Published online Oct 6, 2022. doi: 10.12998/wjcc.v10.i28.10236
Peer-review started: May 3, 2022
First decision: June 16, 2022
Revised: June 29, 2022
Accepted: August 24, 2022
Article in press: August 24, 2022
Published online: October 6, 2022
Processing time: 146 Days and 23.4 Hours
Hepatoid adenocarcinoma of the lung (HAL) is an extremely rare malignant tumor, and many patients with HAL exhibit high levels of alpha-fetoprotein (AFP) expression. Currently, there is no standardized treatment strategy for advanced HAL and its prognosis is poor.
We report a 55-year-old man with unresectable AFP-related HAL. The largest cross-sectional area of the mass in the upper lobe of the left lung at the beginning of treatment was 8.46 cm × 6.53 cm. The patient’s serum AFP level was 9283 ng/mL. The mass increased in size to 8.86 cm × 8.21 cm after two courses of platinum-based combination chemotherapy and immunotherapy, and serum AFP reached its highest level (71232.2 ng/mL). The patient was treated with sorafenib (400 mg twice daily, per os). Forty days later, the mass was reduced to 5.63 cm × 5.29 cm and serum AFP level dropped to 786.8 ng/mL. The patient achieved partial remission for > 9 mo with sorafenib and an excellent biomarker response, as well as survival > 13 mo, which is among the longest reported for unresectable stage IV HAL.
This is the first report to document successful treatment of unresectable AFP-related HAL with single-agent sorafenib after multiline therapy.
Core Tip: Hepatoid adenocarcinoma of the lung (HAL) is an extremely rare malignant tumor with poor prognosis and no standardized treatment strategy. To our knowledge, this is the first report to document a patient with unresectable alpha-fetoprotein-related HAL who benefited from single-agent sorafenib after multiline treatment. Sorafenib may be a viable option for inoperable, previously treated, advanced HAL.
- Citation: Xu SZ, Zhang XC, Jiang Q, Chen M, He MY, Shen P. Alpha-fetoprotein-producing hepatoid adenocarcinoma of the lung responsive to sorafenib after multiline treatment: A case report. World J Clin Cases 2022; 10(28): 10236-10243
- URL: https://www.wjgnet.com/2307-8960/full/v10/i28/10236.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i28.10236
Hepatoid adenocarcinoma (HAC) of the lung (HAL) is an extremely rare malignant tumor with a morphology similar to hepatocellular carcinoma (HCC) and high levels of alpha-fetoprotein (AFP) expression in most cases[1,2]. In 1990, Ishikura et al[3] formally defined HAL, which was later described in the form of case reports[4,5]. In 2021, Hou et al[1] published a systematic review of the literature published from 1981 to 2020, summarizing the characteristics of high malignancy, strong invasion, and poor prognosis of HAL. Currently, there is no standardized or effective treatment for advanced HAL. The first option is surgery, followed by chemotherapy and/or radiotherapy. Targeted therapy and immunotherapy have been applied to HAL in recent years[6,7]. Herein, we present a patient with unresectable AFP-related HAL who successfully achieved partial remission (PR) with an excellent biomarker response when treated with sorafenib after failing multiline chemotherapy and immunotherapy. Progression-free survival (PFS) and overall survival (OS) exceeded 9 and 13 mo, respectively.
In March 2021, a 55-year-old man was admitted to a local hospital with the chief complaint of chest pain for four days.
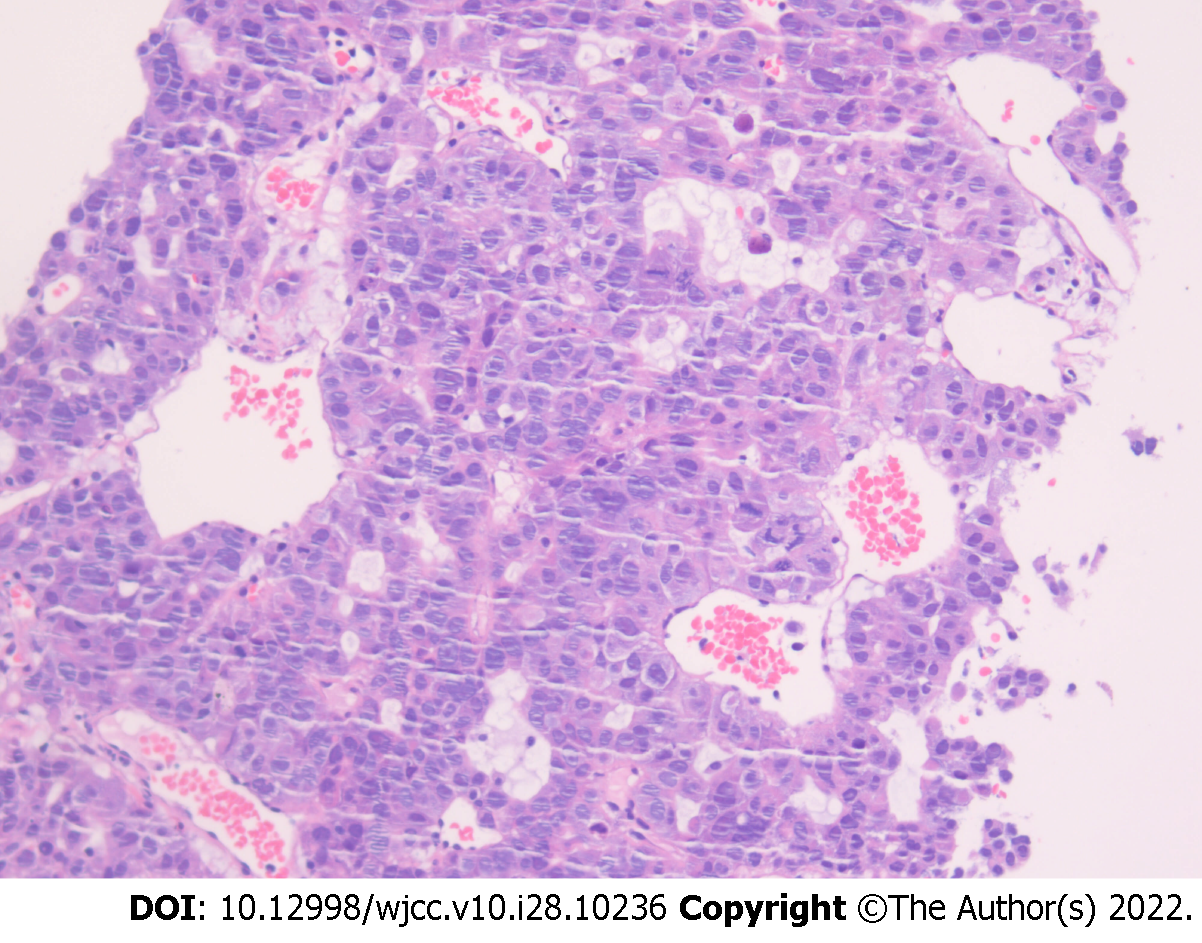
The patient presented with a four-day history of chest pain. On admission, enhanced chest computed tomography (CT) revealed uneven masses on the left upper chest wall, small pleural nodules, and significantly enlarged hilar and mediastinal lymph nodes. Coarse-needle puncture biopsy of the left upper lobe mass revealed a malignant tumor with necrosis. The AFP level was significantly elevated [9283 ng/mL (normal range, 0-20 ng/mL)]. The patient was admitted to our hospital for further pathological confirmation. Pathological consultation following puncture of the left lung mass reported a non-small-cell poorly differentiated carcinoma with neuroendocrine differentiation; and primary lung cancer should be considered after clinical exclusion of HCC metastasis(Figure 1). Immunohistochemical staining results were summarized in Table 1. Ki-67 index was 40%. The patient then underwent another CT-guided percutaneous lung biopsy. Pathological findings from the left lung biopsy specimen were inflammatory cell infiltration with necrosis, and only a few degenerative heteromorphic cells were found. Immunohistochemistry findings were as follows (Figure 2): GPC-3 (+), CK18 (+), AFP (+), CK (Pan) (+), CK5/6 (-), TTF-1 (-), Pax-8 (-), hepatocyte (+), syn (-), P40 (-), P504S (-), PAS (-), and Ki-67 [+ (20%)]. Genetic test results revealed that the driver genes (EGFR, ALK, ROS1, NRAS, BRAF, HER2, KRAS, MET, RET, and PIK3CA) were all wild-type. Furthermore, fluorodeoxyglucose positron emission tomography/CT did not reveal any evidence of hepatic hypermetabolic lesions. Based on these findings, the patient was diagnosed with stage IV AFP-related HAL.
| Immunohistochemical markers | Results |
| CAM5.2, SYN, CK18, CK8 | Strongly positive |
| AFP, P16, CK, GPC-3 | Focally positive |
| NSE, CD56, VM, PLAP, CD5, CD117, WT1, D2-40, CR, CD34, CD56, CgA, SOX-1, CK5/6, P63, P53, HEP, TTF-1, NapsinA, CK20, CK7 | Negative |
The patient had no previous medical history.
The patient smoked 70 cigarettes daily for > 20 years. None of the family members had any similar diseases.
The Eastern Cooperative Oncology Group (ECOG) score ranged from 0 to 1, and the numerical pain intensity scale score was 1. Decreased respiratory sounds were heard in the upper left lung.
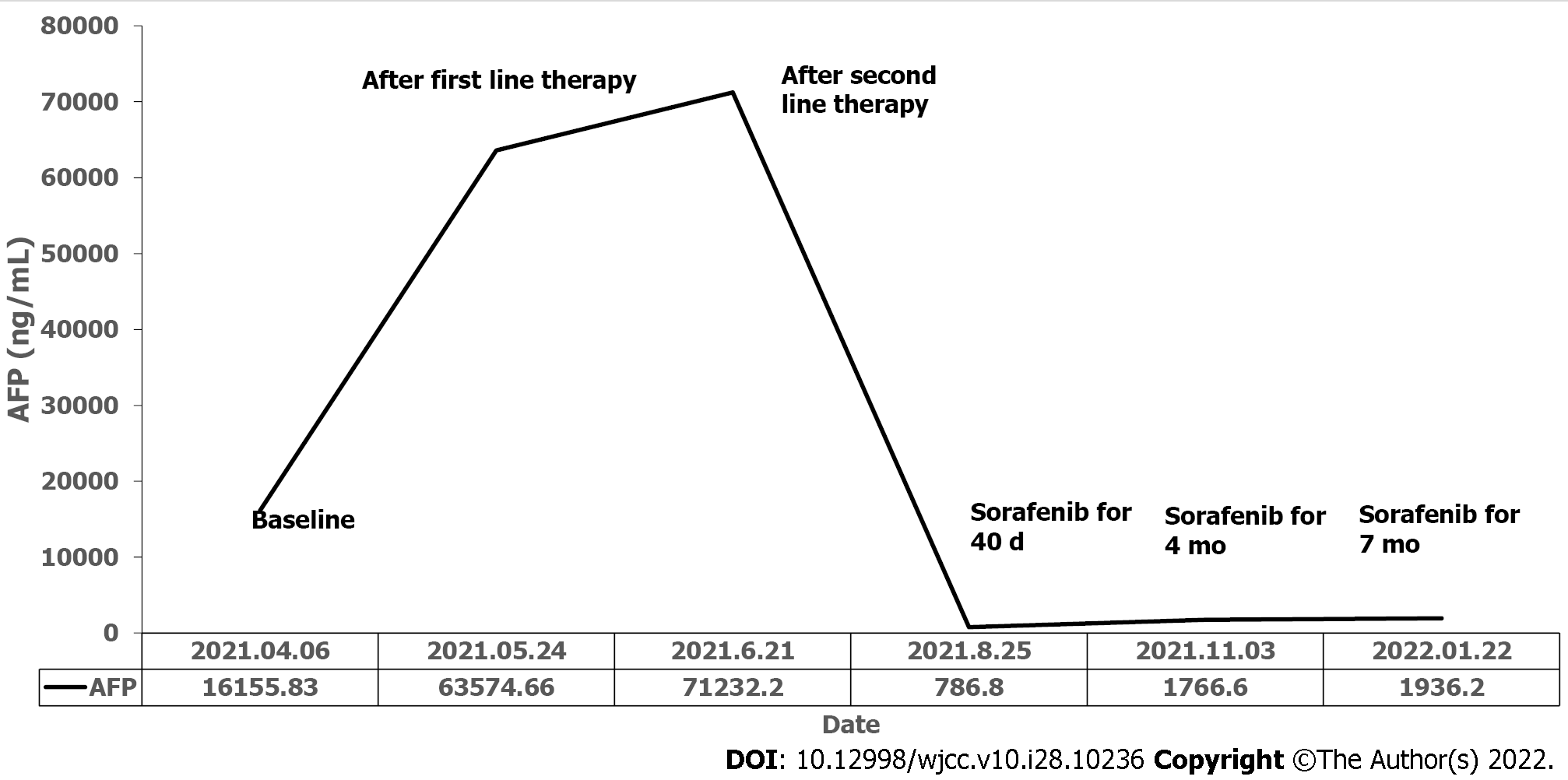
No obvious abnormalities were found in routine laboratory examinations. The patient’s serum AFP level was 16155.83 ng/mL before treatment (Figure 3).
Chest CT revealed a mass, measuring 8.46 cm × 6.53 cm (Figure 4A), on the left upper chest wall, small pleural nodules, and significantly enlarged lymph nodes in the hilar and mediastinum.
The final diagnosis in this case was HAL (cT4N3M1a, stage IVA).
Because the primary lung lesion was surgically unresectable, the patient underwent chemotherapy (albumin-bound paclitaxel plus carboplatin) and immunotherapy (pembrolizumab), both of which are recommended as first-line treatments for non-small cell lung cancer (NSCLC). Plain scan chest CT after two cycles of therapy revealed a huge subpleural mass (8.81 cm × 7.66 cm) in the upper lobe of the left lung, which was larger than that before treatment (8.46 cm × 6.53 cm) (Figure 4B). His serum AFP level increased to an abnormal level (63574.66 ng/mL) (Figure 3). Due to poor treatment outcomes, it was decided to switch to second-line chemotherapy (gemcitabine plus oxaliplatin) and continue immunotherapy (pembrolizumab). However, the mass increased to 8.86 cm × 8.21 cm in size (Figure 4C), and serum AFP reached its highest level (71232.2 ng/mL) (Figure 3). Sorafenib was then administered as a third-line therapy (400 mg orally twice daily). Forty days later, chest CT revealed significant reduction in the size of the subpleural mass (5.63 cm × 5.29 cm) (Figure 4D) and a decrease in serum AFP to 786.8 ng/mL (Figure 3).
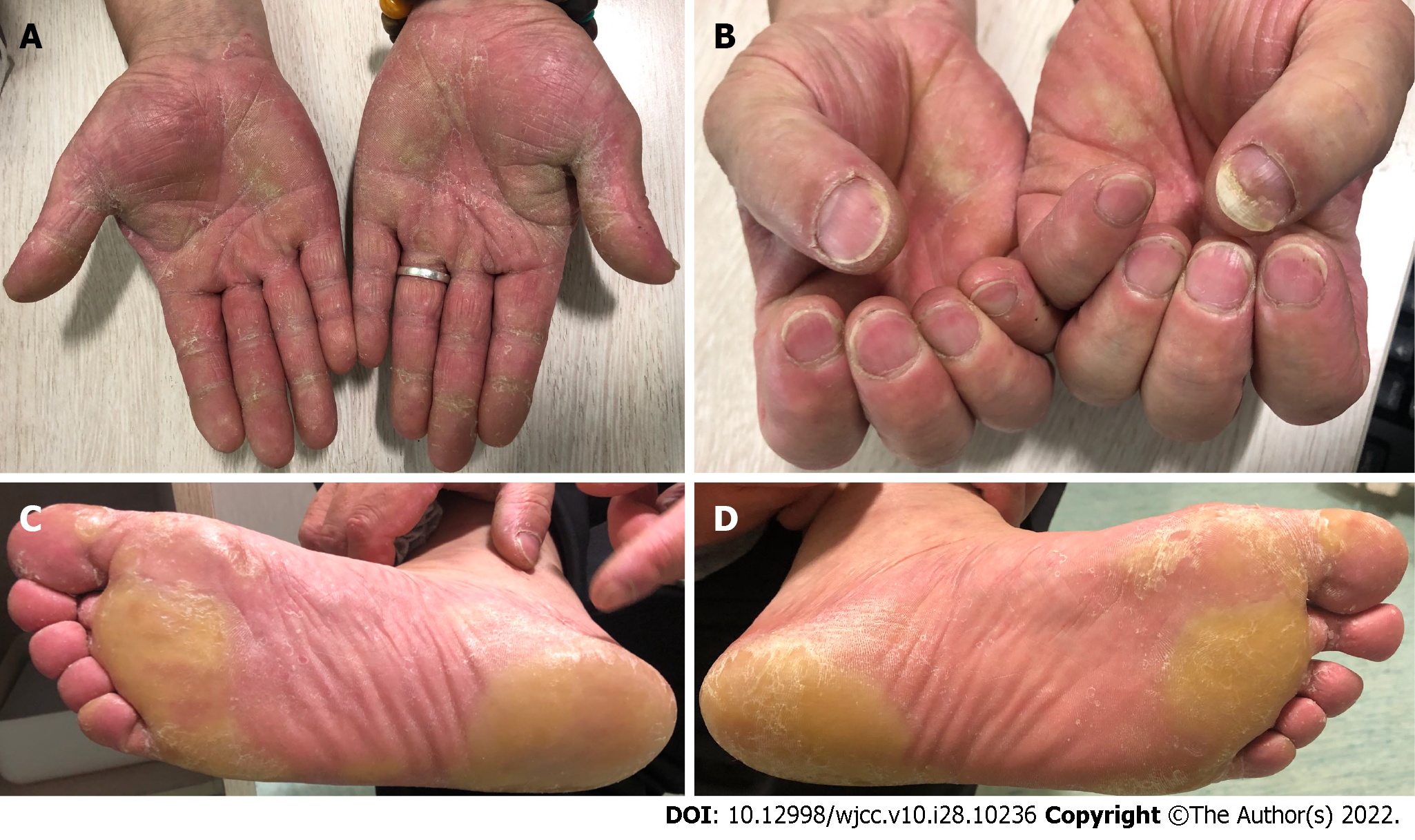
The patient successfully achieved sustained PR after oral administration of sorafenib for 4 mo and 7 mo (Figure 4E and F). However, he simultaneously developed grade II hand-foot syndrome (Figure 5); therefore, sorafenib was reduced to 400 mg once daily. At the time of manuscript submission, the patient had been on sorafenib maintenance for > 9 mo, and the OS had exceeded 13 mo, while maintaining an ECOG performance status of 0 to 1.
HAC is an invasive extrahepatic tumor that resembles HCC in morphology, but is an uncommon clinical entity. Metzgeroth et al[8] summarized data from 261 cases of HAC in 2010[8]. The stomach (63%), ovary (10%), lungs (5%), gallbladder (4%), pancreas (4%), and uterus (4%) were the most common disease sites. HAL is a rare primary adenocarcinoma of the lung that contains hepatocytes and can be associated with elevated AFP levels[1,2]. Haninger et al[9] modified the diagnostic criteria for HAL in 2014, which are currently described as follows: HAL can be a simple HAC, but it can also be typical acinar or papillary adenocarcinoma, signet ring cell carcinoma, or neuroendocrine carcinoma. Positive expression of liver differentiation markers and AFP is not required for diagnosis and it has morphological characteristics of hepatocytes[9].
Reviews summarizing the characteristics of HAL have been published[1,2]. HAL is an aggressive tumor with no standard treatment and a poor prognosis. HAL has a reported median OS (mOS) of 14-18 mo. Patient one-, two-, three-, and five-year OS rates were reported to be approximately 40.0%, 35.3%, 35.0%, and 8%-19%, respectively[1,2]. Patients who used a surgery-based strategy, chemotherapy-based strategy, or another strategy had different prognoses; the one-year OS rates were 53%, 30%, and 0%, respectively[2]. The median survival for patients with unresectable HAL ranges from 6 to 11 mo[1]; overall, however, the prognosis of HAL is poor. Additionally, surgery is a significant prognostic factor for HAL, and is the primary treatment for patients with stages I-III disease. On the other hand, stage IV patients are primarily treated with chemotherapy and radiotherapy. The use of targeted therapy and immunotherapy for HAL has recently increased[6,7].
Because HAC is morphologically similar to HCC, we suspect that HCC treatment is also effective for HAC. The Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol (SHARP) trial found that, as a first-line standard treatment, sorafenib can improve the prognosis of HCC patients[10]. The sorafenib and placebo groups experienced mOS of 10.7 mo and 7.9 mo, mPFS of 5.5 mo and 2.8 mon, and one-year survival rates of 44% and 33%, respectively. Furthermore, oral sorafenib has been used to treat some HAC cases[6,8,11]. One patient with peritoneal HAC treated with sorafenib after first-line chemotherapy experienced two months of clinical stability[8]. A rare case of metastatic pancreatic HAC treated with sorafenib resulted in PFS > 7 mo[11]. Furthermore, Gavrancic et al[6] reported that sorafenib combined with platinum-based doublet chemotherapy resulted in a partial response and sorafenib monotherapy led to stable disease in AFP-related EGFR wild-type HAL. Therefore, the patient survived for an additional 11 mo.
In contrast, HAL is a type of NSCLC; moreover, some studies have attempted to use sorafenib for the treatment of NSCLC. In a phase II, single-arm, multicenter study involving 51 patients with relapsed or refractory advanced NSCLC, the mPFS was 2.7 mo and the mOS was 6.7 mo, stable disease was achieved in 30 (59%) patients who had a mPFS of 5.5 mo, and tumor shrinkage was observed in 15 (29%) patients[12]. After receiving sorafenib for two months, patients were randomly assigned to the sorafenib group or placebo group if they had stable disease in a double-blind randomized, discontinuation, phase II study (ECOG 2501) of sorafenib vs placebo in previously treated NSCLC[13]. The disease control rate in sorafenib group was significantly higher than in the placebo group (47% vs 19%; P = 0.01), and the mPFS (3.6 mo vs 1.9 mo; P = 0.01) was also significantly higher in the sorafenib group (3.6 mo vs 1.9 mo; P = 0.01). Furthermore, a phase III, multicenter, placebo-controlled trial of sorafenib in patients with relapsed or refractory advanced NSCLC after two or three lines of treatment (MISSION), failed to demonstrate that sorafenib improved OS (8.2 mo vs 8.3 mo; P = 0.47) for NSCLC, but increased PFS (2.8 mo vs 1.4 mo; P < 0.0001)[14].
Sorafenib was chosen to treat a patient with unresectable AFP-related HAL which had progressed on two lines of chemotherapy and immunotherapy after reviewing all reported cases and studying the above trials. In the present case, the patient developed grade II hand-foot syndrome and tolerated sorafenib 400 mg once daily. Subsequently, the patient successfully achieved PR with an excellent biomarker response, the PFS is already > 9 mo and the OS is > 13 mo, which is among the longest reported for inoperable stage IV HAL.
Results of this study suggest that systematic therapeutic drugs for primary liver cancer, such as sorafenib, could be viable treatment options for previously treated advanced HAL. Nevertheless, accumulation of more data from more cases and enriched treatment methods to improve the poor prognosis of HAL are needed.
We would like to thank the patient for providing written informed consent for publication of this report and all research staff involved in this case study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kai K, Japan; Leowattana W, Thailand S-Editor: Chen YL L-Editor: Ma JY-MedE P-Editor: Chen YL
| 1. | Hou Z, Xie J, Zhang L, Dai G, Chen Y, He L. Hepatoid Adenocarcinoma of the Lung: A Systematic Review of the Literature From 1981 to 2020. Front Oncol. 2021;11:702216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Mao JX, Liu C, Zhao YY, Ding GS, Ma JQ, Teng F, Guo WY. Merged hepatopulmonary features in hepatoid adenocarcinoma of the lung: a systematic review. Am J Transl Res. 2021;13:898-922. [PubMed] |
| 3. | Ishikura H, Kanda M, Ito M, Nosaka K, Mizuno K. Hepatoid adenocarcinoma: a distinctive histological subtype of alpha-fetoprotein-producing lung carcinoma. Virchows Arch A Pathol Anat Histopathol. 1990;417:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 105] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Papatsimpas G, Kamposioras K, Goula K, Papaparaskeva K, Loukides S, Kotoulas C, Kelekis N, Xiros N, Pectasides D, Koumarianou A. Hepatoid pancoast tumor. A case report and review of the literature. Lung Cancer. 2012;77:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Hayashi Y, Takanashi Y, Ohsawa H, Ishii H, Nakatani Y. Hepatoid adenocarcinoma in the lung. Lung Cancer. 2002;38:211-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Gavrancic T, Park YH. A novel approach using sorafenib in alpha fetoprotein-producing hepatoid adenocarcinoma of the lung. J Natl Compr Canc Netw. 2015;13:387-91; quiz 391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Chen L, Han X, Gao Y, Zhao Q, Wang Y, Jiang Y, Liu S, Wu X, Miao L. Anti-PD-1 Therapy Achieved Disease Control After Multiline Chemotherapy in Unresectable KRAS-Positive Hepatoid Lung Adenocarcinoma: A Case Report and Literature Review. Onco Targets Ther. 2020;13:4359-4364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Metzgeroth G, Ströbel P, Baumbusch T, Reiter A, Hastka J. Hepatoid adenocarcinoma - review of the literature illustrated by a rare case originating in the peritoneal cavity. Onkologie. 2010;33:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Haninger DM, Kloecker GH, Bousamra Ii M, Nowacki MR, Slone SP. Hepatoid adenocarcinoma of the lung: report of five cases and review of the literature. Mod Pathol. 2014;27:535-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10268] [Article Influence: 604.0] [Reference Citation Analysis (2)] |
| 11. | Petrelli F, Ghilardi M, Colombo S, Stringhi E, Barbara C, Cabiddu M, Elia S, Corti D, Barni S. A rare case of metastatic pancreatic hepatoid carcinoma treated with sorafenib. J Gastrointest Cancer. 2012;43:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Blumenschein GR Jr, Gatzemeier U, Fossella F, Stewart DJ, Cupit L, Cihon F, O'Leary J, Reck M. Phase II, multicenter, uncontrolled trial of single-agent sorafenib in patients with relapsed or refractory, advanced non-small-cell lung cancer. J Clin Oncol. 2009;27:4274-4280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 13. | Wakelee HA, Lee JW, Hanna NH, Traynor AM, Carbone DP, Schiller JH. A double-blind randomized discontinuation phase-II study of sorafenib (BAY 43-9006) in previously treated non-small-cell lung cancer patients: eastern cooperative oncology group study E2501. J Thorac Oncol. 2012;7:1574-1582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Paz-Ares L, Hirsh V, Zhang L, de Marinis F, Yang JC, Wakelee HA, Seto T, Wu YL, Novello S, Juhász E, Arén O, Sun Y, Schmelter T, Ong TJ, Peña C, Smit EF, Mok TS. Monotherapy Administration of Sorafenib in Patients With Non-Small Cell Lung Cancer (MISSION) Trial: A Phase III, Multicenter, Placebo-Controlled Trial of Sorafenib in Patients with Relapsed or Refractory Predominantly Nonsquamous Non-Small-Cell Lung Cancer after 2 or 3 Previous Treatment Regimens. J Thorac Oncol. 2015;10:1745-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |