Published online Oct 16, 2022. doi: 10.12998/wjcc.v10.i29.10516
Peer-review started: May 16, 2022
First decision: July 13, 2022
Revised: July 26, 2022
Accepted: August 30, 2022
Article in press: August 30, 2022
Published online: October 16, 2022
Processing time: 136 Days and 4 Hours
New and more severe clinical manifestations associated with the coronavirus disease 2019 (COVID-19) are emerging constantly in the pediatric age group. Patients in this age group are also primary carriers of the influenza virus and are at a higher risk of developing severe infection. However, studies comparing influenza and COVID-19 to show which condition causes a more severe form of disease amongst the pediatric age group are scarce.
To compare the laboratory results, clinical symptoms and clinical outcomes in pediatric patients with COVID-19 and influenza.
A systematic and comprehensive search was carried out in databases and search engines, including EMBASE, Cochrane, MEDLINE, ScienceDirect and Google Scholar from 1964 until January 2022. A meta-analysis was carried out using a random-effects model and pooled odds ratio (OR) or standardized mean difference (SMD) and 95%CI.
A total of 16 studies satisfied the inclusion criteria. Pediatric COVID-19 patients had a significantly reduced risk of cough (pooled OR = 0.16; 95%CI: 0.09 to 0.27), fever (pooled OR = 0.23; 95%CI: 0.12 to 0.43), and dyspnea (pooled OR = 0.54; 95%CI: 0.33 to 0.88) compared to influenza patients. Furthermore, total hemoglobin levels (pooled SMD = 1.22; 95%CI: 0.29 to 2.14) in COVID-19 patients were significantly higher as compared to pediatric influenza patients. There was no significant difference in symptoms such as sore throat, white blood cell count, platelets, neutrophil and lymphocytes levels, and outcomes like mortality, intensive care unit admission, mechanical ventilation or length of hospital stay.
COVID-19 is associated with a significantly lower rate of clinical symptoms and abnormal laboratory indexes compared to influenza in the pediatric age group. However, further longitu
Core Tip: Developing new strategies for prevention, early diagnosis and adequate management of pediatric patients infected with coronavirus disease 2019 (COVID-19) or influenza is crucial. It is still not clear which of these two viruses is more severe in pediatric patients and requires intensive interventions. In addition, co-circulation of influenza and COVID-19 present certain diagnostic and therapeutic difficulties, given the similarities in the clinical features, and may lead to adverse treatment outcomes. Our review is the first attempt to compare the various clinical features, laboratory parameters and outcomes between COVID-19 and influenza pediatric patients. However, longitudinal evidence is required to identify reliable effect sizes and to make evidence-based recommendations for developing interventions in the hospital setting.
- Citation: Yu B, Chen HH, Hu XF, Mai RZ, He HY. Comparison of laboratory parameters, clinical symptoms and clinical outcomes of COVID-19 and influenza in pediatric patients: A systematic review and meta-analysis. World J Clin Cases 2022; 10(29): 10516-10528
- URL: https://www.wjgnet.com/2307-8960/full/v10/i29/10516.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i29.10516
The recent coronavirus disease 2019 (COVID-19) pandemic resulted in millions of deaths around the world. When the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) started circulating late in 2019, it was immediately compared to seasonal and pandemic influenza viruses due to the fairly similar features shared by these viruses[1]. The disease caused by both SARS-CoV-2 and influenza viruses have similar clinical manifestations (fever, respiratory symptoms like cough and sore throat), and may lead to severe forms of lung infections[2]. Both viruses have also demonstrated a similar route of human-to-human transmission via respiratory droplets[3]. However, COVID-19 has distinct clinical characteristics, such as anosmia and hypogeusia[3]. While efficacious vaccines are available for COVID-19, the search for the optimal treatment is still ongoing. In contrast, influenza is easily detectable, treatable and vaccine-preventable[2]. Recent reports suggested that pediatric COVID-19 patients are at a higher risk of the multi-system organ than the adult population. Therefore, it is not possible to manage the pediatric patients based only on the adult data[3-5].
Despite recent advances in developing experimental antiviral medications, supportive treatment remains the primary mode of management for COVID-19 patients[6,7]. However, new and more severe clinical manifestations associated with the COVID-19 are emerging constantly in the pediatric age group. Developing new strategies for prevention, early diagnosis and adequate management of pediatric patients infected with COVID-19 or influenza is crucial. It is still not clear which of these two viruses is more severe in pediatric patients and requires intensive interventions. In addition, co-circulation of influenza and COVID-19 present certain diagnostic and therapeutic difficulties, given the similarities in the clinical features, and may lead to adverse treatment outcomes. However, there are still not enough studies that compare influenza and COVID-19 in pediatric patients. To the best of our knowledge, there are no pooled data on the difference in laboratory results, clinical symptoms and clinical outcomes between COVID-19 and influenza patients of this age group. The purpose of the present review is to pool data from individual studies to examine the possible differences in laboratory results, clinical symptoms and clinical outcomes between pediatric COVID-19 and influenza patients.
Type of study design: Observational studies (cross-sectional/cohort/case-control) that satisfied the inclusion criteria were included. Full-text articles were included, while conference abstracts, case series, case reports or grey literature were excluded.
Type of participants: Studies that provided data of both pediatric (< 18 years) influenza and COVID-19 patients independently.
Type of exposure and comparator group: Studies evaluating the differences in laboratory results, clinical symptoms and clinical outcomes between pediatric COVID-19 and influenza patients.
Type of outcomes: Clinical symptoms: Fever, cough, dyspnea, sore throat, and fatigue; Laboratory results: Hemoglobin, white blood cell (WBC), platelets, neutrophils and lymphocytes; Clinical outcomes: Mortality, intensive care unit (ICU) admission, need for mechanical ventilation and length of hospital stay
A comprehensive search in the databases such as EMBASE, Cochrane library, MEDLINE, and search engines like Google Scholar and ScienceDirect was carried out (Supplementary Table 1). The search strategy included combined medical subject headings (MeSH) and free-text terms and Boolean operators (“AND” & “OR”). The following filters were applied during the search: time point [January 1964 (inception of Medline database) to January 2022], language (English only), and design (observational study). References from the identified articles were further searched for additional relevant studies.
The selection process involved 3 stages: Two independent investigators (XH and RM) screened the titles and abstracts. Full-text studies were retrieved after shortlisting based on the inclusion criteria; Retrieved full texts were then screened by the same set of investigators (XH and RM) and assessed against inclusion criteria, and the reasons for exclusion were recorded for the excluded studies; Disagreements were resolved by discussion with the third investigator (HH).
The review was reported according to the PRISMA statement 2020[8]. Prospero registration ID: CRD42022302686.
Data were manually extracted using a pre-defined structured data extraction form and included the following: authors, the title of study, year of publication, study period, study design, setting, country/region, total sample size, outcome assessment details, average age, primary and secondary outcomes in each group. Data entry was completed by the first author (BY) and the entry was reviewed for correctness by the second author (HC).
The risk of bias was assessed by two independent authors (RM and HH) using the Newcastle Ottawa scale for the observational studies. This assessment includes the following domains: Selection (four stars), comparability (two stars) and outcome (two stars). The final score ranges from zero to eight starts, and studies ranging from 7 to 8 stars indicate “good”, 5 to 6 stars indicate “satisfactory”, and 0-4 stars indicate “unsatisfactory” quality[9].
Pooled effect estimation and visualization: The meta-analysis was carried out using STATA version 14.2 (StataCorp, CollegeStation, TX, United States). The binary outcomes, number of events and sample size in each group were presented as pooled odds ratio (OR) with a 95%CI. For the continuous outcomes, the mean ± SD in each group was entered and the final estimate was interpreted in terms of standardized mean difference (SMD) with 95%CI. Random effects model with inverse-variance was performed to account for the methodological heterogeneity[10]. A forest plot was used to visually depict the study-specific estimate & pooled estimate.
Investigation of statistical heterogeneity: Heterogeneity was evaluated using a chi-square test and the amount of inconsistency was quantified using the I2 statistic. The interpretation of I2 was as follows: I2 < 25% indicated mild, 25%-75% moderate and > 75% substantial heterogeneity[10].
Additional analysis: Sensitivity analysis was executed to evaluate the robustness of the pooled estimate. The assessment of publication bias was analyzed using a funnel plot and Egger’s test was performed for outcomes with more than 10 studies[10].
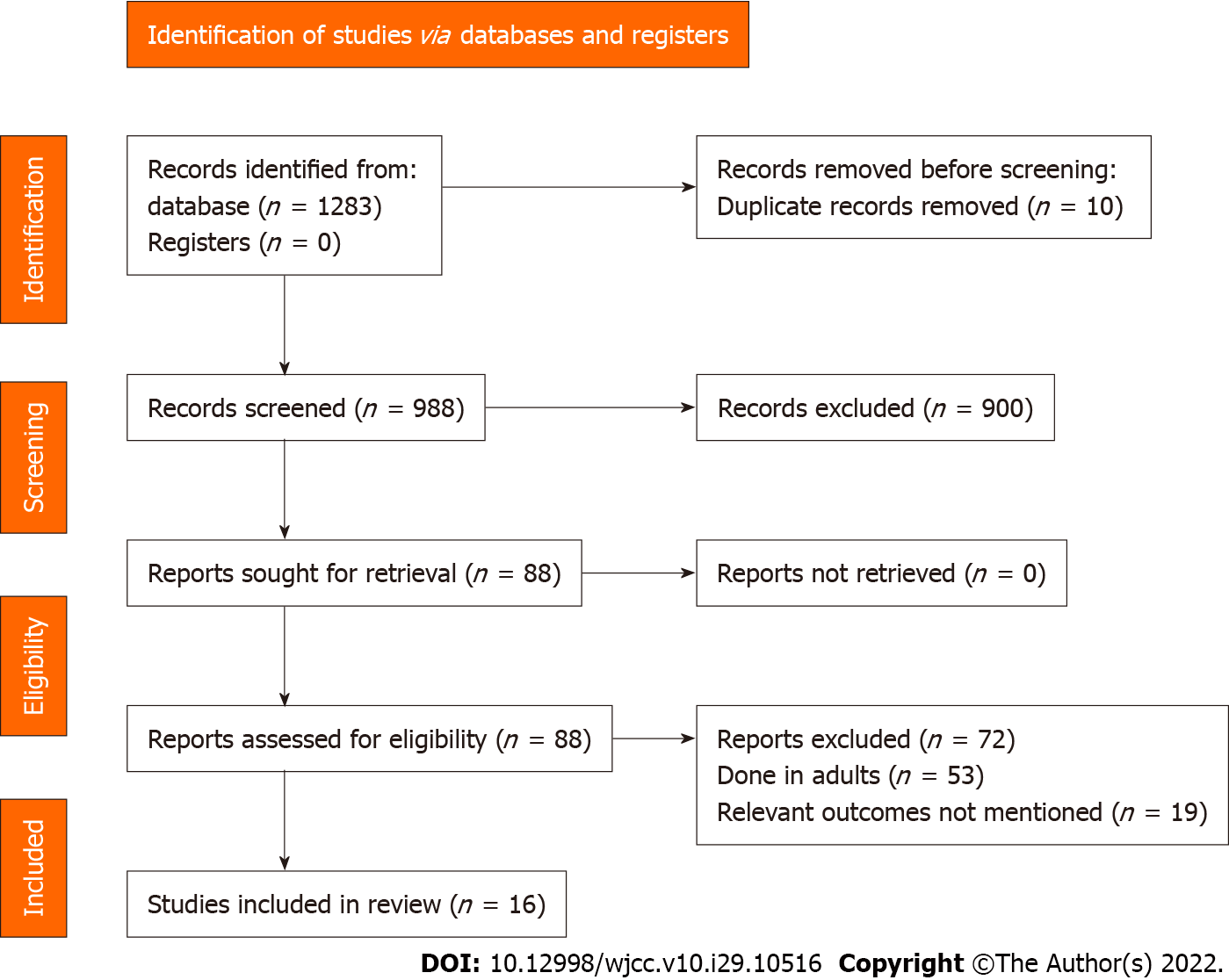
A total of 1283 articles were identified through the literature search. Of them, 88 studies met the criteria for the full-text analysis. Three additional articles were identified by screening the references of the retrieved full texts. After the final screening against eligibility criteria, 16 studies containing 17529 participants were included in the review (Figure 1)[11-26].
Most of studies (except Pokorska-Śpiewak et al[21]) were retrospective. Most (6 out of 16) were conducted in China followed by Turkey (3 studies). The sample sizes ranged from 39 to 10169. The mean age of the children in the COVID-19 group ranged from 12 to 128 mo and in the influenza group-from 12 to 112 mo. Most of the studies were conducted on patients with influenza A and B (Supplementary Table 2).
All the studies except for the study by Pokorska-Śpiewak et al[21] had a higher risk of bias in terms of the representativeness of the sample, and 13 out of 16 studies had a higher risk of bias in terms of the sample size justification. Seven studies had reported on non-response rates. Nine studies reported on ascertainment of exposure and 14 studies reported on the assessment of outcome. However, only five studies did appropriate control of confounding. To summarize, most of the included studies (11 out of 16 studies) had poorer quality (Supplementary Table 3).
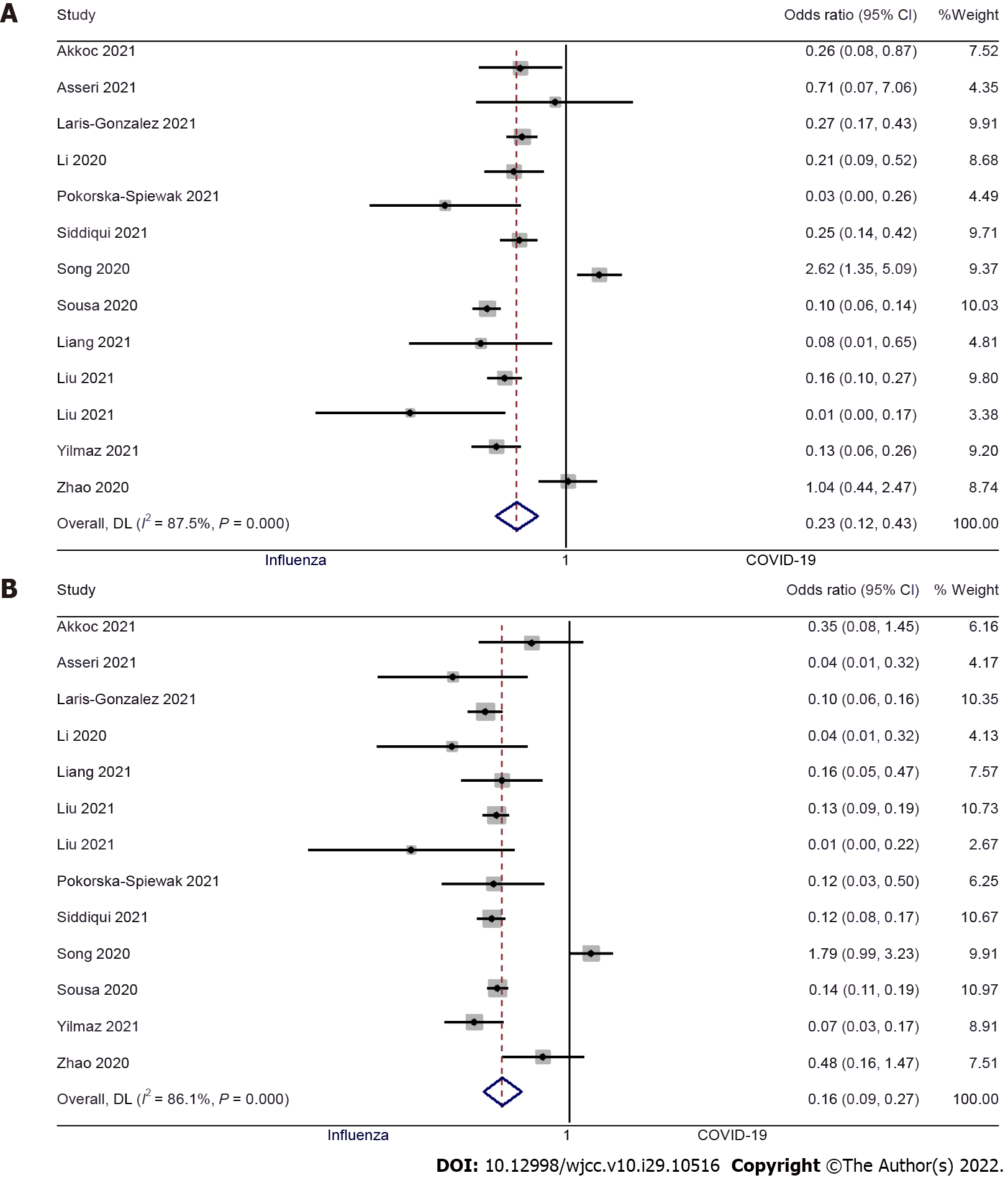
Fever: Thirteen studies examined the difference in fever between pediatric COVID-19 and influenza patients[9,10,12-15,17,19-24]. The pooled OR was 0.23 (95%CI: 0.12 to 0.43; I2 = 87.5%), indicating that there was a significantly lower risk of having a fever in pediatric COVID-19 patients when compared to pediatric influenza patients (P < 0.001) (Figure 2A). We did not find significant publication bias (Egger’s P value = 0.93), as further confirmed by the symmetrical funnel plot (Supplementary Figure 1).
Cough: Thirteen studies examined the difference in having a cough between pediatric patients with COVID-19 and influenza[9,10,12-15,17,19-24]. The pooled OR was 0.16 (95%CI: 0.09 to 0.27; I2 = 86.1%), indicating that the pediatric patients with COVID-19 had an 84% lower risk of having a cough when compared to pediatric influenza patients (P < 0.001) (Figure 2B). No significant publication bias was found for this outcome (Egger’s P value = 0.98), as confirmed by the symmetrical funnel plot (Supplementary Figure 2).
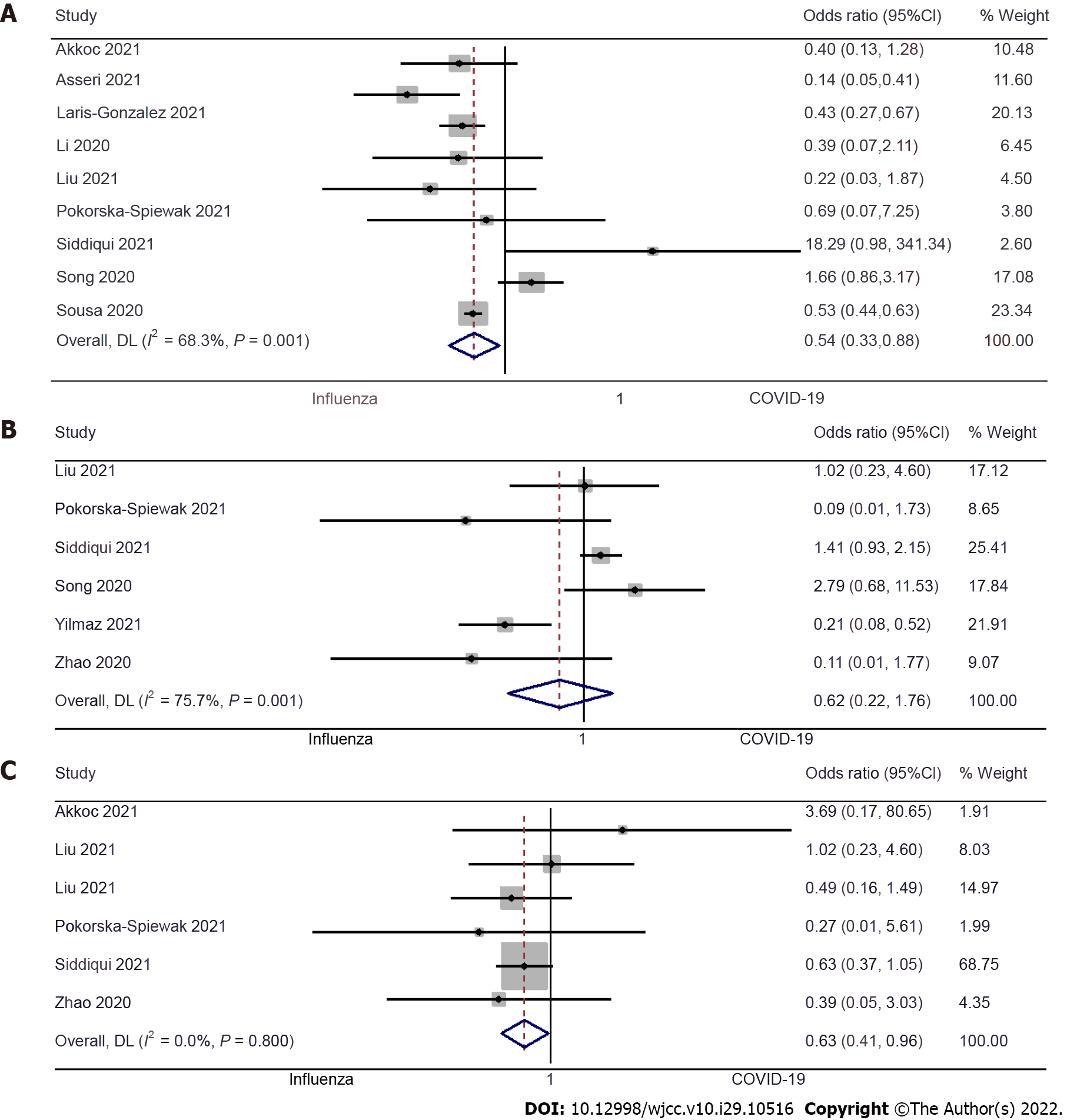
Dyspnea: Nine studies examined the difference in dyspnea between pediatric COVID-19 and influenza patients[9,10,12,13,15,19-22]. The pooled OR was 0.54 (95%CI: 0.33 to 0.88; I2= 68.3%), indicating that the pediatric patients with COVID-19 had a 46% lower risk of having dyspnea when compared to pediatric influenza patients (P = 0.01) (Figure 3A).
Sore throat: Six studies examined the difference in having a sore throat between pediatric COVID-19 and influenza patients[17,19-21,23,24]. The pooled OR was 0.62 (95%CI: 0.22 to 1.76; I2 = 75.7%), which suggests no significant difference in incidences of the sore throat between pediatric patients with COVID-19 or influenza (P = 0.37) (Figure 3B).
Fatigue: Six studies examined the difference in fatigue between pediatric COVID-19 and influenza patients[9,15,17,19,20,24]. The pooled OR was 0.63 (95%CI: 0.41 to 0.96; I2 = 0%). This shows that the pediatric patients with COVID-19 have a 37% lower risk of fatigue when compared to pediatric influenza patients (P = 0.03) (Figure 3C).
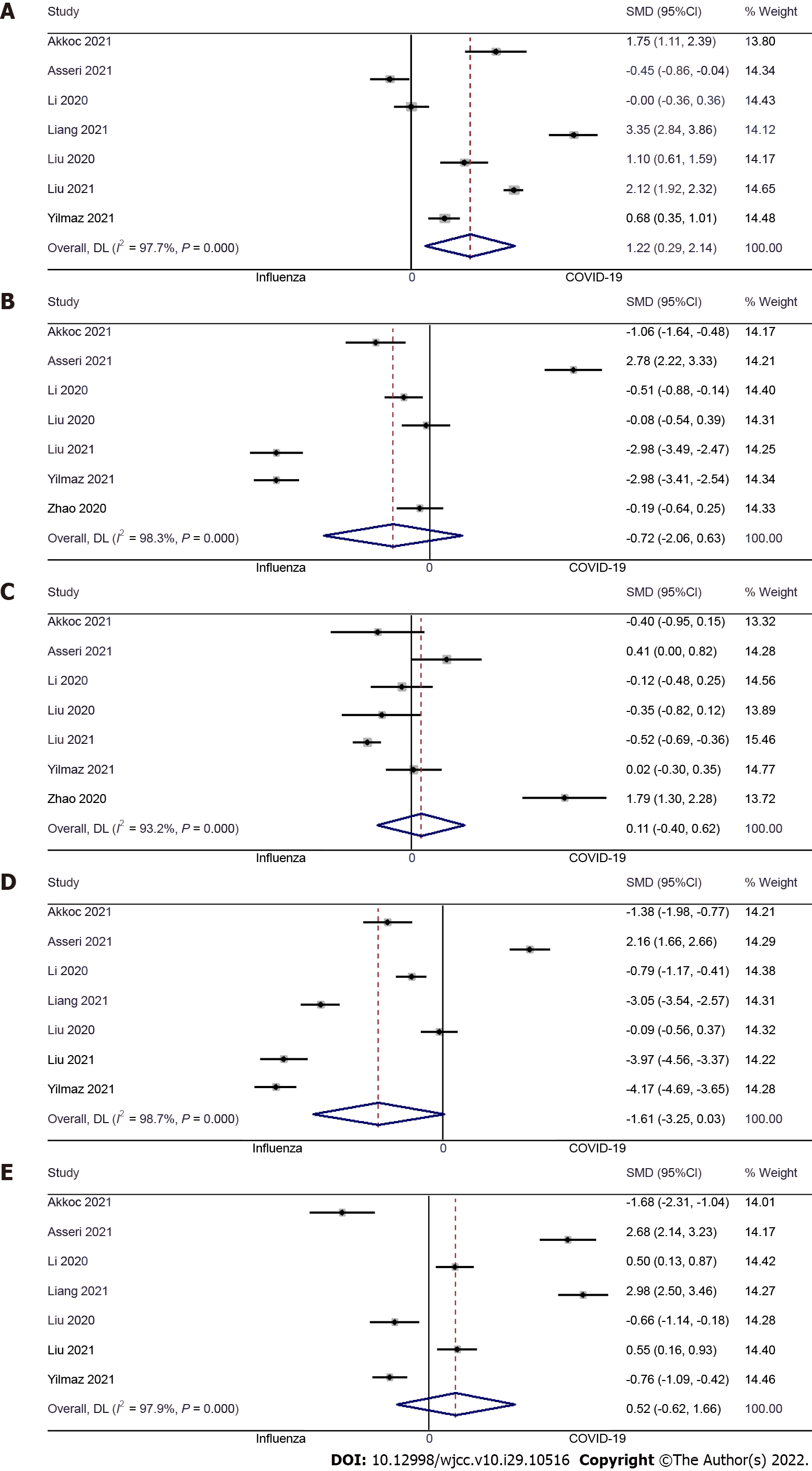
Hemoglobin: Seven studies examined the difference in hemoglobin level between pediatric COVID-19 and influenza patients[9,10,13,14,16,17,23]. The pooled SMD was 1.22 (95%CI: 0.29 to 2.14; I2 = 97.7%), indicating that COVID-19 causes significantly higher hemoglobin levels when compared to pediatric influenza patients (P = 0.01) (Figure 4A).
WBC: Seven studies examined the difference in WBC concentrations between pediatric COVID-19 and influenza patients[9,10,13,16,17,23,24]. The pooled SMD was -0.72 (95%CI: -2.06 to 0.63; I2 = 98.3%), indicative of no significant difference in WBC count between pediatric COVID-19 patients and pediatric influenza patients (P = 0.30) (Figure 4B).
Platelet count: Seven studies examined the difference in platelet count between pediatric COVID-19 and influenza patients[9,10,13,16,17,23,24]. The pooled SMD was 0.11 (95%CI: -0.40 to 0.62; I2 = 93.2%), with no significant difference in platelet count between pediatric COVID-19 patients and pediatric influenza patients (P = 0.68) (Figure 4C).
Neutrophils: Seven studies examined the difference in neutrophil count between pediatric COVID-19 and influenza patients[9,10,13,14,16,17,23]. The pooled SMD was -1.61 (95%CI: -3.25 to 0.03; I2 = 98.7%), indicating that the neutrophil count was similar in pediatric COVID-19 and influenza patients (P = 0.06) (Figure 4D).
Lymphocytes: Seven studies examined the difference in lymphocyte count between pediatric COVID-19 and influenza patients[9,10,13,14,16,17,23]. The pooled SMD was 0.52 (95%CI: -0.62 to 1.66; I2 = 97.9%), indicating that there was no significant difference in terms of lymphocyte count between pediatric COVID-19 patients and pediatric influenza patients (P = 0.37) (Figure 4E).
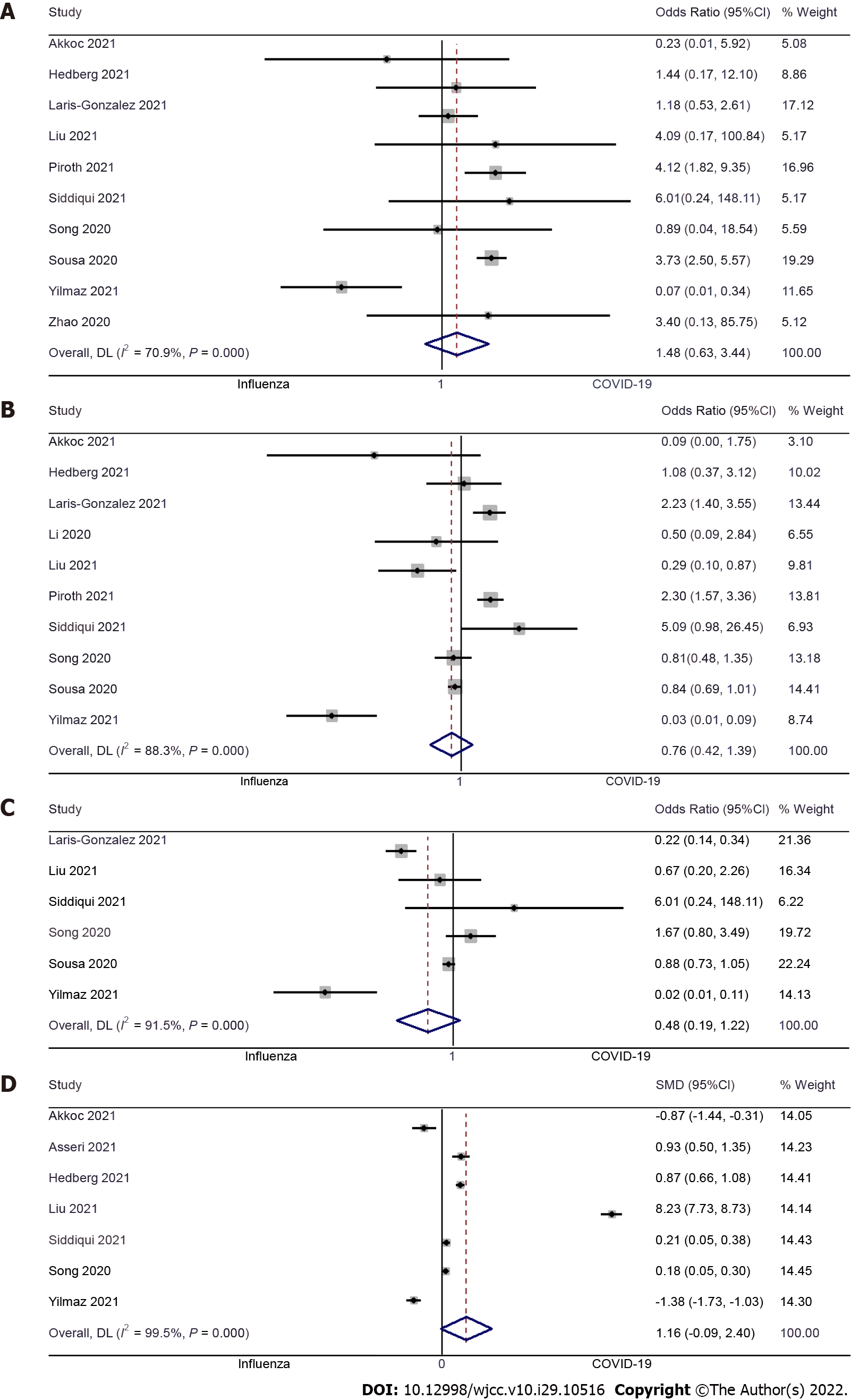
Length of hospital stay: Seven studies examined the difference in length of hospital stay between pediatric COVID-19 and influenza patients[9-11,17,20,21,23]. The pooled SMD was 1.16 (95%CI: -0.09 to 2.40; I2 = 99.5%), with no significant difference between pediatric COVID-19 patients and pediatric influenza patients (P = 0.37) (Figure 5A).
ICU admission: Ten studies examined the difference in ICU admission between pediatric COVID-19 and influenza patients[9,11-13,17,18,20-23]. The pooled OR was 0.76 (95%CI: 0.42 to 1.39; I2 = 88.3%), with no significant difference in terms of ICU admission between pediatric patients with COVID-19 or influenza (P = 0.37) (Figure 5B). No significant publication bias was detected for this outcome (Egger’s P value = 0.59), as further confirmed by the symmetrical funnel plot (Supplementary Figure 3).
Mechanical ventilation: Six studies examined the difference in mechanical ventilation requirements between pediatric COVID-19 and influenza patients[12,17,20-23]. The pooled OR was 0.48 (95%CI: 0.19 to 1.22; I2 = 91.5%), indicating that there is no significant difference between both groups of patients (P = 0.12) (Figure 5C).
Mortality: Ten studies examined the difference in mortality between pediatric COVID-19 and influenza patients[9,11,12,17,18,20-24]. We found no significant difference in terms of mortality in both groups, with the pooled OR of 1.48 (95%CI: 0.63 to 3.44; I2 = 70.9%) (P = 0.37) (Figure 5D), and no significant publication bias (Egger’s P value = 0.20) which was further confirmed by symmetrical funnel plot (Supplementary Figure 4).
A sensitivity analysis revealed no significant variation in the effect size (in terms of magnitude and direction). This indicates a lack of a single study effect on the overall estimate for any of the outcomes.
Our results show that pediatric COVID-19 patients have a significantly lower risk of having fever, cough, and dyspnea when compared to children diagnosed with influenza. Hemoglobin was the only peripheral blood index significantly elevated in pediatric COVID-19 patients. Finally, there were no differences in clinical outcomes between pediatric COVID-19 and influenza patients.
A total of 16 studies fulfilled the eligibility criteria of this review. Most of these studies were done in China followed by European countries such as Turkey, Sweden, France and Poland. Almost all of the studies (except Pokorska-Śpiewak et al[21]) followed a retrospective design. Most studies were of poor quality with a high risk of bias.
The results presented here showed that there is a significantly reduced risk of having fever, cough and dyspnea among pediatric COVID-19 patients when compared to pediatric influenza patients. Sensitivity analysis did not reveal any significant single-study effect on the magnitude or direction of this association. While there were no previous pediatric patient reviews to compare the current study findings, the obtained results are in line with the frequency of symptoms in adults. As shown by numerous studies, fever, cough and dyspnea occurred less frequently in COVID-19 patients when compared to patients with influenza[27-29]. Our results could be explained by the difference in the pathophysiology of the entry receptors for both viruses. The human influenza A virus tends to bind to the alpha 2,6-linked sialic acid cell receptors that are expressed within the respiratory tract, specifically in the nasopharynx, trachea and bronchi, but not in the alveoli, where the 2,3-linked sialic acid receptors are predominant[30]. In contrast, SARS-CoV-2 uses another functional receptor, Angiotensin-converting enzyme-2 (ACE-2) protein (key regulator enzyme of the renin-angiotensin–aldosterone system) to enter the cell. The tissue distribution of the ACE-2 protein is higher in the small intestinal and lung alveolar epithelial cells and endothelial cells, but it is poorly expressed on the nasopharyngeal cell surface[31]. This differential expression of receptors may be responsible for the difference in clinical symptoms between influenza and COVID-19. The observed difference in clinical symptoms might help, therefore, in the initial screening and identification of different viral infections in pediatric patients.
Rahi et al[32] and Mokhtari et al[33] have reported that the COVID-19 infection may significantly affect the coagulation cascade, causes cytokine storms and eventual intravascular thrombosis. Studies in adult patients also indicated that COVID-19 can cause arterial and venous thrombosis, such as pulmonary embolism and pulmonary micro-thromboses[32-34]. A systematic review consisting of 624 children with COVID-19 showed a wide variability in leukocyte indices in pediatric patients with mild and severe COVID-19. These results suggest that these indices are the least reliable in terms of determining the disease severity in children[35].
An up-to-date review of the existing pediatric studies performed to date revealed lower WBC and neutrophil counts and higher lymphocyte counts in COVID-19 patients compared to patients with influenza[15,26]. The results of our study are in agreement with these observations.
Almost all the peripheral blood parameters, except hemoglobin, were similar in both groups of patients in our study, which was consistent with the observed levels in the adult population[27]. Higher hemoglobin levels in COVID-19 patients could be because by binding to the sialic acid receptors of the host cells, the influenza virus causes agglutination of the erythrocytes[36]. It is important to note that, unlike the adults, many more studies are required to make unequivocal and clearer statements on such issues in children, and all the changes in the acute phase reactants need to be carefully monitored by the clinicians.
There was no significant difference in clinical outcomes (death, ICU admission, mechanical ventilation and length of hospital stay) in pediatric COVID-19 and influenza patients. However, previous studies in adult patients have shown a higher mortality, ICU admissions, and need for mechanical ventilation with delayed hospital discharge, especially in the adults older than 50 years[25-27]. This difference may be related to the associated comorbidities like diabetes mellitus, hypertension or heart disease conditions among the middle-aged and elderly adult age group. Further longitudinal studies are needed to compare the outcomes between influenza and COVID-19 pediatric patients.
The major strength of this review is in the rigorous methodology and comprehensive literature search. In addition, this review adds to the limited evidence that is currently available on the comparison of clinical symptoms, laboratory results and clinical outcomes between pediatric COVID-19 and influenza patients. Sensitivity analysis did not detect significant changes in the magnitude or direction of the association and no significant publication bias was found for any of the assessed outcomes. This might further enhance the credibility of the study results. However, there are some limitations to this study. Substantial between-study variability was found for most of the outcomes. Most of the included studies were of poorer quality and limited heterogeneity. This might affect the external validity (generalizability), reliability and stability of the findings. Though we have included 16 studies with more than 17000 participants, most clinical features, laboratory parameters and outcomes had a limited number of samples, which might affect the preciseness of the estimates. Almost all the studies were retrospective, making it difficult to establish the causal association.
Our review is the first attempt to compare the various clinical features, laboratory parameters and outcomes between COVID-19 and influenza pediatric patients. We found that in the pediatric age group, COVID-19 has caused a significantly lower rate of clinical symptoms and abnormalities in laboratory indices compared to influenza. However, longitudinal evidence is required to identify reliable effect sizes and to make evidence-based recommendations for developing interventions in the hospital setting.
Patients in the pediatric age group are primary carriers of the influenza virus and are at a higher risk of developing severe infection. However, studies, comparing influenza and coronavirus disease 2019 (COVID-19) to show which condition causes a more severe form of disease amongst the pediatric age group, are scarce. This study aims to compare the laboratory results, clinical symptoms and clinical outcomes in pediatric patients with COVID-19 and influenza.
To the best of our knowledge, there are no pooled data on the difference in laboratory results, clinical symptoms and clinical outcomes between COVID-19 and influenza patients of this age group.
The purpose of the present review is to pool data from individual studies to examine the possible differences in laboratory results, clinical symptoms and clinical outcomes between pediatric COVID-19 and influenza patients.
A comprehensive search in the databases such as EMBASE, Cochrane library, MEDLINE, and search engines like Google Scholar and ScienceDirect was carried out. The following filters were applied during the search: time point [January 1964 (inception of Medline database) to January 2022], language (English only), and design (observational study).
Pediatric COVID-19 patients had a significantly reduced risk of cough [pooled odds ratio (OR) = 0.16; 95%CI: 0.09 to 0.27], fever (pooled OR = 0.23; 95%CI: 0.12 to 0.43) and dyspnea (pooled OR = 0.54; 95%CI: 0.33 to 0.88) compared to influenza patients. Furthermore, total hemoglobin levels (pooled standardized mean difference = 1.22; 95%CI: 0.29 to 2.14) in COVID-19 patients were significantly higher as compared to pediatric influenza patients. There was no significant difference in symptoms such as sore throat, white blood cell count, platelets, neutrophil and lymphocytes levels, and outcomes like mortality, intensive care unit admission, mechanical ventilation or length of hospital stay.
COVID-19 is associated with a significantly lower rate of clinical symptoms and abnormal laboratory indexes compared to influenza in the pediatric age group.
Further longitudinal studies of the outcomes between influenza and COVID-19 pediatric patients are needed.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious diseases
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dasuqi SA, Saudi Arabia; El Sayed S, Egypt; Singh AK, India S-Editor: Zhang H L-Editor: Filipodia P-Editor: Zhang H
| 1. | Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1527] [Cited by in RCA: 1472] [Article Influence: 294.4] [Reference Citation Analysis (0)] |
| 2. | Centers for Disease Control and Prevention. Similarities and Differences between Flu and COVID-19. 2022. Available from: https://www.cdc.gov/flu/symptoms/flu-vs-covid19.htm. |
| 3. | Bai Y, Yao L, Wei T, Tian F, Jin DY, Chen L, Wang M. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA. 2020;323:1406-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3006] [Cited by in RCA: 2687] [Article Influence: 537.4] [Reference Citation Analysis (0)] |
| 4. | Neuzil KM, Zhu Y, Griffin MR, Edwards KM, Thompson JM, Tollefson SJ, Wright PF. Burden of interpandemic influenza in children younger than 5 years: a 25-year prospective study. J Infect Dis. 2002;185:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 352] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 5. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18194] [Article Influence: 3638.8] [Reference Citation Analysis (0)] |
| 6. | Contini A. Virtual Screening of an FDA Approved Drugs Database on Two COVID-19 Coronavirus Proteins. 2020. [DOI] [Full Text] |
| 7. | Zhavoronkov A, Aladinskiy V, Zhebrak A, Zagribelnyy B, Terentiev V, Bezrukov DS, Polykovskiy D, Shayakhmetov R, Filimonov A, Orekhov P, Yan Y, Popova O, Vanhaelen Q, Aliper A, Ivanenkov Y. Potential COVID-2019 3C-like Protease Inhibitors Designed Using Generative Deep Learning Approaches. ChemRxiv. 2020;. [DOI] [Full Text] |
| 8. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 40322] [Article Influence: 10080.5] [Reference Citation Analysis (2)] |
| 9. | Wells G, Shea B, O’Connell D, Robertson J, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta- Analysis. 2014. |
| 10. | Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions. [cited 31 July 2021]. Available from: https://training.cochrane.org/handbook/current. |
| 11. | Akkoç G, Ağbaş A, Selçuk Duru N. A Comparison of Clinical Findings and Laboratory Test Results Between Hospitalized Children with COVID-19 and Influenza. J Pediatr Res. 2021;8:432-437. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Asseri AA, Shati AA, Al-Qahtani SM, Alzaydani IA, Al-Jarie AA, Alaliani MJ, Ali AS. Distinctive clinical and laboratory features of COVID-19 and H1N1 influenza infections among hospitalized pediatric patients. World J Pediatr. 2021;17:272-279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Hedberg P, Karlsson Valik J, van der Werff S, Tanushi H, Requena Mendez A, Granath F, Bell M, Mårtensson J, Dyrdak R, Hertting O, Färnert A, Ternhag A, Naucler P. Clinical phenotypes and outcomes of SARS-CoV-2, influenza, RSV and seven other respiratory viruses: a retrospective study using complete hospital data. Thorax. 2022;77:154-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Laris-González A, Avilés-Robles M, Domínguez-Barrera C, Parra-Ortega I, Sánchez-Huerta JL, Ojeda-Diezbarroso K, Bonilla-Pellegrini S, Olivar-López V, Chávez-López A, Jiménez-Juárez R. Influenza vs. COVID-19: Comparison of Clinical Characteristics and Outcomes in Pediatric Patients in Mexico City. Front Pediatr. 2021;9:676611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Li Y, Wang H, Wang F, Du H, Liu X, Chen P, Wang Y, Lu X. Comparison of hospitalized patients with pneumonia caused by COVID-19 and influenza A in children under 5 years. Int J Infect Dis. 2020;98:80-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Liang F, Wang X, Shao J, Chen J, Liu L, Li H, Xu Y, He L, Liang H, Li K, Gong S, Xia H. Comparison of clinical features on admission between coronavirus disease 2019 and influenza a among children: a retrospective study in China. BMC Infect Dis. 2021;21:365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Liu M, Han Y, Sun J, Wei X, Zhao X, Wang B, Zhang Y, Ma X, Gai Z. Comparison of the Epidemiological and Clinical Characteristics of Hospitalized Children With Pneumonia Caused by SARS-CoV-2, Influenza A, and Human Adenoviruses: A Case-Control Study. Clin Pediatr (Phila). 2022;61:150-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Liu XP, Guo MM, Liu SF, Kuo HC. Comparison of laboratory data between children with COVID-19 and influenza. Kaohsiung J Med Sci. 2021;37:158-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Liu X, Li W, Zhang B, Guo Y, Hu Z, Peng C, Lei X, Luo Q, Zhang Q, Deng W, Wang J, Tang J, Li Y, Chen J. Comparative study of hospitalized children with acute respiratory distress syndrome caused by SARS-CoV-2 and influenza virus. BMC Infect Dis. 2021;21:412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Piroth L, Cottenet J, Mariet AS, Bonniaud P, Blot M, Tubert-Bitter P, Quantin C. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study. Lancet Respir Med. 2021;9:251-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 407] [Cited by in RCA: 406] [Article Influence: 101.5] [Reference Citation Analysis (0)] |
| 21. | Pokorska-Śpiewak M, Talarek E, Popielska J, Nowicka K, Ołdakowska A, Zawadka K, Kowalik-Mikołajewska B, Tomasik A, Dobrzeniecka A, Lipińska M, Krynicka-Czech B, Coupland U, Stańska-Perka A, Ludek M, Marczyńska M. Comparison of clinical severity and epidemiological spectrum between coronavirus disease 2019 and influenza in children. Sci Rep. 2021;11:5760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Siddiqui M, Gültekingil A, Bakırcı O, Uslu N, Baskın E. Comparison of clinical features and laboratory findings of coronavirus disease 2019 and influenza A and B infections in children: a single-center study. Clin Exp Pediatr. 2021;64:364-369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (7)] |
| 23. | Song X, Delaney M, Shah RK, Campos JM, Wessel DL, DeBiasi RL. Comparison of Clinical Features of COVID-19 vs Seasonal Influenza A and B in US Children. JAMA Netw Open. 2020;3:e2020495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 24. | Sousa BLA, Sampaio-Carneiro M, de Carvalho WB, Silva CA, Ferraro AA. Differences among Severe Cases of Sars-CoV-2, Influenza, and Other Respiratory Viral Infections in Pediatric Patients: Symptoms, Outcomes and Preexisting Comorbidities. Clinics (Sao Paulo). 2020;75:e2273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Yılmaz K, Şen V, Aktar F, Onder C, Yılmaz ED, Yılmaz Z. Does Covid-19 in children have a milder course than Influenza? Int J Clin Pract. 2021;75:e14466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Zhao Y, Sun L, Bouchard HC, Zhang XX, Wan G, Hao YW, He SX, Jiang YY, Pang L. Coronavirus Disease 2019 versus Influenza A in Children: An Observational Control Study in China. Biomed Environ Sci. 2020;33:614-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 27. | Pormohammad A, Ghorbani S, Khatami A, Razizadeh MH, Alborzi E, Zarei M, Idrovo JP, Turner RJ. Comparison of influenza type A and B with COVID-19: A global systematic review and meta-analysis on clinical, laboratory and radiographic findings. Rev Med Virol. 2021;31:e2179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 28. | Chong WH, Saha BK, Medarov BI. A systematic review and meta-analysis comparing the clinical characteristics and outcomes of COVID-19 and influenza patients on ECMO. Respir Investig. 2021;59:748-756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Osman M, Klopfenstein T, Belfeki N, Gendrin V, Zayet S. A Comparative Systematic Review of COVID-19 and Influenza. Viruses. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 30. | Kumlin U, Olofsson S, Dimock K, Arnberg N. Sialic acid tissue distribution and influenza virus tropism. Influenza Other Respir Viruses. 2008;2:147-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 127] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 31. | Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3643] [Cited by in RCA: 4149] [Article Influence: 197.6] [Reference Citation Analysis (0)] |
| 32. | Rahi MS, Jindal V, Reyes SP, Gunasekaran K, Gupta R, Jaiyesimi I. Hematologic disorders associated with COVID-19: a review. Ann Hematol. 2021;100:309-320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 33. | Mokhtari T, Hassani F, Ghaffari N, Ebrahimi B, Yarahmadi A, Hassanzadeh G. COVID-19 and multiorgan failure: A narrative review on potential mechanisms. J Mol Histol. 2020;51:613-628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 320] [Cited by in RCA: 291] [Article Influence: 58.2] [Reference Citation Analysis (0)] |
| 34. | Iba T, Connors JM, Levy JH. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflamm Res. 2020;69:1181-1189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 281] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 35. | de Souza TH, Nadal JA, Nogueira RJN, Pereira RM, Brandão MB. Clinical manifestations of children with COVID-19: A systematic review. Pediatr Pulmonol. 2020;55:1892-1899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 191] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 36. | Trombetta CM, Ulivieri C, Cox RJ, Remarque EJ, Centi C, Perini D, Piccini G, Rossi S, Marchi S, Montomoli E. Impact of erythrocyte species on assays for influenza serology. J Prev Med Hyg. 2018;59:E1-E7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |