Published online Oct 16, 2022. doi: 10.12998/wjcc.v10.i29.10583
Peer-review started: February 16, 2022
First decision: April 10, 2022
Revised: April 20, 2022
Accepted: August 14, 2022
Article in press: August 14, 2022
Published online: October 16, 2022
Processing time: 224 Days and 23.6 Hours
Necrotizing enterocolitis (NEC) is a multifactorial disease that predominantly affects premature neonates. Intestinal dysbiosis plays a critical role in NEC pathogenesis in premature neonates. The main risk factor for NEC in term infants is mesenteric hypoperfusion associated with ductal-dependent congenital heart disease (CHD) that eventually leads to intestinal ischemia. The incidence of NEC in neonates with critical CHD is 6.8%-13%. However, the role of the intestinal microbiome in NEC pathogenesis in infants with ductal-dependent CHD remains unclear.
A male term neonate with right atrial isomerism underwent modified Blalock-Taussig shunt placement on the 14th day of life and had persistent mesenteric hypoperfusion after surgery. The patient had episodes of NEC stage IIA on the 1st and 28th days after cardiac surgery. Fecal microbial composition was analyzed before and after cardiac surgery by sequencing region V4 of the 16S rRNA gene. Before surgery, species belonging to genera Veillonella and Clostridia and class Gammaproteobacteria were detected, Bifidobacteriaceae showed a low abundance. The first NEC episode was associated with postoperative hemodynamic instability, intestinal ischemia-reperfusion injury during cardiopulmonary bypass, and a high abundance of Clostridium paraputrificum (Clostridium sensu stricto I) (56.1%). Antibacterial therapy after the first NEC episode resulted in increased abundance of Gammaproteobacteria, decreased abundance of Firmicutes, and low alpha diversity. These changes in the microbial composition promoted the growth of Clostridium sensu stricto I (72.0%) before the second NEC episode.
A high abundance of Clostridium sensu stricto I and mesenteric hypoperfusion may have contributed to NEC in the present case.
Core Tip: Right atrial isomerism is associated with severe congenital heart disease, abnormal arrangement of internal organs, and asplenia/spleen hypoplasia. Infants with right atrial isomerism palliated with the systemic-to-pulmonary shunt have a “diastolic steal” of mesenteric blood flow, which increases the risk of necrotizing enterocolitis (NEC). The role of the microbiome in cardiogenic NEC remains unclear. This case report highlights possible interactions between dynamic changes in the fecal microbiome before and after cardiac surgery and development of recurrent NEC, based on hemodynamic changes, mesenterial perfusion, and antibacterial treatment in an asplenic infant with right atrial isomerism who underwent palliative cardiac surgery.
- Citation: Kaplina A, Zaikova E, Ivanov A, Volkova Y, Alkhova T, Nikiforov V, Latypov A, Khavkina M, Fedoseeva T, Pervunina T, Skorobogatova Y, Volkova S, Ulyantsev V, Kalinina O, Sitkin S, Petrova N. Intestinal microbiome changes in an infant with right atrial isomerism and recurrent necrotizing enterocolitis: A case report and review of literature. World J Clin Cases 2022; 10(29): 10583-10599
- URL: https://www.wjgnet.com/2307-8960/full/v10/i29/10583.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i29.10583
Necrotizing enterocolitis (NEC) is a severe multifactorial disease that predominantly affects premature infants of gestational age less than 29 wk. NEC in premature neonates represents 70%-90% of all NEC cases[1]. On account of the immaturity of the intestinal epithelium in premature neonates, NEC in neonates is characterized by intestinal inflammation and hyperactivation of Toll-like receptor-4 of the intestinal wall by lipopolysaccharides present in gram-negative bacteria[2].
The first cases of NEC in term neonates with congenital heart disease (CHD) were described by Polin et al[3] in 1976. The incidence of NEC in neonates with ductal-dependent CHD varies between 6.8% and 13%[4], and the mortality rate of NEC in neonates with CHD is twice that in neonates who do not have CHD (24.4% vs 11.8%)[5]. In infants with CHD, the main risk associated with NEC is “diastolic steal” of mesenteric blood flow that leads to intestinal ischemia[6]. In addition, decreased arterial blood oxygenation contributes to intestinal hypoxia[7]. The other known risk factors associated with NEC in neonates with CHD are earlier gestational age, single ventricle defect (SV), hypoplastic left heart syndrome, truncus arteriosus, the need for high doses of prostaglandin E1 (> 50 ng/kg/min), and episodes of systemic hypoperfusion or shock[8].
Many studies have analyzed the role of the intestinal microbiome composition in the development of NEC in premature infants, however, the role of the intestinal microbiome in the pathogenesis of NEC in neonates with CHD remains unclear. A literature search with the search terms “microbiome”, “congenital heart disease”, and “neonates” yielded six papers[6,9-13]: Two of these were reviews that focused on the role of the microbiome in the pathogenesis of NEC[6,11], but none of the six studies focused on the composition of the intestinal microbiome in neonates with CHD and NEC. In adults undergoing heart surgery, intestinal dysbiosis in the preoperative period is associated with the risk of NEC after heart surgery[14].
Some of the most severe cases of CHD are typically characterized by right atrial isomerism associated with SV, common atrium, common atrioventricular valve, pulmonary artery stenosis/atresia, and abnormal atrioventricular connection abnormalities[15]. Right atrial isomerism (Ivemark syndrome or heterotaxy syndrome), which was first described by Ivemark[16] in 1955, is characterized by abnormal arrangement of the internal organs, asplenia or hypoplasia of the spleen, midline liver, and the presence of three lobes in each lung. Ivemark syndrome occurs in 1 out of 10000 to 1 out of 40000 live births[17]. Patients with SV undergo staged cardiac surgery. The first stage in infants with SV and pulmonary artery stenosis is systemic-to-pulmonary shunt placement (modified Blalock-Taussig shunt/mBTS). The mBTS placement could require the use of cardiopulmonary bypass (CPB) depending on the anatomy of CHD. Infants palliated with the systemic-to-pulmonary shunt have reverse diastolic blood flow in the superior mesenteric artery[6], which increases the risk of NEC.
The present study describes the case of a neonate with right atrial isomerism (Ivemark or heterotaxy syndrome) who was palliated with mBTS on the 14th day of life (DOL) and subsequently developed two episodes of NEC IIA after the surgical procedure. The aim of this study was to analyze the dynamics of the intestinal microbiome composition in this infant by sequencing the V4 region of the small subunit ribosomal RNA (16S rRNA) gene of bacteria in serial fecal samples obtained before and after surgery for CHD.
To define the stage of NEC, the criteria of Bell et al modified by Kliegman and Walsh[18] were used. NEC was treated according to clinical guidelines approved by the Russian Federation for the diagnosis and treatment of newborns with NEC[19]. The results of 16S rRNA gene-based microbiota profiling were obtained after the patient’s discharge and did not influence treatment decisions.
A male term infant with right atrial isomerism underwent right-sided mBTS (3.5 mm) placement on DOL 14. The infant developed two episodes of NEC IIA after surgical treatment of CHD: On the first postoperative day (POD) 1 (DOL 15) and POD 28 (DOL 42). Green-colored gastric residuals with a volume of 6 mL were noted on POD 1 (DOL 15); the infant was placed on nil per os (NPO) at the time.
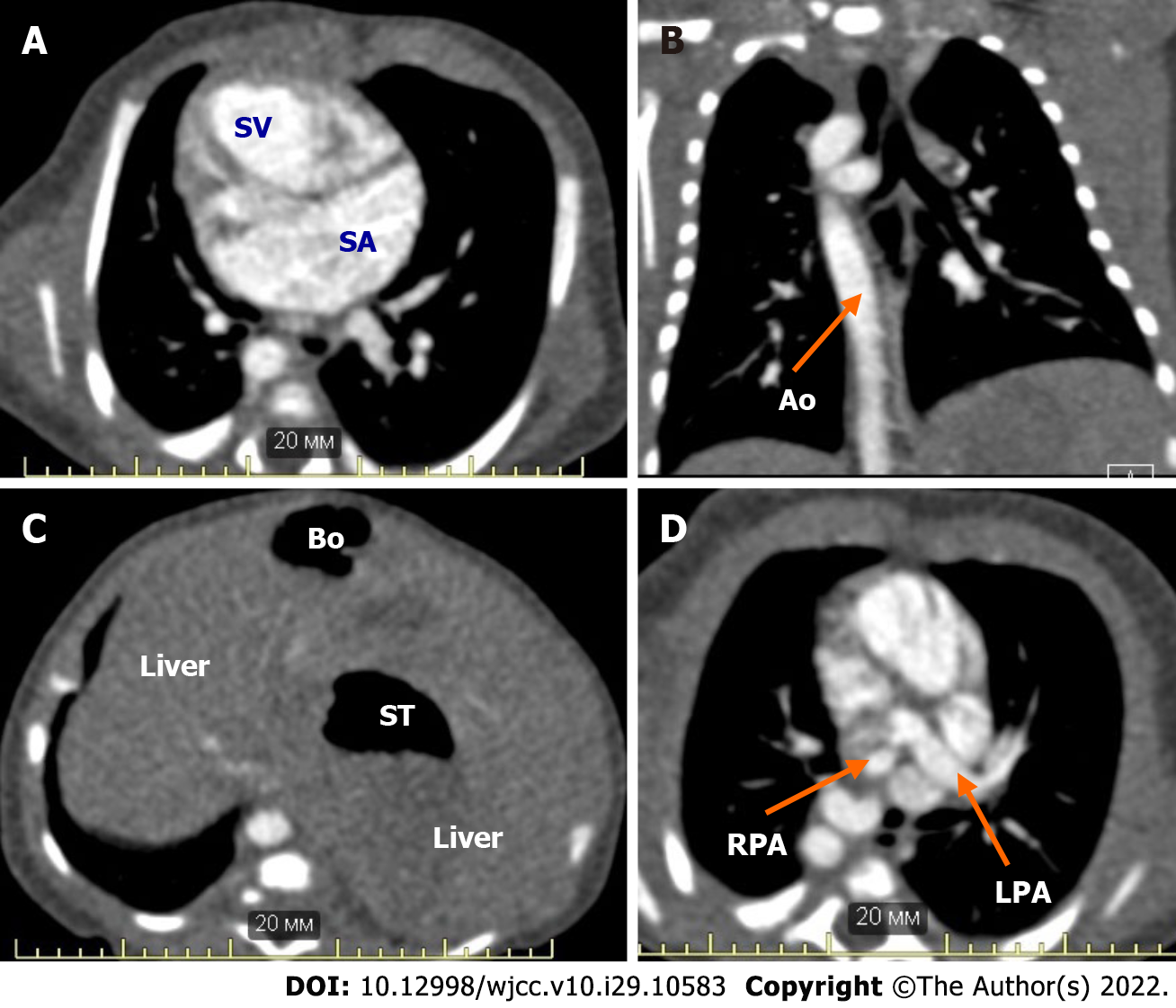
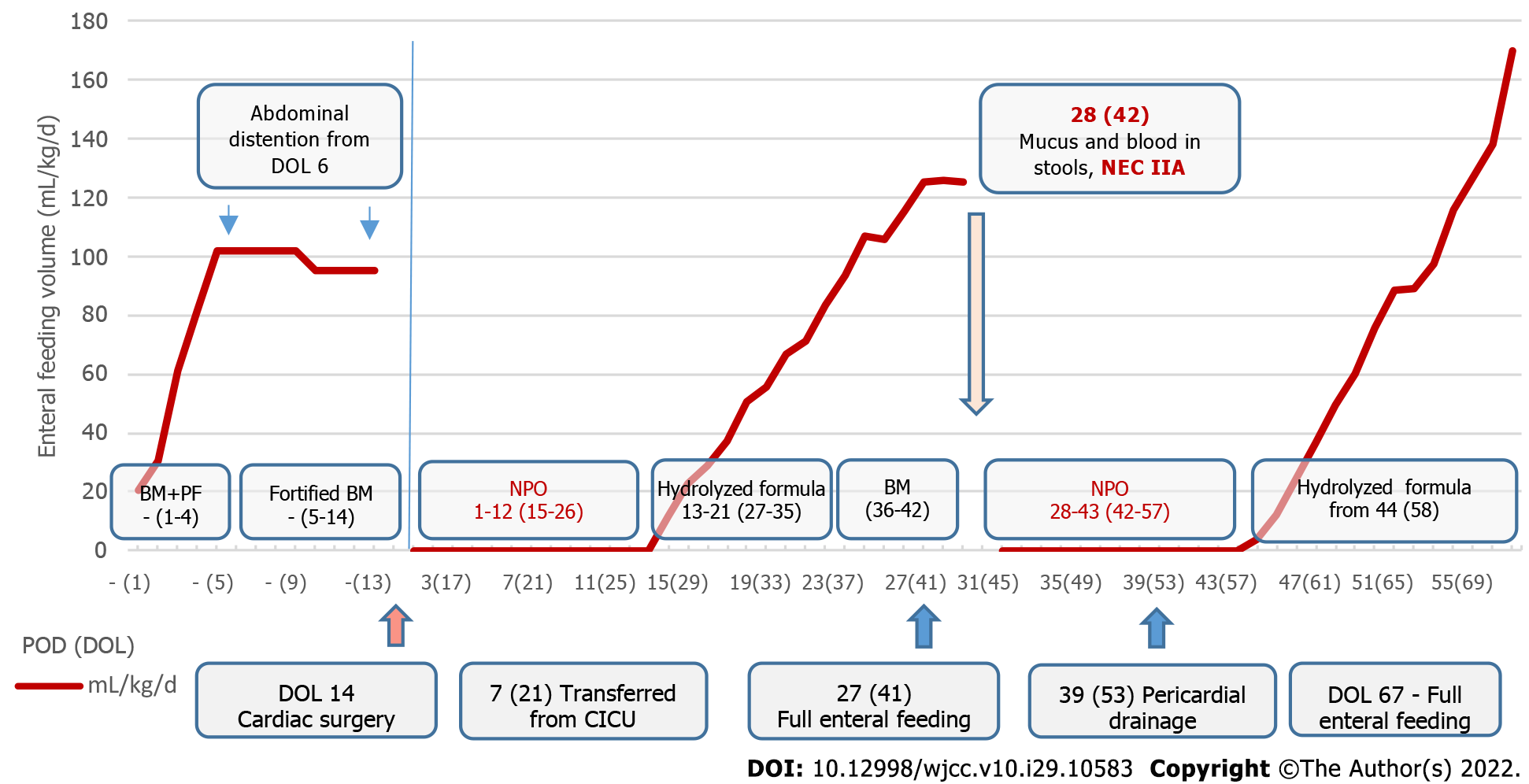
A male neonate with right atrial isomerism was born to a 19-year-old primiparous mother via spontaneous delivery at 40 wk of gestation (Figure 1). The patient was born at Almazov National Medical Research Centre. His Apgar score was 7 and 8 at 1 and 5 min of life, respectively; birth weight, 3430 g; height, 50 cm. Right atrial isomerism (right-sided heart, pulmonary artery atresia, common atrioventricular valve, and asplenia) was suspected based on the prenatal ultrasound findings obtained at the 24th week of gestation. After birth, the neonate was admitted to the neonatal intensive care unit. Postnatal echocardiography and computed tomography confirmed CHD (Table 1 and Figure 2). Intravenous prostaglandin E1 infusion at a dose of 30 ng/kg/min was commenced. The patient did not require respiratory or inotropic support before CHD correction. Enteral feeding was initiated from DOL 1 through oral administration of 10 mL of preterm formula every 3 h to provide supplemental protein and energy. Breast milk (BM) was added on DOL 2, and BM fortification was started on DOL 5. Moderate abdomen distention was noted on DOL 6, and feeding volume was not increased (Figure 3). Complete blood count (CBC) and C-reactive protein (CRP) were within the normal ranges before cardiac surgery [white blood cell (WBC) count, 13.4 × 109/L; CRP, 4 mg/L], and antibiotics were not administered. The results of a stool sample culture done on DOL 10 was negative, but oral swab culture revealed growth of Staphylococcus hominis.
| Characteristics | Description |
| CHD (according to echocardiography and CT results) | Right-sided heart. Double-inlet single ventricle. Common atrium. Malposition of the great arteries. Complete, unbalanced common atrioventricular valve. Right pulmonary artery stenosis. Double superior vena cava. Total, anomalous pulmonary venous return to the left superior vena cava (supracardiac type) |
| Noncardiac congenital anomalies (according to CT and abdominal ultrasound imaging) | Tracheal and esophageal displacement to the vertebral column. Tracheal narrowing to 4 mm × 2 mm at the level of the brachiocephalic trunk. Symmetrical liver. Asplenia. Common origin of the celiac trunk and superior mesenteric artery from the abdominal aorta. Right-sided aortic arch, right descending aorta |
Right-sided mBTS (3.5 mm) placement was performed on DOL 14 (Table 2). During the operation, right atrial isomerism was confirmed. Two open arterial ducts flowing into the pulmonary arteries from the aortic arch and the brachiocephalic trunk were found. Perioperative prophylaxis with cefuroxime was administered intravenously at a dose of 50 mg/kg 30 min before surgery, and after surgery, it was administered at a dose of 50 mg/kg every 8 h (three times), according to relevant national[20] and international guidelines[21].
| Characteristics | Values |
| Cardiopulmonary bypass duration (min) | 105 |
| Aortic cross-clamp during operation | Not performed |
| Mechanical ventilation duration after cardiac surgery (h) | 95 |
| Inotrope therapy duration (number of postoperative days) | 4 |
| Length of stay in the cardiac intensive care unit (d) | 6 |
| Length of stay after cardiac surgery (number of postoperative days) | 55 |
The patient’s condition deteriorated 45 min after the end of mBTS placement and he exhibited signs of cardiogenic shock: Oxygen saturation (SpO2) dropped to 50% and arterial hypotension (58/28 mmHg) occurred during inotropic therapy with a maximal allowable dose of epinephrine (0.5 µg/kg/min) and dobutamine (5 µg/kg/min). Considering the ineffectiveness of the therapy, norepinephrine was added with dose escalation from 0.2 to 0.4 µg/kg/min. This led to a blood pressure increase to 88/40 mmHg, after which the dose of epinephrine was decreased to 0.3 µg/kg/min. SpO2 increased to 71%, but was still low.
During resternotomy, a small amount of liquid blood in the pericardial cavity was revealed, without signs of shunt dysfunction. Delayed closure of the sternotomy wound was performed 22 h later. Diastolic steal of mesenteric blood flow persisted after the palliative cardiac surgery (Table 3). Green-colored gastric content with a residual volume of 6 mL was noted on POD 1 (DOL 15) while the patient was kept at NPO. NEC was suspected.
| Parameters | Postoperative day (day of life) | ||
| - (1) | 1 (15) | 5 (19) | |
| Course of the first episode of NEC stage IIA | |||
| RI AbdAo | > 1.0 | > 1.0 | 1.17 |
| RI celiac trunk | 0.9 | 1.05 | Not evaluated because patient exhibited anxiety |
| RI SMA | 0.86 | 1.05 | |
| Conclusion | Holodiastolic flow reversal in AbdAo | Holodiastolic flow reversal in AbdAo, celiac trunk, SMA | Holodiastolic flow reversal in AbdAo |
This is not applicable in the present case.
The mother and father of the patient were healthy. Bacterial culture of the cervix in the third trimester of pregnancy revealed abundant growth of Escherichia coli (E. coli) Placental histological examination revealed chorio-deciduitis.
The patient was sedated and treated with analgesics after cardiac surgery. His temperature was normal (36.7 °C), but he needed inotropic support and corticosteroid supplementation to maintain hemodynamic stability (epinephrine: 0.15 mg/kg/min, hydrocortisone: 25 mg/kg/d), as well as assisted ventilation. SpO2 was 85%; the target SpO2 is > 75% in infants with SV. The heart rate was 187 beats per minute (tachycardia, target values is 100-180 beats per minute), arterial blood pressure was maintained normal by inotropic support (82/37 mmHg). The abdomen was moderately distended, but not tender on palpation and did not exhibit peristalsis.
The WBC and neutrophil counts and CRP levels were much higher than normal for two days after surgery (Table 4). Gastric culture performed on POD 5 (DOL 19) revealed abundant growth of Enterococcus faecalis (sensitive to ampicillin, gentamicin, and vancomycin). Blood and fecal cultures revealed no microbial growth. Leukocytosis (based on the CBC results) increased on POD 6 and 7 (DOL 20 and 21) (Table 4). A pharynx smear culture on POD 5 (DOL 19) revealed growth of E. coli (sensitive to gentamicin and piperacillin/tazobactam, resistant to ampicillin/sulbactam).
| Parameters | - (9) | Postoperative day (day of life) | |||||||
| 1 (15) | 2 (16) | 3 (17) | 4 (18) | 5 (19) | 6 (20) | 7 (21) | |||
| Course of the first episode of NEC IIA | |||||||||
| Hemoglobin, g/L | 147 | 171 | 148 | 135 | 134 | 148 | 157 | 154 | |
| Red blood cells, 1012/L | 3.95 | 5.54 | 4.85 | 4.39 | 4.38 | 4.83 | 5.19 | 5.11 | |
| Platelets, 109/L | 559 | 154 | 144 | 152 | 158 | 208 | 263 | 289 | |
| White blood cells, 109/L | 13.4 | 19.5 | 24.7 | 18.7 | 11.1 | 12.7 | 17.6 | 18.4 | |
| Normoblasts per 100 white blood cells | 2 | 6 | 2 | ||||||
| Metamyelocytes, % | 1 | ||||||||
| Myelocytes, % | 1 | ||||||||
| Stab neutrophils, % | 11 | 9 | 1 | 2 | 2 | ||||
| Segmented neutrophils, % | 25 | 66 | 80 | 79 | 50 | 40 | 35 | 41 | |
| Eosinophils, % | 3 | 1 | 9 | 2 | 2 | ||||
| Basophils, % | 1 | ||||||||
| Monocytes, % | 16 | 13 | 3 | 7 | 10 | 13 | 11 | 26 | |
| Lymphocytes, % | 53 | 10 | 8 | 14 | 37 | 37 | 49 | 30 | |
| С-reactive protein, mg/L | 4 | 78 | 181 | 61 | 38 | 21 | 16 | 16 | |
Diastolic steal of mesenteric blood flow persisted after palliative cardiac surgery according to Doppler ultrasonography of the visceral arteries (Table 3).
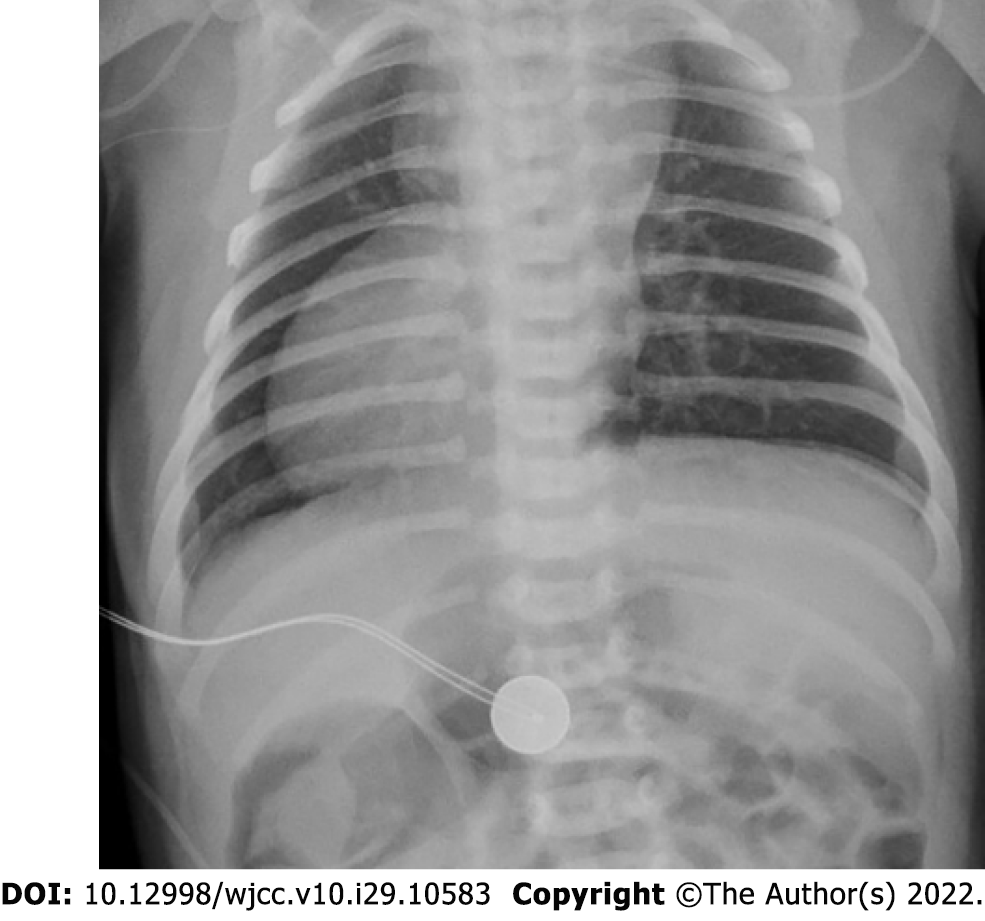
Abdominal ultrasound examination on POD 1 (DOL 15) revealed pneumatosis, thickening of the intestinal wall (up to 2 mm), and absence of peristalsis. There was no free gas in the abdominal cavity according to a radiograph taken on POD 1 (DOL 15). According to abdominal ultrasound data obtained on POD 5 (DOL 19), there were still signs of NEC IIA: Thickening of the intestinal walls to 1.5-2.0 mm and focal pneumatosis of the intestinal wall. However, ultrasound signs of NEC were absent on POD 11 (DOL 25).
Composition analysis of the fecal microbiota: Samples for 16S rRNA gene-based microbiota profiling were obtained before surgical treatment of CHD, on the 7th day of life (DOL 7) and DOL 14, as well as after surgical treatment of CHD, on POD 8 (DOL 22), POD 21 (DOL 35), and POD 29 (DOL 43) (Table 5). Samples were obtained from a diaper, collected in a sterile collection tube, and stored at -40 °C until analysis.
| Examinations | Postoperative day (day of life) | ||||||||||
| - (2) | - (7) | - (10) | - (14) | 5 (19) | 8 (22) | 18 (32) | 21 (35) | 29 (43) | 35 (49) | 40 (54) | |
| 16S rRNA gene-based microbiota profiling | - | + | - | + | - | + | - | + | + | - | - |
| Fecal culture | - | - | + | - | + | + | + | - | - | + | + |
| Gastric content culture | - | - | - | - | + | - | + | - | - | - | - |
| Pharynx smear culture | - | - | + | + | + | - | + | - | - | + | + |
| Blood culture | + | - | - | + | + | - | - | - | + | - | - |
Total DNA was extracted from 0.25 g of feces using the QIAamp Power Fecal DNA Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The fecal microbiota profiles were determined by sequencing the V4 region of the 16S rRNA gene with the NEXTflex™ 16S V4 Amplicon-Seq Kit 2.0 (PerkinElmer, Inc., Waltham, MA, United States). Sequencing was done on the Illumina MiSeq platform using MiSeq Reagent Kit v2 (500 cycles) (Illumina Inc., San Diego, CA, United States).
The sequences obtained were processed using QIIME 2 version 2020.11.1 (https://doi.org/10.1038/s41587-019-0209-9). Amplicon sequence variants were detected using DADA2 (https://doi.org/10.1038/nmeth.3869) via the QIIME plugin, without truncation of the forward and reverse reads and under other default parameters. Taxonomy was assigned using the q2-feature-classifier plugin (https://doi.org/10.1186/s40168-018-0470-z) by applying the pre-trained Naive Bayes classifier on the SILVA v.138 NR 99 database (https://doi.org/10.1093/nar/gks1219) for the V4 region, bound by the 515F/806R primer pair. Interactive visualization of taxonomy was performed with the “QIIME taxa barplot” method. Alpha diversity was calculated using the q2-diversity plugin, Chao1 index, and Shannon diversity index.
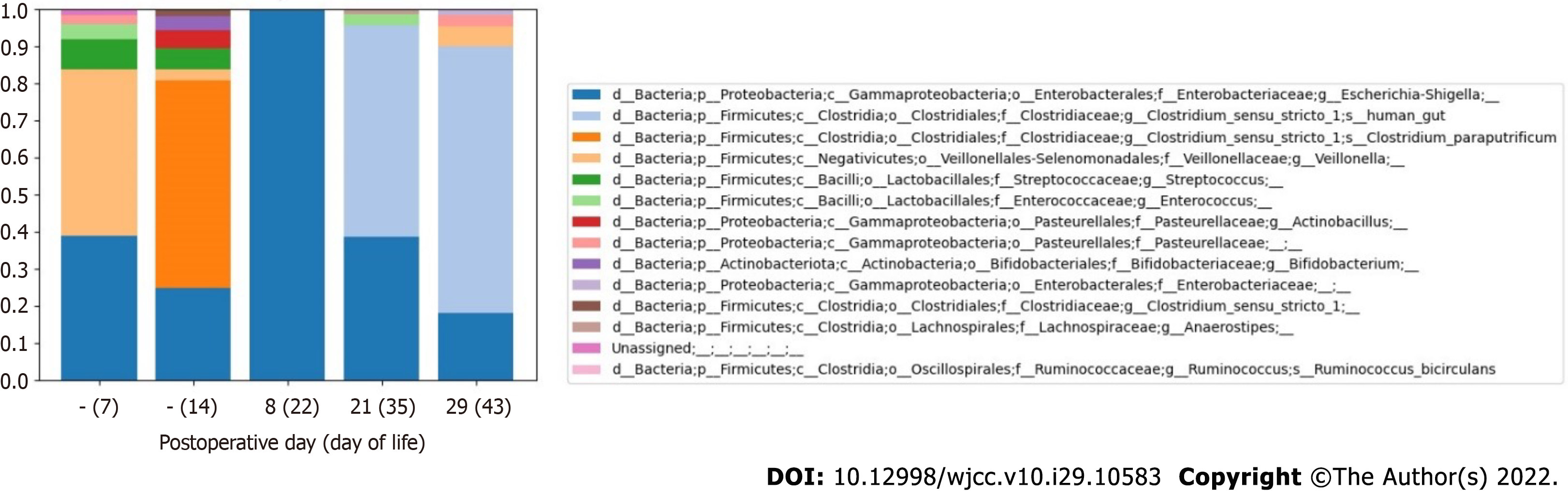
Composition of intestinal microbiota before surgical treatment of CHD and before onset of the first NEC episode: At a genus level, a high abundance of Veillonella (44.9%) was noted on DOL 7, but this decreased to 3.1% by DOL 14 (Figure 4 and Table 6). Despite feeding with expressed BM, a paucity of Bifidobacterium (3.8%) (Actinobacteria) was revealed before surgical treatment of CHD. Escherichia/Shigella species remained in the fecal samples on DOL 7 and DOL 14 (38.8% and 24.8%, respectively). Clostridium bacteria were not detected on DOL 7. However, before the first NEC episode, which developed on POD 1 (DOL 15), the prevalence of Clostridium sensu stricto I (57.8%), represented by Clostridium paraputrificum (C. paraputrificum) (56.1%), was noted on DOL 14.
| POD (DOL) | Class level | Family level | Genus level |
| Before surgical treatment of CHD (DOL 7) – feeding with fortified BM with preterm formula added (because of low BM supply) | |||
| - (7) | Negativicutes 44.9% | Veillonellaceae 44.9% | Veillonella 44.9% |
| Gammaproteobacteria 41.1% | Enterobacteriaceae 38.8% Pasteurellaceae 2.3% | Escherichia/Shigella 38.8% | |
| Bacilli 12.3% | Streptococcaceae 8.0% Enterococcaceae 4.3% | Streptococcus 8.0% Enterococcus 4.3% | |
| Before surgical treatment of CHD (DOL 14) – before the first episode of NEC stage IIA on POD 1 (DOL 15) Feeding with fortified BM with preterm formula added (because of low BM supply) | |||
| - (14) | Clostridia 57.8% | Clostridiaceae 57.8% | Clostridium sensu stricto I 57.8% 1Clostridium paraputrificum 56.1% |
| Gammaproteobacteria 29.6% | Enterobacteriaceae 24.8% Pasteurellaceae 4.8% | Escherichia/Shigella 24.8% Actinobacillus 4.8% | |
| Bacilli 5.7% | Streptococcaceae 5.7% | Streptococcus 5.7% | |
| Actinobacteria 3.8% | Bifidobacteriaceae 3.8% | Bifidobacterium 3.8% | |
| Negativicutes 3.1% | Veillonellaceae 3.1% | Veillonella 3.1% | |
| POD 8 (DOL 22) – after ampicillin/sulbactam therapy (POD 1-7) and metronidazole therapy (POD 2-6) had been completed and piperacillin/tazobactam therapy was started (POD 7-14) (nil per os) | |||
| 8 (22) | Gammaproteobacteria 99.8% | Enterobacteriaceae 99.8% | Escherichia/Shigella 99.8% |
| Clostridia 0.2% | Ruminococcaceae 0.2% | 1Ruminococcus bucirculans 0.2% | |
| POD 21 (DOL 35) – 7 d after the antibacterial therapy was completed on POD 14 Feeding with hydrolyzed formula | |||
| 21 (35) | Clostridia 58.0% | Clostridiaceae 56.9% Lachnospiraceae 1.1% | Clostridium sensu stricto I 56.9% Anaerostripes 1.1% |
| Gammaproteobacteria 38.7% | Enterobacteriaceae 38.7% | Escherichia/Shigella 38.7% | |
| Bacilli 3.2% | Enterococcaceae 3.2% | Enterococcus 3.2% | |
| POD 29 (DOL 43) – on the day after onset of the second episode of NEC IIA Feeding before NEC IIA onset: Expressed BM (from DOL 37) with hydrolyzed formula dotation | |||
| 29 (43) | Clostridia 72.0% | Clostridiaceae 72.0% | Clostridium sensu stricto I 72.0% |
| Gammaproteobacteria 22.7% | Enterobacteriaceae 19.8% Pasteurellaceae 2.9% | Escherichia/Shigella 18.1% | |
| Negativicutes 5.3% | Veillonellaceae 5.3% | Veillonella 5.3% | |
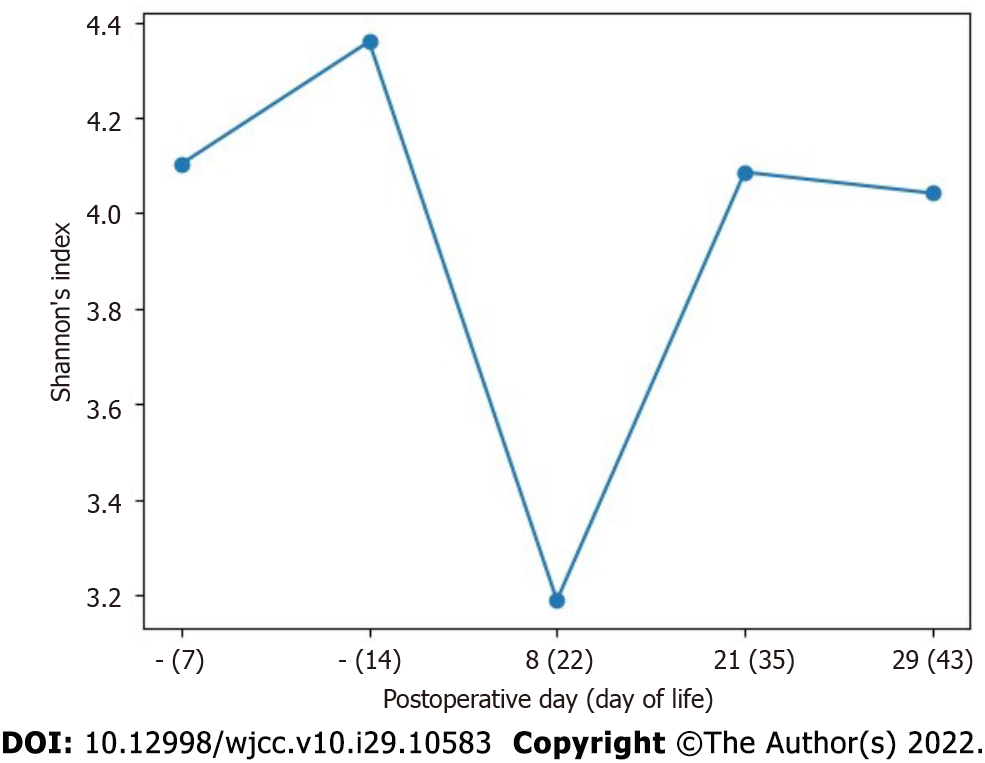
Composition of intestinal microbiota in the early postoperative period after surgical treatment of CHD: Low alpha diversity of bacteria was revealed on POD 8 (DOL 22), during the period in which antibacterial therapy was being administered for the first episode of NEC [after ampicillin/sulbactam (POD 1-7) and metronidazole therapy (POD 2-6) had been completed and piperacillin/tazobactam therapy (POD 7-14) was initiated] (Figure 5). The intestinal microbial composition changed at the phylum level: The abundance of Firmicutes decreased from 66.6% to 0.2%, and the proportion of Proteobacteria increased from 29.6% to 99.8% (Table 6).
Fecal cultures showed no bacterial growth before and after surgical treatment of CHD (Table 7). The absence of growth was probably associated with the predominance of anaerobic bacteria, which cannot be detected by routine culture.
| POD (DOL) | Fecal microbiome at the genus level, 16S rRNA | Fecal culture | Gastric culture | Pharynx smear culture | Blood culture | Antibacterial therapy |
| - (2) | - | - | - | - | No growth | No |
| - (7) | Escherichia/Shigella, Veillonella, Streptococcus, Enterococcus | - | - | - | - | No |
| - (10) | - | No growth | - | Staphylococcus hominis | - | No |
| - (14) | Clostridium sensu stricto I, Escherichia/Shigella, Streptococcus, Actinobacillus, Bifidobacterium, Veillonella | - | - | No growth | No growth | CUR: POD 0-1 (perioperative prophylaxis) |
| 5 (19) | - | No growth | Enterococcus faecalis R: TET | Escherichia coli S: CUR, PIT, GEN R: AMS | No growth | NEC IIA AMS: POD 1-7; MET: POD 2-6 GEN+PIT: POD 7-14 |
| 8 (22) | Escherichia/Shigella, Ruminococcus | No growth | - | - | - | |
| 18 (32) | - | No growth | No growth | Streptococcus oralis | - | No |
| 21 (35) | Clostridium sensu stricto I, Escherichia/Shigella, Enterococcus | - | - | - | - | No |
| 29 (43) | Clostridium sensu stricto I, Escherichia/Shigella, Veillonella | - | - | - | No growth | NEC IIA No |
| 35 (49) | - | No growth | - | Streptococcus mitis, Rothia mucilaginosa | - | No |
| 40 (54) | - | No growth | - | No growth | - | No |
Chief complaints: The infant presented with mucus and blood in the stool and post-feeding anxiety on POD 28 (DOL 42).
History of present illness: After the first NEC episode, enteral feeding was started on POD 13 (DOL 27). Full enteral feeding was achieved on POD 27 (DOL 41) (Figure 3). Before onset of the second NEC episode on POD 28 (DOL 42), the infant received full enteral feeding predominantly with expressed BM (Figure 3).
History of past illness: The patient had the first episode of NEC IIA after surgical treatment of CHD on POD 1 (DOL 15).
Physical examination: The infant presented on POD 28 (DOL 42) with mucus and blood in the stool and post-feeding anxiety. He was hemodynamically stable and did not require inotropic or respiratory support. The patient received oral antiplatelet therapy with aspirin (5 mg/kg/d) to prevent shunt thrombosis. The heart rate was 154 beats per minute, and SpO2 was 84%. The abdomen was bloated and moderately painful on palpation, but was not tender. Peristalsis was noted.
Laboratory examinations: CBC on POD 29 (DOL 43) revealed neutrophilia (60%), but the CRP levels were normal (Table 8). The laboratory findings were normal on POD 32 (DOL 46) and POD 36 (DOL 50). Blood and fecal cultures revealed no microbial growth. Pharynx smear culture revealed moderate growth of Streptococcus mitis and Rothia mucilaginosa.
| Parameters | Postoperative day (day of life)/day after the pericardial drainage procedure | ||||||
| 26 (40) | 29 (43) | 32 (46) | 36 (50) | 40 (54)/1 | 41 (55)/2 | 43 (57)/4 | |
| Course of the second episode of NEC IIA | |||||||
| Hemoglobin, g/L | 136 | 132 | 139 | 155 | 124 | 115 | 122 |
| Red blood cells, 1012/L | 4.6 | 4.4 | 4.6 | 5.2 | 4.1 | 3.8 | 4.1 |
| Platelets, 109/L | 461 | 554 | 139 | 331 | 216 | 141 | 236 |
| White blood cells, 109/L | 7.6 | 9.7 | 13.6 | 8.8 | 17.5 | 21.9 | 14.3 |
| Stab neutrophils, % | 1 | 1 | 1 | ||||
| Segmented neutrophils, % | 27 | 60 | 20 | 19 | 55 | 50 | 28 |
| Eosinophils, % | 3 | 4 | 3 | 1 | 2 | 1 | |
| Basophils, % | 1 | ||||||
| Monocytes, % | 13 | 8 | 10 | 11 | 9 | 7 | |
| Lymphocytes, % | 57 | 28 | 67 | 67 | 39 | 39 | 63 |
| С-reactive protein, mg/L | 4 | 1 | NA | 1 | 53 | 203 | 54 |
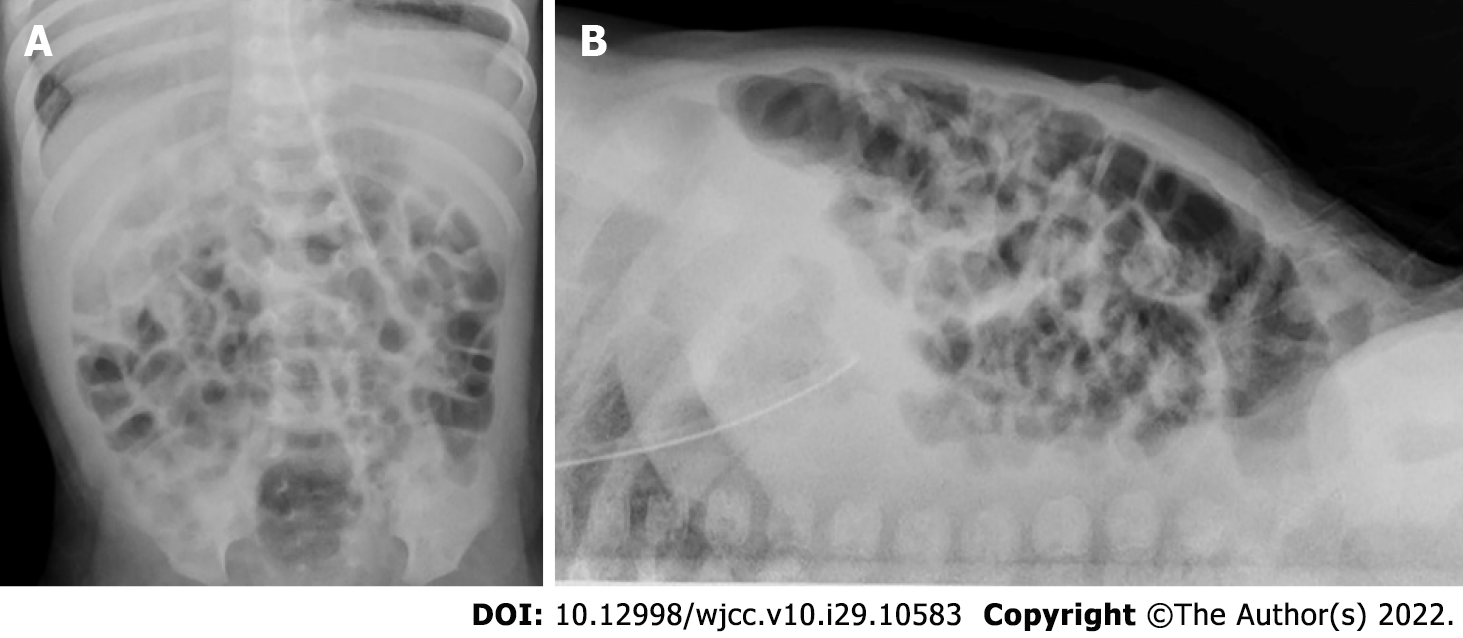
Imaging examinations: Abdomen ultrasound examination performed on POD 29 (DOL 43) revealed signs of NEC IIA: Pneumatosis intestinalis, dilation of the bowel loops to 13-15 mm, and decreased peristalsis. No free gas in the abdominal cavity was detected on a radiograph that was taken (Figure 6). Abdominal ultrasound examination performed on the 16th day after NEC onset (DOL 58) showed no signs of NEC, so enteral feeding with hydrolyzed formula was started.
Composition of intestinal microbiota in the late postoperative period after surgical treatment of CHD and at onset of the second NEC episode: After the end of antibacterial therapy for the first episode of NEC (ampicillin/sulbactam course on POD 1-7, metronidazole on POD 2-6, and piperacillin/ tazobactam and gentamicin POD 7-14), the proportion of Clostridium sensu stricto I increased again on POD 21 (DOL 35) to 56.9%.
The abundance of Clostridium sensu stricto I was the highest (72.0%) on POD 29 (DOL 43), after onset of the second episode of NEC IIA (Figure 4 and Table 6). Clostridium difficile (Clostridioides difficile) was not detected in any of the fecal samples. Two weeks after completion of the antibiotic course, Veillonella species were detected again (5.3%) on POD 29 (DOL 43).
Based on ultrasound findings, clinical data, and the results of 16S rRNA gene-based fecal microbiota profiling, the final diagnosis was recurrent NEC in infant with CHD associated with a high abundance of Clostridium sensu stricto I.
Antibacterial therapy with ampicillin/sulbactam was started (150 mg/kg/d two times a day, intravenously administered) from POD 1[22], and was intensified with metronidazole (an initial dose of 15 mg/kg that was reduced to 7.5 mg/kg and administered every 12 h) according to the national guidelines for the treatment of NEC[19,23]. According to the abdominal ultrasound data obtained on POD 5 (DOL 19), the signs of NEC IIA remained. Further, leukocytosis increased on POD 6 and 7 (DOL 20 and 21) (Table 4). A pharynx smear culture on POD 5 (DOL 19) revealed growth of E. coli (sensitive to gentamicin and piperacillin/tazobactam, resistant to ampicillin/sulbactam). Antibacterial therapy was changed to gentamicin (4 mg/kg every 24 h)[23] and piperacillin/tazobactam (90 mg/kg every 8 h) on POD 7 (DOL 21).
NPO was initiated on POD 28 (DOL 42) when NEC was suspected. The vomiting and bloody stools stopped after NPO was instated, and the patient’s condition improved. Additionally, the laboratory findings were normal on POD 32 (DOL 46) and POD 36 (DOL 50), so antibacterial therapy was avoided.
The antibiotic course was completed on POD 14 (DOL 28). After the first episode of NEC, the patient was kept NPO until POD 11. Ultrasound signs of NEC were absent on POD 11 (DOL 25), so water intake was allowed on POD 12 and was followed by enteral feeding with a hydrolyzed formula on POD 13 (DOL 27). The patient was fed with expressed BM, which was replaced with hydrolyzed formula for 2-3 feedings a day (because of low BM supply) from POD 22 (DOL 36). Full enteral feeding was achieved on POD 27 (DOL 41) (Figure 3).
On POD 39 (DOL 53), the patient showed initial signs of hydropericardium, so pericardial drainage was performed. Before the pericardial drainage procedure and within 48 h after the procedure, antibacterial prophylaxis with cefuroxime (50 mg/kg) was administered every 8 h according to an internal protocol.
On the second day after the pericardial drainage procedure (DOL 55), CRP increased to 203 mg/L (Table 8). Additionally, ultrasound signs of NEC IIA were still present on the 13th day after NEC onset (DOL 55). Therefore, cefuroxime therapy was continued for 3 d after the pericardial drainage procedure, and the CRP level decreased to 54 mg/L on DOL 57.
Abdominal ultrasound examination performed on the 16th day after NEC onset (DOL 58) showed no signs of NEC, so enteral feeding with hydrolyzed formula was started. Full enteral feeding was achieved by the 25th day after NEC onset (DOL 67).
The patient was discharged on the 55th day after surgical treatment of CHD (DOL 69).
This study describes the case of an infant with Ivemark syndrome who underwent palliative surgical treatment of CHD and had two episodes of NEC in different premorbid states. The first NEC episode occurred after significant acute hemodynamic instability during the early postoperative period, at which point the infant had not received prior antibacterial therapy. The second NEC episode developed a month after palliative cardiac surgery with the patient on full enteral nutrition and in a hemodynamically stable state, but with persistent mesenteric hypoperfusion. The patient had a history of antibacterial therapy at the time of the second NEC episode.
In the present study, fecal microbiota profiling based on sequencing of the V4 region of the 16S rRNA gene revealed several interesting features. The infant had a considerably smaller proportion of Bifidobacteriaceae (3.8%) than is usually present in healthy breastfed infants (60%-80%) and infants that are formula fed (40%)[24]. The paucity of Bifidobacteriaceae might have been a result of mixed feeding, but the influence of CHD-related oxygenation issues cannot be ruled out. In fact, Ellis et al[9] reported a decrease in the Bifidobacteriaceae count in the fecal samples of infants with CHD.
Before cardiac surgery, we found a high abundance of Veillonella (Negativicutes) in combination with Escherichia/Shigella (Gammaproteobacteria) on DOL 7. The prevalence of Gammaproteobacteria[2,25] and decreased abundance of Negativicutes[26] are known to be associated with NEC in preterm neonates. Additionally, the combination of Veillonella and Klebsiella (Enterobacteriaceae) has been reported in preterm neonates without NEC[27]. In particular, a high abundance of Veillonella was found in preterm neonates without NEC who did not require antibacterial therapy, while a paucity of Veillonella was observed in infants recovering from NEC[28]. Therefore, according to the results of previous studies, the microbiome findings in the first week of life in the present patient were probably not associated with NEC risk.
Before the first NEC episode, the patient had a high abundance of C. paraputrificum (Clostridium sensu stricto I) (56.1%) in combination with Escherichia/Shigella (24.8%) on DOL 14, while the proportion of Veillonella decreased by 14.5 times. High abundance of Firmicutes is typical in the intestinal microbiome of adults, while in newborns, the prevalent bacteria are Bifidobacteriaceae (Actinobacteria)[29]. In addition, Firmicutes predominance has also been found in infants with CHD[9]. Further, Clostridium bacteria were found to be more common in the feces of bottle-fed infants who underwent hospitalization after birth and premature neonates[30]. The microbiome composition of the patient at the second week of life was not typical for healthy neonates and possibly could predispose him to NEC development after cardiac surgery. We speculate that feeding with exclusive BM would have increased the Bifdobacteriaceae preoperative abundance and the abundance of Clostridium would not have been as high.
The significant role of hemodynamic instability in NEC development on the first day after cardiac surgery cannot be excluded in our patient, given the fact that he developed cardiogenic shock that required high doses of inotropes. Accordingly, a significantly high incidence of decreased cardiac output and shock has been previously reported in infants with CHD who developed NEC[8]. Additionally, it has been shown that neonates with CHD and NEC had frequent episodes of hypoxia before the development of NEC[31].
In the present case, the prevalence of C. paraputrificum on DOL 14 could also be associated with the first NEC episode on POD 1 (DOL 15). Shinha and Hadi[32] described a case of intestinal necrosis associated with C. paraputrificum bacteremia in an adult patient with acquired immunodeficiency syndrome. The patient in our case had asplenia, which is also a form of immunodeficiency. Therefore, it might be interesting to explore the role of C. paraputrificum in the pathogenesis of NEC in asplenic patients.
After antibacterial therapy initiation in the early postoperative period, bacterial alpha diversity decreased and the intestinal microbial composition changed at the phylum level: The abundance of Firmicutes decreased to 0.2%, and the proportion of Proteobacteria increased to 99.8%. Gammaproteobacteria (Enterobacteriaceae and Trabusiella) predominance was previously shown to be associated with NEC in preterm neonates[2]. In our patient, Clostridium sensu stricto I predominance resumed at 1 wk and 2 wk after the end of antibacterial therapy (Figure 4 and Table 6). The highest abundance of Clostridium sensu stricto I (72%) was detected at the onset of the second NEC episode, and could be an etiological factor.
The Clostridium sensu stricto I genus includes more than 30 species [Clostridium butyricum (C. butyricum), Clostridium paraputrificum, Clostridium perfringens, Clostridium disporicum, Clostridium saudiense, etc.] that produce short-chain fatty acids via the anaerobic fermentation of carbohydrates. The specific metabolite of Clostridium sensu stricto I is butyrate, which is produced from carbohydrates by the phosphotransbutyrylase/butyrate kinase pathway[33]. Butyrate is a principal source of energy for mature colonocytes, which metabolize it via β-oxidation[34]. Butyrate reduces the production of pro-inflammatory cytokines[35], inhibits autoantibody production by B-lymphocytes[36], and affects colonic regulatory T-cell differentiation[37]. For these reasons, specific C. butyricum strains are used as probiotics in Japan, Korea, and China[38].
A number of studies have confirmed the etiological role of Clostridium sensu stricto I in the development of NEC in premature infants[28,39,40]. Infants with late-onset NEC had a significantly higher Escherichia/Shigella abundance six days before NEC onset[41], while early-onset NEC (DOL 22 and earlier) in preterm neonates was found to be related to a higher abundance of Clostridium sensu stricto I (56.4%). Similarly, Rozé et al[40] reported increased abundance of Clostridium sensu stricto I (20.6%) in preterm infants with NEC, but this was lower than the abundance in our term patient at NEC onset. The concentration of butyrate in premature infants with poor intestinal motility can reach toxic levels of 200-300 mM in the proximal colon[42]. This can lower the intestinal pH and potentially cause intestinal mucosal injury, which might play a role in the pathogenesis of NEC[42].
The role of butyrate in NEC pathogenesis is probably associated with its ability to inhibit the proliferation of intestinal stem/progenitor cells located at the base of intestinal crypts[43]. The opposite effect of butyrate on the proliferation of differentiated and stem cells is known as the “butyrate paradox”[44]. The antiproliferative effect of butyrate provides protection from colon carcinogenesis[44]. This pathway is only realized when the base of the intestinal crypt is exposed to high concentrations of butyrate, but this could result in mucosal damage. Alternatively, the crypt structure may prevent contact between luminal butyrate and progenitor cells[43]. Mature colonocytes at the top of the crypt utilize butyrate and, thereby, decrease its concentration in the crypt. Thus, in the absence of mucosal damage, only a small amount of butyrate reaches the stem cells at the base of the crypt, exerting a beneficial anti-inflammatory and tolerogenic effect on T-regulatory cells and macrophages[43]. Based on these findings, we speculate that the effect of butyrate synthesized by abundant Clostridium sensu stricto I (57.8%) on the damaged intestinal mucosa might have acted as an additional risk factor for NEC in our patient.
The second NEC episode in the present case was associated with the highest abundance of Clostridium sensu stricto I (72.0%) under conditions of persistent diastolic flow reversal. Several studies have shown the dose-dependent effect of butyrate. For instance, high concentrations (8-16 mmol/L) have a toxic effect and induce apoptosis of epithelial cells, increase their permeability, disrupt the barrier function of the intestinal epithelium[45], increase gene expression of the pro-inflammatory cytokine IL-6, and reduce the number of viable cells[46]. A low concentration of butyrate (1-2 mmol/L), on the contrary, reduces the permeability of the intestinal epithelium, improves barrier function[45], and reduces IL-6 gene expression[46].
The high abundance of Clostridium sensu stricto I revealed in the patient is not typical for the neonates that could also be associated with bottle-feeding and not exclusive BM feeding of the patient. The increase in Clostridium sensu stricto I abundance in healthy infants from 4 to 12 mo of age is probably associated with the cessation of breast feeding and the start of solid food intake, as these bacteria have the ability to metabolize polysaccharides[47,48]. However, the proportion of Clostridium sensu stricto I in healthy children in the first year of life is not high (2%), and a higher abundance of Bifidobacterium (22.5%), Escherichia/Shigella (11.9%), and Veillonella (5.6%) is more common[48]. It is possible that the continuation of breastfeeding as the main food source after initiation of solid foods may inhibit the growth of Firmicutes capable of producing butyrate[49]. The predominance of Clostridium sensu stricto I in infants is known to be related to maternal antibacterial therapy during delivery, caesarean section, formula and mixed feeding, and stay in the intensive care unit[50]. Appert et al[51] have suggested that the colonization of endospore formers starts during the first months or even days of life, but the endospore community mainly composed of the butyrate-producing Clostridium sensu stricto I only contributes to 0.001% of the total cells.
Many studies have analyzed the role of the intestinal microbiome composition in the development of NEC in premature infants, but there is not enough information on gut microbiome composition in infants with CHD who develop NEC. There is some information on intestinal microbiome composition in infants with CHD without NEC[9,12,13], and several studies have evaluated the role of probiotics in children with CHD[9,10]. Further, Wang et al[52] revealed low alpha diversity of the gut microbiome in the mothers of infants with CHD and distinct overall microbial composition compared with the mothers of infants without CHD. Additionally, Ellis et al[9] revealed alteration of the gut microbiome in infants with CHD without NEC: They found a decrease in the total bacteria, Actinobacteria (predominantly Bifidobacteria), Bacteroidetes, and Enterobacteriaceae, and an increase in Firmicutes (predominantly Enterococci). Infants who underwent CPB had a lower alpha diversity, a high abundance of Proteobacteria and Actinobacteria, and a low level of Bacteroides (which also decreased after surgery)[12]. Infants without CHD who underwent surgery without CPB tended to have a higher level of Firmicutes, represented by families Ruminococcaceae and Lachnospiraceae, to which Clostridia of clusters IV and XIVa belong[12]. The findings in the present case partly collaborate with findings in mentioned studies[9,12]: Low bacterial alpha diversity and predominance of Proteobacteria were revealed in the patient on 8th day after cardiac surgery with CPB, but this also could be associated with previous antibacterial therapy of NEC.
A limitation of the present study is the difference in the timing of the microbiological examinations and the timing of 16S rRNA gene-based microbiota profiling. However, the overall results were not affected, as comparison of the results of microbiological examinations and the results of 16S rRNA gene-based microbiota profiling was not the main aim of the study.
NEC in infants with CHD is a heterogeneous condition, and the risk of NEC in these infants depends on the cardiac anatomy, type of cardiac surgery, early postoperative course, hemodynamic characteristics of the cardiac surgery stages, and antibacterial therapy. Additionally, mesenteric hypoperfusion and intestinal dysbiosis may contribute to NEC development, but their roles need to be clearly elucidated. Further, the influence of hypoxia and persistent mesenteric hypoperfusion on intestinal microbiome formation in infants who undergo palliative cardiac surgery needs further evaluation.
We would like to thank neonatologists of the Perinatal Centre of Almazov National Medical Research Centre: Kim M, Shemyakina O, Kiseleva N, Guryanova N, Islamova K, Treskina N, Klimenko A, Ti Y, and neonatal nurses for help in the investigation. We thank anonymous reviewers for their very useful comments on the first version of the paper.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: European Crohn’s and Colitis Organisation, No. 37495.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Russia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wang J, China; Wang KW, China S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Duchon J, Barbian ME, Denning PW. Necrotizing Enterocolitis. Clin Perinatol. 2021;48:229-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 2. | Lindberg TP, Caimano MJ, Hagadorn JI, Bennett EM, Maas K, Brownell EA, Matson AP. Preterm infant gut microbial patterns related to the development of necrotizing enterocolitis. J Matern Fetal Neonatal Med. 2020;33:349-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Polin RA, Pollack PF, Barlow B, Wigger HJ, Slovis TL, Santulli TV, Heird WC. Necrotizing enterocolitis in term infants. J Pediatr. 1976;89:460-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 85] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Manuri L, Morelli S, Agati S, Saitta MB, Oreto L, Mandraffino G, Iannace E, Iorio FS, Guccione P. Early hybrid approach and enteral feeding algorithm could reduce the incidence of necrotising enterocolitis in neonates with ductus-dependent systemic circulation. Cardiol Young. 2017;27:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Spinner JA, Morris SA, Nandi D, Costarino AT, Marino BS, Rossano JW, Shamszad P. Necrotizing Enterocolitis and Associated Mortality in Neonates With Congenital Heart Disease: A Multi-Institutional Study. Pediatr Crit Care Med. 2020;21:228-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 6. | Kelleher ST, McMahon CJ, James A. Necrotizing Enterocolitis in Children with Congenital Heart Disease: A Literature Review. Pediatr Cardiol. 2021;42:1688-1699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 7. | Bubberman JM, van Zoonen A, Bruggink JLM, van der Heide M, Berger RMF, Bos AF, Kooi EMW, Hulscher JBF. Necrotizing Enterocolitis Associated with Congenital Heart Disease: a Different Entity? J Pediatr Surg. 2019;54:1755-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | McElhinney DB, Hedrick HL, Bush DM, Pereira GR, Stafford PW, Gaynor JW, Spray TL, Wernovsky G. Necrotizing enterocolitis in neonates with congenital heart disease: risk factors and outcomes. Pediatrics. 2000;106:1080-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 268] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 9. | Ellis CL, Bokulich NA, Kalanetra KM, Mirmiran M, Elumalai J, Haapanen L, Schegg T, Rutledge JC, Raff G, Mills DA, Underwood MA. Probiotic administration in congenital heart disease: a pilot study. J Perinatol. 2013;33:691-697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Umenai T, Shime N, Asahara T, Nomoto K, Itoi T. A pilot study of Bifidobacterium breve in neonates undergoing surgery for congenital heart disease. J Intensive Care. 2014;2:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Ellis CL, Rutledge JC, Underwood MA. Intestinal microbiota and blue baby syndrome: probiotic therapy for term neonates with cyanotic congenital heart disease. Gut Microbes. 2010;1:359-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Salomon J, Ericsson A, Price A, Manithody C, Murry DJ, Chhonker YS, Buchanan P, Lindsey ML, Singh AB, Jain AK. Dysbiosis and Intestinal Barrier Dysfunction in Pediatric Congenital Heart Disease Is Exacerbated Following Cardiopulmonary Bypass. JACC Basic Transl Sci. 2021;6:311-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 13. | Patterson S, Davis K, Klausner R, Laws J, Boone HH, Shilts MH, Das SR, Creech CB. Gastrointestinal microbiota diversity and clinical outcomes in congenital heart disease. Crit Care Med. 2020;48:68. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Ferguson LP, Gandiya T, Kaselas C, Sheth J, Hasan A, Gabra HO. Gastrointestinal complications associated with the surgical treatment of heart disease in children. J Pediatr Surg. 2017;52:414-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Mishra S. Cardiac and Non-Cardiac Abnormalities in Heterotaxy Syndrome. Indian J Pediatr. 2015;82:1135-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Ivemark BI. Implications of agenesis of the spleen on the pathogenesis of conotruncus anomalies in childhood; an analysis of the heart malformations in the splenic agenesis syndrome, with fourteen new cases. Acta Paediatr Suppl (Upps). 1955;44:7-110. [PubMed] [DOI] [Full Text] |
| 17. | Noack F, Sayk F, Ressel A, Berg C, Gembruch U, Reusche E. Ivemark syndrome with agenesis of the corpus callosum: a case report with a review of the literature. Prenat Diagn. 2002;22:1011-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Kliegman RM, Walsh MC. Neonatal necrotizing enterocolitis: pathogenesis, classification, and spectrum of illness. Curr Probl Pediatr. 1987;17:213-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 191] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Dorofeeva EI, Podurovskaya YuL, Burov AA, Ryumina II, Narogan MV, Grosheva EV, Ionov OV, Balashova EN, Kirtbaya AR, Degtyarev DN. Diagnostics and conservative treatment of newborns with necrotizing enterocolitis. 2014. Available from: https://www.mrckb.ru/files/nek.pdf. |
| 20. | National Association of Specialists for the Control of Healthcare-Associated Infections. Prevention of surgical site infection. Clinical practice guideline. 2018. Available from: http://antimicrob.net/wp-content/uploads/2018-Profilaktika-IOKHV.pdf. |
| 21. | Gertler R, Gruber M, Wiesner G, Grassin-Delyle S, Urien S, Tassani-Prell P, Martin K. Pharmacokinetics of cefuroxime in infants and neonates undergoing cardiac surgery. Br J Clin Pharmacol. 2018;84:2020-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Ministry of Health of the Russian Federation. Instructions for the medical use of the medicine Ampicillin + Sulbactam. 2018 Preprint. |
| 23. | Shabalov NP. Medicines used to treat neonates. In: Volodin NN. Neonatology. National guidelines. Moscow: GEOTAR-Media, 2009: 813-833. |
| 24. | Tannock GW, Lawley B, Munro K, Gowri Pathmanathan S, Zhou SJ, Makrides M, Gibson RA, Sullivan T, Prosser CG, Lowry D, Hodgkinson AJ. Comparison of the compositions of the stool microbiotas of infants fed goat milk formula, cow milk-based formula, or breast milk. Appl Environ Microbiol. 2013;79:3040-3048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 158] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 25. | Petrova NA, Kaplina AV, Khavkin AI, Pervunina TM, Komlichenko EV, Nikiforov VG, Sitkin SI. Necrotizing enterocolitis: current concepts of etiopathogenesis with an emphasis on microbiome and metabolomics. Vopr prakt pediatr. 2021;16:98-105. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Warner BB, Deych E, Zhou Y, Hall-Moore C, Weinstock GM, Sodergren E, Shaikh N, Hoffmann JA, Linneman LA, Hamvas A, Khanna G, Rouggly-Nickless LC, Ndao IM, Shands BA, Escobedo M, Sullivan JE, Radmacher PG, Shannon WD, Tarr PI. Gut bacteria dysbiosis and necrotising enterocolitis in very low birthweight infants: a prospective case-control study. Lancet. 2016;387:1928-1936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 347] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 27. | Hui Y, Smith B, Mortensen MS, Krych L, Sørensen SJ, Greisen G, Krogfelt KA, Nielsen DS. The effect of early probiotic exposure on the preterm infant gut microbiome development. Gut Microbes. 2021;13:1951113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 28. | Fu CY, Li LQ, Yang T, She X, Ai Q, Wang ZL. Autoinducer-2 May Be a New Biomarker for Monitoring Neonatal Necrotizing Enterocolitis. Front Cell Infect Microbiol. 2020;10:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Mariat D, Firmesse O, Levenez F, Guimarăes V, Sokol H, Doré J, Corthier G, Furet JP. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009;9:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 997] [Cited by in RCA: 1242] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 30. | Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, van den Brandt PA, Stobberingh EE. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1513] [Cited by in RCA: 1528] [Article Influence: 80.4] [Reference Citation Analysis (0)] |
| 31. | van der Heide M, Mebius MJ, Bos AF, Roofthooft MTR, Berger RMF, Hulscher JBF, Kooi EMW. Hypoxic/ischemic hits predispose to necrotizing enterocolitis in (near) term infants with congenital heart disease: a case control study. BMC Pediatr. 2020;20:553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Shinha T, Hadi C. Clostridium paraputrificum Bacteremia Associated with Colonic Necrosis in a Patient with AIDS. Case Rep Infect Dis. 2015;2015:312919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Louis P, Flint HJ. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol. 2017;19:29-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 998] [Cited by in RCA: 1705] [Article Influence: 189.4] [Reference Citation Analysis (0)] |
| 34. | Donohoe DR, Garge N, Zhang X, Sun W, O'Connell TM, Bunger MK, Bultman SJ. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13:517-526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1507] [Cited by in RCA: 1345] [Article Influence: 96.1] [Reference Citation Analysis (0)] |
| 35. | Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1657] [Cited by in RCA: 1845] [Article Influence: 108.5] [Reference Citation Analysis (0)] |
| 36. | Sanchez HN, Moroney JB, Gan H, Shen T, Im JL, Li T, Taylor JR, Zan H, Casali P. B cell-intrinsic epigenetic modulation of antibody responses by dietary fiber-derived short-chain fatty acids. Nat Commun. 2020;11:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 237] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 37. | Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2951] [Cited by in RCA: 3771] [Article Influence: 314.3] [Reference Citation Analysis (0)] |
| 38. | Cassir N, Benamar S, La Scola B. Clostridium butyricum: from beneficial to a new emerging pathogen. Clin Microbiol Infect. 2016;22:37-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 146] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 39. | Romano-Keeler J, Shilts MH, Tovchigrechko A, Wang C, Brucker RM, Moore DJ, Fonnesbeck C, Meng S, Correa H, Lovvorn HN 3rd, Tang YW, Hooper L, Bordenstein SR, Das SR, Weitkamp JH. Distinct mucosal microbial communities in infants with surgical necrotizing enterocolitis correlate with age and antibiotic exposure. PLoS One. 2018;13:e0206366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Rozé JC, Ancel PY, Lepage P, Martin-Marchand L, Al Nabhani Z, Delannoy J, Picaud JC, Lapillonne A, Aires J, Durox M, Darmaun D, Neu J, Butel MJ; Nutrition EPIPAGE 2 study group; EPIFLORE Study Group. Nutritional strategies and gut microbiota composition as risk factors for necrotizing enterocolitis in very-preterm infants. Am J Clin Nutr. 2017;106:821-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 41. | Zhou Y, Shan G, Sodergren E, Weinstock G, Walker WA, Gregory KE. Longitudinal analysis of the premature infant intestinal microbiome prior to necrotizing enterocolitis: a case-control study. PLoS One. 2015;10:e0118632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 142] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 42. | Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1896] [Cited by in RCA: 2184] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 43. | Kaiko GE, Ryu SH, Koues OI, Collins PL, Solnica-Krezel L, Pearce EJ, Pearce EL, Oltz EM, Stappenbeck TS. The Colonic Crypt Protects Stem Cells from Microbiota-Derived Metabolites. Cell. 2016;165:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 475] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 44. | Salvi PS, Cowles RA. Butyrate and the Intestinal Epithelium: Modulation of Proliferation and Inflammation in Homeostasis and Disease. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 250] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 45. | Peng L, He Z, Chen W, Holzman IR, Lin J. Effects of butyrate on intestinal barrier function in a Caco-2 cell monolayer model of intestinal barrier. Pediatr Res. 2007;61:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 368] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 46. | Liu J, Zhu H, Li B, Lee C, Alganabi M, Zheng S, Pierro A. Beneficial effects of butyrate in intestinal injury. J Pediatr Surg. 2020;55:1088-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 47. | Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, Li Y, Xia Y, Xie H, Zhong H, Khan MT, Zhang J, Li J, Xiao L, Al-Aama J, Zhang D, Lee YS, Kotowska D, Colding C, Tremaroli V, Yin Y, Bergman S, Xu X, Madsen L, Kristiansen K, Dahlgren J, Wang J. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe. 2015;17:690-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1477] [Cited by in RCA: 1974] [Article Influence: 197.4] [Reference Citation Analysis (0)] |
| 48. | Guo M, Miao M, Wang Y, Duan M, Yang F, Chen Y, Yuan W, Zheng H. Developmental differences in the intestinal microbiota of Chinese 1-year-old infants and 4-year-old children. Sci Rep. 2020;10:19470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 49. | Pannaraj PS, Li F, Cerini C, Bender JM, Yang S, Rollie A, Adisetiyo H, Zabih S, Lincez PJ, Bittinger K, Bailey A, Bushman FD, Sleasman JW, Aldrovandi GM. Association Between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome. JAMA Pediatr. 2017;171:647-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 680] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 50. | Moore RE, Townsend SD. Temporal development of the infant gut microbiome. Open Biol. 2019;9:190128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 179] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 51. | Appert O, Garcia AR, Frei R, Roduit C, Constancias F, Neuzil-Bunesova V, Ferstl R, Zhang J, Akdis C, Lauener R, Lacroix C, Schwab C. Initial butyrate producers during infant gut microbiota development are endospore formers. Environ Microbiol. 2020;22:3909-3921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 52. | Wang T, Chen L, Huang P, Yang T, Zhang S, Zhao L, Ye Z, Luo L, Qin J. Association of maternal gut microbiota and plasma metabolism with congenital heart disease in offspring: a multi-omic analysis. Sci Rep. 2021;11:5339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |