Published online Nov 6, 2022. doi: 10.12998/wjcc.v10.i31.11493
Peer-review started: May 7, 2022
First decision: June 27, 2022
Revised: July 27, 2022
Accepted: September 23, 2022
Article in press: September 23, 2022
Published online: November 6, 2022
Processing time: 172 Days and 5.6 Hours
Aorto-esophageal fistula is an extremely rare cause of acute upper gastrointestinal bleeding (UGIB).
We present a case of an 80-year-old woman with esophageal cancer who was admitted to our department with hemorrhagic shock due to UGIB. During the diagnostic procedure, emergency computed tomography angiography was performed and confirmed aorto-esophageal fistula. Interventional radiologists inserted a stent graft into the aorta, successfully closing the fistula. Unfortunately, the patient later died of heart failure following irreversible hemorrhagic shock. Autopsy confirmed the aorto-esophageal fistula, which formed 1 cm below the distal edge of the stent previously inserted into the esophagus for a malignant stricture.
There are very rare causes of UGIB. Although clinical decisions are made during the diagnostic workup of these patients, we must be aware of the limitations of various therapeutic options, even the most contemporary.
Core Tip: Acute upper gastrointestinal bleeding (UGIB) is a life-threatening condition. Although most UGIB cases have a benign course, only a few have a severe and fatal outcome. Improved diagnostic and therapeutic options have been made possible, in particular, by technological advances in interventional endoscopy, radiology and minimally invasive, laparoscopic surgery. In the paper, the authors present a patient with UGIB caused by an aorto-esophageal fistula, which formed due to a stent inserted into the middle third of the esophagus for advanced cancer. Despite appropriate and timely clinical decisions, the outcome of treatment was fatal.
- Citation: Ćeranić D, Nikolić S, Lučev J, Slanič A, Bujas T, Ocepek A, Skok P. Fatal bleeding due to an aorto-esophageal fistula: A case report and literature review . World J Clin Cases 2022; 10(31): 11493-11499
- URL: https://www.wjgnet.com/2307-8960/full/v10/i31/11493.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i31.11493
Acute upper gastrointestinal bleeding (UGIB) is a life-threatening condition we encounter every day in emergency medicine. Despite the progress made in the pharmacological treatment of ulcer diseases, peptic duodenal or stomach ulcers remain the leading cause of UGIB[1,2]. Other common causes of UGIB include ruptured esophageal and/or stomach varices, Mallory-Weiss syndrome (laceration at the site of esophago-gastric junction), and hemorrhagic erosion of the upper gastrointestinal mucosa. Dieulafoy’s lesion, angiodysplasia, and aorto-enteric fistula are rare causes of UGIB[3-5]. Acute UGIB is a condition that requires careful clinical evaluation, effective early symptomatic treatment, targeted diagnostic procedures to identify the cause of bleeding, and effective hemostatic techniques[2]. Despite the established recommendations for the treatment of these patients as well as improved diagnostic and therapeutic options enabled by advanced interventional methods in the fields of endoscopy, radiology and minimally invasive surgery, we occasionally face challenges that cannot always be overcome successfully and in a timely manner[6-8].
We present a patient with fatal UGIB caused by an aorto-esophageal fistula, which formed after a stent was inserted into the middle third of the esophagus for advanced cancer.
An 80-year-old female patient with carcinoma of the middle third of the esophagus was admitted to the Department of Gastroenterology for hemorrhagic shock, following UGIB presenting with hematemesis.
Four months prior to admission, the patient was diagnosed with squamous cell carcinoma, assessed as stage T4N2M1 in the middle third of the esophagus. Due to the patient’s age and associated diseases, conservative nonsurgical treatment was decided by the multidisciplinary council. Palliative radiotherapy was performed TD 36 Gy in total after dose fractionation 12 × 3 Gy. Immediately after the completion of palliative radiotherapy, an esophageal self-expandable, fully covered nitinol stent preloaded in a delivery system (type SX-ELLA 85) was inserted across the malignant stricture.
In recent years, the patient was treated for heart failure, atrial fibrillation, arterial hypertension, and hyperlipidemia. Her regular therapy regimen included bisoprolol fumarate, enalapril, fluvastatin, and warfarin, which is an anticoagulant medication.
In the past, the patient was successfully treated with surgery and radio-chemotherapy for breast cancer 15 years ago. During follow-up, no signs of disease recurrence were confirmed.
At admission, the patient was found to have relative hypotension (blood pressure: 114/51), absolute arrhythmia (heart rate: 88 beats/min), signs of anemia, traces of blood in her mouth, and massive melena with blood clots in the rectal ampulla.
A complete blood count (CBC) confirmed anemia: Red blood cell count (RBC) was 2.6 × 1012, hemoglobin (HGB) value was 75 g/L, prothrombin time was 0.35, and international normalized ratio was 2.03.
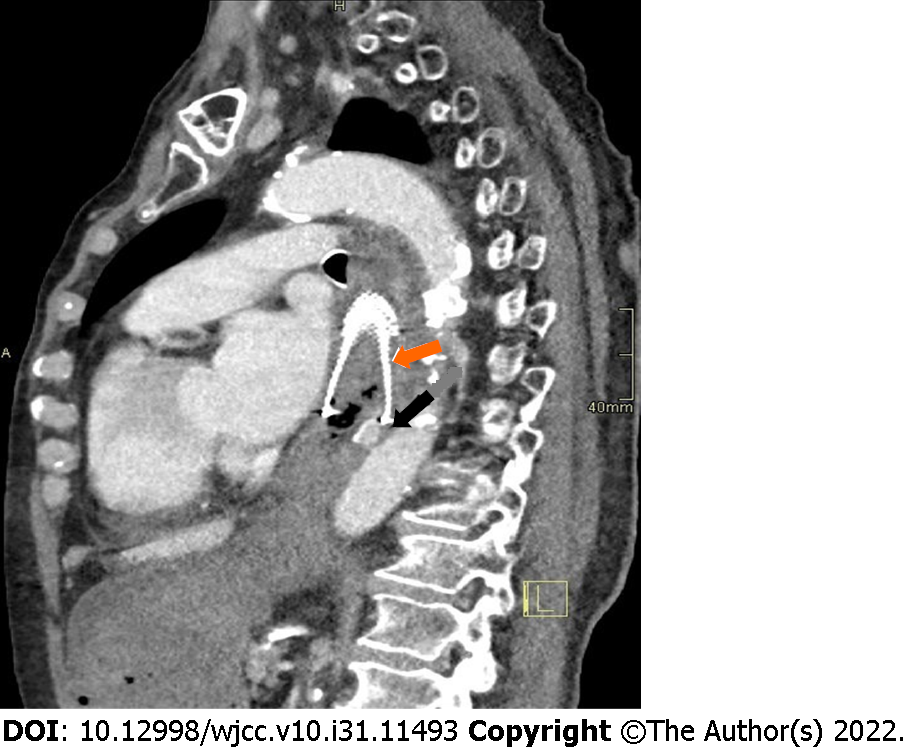
After initial resuscitation, including the placement of central venous access through the right femoral vein, delivery of crystalloid solutions, intravenous medications (the proton pump inhibitor pantoprazole, the antifibrinolytic agent tranexamic acid, the antiemetic thiethylperazine, and vitamin K preparation), transfusions, and infusions of fresh frozen plasma, we performed emergency computed tomography (CT) angiography. CT imaging confirmed bleeding through the aorto-esophageal fistula 1 cm distal to the distal edge of the esophageal stent (Figure 1). The recommended treatment was a stent graft placed into the aorta to close the fistula.
CT imaging confirmed bleeding through the aorto-esophageal fistula one centimeter distal to the distal edge of the esophageal stent (Figure 1).
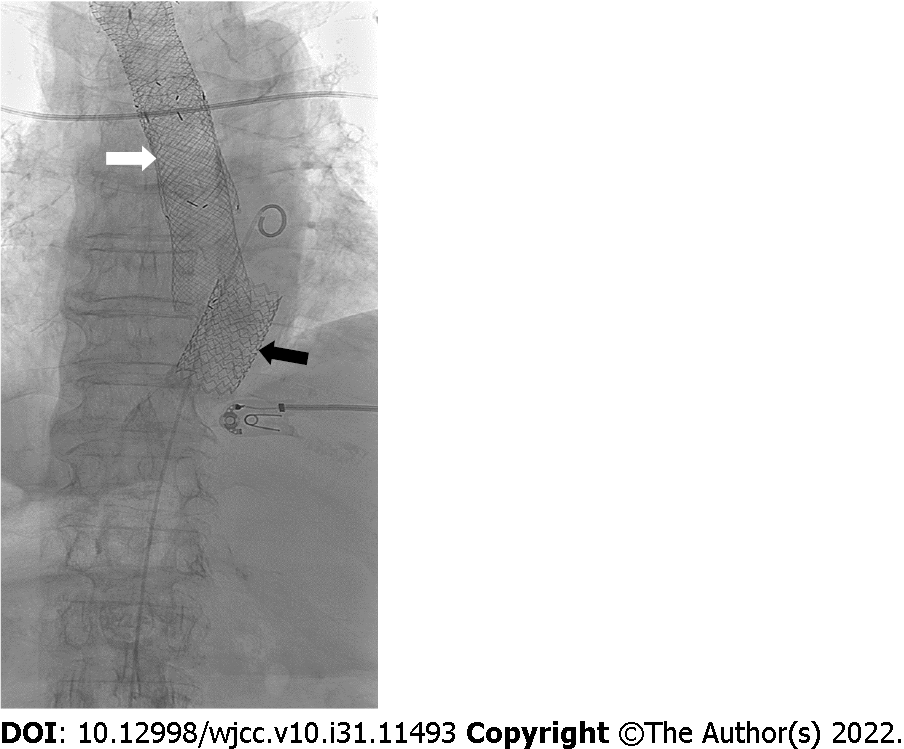
The recommended treatment was a stent graft placed into the aorta, to close the fistula. Using a local anesthetic, we performed ultrasound (US)-guided retrograde femoral artery puncture and successfully placed a stent, type BeGraft, Bentley (introducer sheath 16 FR, graft BeGraft Bentley 22 mm × 48 mm), at the bleeding site in the distal part of the descending thoracic aorta (Figure 2). A control CBC prior to intervention showed that anemia had progressed, with an RBC of 2.03 × 1012 and an HGB value of 59 g/L. During the procedure, which took 15 min from puncture of the femoral artery to insertion of the stent, the patient was supervised by the anesthesiologist and received intravenous vasopressors phenylephrine and ephedrine. Once the procedure was completed and successful closure of the fistula was confirmed angiographically, we continued to treat the patient at the Department of Gastroenterology.
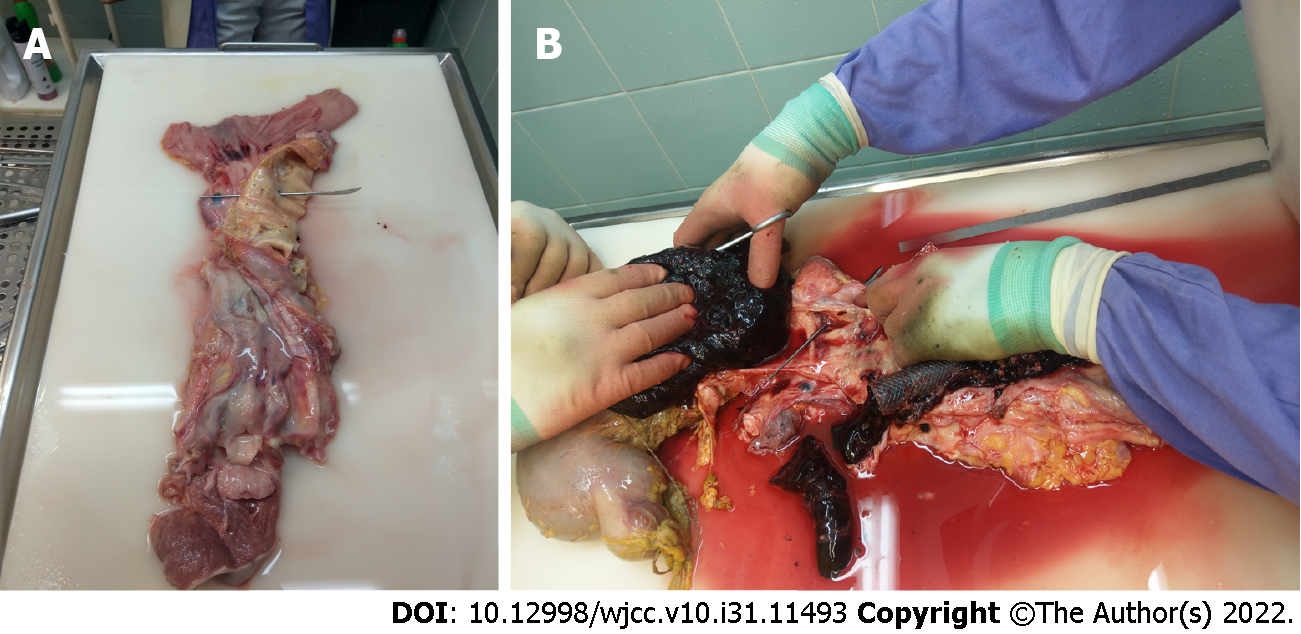
Despite continued resuscitation attempts and intensified symptomatic treatment, the patient’s condition gradually deteriorated during the next few hours. She died with signs of heart failure due to irreversible hemorrhagic shock. Autopsy confirmed an aorto-esophageal fistula in addition to advanced esophageal carcinoma (Figure 3A) and an abundance of blood clots in the stomach and upper gastrointestinal tract (Figure 3B).
Aorto-enteric fistula is an extremely rare cause of acute UGIB[9-13]. It is a pathologic communication between the aorta and gastrointestinal tract. An aorto-esophageal fistula represents the communication between the thoracic aorta and esophagus, is a potentially fatal health condition and is an extremely rare cause of acute UGIB. Based on the mechanism of their formation, aorto-enteric fistulas are classified as primary and secondary fistulas. A primary aorto-enteric fistula (PAF) develops when a connection is created between the aorta and the gastrointestinal tract, usually due to an aortic aneurysm, whereas a secondary aorto-enteric fistula (SAF) develops after the placement of endovascular prostheses or stent grafts into the aorta[10,11]. A PAF caused by an aneurysm was first described by the famous surgeon Astley Paton Cooper in 1892, who significantly influenced the development of vascular surgery, anatomy and pathology in the 19th century[13]. According to the literature, the incidence of PAF has not significantly changed during recent decades (0.04%–0.07%). The prevalence of SAF has increased and is estimated to be between 0.6% and 2.3%, and is associated with the increasing number of interventions and aortic surgeries[8,10,11]. Most primary fistulas (73%) develop due to atherosclerotic aneurysms of the infrarenal abdominal aorta. Cases of fistulas forming due to a thoracic-abdominal aortic aneurysm are rare. Approximately 26% of fistulas develop after an injury or because of a mycotic aneurysm. SAFs are rarely caused by radiation, advanced malignant disease or abdominal inflammation[11,14-16]. The location of most SAFs is the infrarenal segment of the abdominal aorta, where aneurysms are more frequent, and the number of surgical and radiological interventions (placement of endovascular prostheses) has increased. Aorto-enteric fistulas are most common in the segment of the duodenum crossing the aorta (horizontal segment, 57%); other duodenal segments are less commonly affected (9%)[4,5,8]. Fistulas can also be found in other segments of the gastrointestinal tract, including the small intestine (8%), the large intestine (4%), and the stomach or esophagus (4%)[8]. Most authors explain the development of an aorto-esophageal fistula as the result of multicausal esophageal ischemia caused by pressure on the esophageal artery, with simultaneous increased pressure due to an aneurysm on the posterior mediastinum, or, alternatively, inflammation during hematoma resorption as well as anatomical changes in the aortic arch and descending aorta after placement of implants–prostheses such as thoracic endovascular aortic repair (TEVAR)[11,13,15,17,18]. Others believe that a perioperative infection of the prosthesis leads to the development of a pseudoaneurysm, which creates a connection with the gastrointestinal tract by exerting pressure[9,10,14]. Another possible explanation is that a periprosthetic fistula forms at the site of a hematoma along the suture line, which leads to an intestinal infection and enables the formation of a connection between the aorta and the digestive system[11,12,17].
Our patient developed a fistula 1 cm distal to the esophageal stent, which was inserted for advanced carcinoma that resulted in malignant stenosis. The mechanism of fistula formation was most likely twofold: A fatal combination of aortic pulsations transferring to the esophageal wall, creating a pseudoaneurysm below the stent, and tumor overgrowth through the esophageal wall. We speculated about the possible influence of radiotherapy, but this was not confirmed at autopsy. According to the data available in the literature, aorto-esophageal fistulas are extremely rare and are most frequently described as the result of aortic disease, aneurysm, a pointed foreign body in the esophagus, or advanced malignant disease, as described in our case[4,8,18]. The international literature also mentions less common causes, such as aortic wall hematoma or aortic ulcers, Takayasu aortitis, consumption of Dieffenbachie picte (ornamental pot plant), radiation, lymphoma, anatomical variations in the aortic arch and long-term nasogastric tube use[18-22].
Gastrointestinal bleeding followed by abdominal pain, which can radiate into the lower back and lumbar region, is the most common sign in the clinical presentation of patients with an aorto-enteric fistula[8]. A pulsating mass in the abdomen is a rare sign found in 20% of patients[4,5]. Another common clinical sign is a sudden and unexplained febrile condition resulting from the direct migration of microorganisms from the intestinal lumen into the vascular system[5,8,10]. The diagnostic procedure often requires urgent upper gastrointestinal endoscopy to determine the cause of bleeding, which has limitations. First, we can assume the cause of bleeding, but if traces of blood are found in the lumen or a clot is attached to the wall, we must always consider the possibility of a fistula and should not remove the clot. The sensitivity of this investigation method is supposed to range between 25% and 80%[22]. We did not opt for urgent endoscopy in our patient because of cardiocirculatory instability, danger of aspiration with frequent vomiting of bloody contents and placed esophageal stents, which could interfere with or even prevent certain hemostatic procedures (e.g., hemostasis with various clips). Modern CT angiography is one of the most precise methods for detecting aorto-enteric fistula (sensitivity up to 93%), and compared to endoscopy, it offers certain advantages, namely, it is fast, noninvasive and displays high-resolution images of the abdominal organs[23-25]. Angiography of the abdominal vessels has limitations and often does not confirm a connection between the gastrointestinal tract and the aorta because a blood clot covers the fistula opening. Other imaging examinations, such as US, have limitations and are not recommended in the urgent treatment of a hemodynamically unstable patient[8]. Patients with aorto-enteric fistula are generally surgically treated, depending on the cause of the connection and the changes in the aorta and gastrointestinal tract. Unfortunately, surgery carries a high mortality rate, ranging between 20% and 93%[4,8,22].
Recently, more data on successful endovascular treatment have been published in the international literature. This type of treatment successfully stops bleeding, but surgical correction of the gastrointestinal wall is ultimately necessary to avoid septic complications[26-30]. A novel method of treating thoracic aortic diseases is a minimally invasive surgical procedure called TEVAR[12,22,25-27]. It is a method used in aneurysms, traumatic aortic transections, type B dissections, ulcers and aortic intramural hematomas[25]. Its advantage is a rapid setting and therefore a rapid cessation of bleeding, while its disadvantage is the recurrence of bleeding and the possibility of reinfection, especially if the previous TEVAR infection is not completely eliminated[28-32]. Since this is a very rare complication, most available literature on aorto-enteric fistulas are individual case reports, and only analyses of a cohort of patients over a longer period of time (usually decades) could be performed, but this would mean that the treatment methods would change over time, and the comparison of different therapeutic methods would be difficult[4,5,30-32]. In recent years, the recommended treatment procedures have been minimally invasive surgery and interventional radiologic techniques[29,31,32].
The targeted diagnostic procedure and exemplary cooperation of the medical staff involved in the present case resulted in the diagnosis of an aorto-esophageal fistula and enabled the provision of modern and optimal hemostatic treatment with the successful insertion of a stent graft. Unfortunately, irreversible hemorrhagic shock led to heart failure and resulted in the death of our patient.
The diagnosis and treatment of patients with acute UGIB is usually performed in compliance with the adopted clinical guidelines. However, sometimes it is necessary to think outside the box when determining the etiology of UGIB and conduct targeted diagnostic and therapeutic procedures. Despite accurate and timely clinical decisions, one should know that even the most contemporary interventional therapeutic procedures have limitations in certain rare clinical situations.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: European Society of Gastrointestinal Endoscopy, No. 2636; Slovenian Society for Gastroenterology and Hepatology.
Specialty type: Medicine, research and experimental
Country/Territory of origin: Slovenia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Aoun R, United States; Thomopoulos K, Greece S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Skok P. The epidemiology of hemorrhage from the upper gastrointestinal tract in the mid-nineties--has anything changed? Hepatogastroenterology. 1998;45:2228-2233. [PubMed] |
| 2. | Skok P, Sinkovič A. Upper gastrointestinal haemorrhage: predictive factors of in-hospital mortality in patients treated in the medical intensive care unit. J Int Med Res. 2011;39:1016-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Skok P. Endoscopic hemostasis in exulceratio simplex-Dieulafoy's disease hemorrhage: a review of 25 cases. Endoscopy. 1998;30:590-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Burks JA Jr, Faries PL, Gravereaux EC, Hollier LH, Marin ML. Endovascular repair of bleeding aortoenteric fistulas: a 5-year experience. J Vasc Surg. 2001;34:1055-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 150] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Cendan JC, Thomas JB 4th, Seeger JM. Twenty-one cases of aortoenteric fistula: lessons for the general surgeon. Am Surg. 2004;70:583-7; discussion 587. [PubMed] |
| 6. | Baril DT, Carroccio A, Ellozy SH, Palchik E, Sachdev U, Jacobs TS, Marin ML. Evolving strategies for the treatment of aortoenteric fistulas. J Vasc Surg. 2006;44:250-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Heidemann J, Domagk D, Wessling J, Domschke W, Kucharzik TF. Recurrent obscure gastrointestinal bleeding caused by aorto-enteric fistula. Z Gastroenterol. 2006;44:981-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Skok P. Aortodudenal fistula-fatal complication in a patient with aortic prosthetis. Acta medico-biotechnica: AMB. 2010;3:41-44. |
| 9. | Isasti G, Gómez-Doblas JJ, Olalla E. Aortoesophageal fistula: an uncommon complication after stent-graft repair of an aortic thoracic aneurysm. Interact Cardiovasc Thorac Surg. 2009;9:683-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Hsu WF, Lin CC, Chang KM, Lee TH. Primary aortoesophageal fistula: a rare but fatal cause of upper gastrointestinal bleeding. J Dig Dis. 2013;14:676-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Xi EP, Zhu J, Zhu SB, Zhang Y. Secondary aortoesophageal fistula after thoracic aortic aneurysm endovascular repair: literature review and new insights regarding the hypothesized mechanisms. Int J Clin Exp Med. 2014;7:3244-3252. [PubMed] |
| 12. | Nation DA, Wang GJ. TEVAR: Endovascular Repair of the Thoracic Aorta. Semin Intervent Radiol. 2015;32:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 13. | Karthaus EG, Post IC, Akkersdijk GJ. Spontaneous aortoenteric fistula involving the sigmoid: A case report and review of literature. Int J Surg Case Rep. 2016;19:97-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | McKeating J, Smith S, Kochanck P, Perper J, Orenstein S, Nakayama D. Fatal aortoesophageal fistula due to double aortic arch: an unusual complication of prolonged nasogastric intubation. J Pediatr Surg. 1990;25:1298-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Sivaraman SK, Drummond R. Radiation-induced aortoesophageal fistula: an unusual case of massive upper gastrointestinal bleeding. J Emerg Med. 2002;23:175-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Antoniou GA, Koutsias S, Antoniou SA, Georgiakakis A, Lazarides MK, Giannoukas AD. Outcome after endovascular stent graft repair of aortoenteric fistula: A systematic review. J Vasc Surg. 2009;49:782-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Zhan Y, Xu Z. Massive hemorrhage from an aortoesophageal fistula caused by esophageal stent implantation: A case report and literature review. Medicine (Baltimore). 2019;98:e18303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Skok P, Skok K. Urgent endoscopy in patients with "true foreign bodies" in the upper gastrointestinal tract - a retrospective study of the period 1994-2018. Z Gastroenterol. 2020;58:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Bagshaw SM, Crabtree T, Green F, Stewart DA. ALK-positive anaplastic large T-cell lymphoma preceded by Epstein-Barr virus infection complicated by development of an aorto-esophageal fistula. Leuk Lymphoma. 2002;43:915-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Reddi A, Chetty R. Primary aorto-esophageal fistula due to Takayasu's aortitis. Cardiovasc Pathol. 2003;12:112-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Snajdauf J, Mixa V, Rygl M, Vyhnánek M, Morávek J, Kabelka Z. Aortoesophageal fistula--an unusual complication of esophagitis caused by Dieffenbachia ingestion. J Pediatr Surg. 2005;40:e29-e31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Roten R, Peterfy R. Aortoesophageal Fistula. Clin Pract Cases Emerg Med. 2017;1:260-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Rawala MS, Badami V, Rizvi SB, Nanjundappa A. Aortoesophageal Fistula: A Fatal Complication of Thoracic Endovascular Aortic Stent-Graft Placement. Am J Case Rep. 2018;19:1258-1261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Chen C, Kim JW, Shin JH, Kwon Y, Kim J, Lee IJ. Management of life-threatening aortoesophageal fistula: experiences learned from eight patients. Acta Radiol. 2021;62:447-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Lee S, N Srinivasa R, A Rigberg D, Yanagawa J, Benharash P, M Moriarty J. Aortoesophageal fistula involving the central aortic arch salvaged with emergent percutaneous TEVAR, great vessel coverage and in vivo graft fenestration. Diagn Interv Radiol. 2021;27:122-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Oliva VL, Bui BT, Leclerc G, Gravel D, Normandin D, Prenovault J, Guimond JG. Aortoesophageal fistula: repair with transluminal placement of a thoracic aortic stent-graft. J Vasc Interv Radiol. 1997;8:35-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Marone EM, Coppi G, Kahlberg A, Tshomba Y, Chiesa R. Combined endovascular and surgical treatment of primary aortoesophageal fistula. Tex Heart Inst J. 2010;37:722-724. [PubMed] |
| 28. | Numan F, Gulsen F, Cantasdemir M, Solak S, Arbatli H. Percutaneous treatment of an infected aneurysmal sac secondary to aortoesophageal fistula with a history of stent-graft treatment for thoracic aortic aneurysm. Cardiovasc Intervent Radiol. 2012;35:690-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | So K, Smith CR, Faroqui NM, Simone N, Senkowski A, Patel SK, Bailey BM. Control of Aortoesophageal Fistula Using Endoscopic and Endovascular Techniques: A Palliative Intervention. Am Surg. 2018;84:e47-e49. [PubMed] |
| 30. | Kakkos SK, Papadoulas S, Tsolakis IA. Endovascular management of arterioenteric fistulas: a systemic review and meta-analysis of the literature. J Endovasc Ther. 2011;18:66-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Iwabu J, Namikawa T, Yokota K, Kitagawa H, Kihara K, Hirose N, Hanazaki K. Successful management of aortoesophageal fistula caused by esophageal cancer using thoracic endovascular aortic repair. Clin J Gastroenterol. 2020;13:678-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 32. | Takeno S, Ishii H, Nanashima A, Nakamura K. Aortoesophageal fistula: review of trends in the last decade. Surg Today. 2020;50:1551-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |