Published online Nov 6, 2022. doi: 10.12998/wjcc.v10.i31.11500
Peer-review started: May 13, 2022
First decision: August 4, 2022
Revised: August 9, 2022
Accepted: October 9, 2022
Article in press: October 9, 2022
Published online: November 6, 2022
Processing time: 166 Days and 15 Hours
Polycystic kidney disease (PKD) is a genetic disorder characterized by the growth of numerous cysts within the kidneys. Disease progress of some patients often occurs at the early stage. Thus, managing and controlling disease progress is important to slow the kidney function decline especially for the patient with other disorders.
One 80-year-old male autosomal dominant polycystic kidney disease (ADPKD) patient with chronic kidney disease and other clinical disorders was treated with tolvaptan and edoxaban. Estimated glomerular filtration rate, creatinine and uric acid were monitored during the treatment. In addition, the whole exome sequencing was performed to screen ADPKD genetic variants. The kidney function decline was prevented after using tolvaptan and edoxaban treatment and in the meantime, a venous thromboembolism was removed and leg and pedal edema were alleviated. One mutation c.10102G>A /p.D3368N in the PKD1 gene was identified.
Tolvaptan combined with edoxaban administration could delay kidney function decline and eliminate the edema caused by the thromboembolism.
Core Tip: Autosomal dominant polycystic kidney disease is a genetic and heritable kidney disease. It appears with cyst formation and growth, kidney enlargement and eventually leads to end stage renal disease. Some patients with severe polycystic kidney disease and end stage kidney disease have been treated with nephrectomy and early dialysis therapy, however, the life quality of these autosomal dominant polycystic kidney disease (ADPKD) patients declines due to the above treatment. In this case report, tolvaptan combined with edoxaban administration improved kidney function for one elderly ADPKD patient while avoiding the decreased life quality from nephrectomy or dialysis therapy.
- Citation: Zhou L, Tian Y, Ma L, Li WG. Tolvaptan ameliorated kidney function for one elderly autosomal dominant polycystic kidney disease patient: A case report. World J Clin Cases 2022; 10(31): 11500-11507
- URL: https://www.wjgnet.com/2307-8960/full/v10/i31/11500.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i31.11500
Autosomal dominant (AD) polycystic kidney disease (PKD) is a genetic and heritable kidney disease. This disease appears with cyst formation and growth, kidney enlargement and eventually leads to end stage renal disease. Most cases are caused by the heterozygosity of the pathogenic variant PKD1 or PKD2[1]. In addition, heterozygosity of variants in GANAB and DNAJB11 were also reported to cause autosomal dominant polycystic kidney disease in 0.3% and 0.1% of families, respectively[2,3]. However, the molecular mechanisms underlying the renal dysfunction resulted from mutations in ADPKD genes are still unclear and needs to be further investigated.
Some patients with severe PKD and end stage kidney disease have been treated with nephrectomy and early dialysis therapy. However, nephrectomy has a high risk due to refractory hypotension because of post-operative loss of renin secretion[4]. The life quality of the ADPKD patients declines due to restraint time and access surgery for dialysis therapy. The surgery and the dialysis treatment also add the medical expenses for the patients and their family[5]. Therefore, delayed initiation of dialysis in chronic kidney disease (CKD) patients is thought meaningful. It is critical to choose and determine the appropriate administration strategy for patients with aggressive, progressive kidney disease to prevent kidney function decline and complication occurrence.
Tolvaptan (TLV) is an oral selective arginine vasopressin type V2 receptor antagonist that has shown an ability to slow the rapidly progressing PKD and has been approved to treat ADPKD in the European Union, Japan, South Korea, Canada and the United States[6]. The efficacy of TLV has been reported for patients with CKD in other studies[7,8]. Recently, the administration of TLV also demonstrated that it decreased the worsening of renal function[9,10]. Furthermore, TLV therapy was more effective in maintaining renal function than treatment with increasing doses of loop diuretics because TLV has a strong diuretic effect. It can reduce edema and won’t destroy the blood electrolyte balance[11,12]. In this case report, one elderly male ADPKD patient who had been diagnosed with CKD and other complications, including venous thromboembolism, was treated with TLV. Considering the patient had leg edema plus venous thrombosis of the lower extremities, edoxaban was also administered to this patient since edoxaban could reduce thromboembolism in the patient with CKD as an oral anticoagulant[13]. Follow up therapeutic results of this patient demonstrated that the combined administration of TLV and edoxaban could improve kidney function for this elderly patient and reduce the edema accompanied with venous thromboembolism alleviation.
One 80-year-old, male ADPKD patient with leg edema and pedal edema presented at China-Japan Friendship Hospital in November 2020.
The patient had a distended abdomen accompanied by leg edema and pedal edema. He had CKD.
This patient had 14 years of a cerebral infarction, 30 years of PKD, and 20 years of hypertension. In addition, this patient had other clinical disorders such as renal anemia, hyperlipidemia, arteriosclerosis obliterans, lacunar infarction and type 2 diabetes.
This patient had ADPKD. One family member, the patient’s daughter, was included in this case report study for the whole exome sequencing in order to screen the ADPKD genetic variants.
This patient’s body weight was 62.5 kg and his height was 170 cm. Blood pressure was 140/80 mmHg. The distended abdomen was observed and both leg edema and pedal edema were also found.
Estimated glomerular filtration rate (eGFR) was calculated according to the CKD-EPI creatinine (CR) equation[14]. Hemoglobin (HGB), albumin and total protein were examined. Liver function and kidney function were evaluated by measuring alanine aminotransferase (ALT), aspartate transaminase (AST), CR and uric acid (UA). Coagulation-associated molecules, such as fibrinogen, D-dimer, fibrin degra
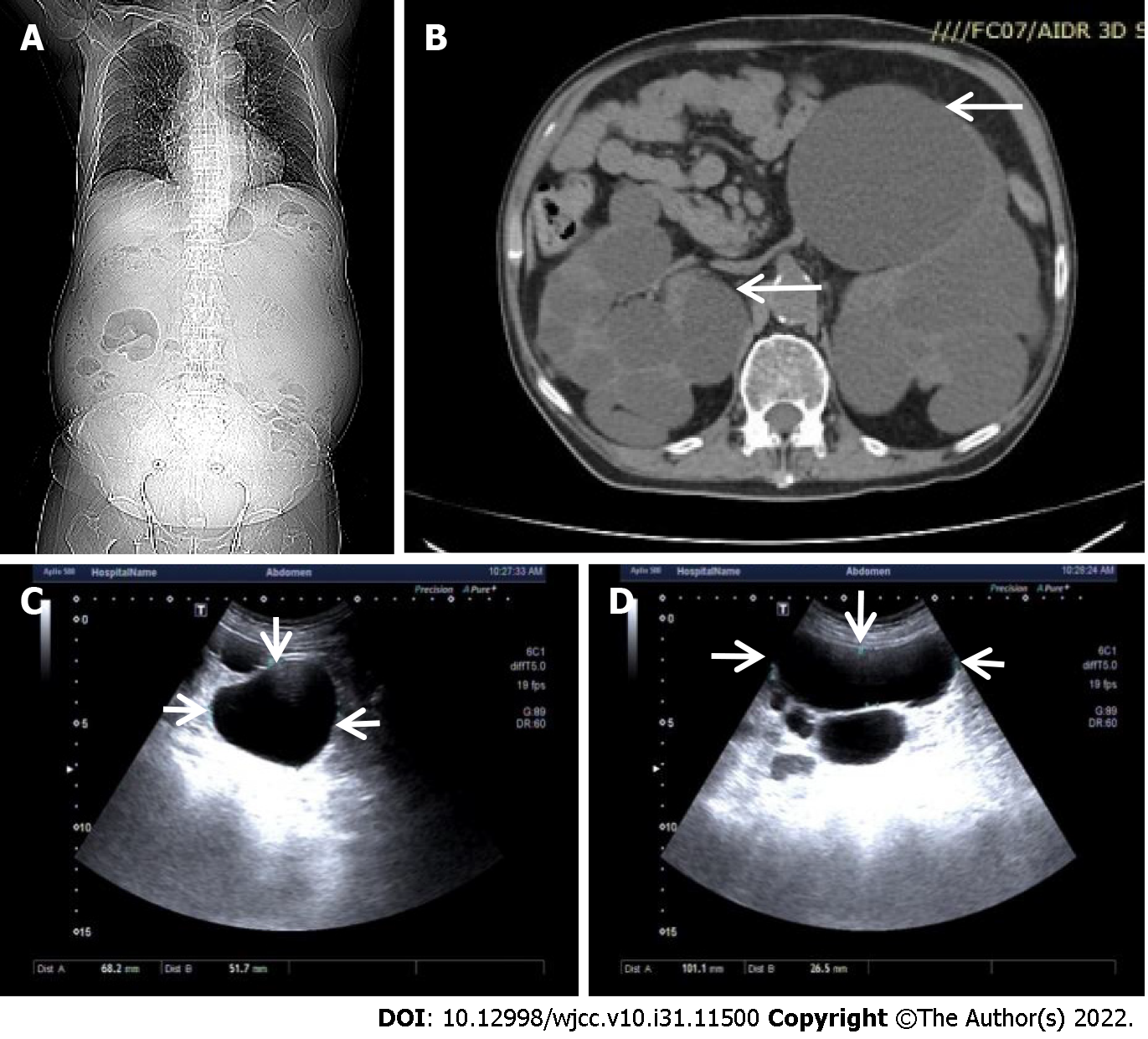
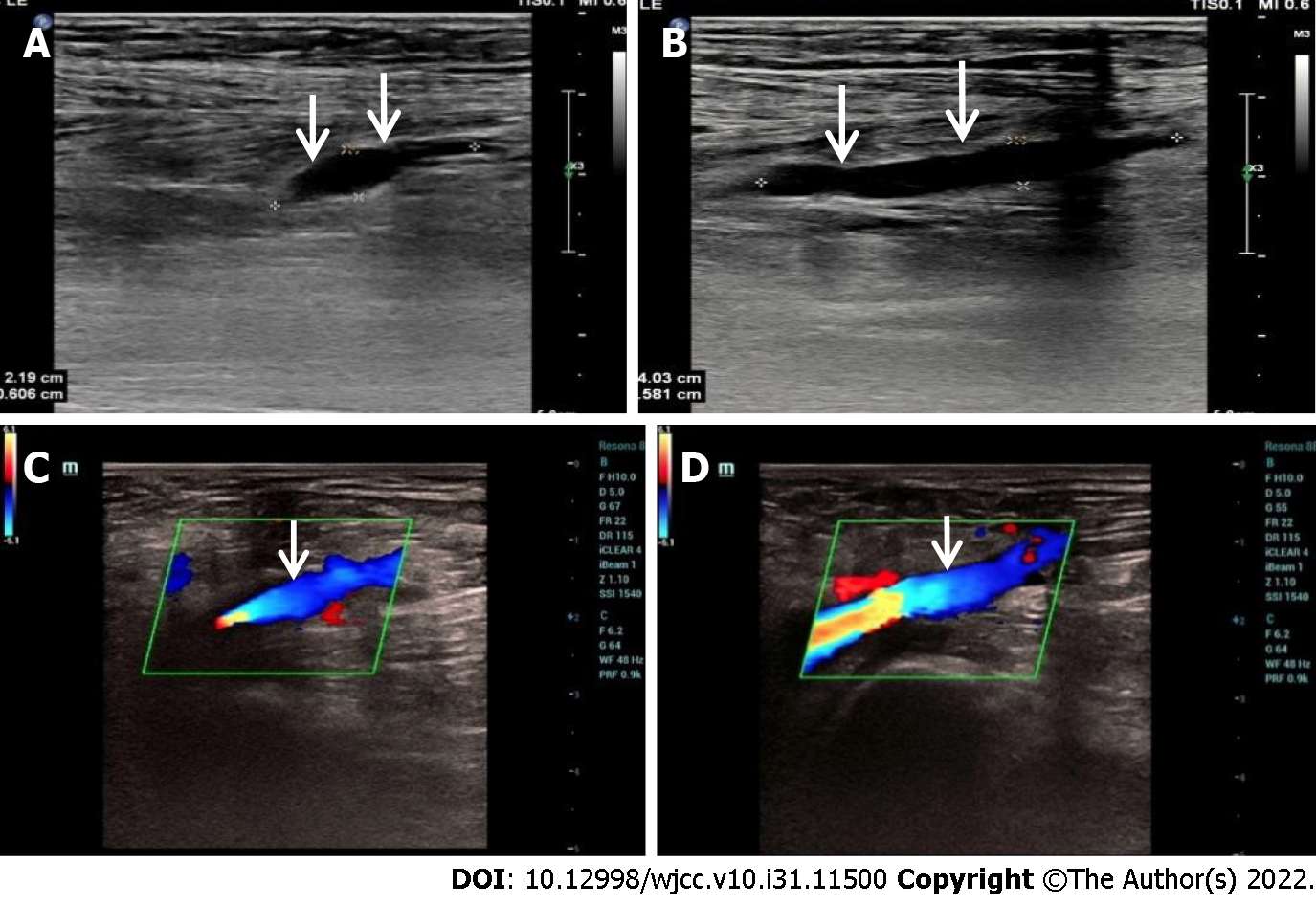
Computed tomography and abdominal ultrasound were performed on this patient and the kidney size and multiple cysts were measured (Figure 1). Venous thrombosis of the lower extremities was also examined by ultrasound (Figure 2).
The peripheral blood of this patient and one family member was collected for the whole exome sequencing analysis to screen ADPKD associated single nucleotide polymorphism (SNP), inser
Chronic kidney dysfunction accompanied by venous thrombosis of the lower extremities.
TLV was used as a diuretic to enhance the ability of the kidneys to process water. In the meantime, edoxaban was used as an oral anticoagulant to treat venous thrombosis to alleviate the lower extremity and pedal edema. Besides TLV and edoxaban, Roxadustat was used to treat anemia and other medicines were also administered to cure other diseases.
The doses of TLV and edoxaban were adjusted according to the clinical symptoms and the corresponding administration strategy. At the treatment initiation, 7.5 mg of TLV and 15 mg of edoxaban were used daily. In the second month, edoxaban dosage was increased to 30 mg daily due to a new venous thrombosis formation. After that, 30 mg of edoxaban was the daily maintenance dose without change. At the beginning of the third month, TLV dosage was adjusted to 15 mg per day because the CR level was still high. Five months later, kidney function tended to be stable and both the edema and thrombosis had disappeared. TLV dosage was increased to restrict the growth of cysts. TLV was used twice per day, 15 mg in the morning and 7.5 mg in the evening. After one year, TLV was administered twice per day, 15 mg each time.
After the combination treatment of TLV and edoxaban, the patient’s health status was improved. Total protein, albumin and HGB were within normal ranges and renal anemia had been cured. The bilateral lower extremity edema had disappeared. So far, this patient has been followed up for 15 mo.
Sequencing data was about 10G and the average sequencing depth was 140X. Based on the whole exome sequencing data of this patient and one family member, one mutation was found for the gene PKD1 (NM_001009944.2) c.10102G>A /p.D3368N. According to the ACMG Criteria and Guidelines for variant analysis, this genetic variant has been recorded in the HGMD database, and the frequency of this variant in the GnomAD database is 0.000250 (63/251880). MutationTaster predicted that this variant may be harmful, while Polyphen-2_HVAR and SIFT predicted it is not a harmful variant and this variant was defined as a variant of uncertain significance (VUS). All variants defined as VUS were shown in Table 1. Raw data is available via https://www.biosino.org/download/node/data/OED732748.
| Gene name (reference transcript) | Chromosome location (GRCh37/hg19) | Nucleotide/amino acid variant | Allele genotype | Variant classification | |
| Patient | PKD1 (NM_001009944.2) | Chr16:2147934 | c.10102G>A/p.D3368N | GA | VUS |
| PKHD1 (NM_128694.3) | Chr6:51890599 | c.4009G>A/p.D1337N | GA | VUS | |
| Family member | PKD1 (NM_001009944.2) | Chr16:2147934 | c.10102G>A/p.D3368N | GA | VUS |
| PKD1 (NM_001009944.2) | Chr16:2169353 | c.242C>T/p.A81V | CT | VUS | |
| PKHD1 (NM_128694.3) | Chr6:51890599 | c.4009G>A/p.D1337N | GA | VUS |
CT imaging demonstrated a relatively small chest and a distended abdomen with massively enlarged kidneys, shown in Figure 1A. Multiple cysts were observed within the bilateral kidneys, representative imaging shown in Figure 1B. The abdominal ultrasound also showed the enlarged echogenic kidney with multiple cysts; right kidney size is 19.2 cm × 13.8 cm 12.0 cm and left kidney size is 21.8 cm × 12.8 cm × 12.7 cm. The largest cyst on the right kidney is 6.8 cm × 5.2 cm; the largest cyst on the left kidney is 10.1 cm × 2.7 cm, as shown in Figure 1C and D. Venous thrombosis of the lower extremities was observed, shown in Figure 2A and B; leg edema and pedal edema were attributed to the obstructed venous return. The thrombosis disappeared after a few months of treatment, shown in Figure 2C and D.
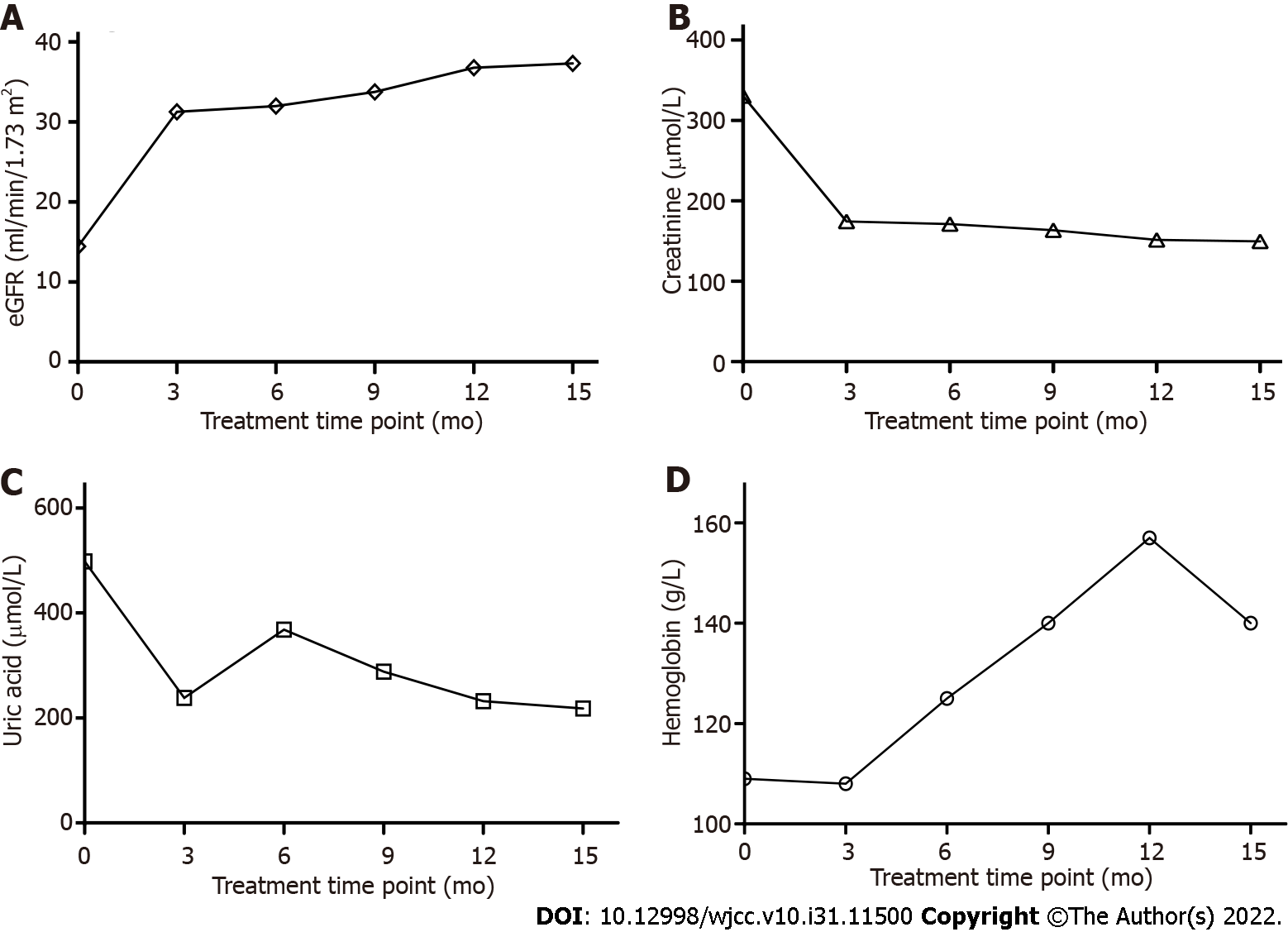
After the patient was administered TLV and edoxaban for 3 mo, the venous thrombosis in the lower extremities was removed as shown in Figure 2C and D. The eGFR was raised from 14.43 mL/min/1.73 m2 to 31.25 mL/min/1.73 m2, which is shown in Figure 3A. CR and UA were obviously reduced shown in Figure 3B and C. In addition, HGB was increased compared with the beginning of treatment which meant the renal anemia was relieved and the data was shown in Figure 3D.
Autosomal dominant PKD is one common monogenic disease which can lead to end stage renal failure and occurs in 1:400 to 1:1000 individuals worldwide. It is difficult to estimate the precise prevalence for ADPKD because its progression rate and severity vary among the patients[15]. The cysts compress and destroy the kidney tissue resulting in a progressive loss of function in a few decades. About 50% of patients at the age of 60 to 70 progress to end stage kidney disease[16]. For the patients with end stage kidney disease, kidney transplantation is a good therapeutic option to improve the quality of life. Since the incidence of renal cancer of the PKD patients is higher when comparing with the healthy population, it would be important to implement tumor screening for PKD patients. Moreover, there is a potential risk of transmission of cancer from donor to recipient[17]. Thus, it may be necessary to obtain and estimate the donor history when performing kidney transplantation for the patient with ESRD.
In this case report, the patient has 30 years of autosomal dominant PKD with CKD. According to the whole exome sequencing result, only c.10102G>A /p.D3368N variant on PKD1 and c.4009G>A /p.D1337N on PKHD1 were identified as variants of uncertain significance. However, whether they are potentially pathogenic is unclear because one family member of this patient with the same variants does not have PKD. The kidney function examination results indicated there was acute kidney injury with higher CR and UA, moreover severe leg and pedal edema was also observed. The edema was usually managed with furosemide, however, taking this medicine may lead to kidney overload and further impair renal function. Since TLV has been approved as a diuretic drug to combat hyponatremia and slow kidney function decline, TLV was used to treat this patient to protect the kidneys against further damage.
TLV was started at 7.5 mg per day. Water intake was limited at 1.5 to 2 liters per day. Because of hypernatremia concerns, the TLV dose was slowly increased to 15 mg. Serum sodium and potassium were consistently within normal range. Because TLV may cause liver damage side effect[18], the liver function was evaluated by measuring ALT and AST, both of them were within normal ranges during treatment. At the beginning of treatment, serum CR concentration was remarkably exceeding normal range, indicating acute kidney injury occurrence. After 3 mo of TLV administration, CR had fallen to half the level at the treatment initiation and decreased to a normal range after 15 mo.
eGFR is one important indicator for realistic reflection of kidney function and is most commonly used for determining stages for CKD[19]. eGFR was less than 15 ml/min/1.73 m2 at the treatment initiation, demonstrating this patient was at Stage 5 of CKD, according to National Kidney Foundation developed criteria, Kidney Disease Outcomes Quality Initiative (NKF KDOQI™)[20]. After 15 mo, eGFR increased to 37.32 mL/min/1.73 m2 which is within eGFR range at Stage 3 of CKD. Thus, after TLV treatment, the kidney function of this patient was recovered and protected against further injury, though kidney size and cyst size had no obvious change according to the ultrasound results, which may indicate that the increased TLV could limit the cyst growth. Kidney size and cyst size are unlikely to get smaller as this elderly patient has a longer ADPKD history which usually makes the kidney and cyst enlargement irreversible. For this elderly patient, preserving residual renal function (RRF) is an important aim. The loop diuretic furosemide is typically used in the treatment of heart failure and CKD but reducing RRF is often observed[21]. Thus, TLV has advantages over furosemide to administer to this elderly patient with end stage renal disease (ESRD).
Ultrasound examination on bilateral legs showed right tibial and peroneal venous thrombosis and right lower extremity atherosclerosis with plaque formation at the beginning of treatment, which was attributed to the obstruction of venous return leading to the edema. In the meantime, edoxaban was started at 15 mg per day to treat venous embolism and the dose was adjusted based on the embolus size and the patient’s condition. After the patient took TLV and edoxaban for a few months, the blood flow in the deep veins of the bilateral lower extremities ran smoothly and no obvious thrombosis was found in the deep veins of the bilateral lower extremities by ultrasound examination. The leg and pedal edemas disappeared. Edoxaban exhibits several advantages for the treatment and prevention of venous thromboembolism[22,23]. Especially, it exhibited safety and efficacy when used to treat an over 80- year- old patient with poor renal function. The patient recovered from CKD and his condition improved.
In this case report, the patient was administered TLV and edoxaban for 15 mo. His kidney function decline was delayed, acute kidney injury was recovered, kidney failure was prevented and dialysis therapy was avoided. The life quality of the patient was maintained and the aggressive progress of CKD to the end stage of kidney disease was prevented. TLV was safe and effective to slow CKD progress when it was used to treat this elderly ADPKD patient with chronic kidney dysfunction and deep vein thrombosis in the legs. Therefore, TLV could be useful for elderly ADPKD patients to protect kidney disease progress and alleviate complications.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Alsaidan A, Saudi Arabia; Eccher A, Italy; Moreno-
| 1. | Harris PC, Torres VE. Polycystic kidney disease. Annu Rev Med. 2009;60:321-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 681] [Cited by in RCA: 605] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 2. | Porath B, Gainullin VG, Cornec-Le Gall E, Dillinger EK, Heyer CM, Hopp K, Edwards ME, Madsen CD, Mauritz SR, Banks CJ, Baheti S, Reddy B, Herrero JI, Bañales JM, Hogan MC, Tasic V, Watnick TJ, Chapman AB, Vigneau C, Lavainne F, Audrézet MP, Ferec C, Le Meur Y, Torres VE; Genkyst Study Group, HALT Progression of Polycystic Kidney Disease Group; Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease, Harris PC. Mutations in GANAB, Encoding the Glucosidase IIα Subunit, Cause Autosomal-Dominant Polycystic Kidney and Liver Disease. Am J Hum Genet. 2016;98:1193-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 325] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 3. | Cornec-Le Gall E, Olson RJ, Besse W, Heyer CM, Gainullin VG, Smith JM, Audrézet MP, Hopp K, Porath B, Shi B, Baheti S, Senum SR, Arroyo J, Madsen CD, Férec C, Joly D, Jouret F, Fikri-Benbrahim O, Charasse C, Coulibaly JM, Yu AS, Khalili K, Pei Y, Somlo S, Le Meur Y, Torres VE; Genkyst Study Group; HALT Progression of Polycystic Kidney Disease Group; Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease, Harris PC. Monoallelic Mutations to DNAJB11 Cause Atypical Autosomal-Dominant Polycystic Kidney Disease. Am J Hum Genet. 2018;102:832-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 215] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 4. | Desai PJ, Castle EP, Daley SM, Swanson SK, Ferrigni RG, Humphreys MR, Andrews PE. Bilateral laparoscopic nephrectomy for significantly enlarged polycystic kidneys: a technique to optimize outcome in the largest of specimens. BJU Int. 2008;101:1019-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Gilbert RD, Sukhtankar P, Lachlan K, Fowler DJ. Bilineal inheritance of PKD1 abnormalities mimicking autosomal recessive polycystic disease. Pediatr Nephrol. 2013;28:2217-2220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Chebib FT, Perrone RD, Chapman AB, Dahl NK, Harris PC, Mrug M, Mustafa RA, Rastogi A, Watnick T, Yu ASL, Torres VE. A Practical Guide for Treatment of Rapidly Progressive ADPKD with Tolvaptan. J Am Soc Nephrol. 2018;29:2458-2470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 174] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 7. | Muto S, Okada T, Shibasaki Y, Ibuki T, Horie S. Effect of tolvaptan in Japanese patients with autosomal dominant polycystic kidney disease: a post hoc analysis of TEMPO 3:4 and TEMPO Extension Japan. Clin Exp Nephrol. 2021;25:1003-1010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Tanaka A, Katsuno T, Ozaki T, Sakata F, Kato N, Suzuki Y, Kosugi T, Kato S, Tsuboi N, Sato W, Yasuda Y, Mizuno M, Ito Y, Matsuo S, Maruyama S. The efficacy of tolvaptan as a diuretic for chronic kidney disease patients. Acta Cardiol. 2015;70:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Tanaka A, Hiramatsu E, Watanabe Y, Ito C, Shinjo H, Otsuka Y, Takeda A. Efficacy of Long-Term Treatment With Tolvaptan to Prolong the Time Until Dialysis Initiation in Patients With Chronic Kidney Disease and Heart Failure. Ther Apher Dial. 2019;23:319-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Futamura Y, Watanuki H, Okada M, Sugiyama K, Matsuyama K. The Efficacy and Renal Protective Effect of Tolvaptan in Chronic Kidney Disease Patients after Open-Heart Surgery. Ann Thorac Cardiovasc Surg. 2021;27:317-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Hanatani A, Shibata A, Kitada R, Iwata S, Matsumura Y, Doi A, Sugioka K, Takagi M, Yoshiyama M. Administration of tolvaptan with reduction of loop diuretics ameliorates congestion with improving renal dysfunction in patients with congestive heart failure and renal dysfunction. Heart Vessels. 2017;32:287-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Torres VE, Gansevoort RT, Perrone RD, Chapman AB, Ouyang J, Lee J, Japes H, Nourbakhsh A, Wang T. Tolvaptan in ADPKD Patients With Very Low Kidney Function. Kidney Int Rep. 2021;6:2171-2178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Grandone E, Aucella F, Barcellona D, Brunori G, Forneris G, Gresele P, Marietta M, Poli D, Testa S, Tripodi A, Genovesi SC. Position paper on the safety/efficacy profile of direct oral anticoagulants in patients with chronic kidney disease. Consensus document from the SIN, FCSA and SISET. Blood Transfus. 2020;18:478-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 14. | Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS; CKD-EPI Investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2913] [Cited by in RCA: 3164] [Article Influence: 243.4] [Reference Citation Analysis (0)] |
| 15. | Suwabe T, Shukoor S, Chamberlain AM, Killian JM, King BF, Edwards M, Senum SR, Madsen CD, Chebib FT, Hogan MC, Cornec-Le Gall E, Harris PC, Torres VE. Epidemiology of Autosomal Dominant Polycystic Kidney Disease in Olmsted County. Clin J Am Soc Nephrol. 2020;15:69-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 16. | Chapman AB. Approaches to testing new treatments in autosomal dominant polycystic kidney disease: insights from the CRISP and HALT-PKD studies. Clin J Am Soc Nephrol. 2008;3:1197-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Eccher A, Girolami I, Motter JD, Marletta S, Gambaro G, Momo REN, Nacchia F, Donato P, Boschiero L, Boggi U, Lombardini L, Cardillo M, D'Errico A, Neil D, Segev DL, Zaza G. Donor-transmitted cancer in kidney transplant recipients: a systematic review. J Nephrol. 2020;33:1321-1332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Watkins PB, Lewis JH, Kaplowitz N, Alpers DH, Blais JD, Smotzer DM, Krasa H, Ouyang J, Torres VE, Czerwiec FS, Zimmer CA. Clinical Pattern of Tolvaptan-Associated Liver Injury in Subjects with Autosomal Dominant Polycystic Kidney Disease: Analysis of Clinical Trials Database. Drug Saf. 2015;38:1103-1113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 152] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 19. | Turin TC, Hemmelgarn BR. Improvement in kidney function: a real occurrence. J Am Soc Nephrol. 2012;23:575-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1731] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 21. | Mori T, Oba I, Koizumi K, Kodama M, Shimanuki M, Tanno M, Chida M, Saito M, Kiyomoto H, Miyazaki M, Ogawa S, Sato H, Ito S. Beneficial role of tolvaptan in the control of body fluids without reductions in residual renal function in patients undergoing peritoneal dialysis. Adv Perit Dial. 2013;29:33-37. [PubMed] |
| 22. | Feldberg J, Patel P, Farrell A, Sivarajahkumar S, Cameron K, Ma J, Battistella M. A systematic review of direct oral anticoagulant use in chronic kidney disease and dialysis patients with atrial fibrillation. Nephrol Dial Transplant. 2019;34:265-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Su X, Yan B, Wang L, Lv J, Cheng H, Chen Y. Oral Anticoagulant Agents in Patients With Atrial Fibrillation and CKD: A Systematic Review and Pairwise Network Meta-analysis. Am J Kidney Dis. 2021;78:678-689.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |