Published online Jan 21, 2022. doi: 10.12998/wjcc.v10.i3.1077
Peer-review started: June 29, 2021
First decision: September 1, 2021
Revised: September 8, 2021
Accepted: December 22, 2021
Article in press: December 22, 2021
Published online: January 21, 2022
Processing time: 199 Days and 17.2 Hours
Charcot neuroarthropathy (CN) is a systemic disease characterized by progressive bone loss and destruction, which is usually closely related to diabetes, HIV, etc. However, CN caused by syringomyelia accounts for only 5% of CN cases; the shoulder and elbow are most often involved, and the hip joint is rarely affected. As a rare factor, cervical spondylotic myelopathy (CSM) can be associated with syringomyelia, which is scarcely reported in the literature. Here, we present the first case report to date of CN of the hip caused by syringomyelia secondary to CSM.
We describe a 76-year-old male patient who was diagnosed with CSM due to neck pain and weakness of limbs 16 years ago. Four years ago, he noticed recurrent swelling of the right hip with pain and was diagnosed with degenerative arthritis. Recently, however, his symptoms gradually worsened, and because of progressive pain, destabilization and weakness of the right hip, he was admitted to our hospital. Through systematic physical, radiographic and laboratory examinations, we finally reached a diagnosis: CN of the right hip associated with syringomyelia secondary to CSM. After comprehensive evaluation of the patient's condition, we performed right total hip arthroplasty. During the follow-up, the patient felt well clinically and could walk independently with a knee brace.
We suggest a possible etiological association between CSM and syringomyelia, which may reflect a potential pathogenesis of CN. We encourage clinicians to actively carry out a detailed medical history and comprehensive physical and imaging examinations in patients with joint lesions, especially chronic shoulder neck pain, to rule out the possibility of this association, which plays a crucial role in the early diagnosis of CN. Arthroplasty may no longer be an absolute contraindication to surgical treatment of CN. Reasonable selection of the surgical strategy can markedly improve the clinical symptoms and quality of life of patients.
Core Tip: We report an unprecedented case of Charcot neuroarthropathy (CN) of the right hip associated with syringomyelia secondary to cervical spondylotic myelopathy (CSM). After comprehensive evaluation of the patient's condition, we performed right total hip arthroplasty. During the follow-up, the patient felt well clinically and could walk independently with a knee brace. We suggest a potential etiological association between CN and CSM complicated by syringomyelia, which reminds clinicians to actively carry out comprehensive physical and imaging examinations in patients with joint lesions, especially chronic shoulder neck pain, to rule out the possibility of this association. Detailed medical history acquisition and comprehensive examination play a crucial role in the early diagnosis of CN. Arthroplasty may no longer be an absolute contraindication to surgical treatment of CN. Reasonable selection of the surgical strategy can markedly improve the clinical symptoms and quality of life of patients.
- Citation: Lu Y, Xiang JY, Shi CY, Li JB, Gu HC, Liu C, Ye GY. Cervical spondylotic myelopathy with syringomyelia presenting as hip Charcot neuroarthropathy: A case report and review of literature. World J Clin Cases 2022; 10(3): 1077-1085
- URL: https://www.wjgnet.com/2307-8960/full/v10/i3/1077.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i3.1077
Charcot neuroarthropathy (CN), or Charcot joint, is a systemic disease characterized by progressive bone loss and destruction of the joints and/or spine usually accompanied by dysfunctions of sensitive and autonomic nerves and is reported to be closely associated with diabetes mellitus, HIV, chronic alcoholism, end-stage renal disease, gigantism, intra-articular steroid injections, peripheral neuropathy, meningomyelocele, multiple sclerosis, myelodysplasia, leprosy, amyloidosis, and congenital pain insensitivity[1-3]. However, CN caused by syringomyelia accounts for approximately 5% of CN cases[4]. A retrospective study showed that syringomyelia-associated CN most often involves the shoulder (17 of 33, 50.0%) and elbow (17 of 33, 50.0%), while the hip joint is rarely involved (1 of 33, 2.94%)[5]. To date, a few cases of CN associated with syringomyelia have been reported, but a case report of CN of the hip caused by syringomyelia secondary to cervical spondylotic myelopathy (CSM) has never been published. Therefore, the purpose of this report is to discuss this rare pathological association and to provide new insights into the surgical treatment of CN.
A 76-year-old male patient presented to the Department of Orthopedics of our hospital complaining of worsening progressive right hip pain, a limp when walking and weakness of the right hip. He was admitted to our hospital.
The patient’s symptoms started four years ago with recurrent swelling of the right hip with mild pain, which had worsened in the last 48 h.
The patient initially presented with neck pain and weakness in all four limbs 16 years ago and was diagnosed with cervical spondylosis, which was treated conservatively.
The patient had an unremarkable personal and family history.
The patient reported mild pain with flexion and extension of the cervical spine and activity limitation. Apparent swelling was observed in the left hip, and more than 110 mL of clear yellowish joint fluid was extracted. The active range of motion of the right hip was recorded as follows: flexion 90°, abduction 30°, internal rotation 20°, and external rotation 35°, with pain in all directions. The visual analog score was 6/10 points, and the Harris hip score (HHS) was 56 points. Neurologically, the sensation of pain and temperature in the upper and lower extremities was decreased, and proprioception and position sensation were normal. A pathologic reflex was not elicited. Both upper limbs and the right lower limb exhibited weakness with a muscle strength of 4/5, the muscle strength of the left lower limb was normal at 5/5, the right abductor had a muscle strength score of 5/5, and the modified Japanese Orthopedic Association score for CSM was 9/17 points. The bone mineral density (BMD) of the hip was 0.45 g/cm2, and the T score was -2.9. Laboratory results were nonspecific.
Blood biochemistry and urine analyses were normal. Electrocardiogram, chest X-ray and arterial blood gases were also normal. The BMD of the hip was 0.45 g/cm2, and the T score was -2.9.
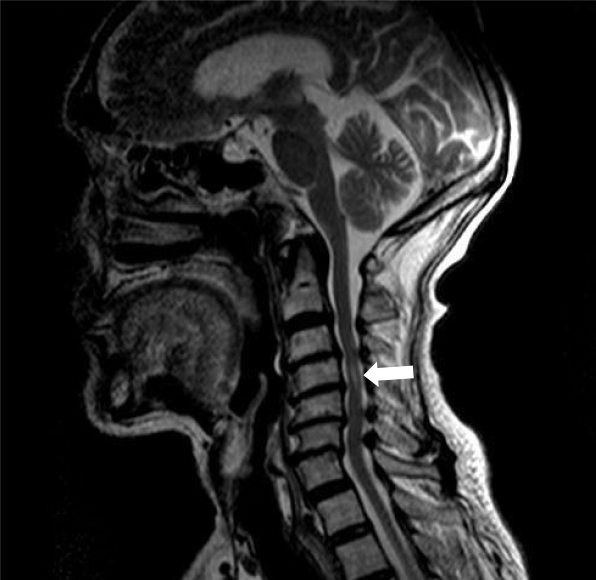
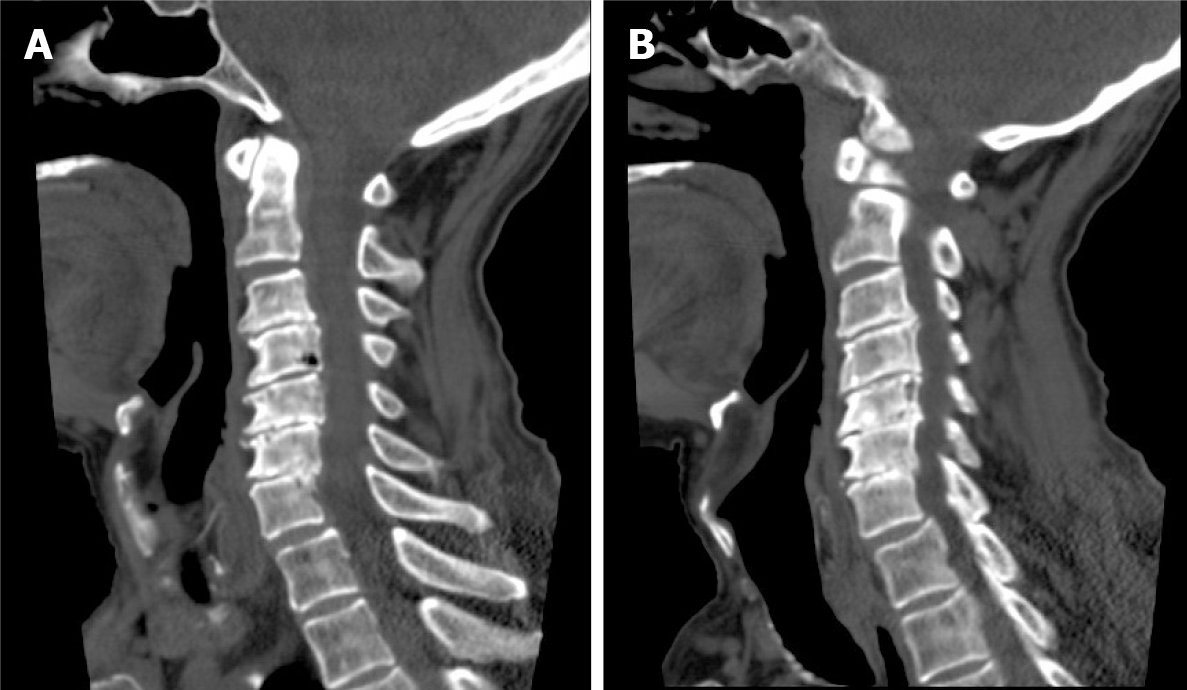
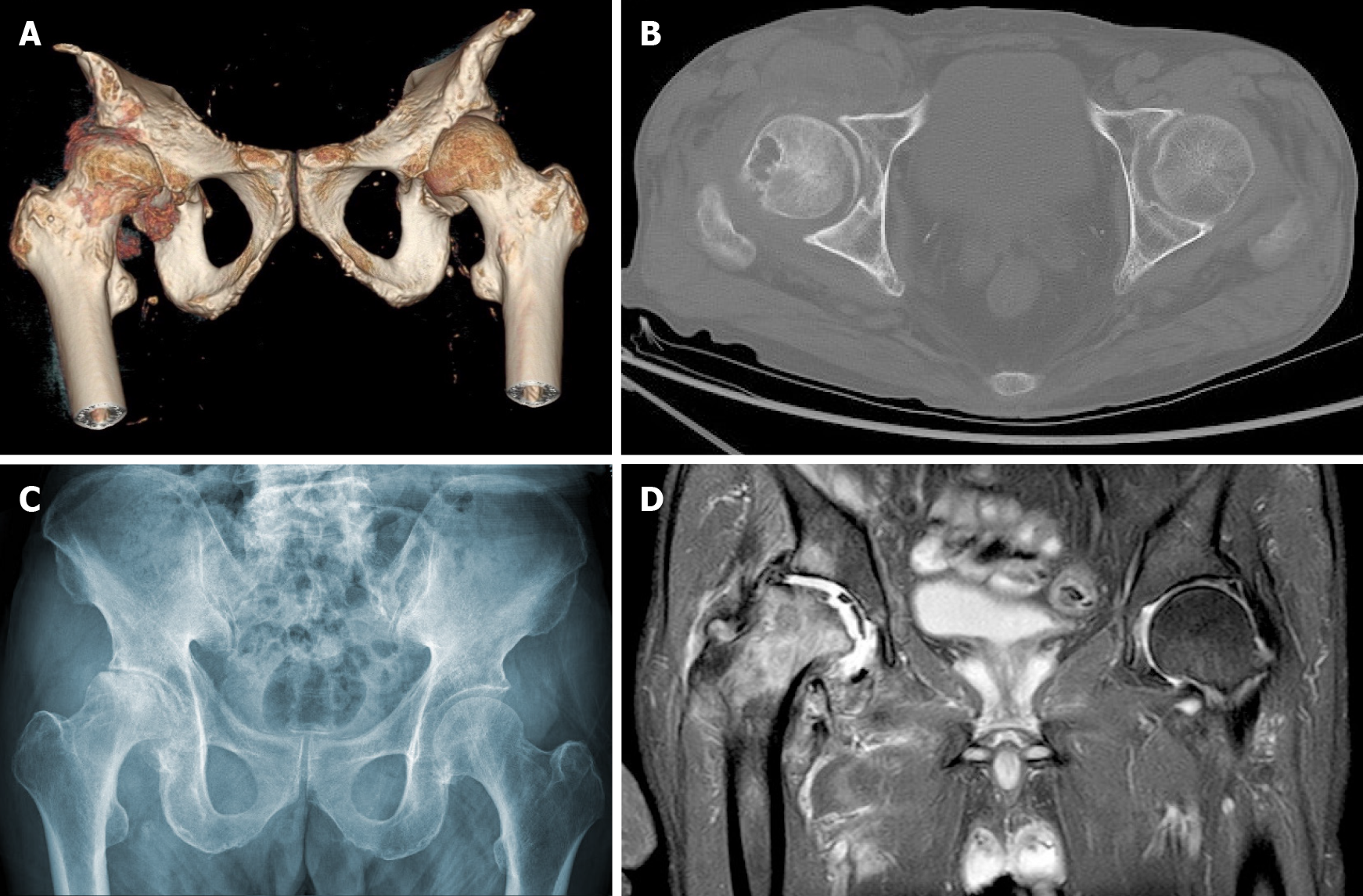
Magnetic resonance imaging (MRI) of the cervical spine showed cervical syringomyelia at C4, cervical disc herniation and spinal canal stenosis from the C3 to the C7 levels (Figure 1). Cervical computed tomography (CT) revealed destruction of the vertebral body at C4 and C5–7 vertebral body assimilation (Figure 2). Three-dimensional CT reconstruction, CT scans, and X-rays of the right hip joint showed joint space loss, articular surface collapse, and destructive changes in the acetabulum and femoral head (Figure 3A-C). T2W1 MRI of the right hip showed articular cartilage loss, degeneration of the joint, disordered soft tissue, and apparent joint fluid (Figure 3D).
The final diagnosis was CN of the hip associated with syringomyelia and CSM.
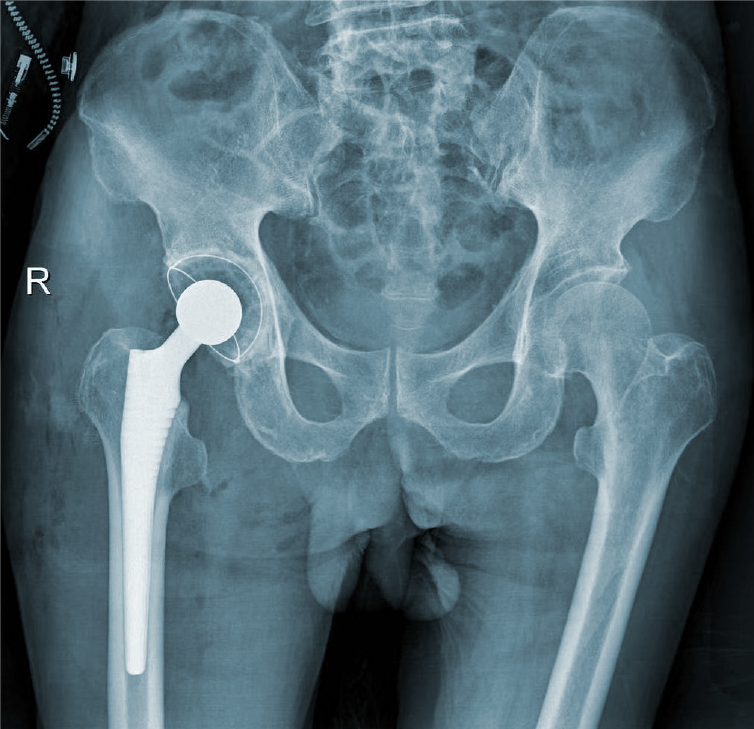
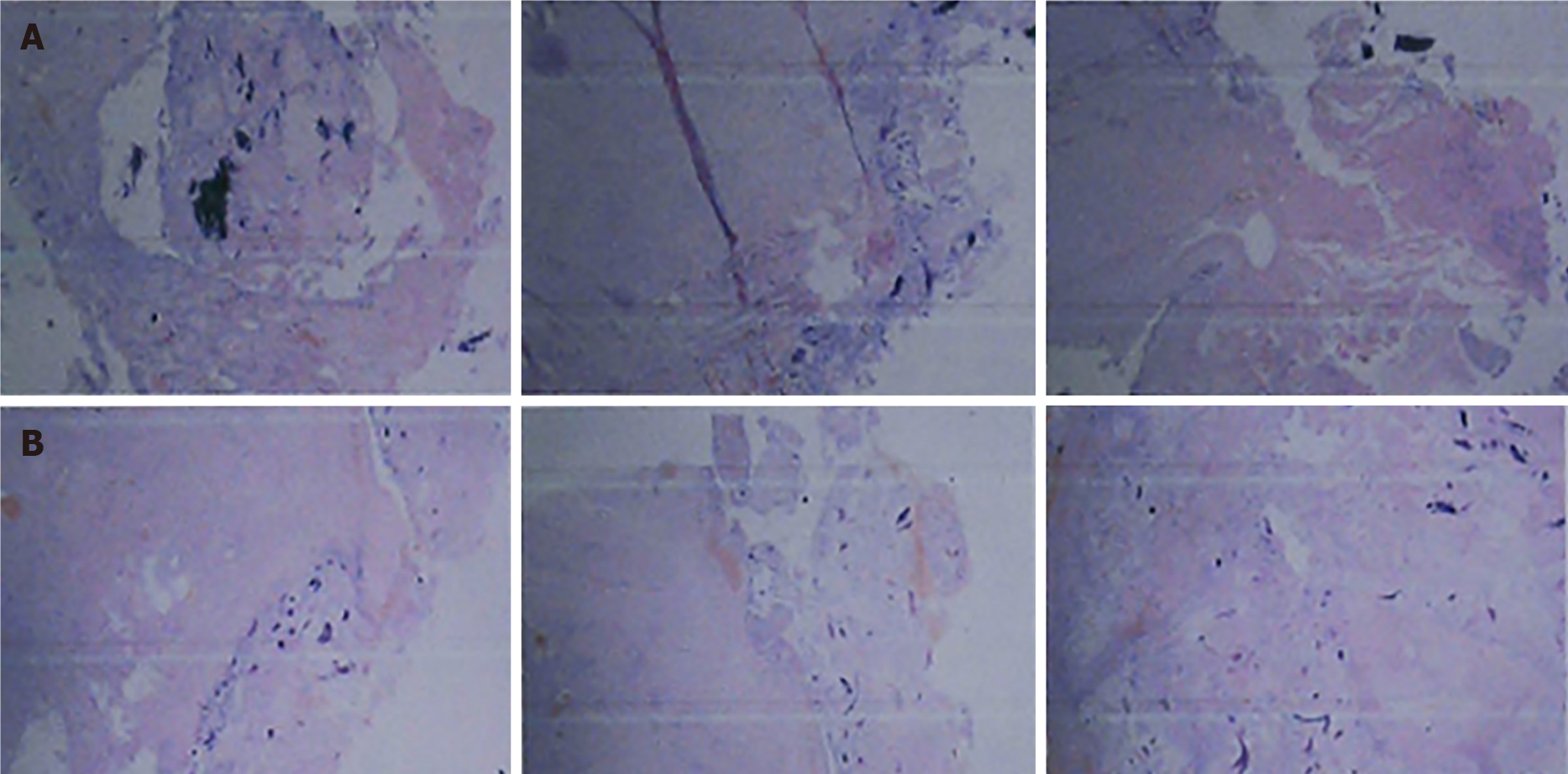
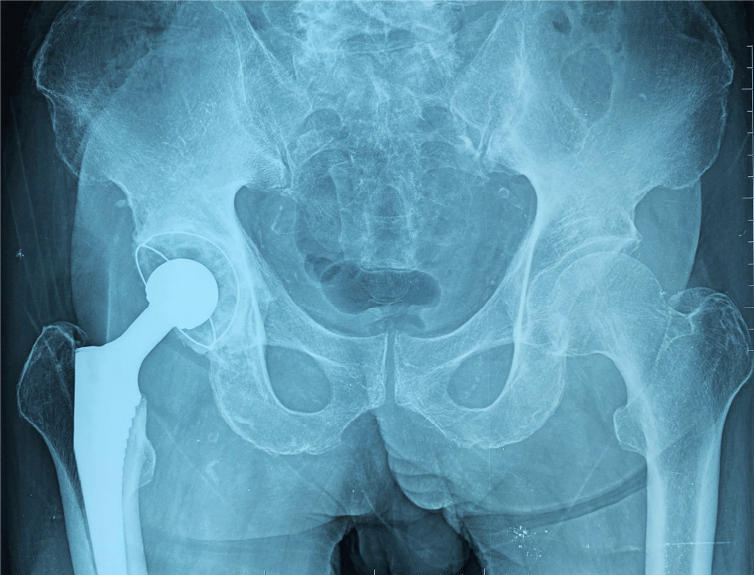
We performed total hip arthroplasty (THA) on the right hip (Figure 4) with a cement-type Lubinus anti-dislocation acetabular cup, D = 50 mm (Walde mar Link, Germany). We conducted pathological examinations on specimens of the right femoral head and synovial tissue collected intraoperatively. Tissue degeneration, calcification, small vascular proliferation and a multicore giant cell response were observed in some regions of femoral head tissue sections, and capillary hyperplasia with acute and chronic inflammatory cell infiltration, local tissue denaturation, and calcification were observed in synovial tissue sections (Figure 5). The pathologist considered that the pathological changes were consistent with CN.
At the 6-mo follow-up, the patient felt clinically well, with no pain, joint fluid collection, or hip dislocation, and could walk independently with a knee brace. The right lower limb had a muscle strength score of 4/5, and the HHS score was 84 points. The 6-mo follow-up X-rays revealed that the acetabular inclination and anteversion were well maintained, without clinical manifestations of implant loosening compared with postoperative observations (Figure 6).
CN is considered a multifactorial disease that may lead to progressive bone resorption and destruction of the joints and further develop into joint instability, deformity, and structural collapse with or without pain[6,7]. The possible pathogenesis includes the following: (1) Inflammatory cascading reactions caused by excessive expression of proinflammatory cytokines lead to an increase in osteoclastogenesis; (2) Abnormal neurovascular reflexes at joints promote osteoclast activation, leading to osteopenia; and (3) Painful sensory neuropathy and local temperature increases lead to the accumulation of microtrauma, resulting in joint dislocation and collapse[8,9]. Diabetes is considered the most common cause of CN and usually affects the foot and ankle joints[10]. However, syringomyelia-associated CN accounts for only approximately 5% of CN cases and easily causes upper limb CN[4,11], which most frequently involves the shoulder and elbow joints[3,12]. Only a few studies have reported that CN of the hip and knee joint is a rare complication of lumbar syringomyelia caused by epidural anesthesia[13,14].
Syringomyelia is a chronic progressive condition in which cystic tubular cavitation of the spinal cord is commonly caused by disorders of normal cerebrospinal fluid (CSF) flow dynamics. The possible mechanisms of syringomyelia include: (1) CSF transfer from the fourth ventricle to the central canal[15,16]; and (2) CSF filtration through medullary channels through perivascular and interstitial metastasis[17]. While the etiology of syringomyelia is not completely understood, Chiari 1 malformation is generally considered the most significant etiology. Other possible causes include Chiari 2 malformation, myelomeningocele, tethered cord syndrome, hydrocephalus, infection, inflammatory conditions, trauma, extramedullary tumors, arachnoid cysts and spinal canal stenosis[18,19]. As a rare factor, CSM associated with syringomyelia is scarcely reported in the literature[20]. Four potential pathologic mechanisms have been considered for cervical syringomyelia secondary to CSM: Ischemia causing degeneration, microtrauma caused by persistent oppression, hydrodynamic changes in CSF, and the dynamic pincer effect[21]. In addition, enhanced blood flow signals in compressed segments of the cervical spinal cord suggest that dyskinesia of CSF movement may play a role in the process of lesion development[22,23]. The typical clinical manifestation of syringomyelia is weakening or loss of pain and temperature sensation, but proprioception and position sensation are maintained, which is known as "free anesthesia", with muscular atrophy and undernourishment at the level of lesions. With loss of the normal protective reflex, joints may suffer from unrecognized trauma or repeated injury, eventually leading to the occurrence of CN.
In this case, the patient had no significant previous medical history except for CSM diagnosed 16 years prior. According to the MRI results, we found that the patient had CSM and cervical syringomyelia at C4, without other special lesions. Therefore, we suspected that cervical syringomyelia at C4 was associated with long-term CSM, although this is an extremely rare pathological association. Meanwhile, a typical X-ray showed severe hip deterioration, bone loss, and destruction. Interestingly, we carried out systemic physical, laboratory and imaging examinations on the patient, which confirmed that the patient's blood glucose and laboratory results were normal, indicating no other systemic diseases. Therefore, we excluded the differential diagnoses of congenital pain insensitivity, infection and inflammatory joint disease. Finally, after discussion with neurologists and radiologists, the patient was diagnosed with CN of the right hip caused by cervical syringomyelia secondary to CSM.
Early CN is difficult to distinguish from other diseases due to a lack of characteristic clinical manifestations, typical X-ray findings, laboratory tests, and severe underlying neurological disease that can facilitate clinical diagnosis. The management goal for CN patients is early diagnosis and intervention to maintain joint and limb function and active treatment of primary underlying diseases. The surgical strategy for CN of the hip remains controversial.
In the past, THA was considered a contraindication in the absence of complete remission of the primary disease[24,25]. However, a recent study suggested that the clinical prognosis of patients undergoing primary THA for CN was significantly improved, including pain relief, mobility recovery, and joint stability enhancement. Notably, a higher risk of early complications, such as recurrent dislocation, femoral body loosening and periprosthetic fracture, is evident due to the loss of protective sensations and reflexes, osteopenia, and ligament and muscle weakness[26,27]. According to the study of Parvizi et al[28], in the largest series of TKA (non-THA) treatment for CN, the survival rate without mechanical failure within 8 years was 82.5%, the aseptic loosening rate was 5.3%, and the instability rate was 2.6%.
Therefore, adopting reasonable precautions to provide a fixed prosthesis and increase the stability of the hip joint is particularly important to avoid early complications, including: (1) The use of a larger diameter femoral head to reduce the risk of postoperative dislocation; (2) The use of a highly cross-linked polyethylene lining to reduce wear; (3) The use of 3D CT template software to produce femoral stems with appropriate anteversion and femoral offset; (4) The use of a CT navigation system to reproduce the preoperative plan as much as possible; (5) Judicious use of dual mobility constructs; and (6) Revision of the acetabular component and the use of multiple acetabular screws to enhance the firmness of the component[26,29,30]. In addition, we should adhere to conservative treatments during the perioperative period, such as weight-bearing, immobilization, passive stretching, physical therapy and nonsteroidal anti-inflammatory drugs[5].
In our case, the patient refused to undergo cervical decompression surgery. To relieve the pain in the hip joint and restore the motor function of the limbs, we performed right THA. In the preoperative evaluation, the patient suffered from osteoporosis and bone fragmentation and loss around the hip. We suspect that the mass and strength of bone around the hip joint cannot provide sufficient stability at the bone-implant interface if a biotype acetabular cup is adopted. Moreover, the cement-type prosthesis possesses a short curing time, and strong fixation is achieved immediately after curing. Two to three days postoperatively, the patient can begin to bear partial weight with support, which reduces perioperative complications. During the operation, to reduce the risk of dislocation, we used the Hardinge approach to preserve the external rotator muscle group, which can reduce hip joint stability impairment with close suturing of the joint tendon. The cement-type Lubinus anti-dislocation acetabular cup and a biological fully coated biconical femoral stem were used to improve stability and promote bone ingrowth. After surgery, the patient's symptoms improved, without complications such as recurrent dislocation, loosening of the femoral prosthesis, and fracture around the prosthesis.
We suggest a possible etiological association between CSM and syringomyelia, which may reflect a potential pathogenesis of CN. A differential diagnosis should be established for patients with joint swelling, pain and limited mobility, especially patients with chronic shoulder and neck pain. Imaging examinations should be actively performed to rule out the possibility of syringomyelia secondary to CSM. Therefore, the key to successful treatment of patients with CN is comprehensive medical history acquisition, examination and early diagnosis. In addition, arthroplasty may no longer be an absolute contraindication to surgical treatment of CN. Reasonable selection of the surgical strategy can markedly improve the clinical symptoms and quality of life of patients.
We thank the staff at the Department of Radiology and the Department of Pathology in Yunnan Provincial Hospital of Traditional Chinese Medicine.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Oommen AT S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Johnson JT. Neuropathic fractures and joint injuries. Pathogenesis and rationale of prevention and treatment. J Bone Joint Surg Am. 1967;49:1-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 175] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Wukich DK, Sung W. Charcot arthropathy of the foot and ankle: modern concepts and management review. J Diabetes Complications. 2009;23:409-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 143] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 3. | Alpert SW, Koval KJ, Zuckerman JD. Neuropathic Arthropathy: Review of Current Knowledge. J Am Acad Orthop Surg. 1996;4:100-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 52] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Sahoo SK, Salunke P. Charcot arthropathy of the elbow joint as a presenting feature of Chiari malformation with syringomyelia. Br J Neurosurg. 2014;28:811-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Wang X, Li Y, Gao J, Wang T, Li Z. Charcot arthropathy of the shoulder joint as a presenting feature of basilar impression with syringomyelia: A case report and literature review. Medicine (Baltimore). 2018;97:e11391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Anders LF. Charcot neuroathropathy of the foot. In: Bowker J, Pfeifer M, editors. The diabetic foot. 6th edition. St. Louis: Mosby, 2001: 439-466. |
| 7. | Chantelau E, Richter A, Ghassem-Zadeh N, Poll LW. "Silent" bone stress injuries in the feet of diabetic patients with polyneuropathy: a report on 12 cases. Arch Orthop Trauma Surg. 2007;127:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Chisholm KA, Gilchrist JM. The Charcot joint: a modern neurologic perspective. J Clin Neuromuscul Dis. 2011;13:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Rajbhandari SM, Jenkins RC, Davies C, Tesfaye S. Charcot neuroarthropathy in diabetes mellitus. Diabetologia. 2002;45:1085-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 157] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | Deng X, Wu L, Yang C, Xu Y. Neuropathic arthropathy caused by syringomyelia. J Neurosurg Spine. 2013;18:303-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Williams B. Orthopaedic features in the presentation of syringomyelia. J Bone Joint Surg Br. 1979;61-B:314-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 55] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Kenan S, Lewis MM, Main WK, Hermann G, Abdelwahab IF. Neuropathic arthropathy of the shoulder mimicking soft tissue sarcoma. Orthopedics. 1993;16:1133-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Paliwal VK, Singh P, Rahi SK, Agarwal V, Gupta RK. Charcot knee secondary to lumbar spinal cord syringomyelia: complication of spinal anesthesia. J Clin Rheumatol. 2012;18:207-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Memarpour R, Tashtoush B, Issac L, Gonzalez-Ibarra F. Syringomyelia with Chiari I malformation presenting as hip charcot arthropathy: a case report and literature review. Case Rep Neurol Med. 2015;2015:487931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Gardner WJ. Hydrodynamic mechanism of syringomyelia: its relationship to myelocele. J Neurol Neurosurg Psychiatry. 1965;28:247-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 409] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Williams B, Terry AF, Jones F, McSweeney T. Syringomyelia as a sequel to traumatic paraplegia. Paraplegia. 1981;19:67-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Ball MJ, Dayan AD. Pathogenesis of syringomyelia. Lancet. 1972;2:799-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 255] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Vandertop WP. Syringomyelia. Neuropediatrics. 2014;45:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Jones BV. Cord Cystic Cavities: Syringomyelia and Prominent Central Canal. Semin Ultrasound CT MR. 2017;38:98-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Bhagavathula Venkata SS, Arimappamagan A, Lafazanos S, Pruthi N. Syringomyelia secondary to cervical spondylosis: Case report and review of literature. J Neurosci Rural Pract. 2014;5:S78-S82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Chang HS, Nejo T, Yoshida S, Oya S, Matsui T. Increased flow signal in compressed segments of the spinal cord in patients with cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2014;39:2136-2142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Baron EM, Young WF. Cervical spondylotic myelopathy: a brief review of its pathophysiology, clinical course, and diagnosis. Neurosurgery. 2007;60:S35-S41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 23. | Fehlings MG, Skaf G. A review of the pathophysiology of cervical spondylotic myelopathy with insights for potential novel mechanisms drawn from traumatic spinal cord injury. Spine (Phila Pa 1976). 1998;23:2730-2737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 179] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 24. | Jostel A, Jude EB. Medical treatment of Charcot neuroosteoarthropathy. Clin Podiatr Med Surg. 2008;25:63-69, vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Fullerton BD, Browngoehl LA. Total knee arthroplasty in a patient with bilateral Charcot knees. Arch Phys Med Rehabil. 1997;78:780-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Chalmers BP, Tibbo ME, Trousdale RT, Lewallen DG, Berry DJ, Abdel MP. Primary Total Hip Arthroplasty for Charcot Arthropathy is Associated With High Complications but Improved Clinical Outcomes. J Arthroplasty. 2018;33:2912-2918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Snoddy MC, Lee DH, Kuhn JE. Charcot shoulder and elbow: a review of the literature and update on treatment. J Shoulder Elbow Surg. 2017;26:544-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Parvizi J, Marrs J, Morrey BF. Total knee arthroplasty for neuropathic (Charcot) joints. Clin Orthop Relat Res. 2003;145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Inoue D, Kabata T, Maeda T, Kajino Y, Fujita K, Hasegawa K, Yamamoto T, Tsuchiya H. Value of computed tomography-based three-dimensional surgical preoperative planning software in total hip arthroplasty with developmental dysplasia of the hip. J Orthop Sci. 2015;20:340-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Kajino Y, Kabata T, Maeda T, Iwai S, Kuroda K, Tsuchiya H. Does degree of the pelvic deformity affect the accuracy of computed tomography-based hip navigation? J Arthroplasty. 2012;27:1651-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |