulis Spatholobi in Treatment of AS Based on Network Pharmacology. Liao Ning Zhong Yi Yao Da Xue Xue Bao. 2019;21:100-103. [DOI] [Full Text] |
| 37. | Giombini A, Giovannini V, Di Cesare A, Pacetti P, Ichinoseki-Sekine N, Shiraishi M, Naito H, Maffulli N. Hyperthermia induced by microwave diathermy in the management of muscle and tendon injuries. Br Med Bull. 2007;83:379-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 38. | Talbot K, Madden VJ, Jones SL, Moseley GL. The sensory and affective components of pain: are they differentially modifiable dimensions or inseparable aspects of a unitary experience? Br J Anaesth. 2019;123:e263-e272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 39. | Baliki MN, Apkarian AV. Nociception, Pain, Negative Moods, and Behavior Selection. Neuron. 2015;87:474-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 463] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 40. | Auvray M, Myin E, Spence C. The sensory-discriminative and affective-motivational aspects of pain. Neurosci Biobehav Rev. 2010;34:214-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 41. | Rasmussen PV, Sindrup SH, Jensen TS, Bach FW. Symptoms and signs in patients with suspected neuropathic pain. Pain. 2004;110:461-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 183] [Article Influence: 8.7] [Reference Citation Analysis (0)] |