Published online Oct 26, 2022. doi: 10.12998/wjcc.v10.i30.11172
Peer-review started: July 6, 2022
First decision: August 4, 2022
Revised: August 24, 2022
Accepted: September 19, 2022
Article in press: September 19, 2022
Published online: October 26, 2022
Processing time: 106 Days and 13.8 Hours
Hepatic myelopathy (HM) is a rare neurological complication of advanced cirrhosis. Prognosis of patients with HM is generally poor without timely liver transplantation or interventional therapy. Self-resolving HM in patients with alcoholic cirrhosis has never been reported.
A 53-year-old man with alcoholic cirrhosis and recurrent overt hepatic encephalopathy for 1 year was admitted for lower extremity weakness, slow movement, and stumbling gait. The patient was diagnosed with HM after excluding other causes of spastic paraparesis. The patient refused liver transplantation. However, the patient kept total abstinence and received a multidisciplinary treatment for complications of decompensated cirrhosis. The symptoms of HM resolved gradually after 2 years of treatment. All complications of alcoholic cirrhosis resolved after 4 years of follow-up.
The case demonstrates that HM can resolve in patients without liver transplan-tation after total abstinence and systemic management of complications.
Core Tip: Hepatic myelopathy (HM) is a rare neurological complication of advanced cirrhosis. Prompt liver transplantation or interventional therapy may reverse the symptoms of HM. Self-resolving HM in patients with alcoholic cirrhosis has never been reported. Our report presents that self-resolving HM in a patient with alcoholic cirrhosis is possible without any liver transplantation and interventional therapy after promptly controlling the etiology and systemic management of complications. This case provides new insight into the self-remission of patients with HM.
- Citation: Chang CY, Liu C, Duan FF, Zhai H, Song SS, Yang S. Spontaneous remission of hepatic myelopathy in a patient with alcoholic cirrhosis: A case report. World J Clin Cases 2022; 10(30): 11172-11177
- URL: https://www.wjgnet.com/2307-8960/full/v10/i30/11172.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i30.11172
Hepatic myelopathy (HM) is a rare neurological complication of advanced cirrhosis. The clinical manifestations of HM are progressive spasmodic paralysis of the limbs and do not involve sensory or sphincter motor symptoms, commonly in patients with recurrent hepatic encephalopathy (HE)[1]. Other causes of spastic paraparesis and partial transverse myelopathy should be ruled out before establishing the diagnosis[2]. Although the first case of HM was reported 30 years ago, the prognosis profiles of patients with HM, especially the rare cases, remain obscure[3]. Limited data have demonstrated that prompt liver transplantation or interventional therapy may reverse the symptoms of HM[4]. In 2017, di Biase et al[5] has reported the first case of self-resolving HM in patients with hepatitis C virus (HCV)-related cirrhosis after HCV treatment. Since then, no case of self-resolving HM was reported. To the best of our knowledge, no case of self-resolving HM for alcoholic cirrhosis has been reported. Herein, we report the first case of self-resolving HM from our large cohort of patients with alcoholic cirrhosis[6]. We also reviewed the treatment of patients with HM.
A 53-year-old man with alcoholic cirrhosis was admitted to Beijing Ditan Hospital of Capital Medical University for lower extremity weakness, slow movement, and stumbling gait that required walking assistance with a crutch in January 2015.
The patient was diagnosed with decompensated alcoholic cirrhosis with ascites in September 2011. In December 2011, he was admitted to the hospital due to gastroesophageal variceal bleeding and received splenectomy combined with a gastroesophageal devascularization surgery. In April 2013, he was hospitalized for comorbid acute hepatitis B and suffered from recurrent overt HE since then. Since January 2015, the patient gradually developed weakness in both lower limbs, slow movement, and hobbling gait.
The patient had no relevant medical history.
The patient had a history of heavy drinking for 25 years, with an average alcohol intake of 200 g per day.
Abdominal examination suggested hepatomegaly and positive shifting dullness. Neurological system examinations demonstrated slurring speech, normal cranial nerves, increased muscle tension and grade 4/5 power of lower limbs, exaggerated deep tendon reflexes, and no sensory deficit or sphincteric involvement.
Serial results of liver function and whole blood count are presented in Table 1. Blood ammonia concentration fluctuated between 50 and 87 μmol/L during the occurrence of overt HE. In January 2015, hospitalization, hepatitis B surface antigen, and anti-HCV tests were negative. Additionally, human immunodeficiency virus (HIV), Syphilis, Epstein-Barr virus (EBV), and cytomegalovirus tests were negative. Serum vitamin B-12 level was normal. Cerebrospinal fluid analysis was normal.
| September, 2011 | September, 2012 | September, 2014 | February, 2015 | August, 2019 | October, 2021 | |
| ALT (U/L) | 25 | 18 | 717 | 28 | 42 | 25 |
| AST (U/L) | 42 | 26 | 976 | 46 | 62 | 32 |
| ALB (g/L) | 30.0 | 32.0 | 33.2 | 32.2 | 36.1 | 41.3 |
| TBIL (μmol/L) | 53.1 | 15.7 | 109.4 | 19.3 | 47.0 | 16.7 |
| Hb (g/L) | 108 | 82 | 127 | 139.0 | 159 | 153 |
| WBC (109/L) | 6.4 | 8.5 | 9.4 | 8.8 | 7.5 | 7.6 |
| PLT (109/L) | 71 | 278 | 159 | 79 | 100 | 121 |
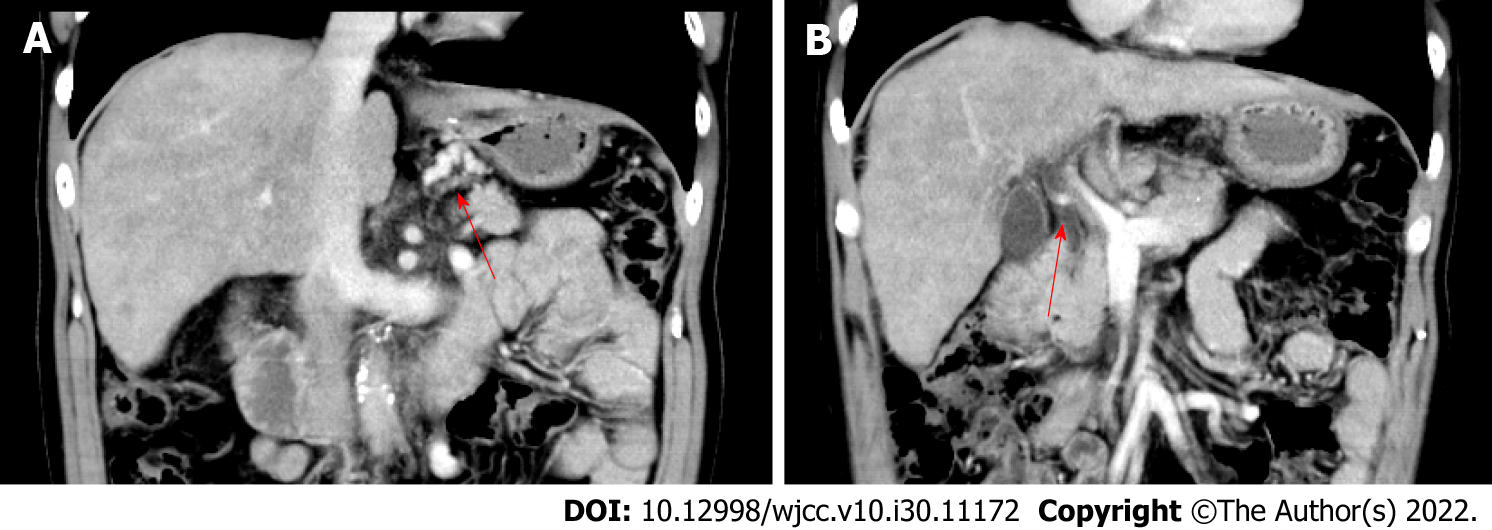
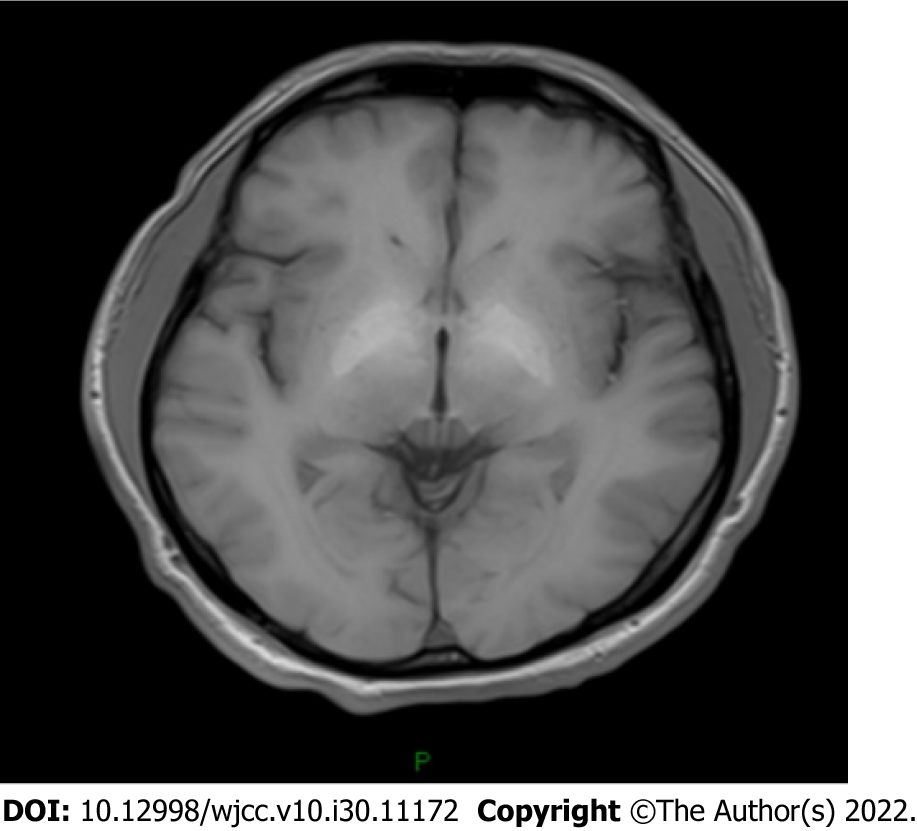
Contrast abdominal computed tomography revealed liver cirrhosis, esophageal and gastric varices, gastro-left renal shunt, and portal vein thrombosis (Figure 1). Magnetic resonance imaging (MRI) of the brain indicated hyperintensities in the bilateral globus pallidus (Figure 2). Moreover, whole spinal MRI and lumbosacral MRI were performed and revealed normal results. The electromyogram showed normal nerve conduction velocity in the bilateral tibial nerves. Somatosensory evoked potentials of the lower limbs were normal. Motor evoked potential was abnormal in both lower limbs.
Multidisciplinary expert consultation was performed; this included experts in hepatology, neurology, infectious diseases, and radiology, to find for the cause of the spastic paraparesis. The cranial and spinal MRI showed no intracranial or spinal space occupation. Normal serum vitamin B-12 levels allowed subacute combined degeneration of the spinal cord to be ruled out. Primary lateral sclerosis was not considered since spastic paraparesis in this patient get spontaneously resolved and does not involve the upper limbs. Spinal multiple sclerosis was excluded based on the normal spinal MRI and lack of sensory deficit or sphincteric involvement. Myelopathy related to HIV, EBV or other pathogens infections was ruled out based on the normal infection biomarkers and normal cerebrospinal fluid status. Moreover, hereditary spastic paraplegia, Wilson’s disease, radiation myelopathy, vascular spinal cord disease, and other causes of spastic paraparesis were ruled out due to the lack of specific neurological features and lack of characteristic distinguishing abnormalities on neuroimaging. The patient was diagnosed with HM after exclusion of any other potential causes of spastic paraparesis.
The patient rejected liver transplantation for financial reasons. The patient chose abstinence and took furosemide, spironolactone, lactulose, L-ornithine-L-aspartate for ascites and HE. The patient was followed up every 3-6 mo.
The patient chose abstinence and symptomatic treatment. During follow-up, he had less ascites and overt HE attacks. Since 2018, he had reported gradual improvement of his lower limb weakness and hobbling gait. In August 2019, the patient reported that he could walk without the assistance of a crutch. The liver function test revealed normal alanine transaminase, aspartate transaminase and albumin levels. Abdominal ultrasound revealed no signs of ascites and disappearance of the portal vein thrombosis. The patient was regularly followed up until October 2021, and has since demonstrated normal liver function and regular limb movement.
HM is a rare complication of cirrhosis, which is common in patients with portosystemic shunts and recurrent HE. Its main clinical manifestation is progressive spastic paraparesis. Diagnosis of HM needs to exclude other causes for spastic paraparesis, which include amyotrophic lateral sclerosis, hereditary and toxic myelopathy, multiple sclerosis, paraneoplastic syndromes, radiation myelopathy, infectious causes of myelopathy, and vascular spinal cord disease[7]. Regarding this patient, he had a history of cirrhosis and recurrent HE attacks. Contrast abdominal computed tomography showed portosystemic shunting. In addition, MRI of the brain indicated cirrhosis and HE. The diagnosis of HM was established after exclusion other potential causes of spastic paraparesis by multidisciplinary expert consultation.
Early spinal cord injury in HM is characterized by symmetrical demyelination of corticospinal tracts due to nitrogenous toxins such as ammonia. The demyelination is reversable with prompt management of the underlying liver disease and/or portosystemic shunts. As the disease progresses, axonal loss occurs, which may be irreversible[8,9].
Troisi et al[10] reported the first case of a patient with HM in whom myelopathy improved after liver transplantation. Since then, an increasing number of studies have demonstrated that liver transplantation might reverse HM[1,4,11-15], although some studies have reported otherwise[16,17]. When comparing patients in whom HM was reversed after liver transplantation and patients whose HM was not reversed, it is generally recognized that the likelihood of HM reversal may be higher when liver transplantation is performed within 18 mo after the onset of symptomatic HM[4]. This theory was further verified by Koul et al’s report, in which two children with acute HM after hepatitis A infection recovered completely after receiving donor liver transplantation[14].
For HM secondary to transjugular intrahepatic portosystemic shunt (TIPS) or surgical splenorenal shunt, reports have revealed that prompt shunt occlusion or shunt limitation may reverse HM[18-21]. Some studys have reported that shunt limitation, not shunt occlusion, is useful for reversing early-onset HM after TIPS[20,21]. Shunt limiting is preferred, as total shunt occlusion might have a higher risk of adverse events related to the rapid increase of portal hypertension. Moreover, Philips et al[22] reported partial splenic artery embolization (PSAE) for a patient with HM. Neurological function improved rapidly and constantly after PSAE. The authors concluded that PSAE may improve liver function, decrease PHT, and lower portosystemic shunting in this way to ameliorate neurological symptoms. Intestinal microbiota is closely related to HE, and some studies have reported that fecal microbiota transplantations (FMT) might improve HE[23]. Based on this, Sun et al[24] reported a case of HM in a patient who received FMT, and neurological function improved after three repetitions of FMT. More studies have revealed that repairing gut microbiota may decrease portal hypertension and repair the blood-brain barrier[25,26]. Further, there is increasing data to demonstrate the usefulness of FMT for improving HE[27,28]. Considering the shared pathogenesis of HM and HE, FMT for HM seems promising and is worth further investigation.
In 2017, di Biase et al[5] reported an interesting case of self-resolving HM. This patient with HCV-related cirrhosis was treated with sofosbuvir plus ribavirin. HM improved 6 mo after HCV treatment. The case demonstrates that self-resolving HM might be possible after relief of the underlying liver disease. As in our case, HM was relieved with total abstinence, and liver function was restored. Additionally, the 6-year follow-up demonstrated sustained re-compensation of liver cirrhosis in this case.
As the first reported case of self-resolving HM in a patient with alcoholic cirrhosis, the case demon
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ferrarese A, Italy; Surani S, United States S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Koo JE, Lim YS, Myung SJ, Suh KS, Kim KM, Lee HC, Chung YH, Lee YS, Suh DJ. Hepatic myelopathy as a presenting neurological complication in patients with cirrhosis and spontaneous splenorenal shunt. Korean J Hepatol. 2008;14:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Jeske J, Schädlich HJ, Sandmann J, Karbe H, Haupt WF, Karenberg A. [Acute paraparesis in chronic hepatic disease]. Nervenarzt. 1991;62:130-132. [PubMed] |
| 3. | Gospe SM Jr, Caruso RD, Clegg MS, Keen CL, Pimstone NR, Ducore JM, Gettner SS, Kreutzer RA. Paraparesis, hypermanganesaemia, and polycythaemia: a novel presentation of cirrhosis. Arch Dis Child. 2000;83:439-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Caldwell C, Werdiger N, Jakab S, Schilsky M, Arvelakis A, Kulkarni S, Emre S. Use of model for end-stage liver disease exception points for early liver transplantation and successful reversal of hepatic myelopathy with a review of the literature. Liver Transpl. 2010;16:818-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | di Biase L, Picillo M, Freitas ME, Bui E, Fasano A. Hepatitis C Virus-Related Hepatic Myelopathy After Treatment With Sofosbuvir and Ribavirin: A Case Report. Ann Intern Med. 2017;166:379-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Duan F, Liu C, Liu Y, Chang C, Zhai H, Xing H, Cheng J, Yang S. Nomogram to Predict the Survival of Chinese Patients with Alcohol-Related Liver Disease. Can J Gastroenterol Hepatol. 2021;2021:4073503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Ben Amor S, Saied MZ, Harzallah MS, Benammou S. Hepatic myelopathy with spastic paraparesis: report of two cases and review of the literature. Eur Spine J. 2014;23 Suppl 2:167-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Weissenborn K. Portosystemic encephalopathy. Handb Clin Neurol. 2014;120:661-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Utku U, Asil T, Balci K, Uzunca I, Celik Y. Hepatic myelopathy with spastic paraparesis. Clin Neurol Neurosurg. 2005;107:514-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Troisi R, Debruyne J, de Hemptinne B. Improvement of hepatic myelopathy after liver transplantation. N Engl J Med. 1999;340:151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Weissenborn K, Tietge UJ, Bokemeyer M, Mohammadi B, Bode U, Manns MP, Caselitz M. Liver transplantation improves hepatic myelopathy: evidence by three cases. Gastroenterology. 2003;124:346-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Qu B, Liu C, Guo L, Yang Y, Li JH, Yu L, Lv Y. The role of liver transplantation in the treatment of hepatic myelopathy: case report with review of the literature. Transplant Proc. 2009;41:1987-1989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Baccarani U, Zola E, Adani GL, Cavalletti M, Schiff S, Cagnin A, Poci C, Merkel C, Amodio P, Montagnese S. Reversal of hepatic myelopathy after liver transplantation: fifteen plus one. Liver Transpl. 2010;16:1336-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Koul R, Lal BB, Pamecha V, Sarin S, Alam S. Liver Transplantation Reverses Hepatic Myelopathy in 2 Children With Hepatitis A Infection. Child Neurol Open. 2021;8:2329048X20983763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Zhu Z, Liu Y, Wu W, Huang D, Guo Y, Zheng H, Wang N, Xu Z, Li X, Qin J, Liu L, Nashan B. Liver Transplantation Reverses Hepatic Myelopathy in Hepatitis B-Related Decompensated Liver Cirrhosis: Case Report and Review of the Literature. Transplant Proc. 2022;54:158-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Counsell C, Warlow C. Failure of presumed hepatic myelopathy to improve after liver transplantation. J Neurol Neurosurg Psychiatry. 1996;60:590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Nardone R, Buratti T, Oliviero A, Lochmann A, Tezzon F. Corticospinal involvement in patients with a portosystemic shunt due to liver cirrhosis: a MEP study. J Neurol. 2006;253:81-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Conn HO, Rössle M, Levy L, Glocker FX. Portosystemic myelopathy: spastic paraparesis after portosystemic shunting. Scand J Gastroenterol. 2006;41:619-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Wang MQ, Liu FY, Duan F. Management of surgical splenorenal shunt-related hepatic myelopathy with endovascular interventional techniques. World J Gastroenterol. 2012;18:7104-7108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Zhao H, Liu F, Yue Z, Wang L, Fan Z. Evaluation of mid- and long-term efficacy of shunt limiting for hepatic myelopathy after transjugular intrahepatic portosystemic shunt. Clin Res Hepatol Gastroenterol. 2016;40:440-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Zhao H, Yue Z, Wang L, Fan Z, He F, Dong X, Liu F. Benefits of Early Treatment for Patients with Hepatic Myelopathy Secondary to TIPS: A Retrospective Study in Northern China. Sci Rep. 2018;8:15184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Philips CA, Kumar L, Augustine P. Partial splenic artery embolization for severe hepatic myelopathy in cirrhosis. Hepatology. 2018;67:1169-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Tandon P, Madsen K, Kao D. Fecal microbiota transplantation for hepatic encephalopathy: Ready for prime time? Hepatology. 2017;66:1713-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Sun L, Li J, Lan LL, Li XA. The effect of fecal microbiota transplantation on Hepatic myelopathy: A case report. Medicine (Baltimore). 2019;98:e16430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, Korecka A, Bakocevic N, Ng LG, Kundu P, Gulyás B, Halldin C, Hultenby K, Nilsson H, Hebert H, Volpe BT, Diamond B, Pettersson S. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6:263ra158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1699] [Article Influence: 169.9] [Reference Citation Analysis (0)] |
| 26. | Huang HC, Tsai MH, Chang CC, Pun CK, Huang YH, Hou MC, Lee FY, Hsu SJ. Microbiota transplants from feces or gut content attenuated portal hypertension and portosystemic collaterals in cirrhotic rats. Clin Sci (Lond). 2021;135:2709-2728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Afecto E, Ponte A, Fernandes S, Silva J, Gomes C, Correia J, Carvalho J. Fecal microbiota transplantation in hepatic encephalopathy : a review of the current evidence and future perspectives. Acta Gastroenterol Belg. 2021;84:87-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Hassouneh R, Bajaj JS. Gut Microbiota Modulation and Fecal Transplantation: An Overview on Innovative Strategies for Hepatic Encephalopathy Treatment. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |