Published online Sep 6, 2022. doi: 10.12998/wjcc.v10.i25.9087
Peer-review started: April 2, 2022
First decision: May 31, 2022
Revised: June 16, 2022
Accepted: August 1, 2022
Article in press: August 1, 2022
Published online: September 6, 2022
Processing time: 145 Days and 14.6 Hours
Paraneoplastic syndromes remain poorly understood and manifest as multifaceted clinical symptoms, making their diagnosis difficult. Cholestasis can be observed in various malignancies. In rare cases, it can be a paraneoplastic manifestation, most often associated with renal cell carcinoma and other urogenital tumors, as well as with bronchial carcinoma. The classical form of Stauffer syndrome presents with a reversible anicteric increase of cholestatic liver function tests, thrombocytosis, coagulation impairment, and hepatosplenomegaly, without any proven hepatobiliary obstruction or metastases.
We report a patient who presented with elevated liver enzymes, cholestatic jaundice, weight loss and pruritus, in whom renal cell carcinoma was incidentally found during hospitalization. Clinical, laboratory, and imaging tests excluded primary hepatic cause or metastatic disease. Jaundice and laboratory abnor
Despite being rare, Stauffer syndrome is a potentially reversible paraneoplastic condition, when the primary cause is treatable. This syndrome should be considered by clinicians because of the remediable liver disturbance, after successful treatment of the underlying malignancy.
Core Tip: Initial presentation of jaundice, in cases of non-hepatic malignancy, is generally associated with metastatic liver disease. Despite the increased bilirubin levels, surgical treatment, when possible, is an appropriate approach in a case with non-metastatic cholestatic syndrome. Stauffer syndrome is a rare presentation of kidney and urinary tract malignancies and even rarer when presented with jaundice.
- Citation: Popov DR, Antonov KA, Atanasova EG, Pentchev CP, Milatchkov LM, Petkova MD, Neykov KG, Nikolov RK. Renal cell carcinoma presented with a rare case of icteric Stauffer syndrome: A case report. World J Clin Cases 2022; 10(25): 9087-9095
- URL: https://www.wjgnet.com/2307-8960/full/v10/i25/9087.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i25.9087
In 1961, Herbert Maurice Stauffer, an American gastroenterologist, first described a syndrome associated with hypernephroma, low blood levels of albumin, elevated gamma globulins, high alkaline phosphatase (AP) and prolonged prothrombin time, with normalization of liver function tests achieved upon successful treatment of the underlying malignancy[1]. Stauffer syndrome is observed in 3% to 6% of cases of renal cell carcinoma (RCC)[2]. Lymphocytic infiltration and cellular degeneration of the liver clinically present elevated hepatic enzymes and impaired liver function in the absence of liver metastases[3]. At least two theories about the pathogenesis are hypothesized, and include: liver damage due to stimulation of cathepsins and hepatic phosphatases by hepatotoxins or lysosomal enzymes; and direct liver damage and the subsequent immune response[4]. Among the substances produced are granulocyte-macrophage colony stimulating factor and interleukin (IL)-6 are notable. Other admissible speculations include generalized hepatic hypervascularity, amyloid deposition, and autoimmune phenomenon[5]. This case is reported in line with the criteria of the 2020 Guideline: Updating Consensus Surgical CAse REport (SCARE)[6].
A 68-year-old Caucasian male was admitted to our clinic to address ongoing intensive jaundice, fatigue, pruritus, pain, and heaviness in the right upper quadrant of the abdomen that had persisted since September 2020.
The patient had been admitted to another medical center, and acute and chronic viral hepatitis were excluded by the following negative markers: hepatitis B surface antigen; anti-hepatitis B core immunoglobulin M (IgM); anti-hepatitis C virus; anti-hepatitis A virus IgM; cytomegalovirus IgM; hepatitis B virus DNA; and hepatitis C virus RNA. Autoimmune and metabolic liver diseases were excluded as well by negative serologic marker testing for autoimmune hepatitis, and by levels of IgG and IgM within the reference range. Slightly increased ceruloplasmin was observed. Ultrasound examination revealed tumor (45 mm × 32 mm) formation in the right kidney. Native magnetic resonance imaging (MRI)-cholangiopancreatography (MRCP) was performed, which was negative for biliary pathology and confirmed tumor formation with expansive growth and compression of the lower surface of the right liver lobe. For clarification, liver biopsy was performed and showed a histological view most compatible with toxic hepatitis, with no stigma for primary biliary cholangitis, hepatitis, or cirrhosis. Treatment consisting of ademetionine, silymarin, L-ornithine, L-aspartate, methylprednisolone 60 mg per day intravenous (i.v.) for 8 d, ursodeoxycholic acid 1000 mg per day, vitamin K i.v., antibiotics, and antisecretory and i.v. fluids was administered but produced no obvious effect.
The patient had a history of arterial hypertension, ischemic stroke (in 2007), and vertigo (as a result of the latter). A perianal fistula had been present in adolescence.
The patient’s personal and family histories were unremarkable.
On October 2, 2020, the patient was admitted with the above-mentioned complaints for the first time to our Gastroenterology clinic. Physical examination showed jaundice of the skin and sclera, skin excoriations, hepatomegaly, and slight abdominal tenderness.
The preoperative laboratory findings are displayed in Table 1. After reconfirmation of negative virological tests, including anti-hepatitis E virus (HEV) IgM (-), Epstein-Barr virus IgM (-) and anti-HEV IgG (+), a positive result was established. However HEV RNA was undetectable. The levels of lactate dehydrogenase, ceruloplasmin, IgA, IgG and IgM were normal. Chronic poisoning by lead, manganese and arsenic was rejected. Tuberculosis was excluded by QuantiFERON-TB Gold Plus assay (Qiagen, Hilden, Germany). Levels of fetal oncoproteins, total prostate-specific antigen, alpha-fetoprotein and CA 19-9 were within normal ranges. Additionally, dyslipidemia was detected, with total cholesterol at 18.1 mmol/L (reference range: < 5.2 mmol/L), low-density lipoprotein at 16.0 mmol/L (< 3.0), triglycerides at 3.3 mmol/L (< 2.0), and high-density lipoprotein at 0.6 mmol/L (> 0.9 mmol/L). During follow-up after the nephrectomy, lipid panel levels reached normal rates, including that of cholesterol (3.9 mmol/L), low-density lipoprotein (1.7 mmol/L), triglycerides (0.6 mmol/L), and high-density lipoprotein (1.3 mmol/L).
| Parameters | September 4, 2020 | October 1, 2020 | October 12, 2020 |
| Tbil/Dbil in µmol/L | 289/ 160 | 381/ 210 | 467/ 291 |
| AST in U/L | 125 | 232 | 225 |
| ALT in U/L | 225 | 228 | 199 |
| GGT in U/L | 566 | 476 | 1369 |
| AP in U/L | 304 | 299 | 508 |
| CRP in mg/L | 17 | 240 | 21 |
| Creat in µmol/L | 130 | 126 | 84 |
| Alb in g/L | 39 | 39 | 32 |
| INR | 0.95 | 1.1 | 1.19 |
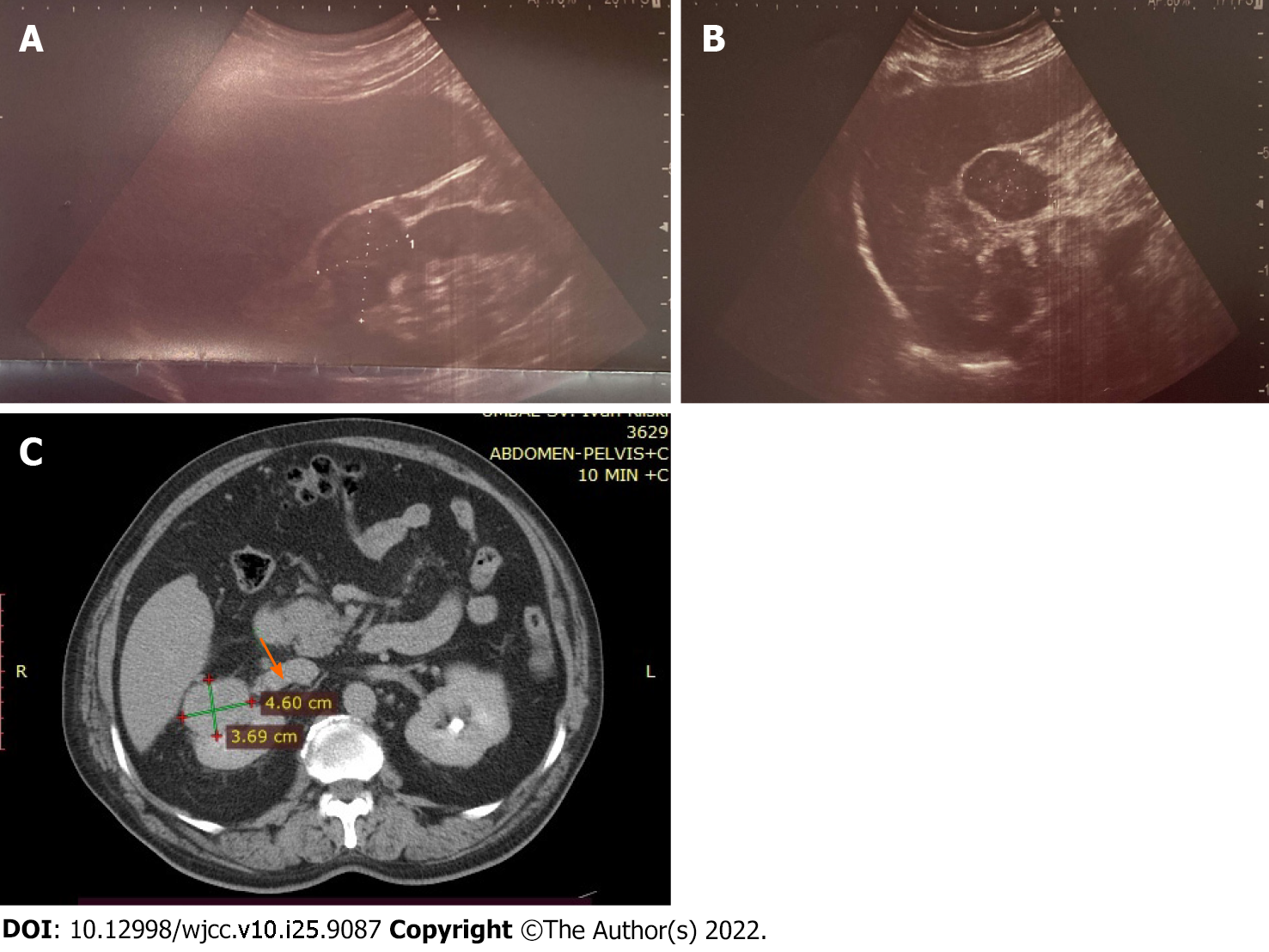
Abdominal ultrasound showed the liver and spleen to be of normal dimensions. No features of biliary or pancreatic morphology changes or portal hypertension were detected. A heterogeneous, rounded lesion (45 mm × 32 mm) was visualized at the upper pole of the right kidney, with detectable arterial signal on Doppler examination (Figure 1A and B). Furthermore, contrast-enhanced computed tomography (CT) of the abdomen was performed. The liver had slightly lobulated contour, hypertrophy of segment I and normal parenchymal density, without pathological lesions or postcontrast enhancement, and non-dilated intrahepatic and extrahepatic bile ducts. Tumor (46 mm × 31 mm) formation in the right kidney was confirmed and found to have invaded the vena cava inferior. The tumor reached the liver parenchyma at segment VI, without infiltration (Figure 1C). Chest X-ray examination revealed no infiltrative or focal lesion.
Findings from the liver biopsy specimens and MRI of the abdomen from the first medical center were reviewed, but provided no other explanation of the observed changes in the liver function tests.
After clinical discussion (with careful consideration of all clinical findings, including results from the laboratory tests and imaging studies and reference to similar cases in the literature), the patient was diagnosed with a paraneoplastic syndrome in urogenital tumors and Stauffer syndrome.
Therapy consisted of ademetionine (administered September 4, 2020 to December 17, 2020), N-acetyl cysteine (October 2, 2020 to present), thiamine and pyridoxine, methylprednisolone (20 mg/d i.v. for 8 d), broad-spectrum antibiotics, gastro protectors, and i.v. fluids, including colloid solutions (i.e. human albumin); unfortunately, no improvement was achieved (according to unchanged laboratory test findings). Instead, the patient experienced a progressive increase in total and direct bilirubin as well as an increase in gamma-glutamyl transferase and AP levels.
As the syndrome is characterized by non-metastatic liver damage caused by hormones and substances secreted by the tumor (including IL-6), we determined that the patient was suitable for surgical treatment. The case was presented to the oncologist, and all of the risks and benefits of potential surgical intervention were carefully assessed. The patient was then referred for nephrectomy in our oncology clinic at The National Oncology Centre.
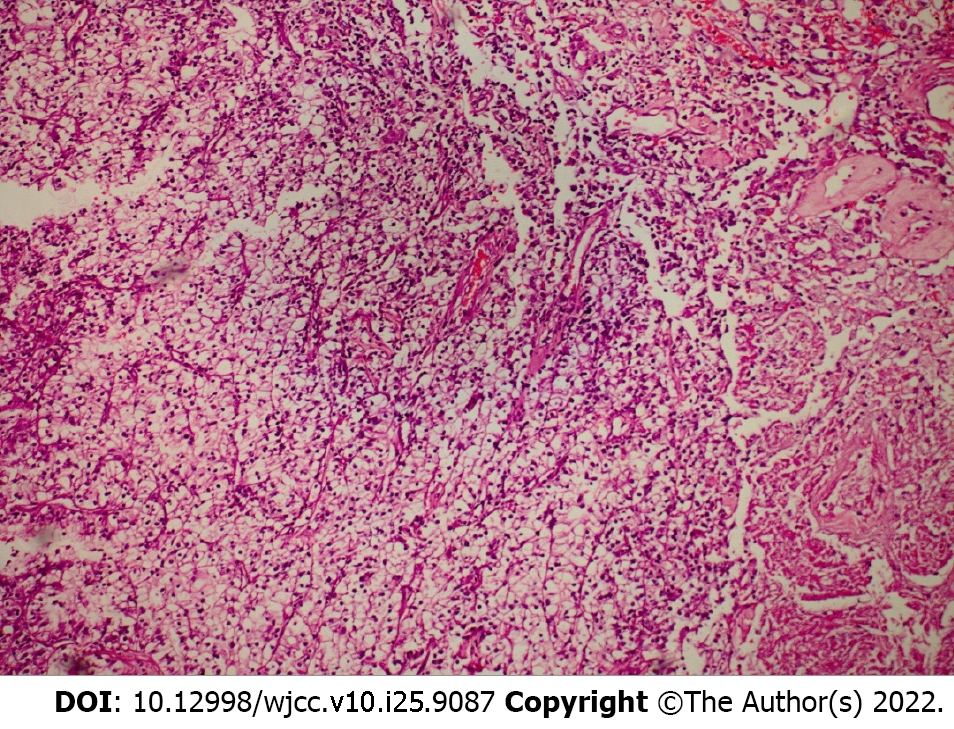
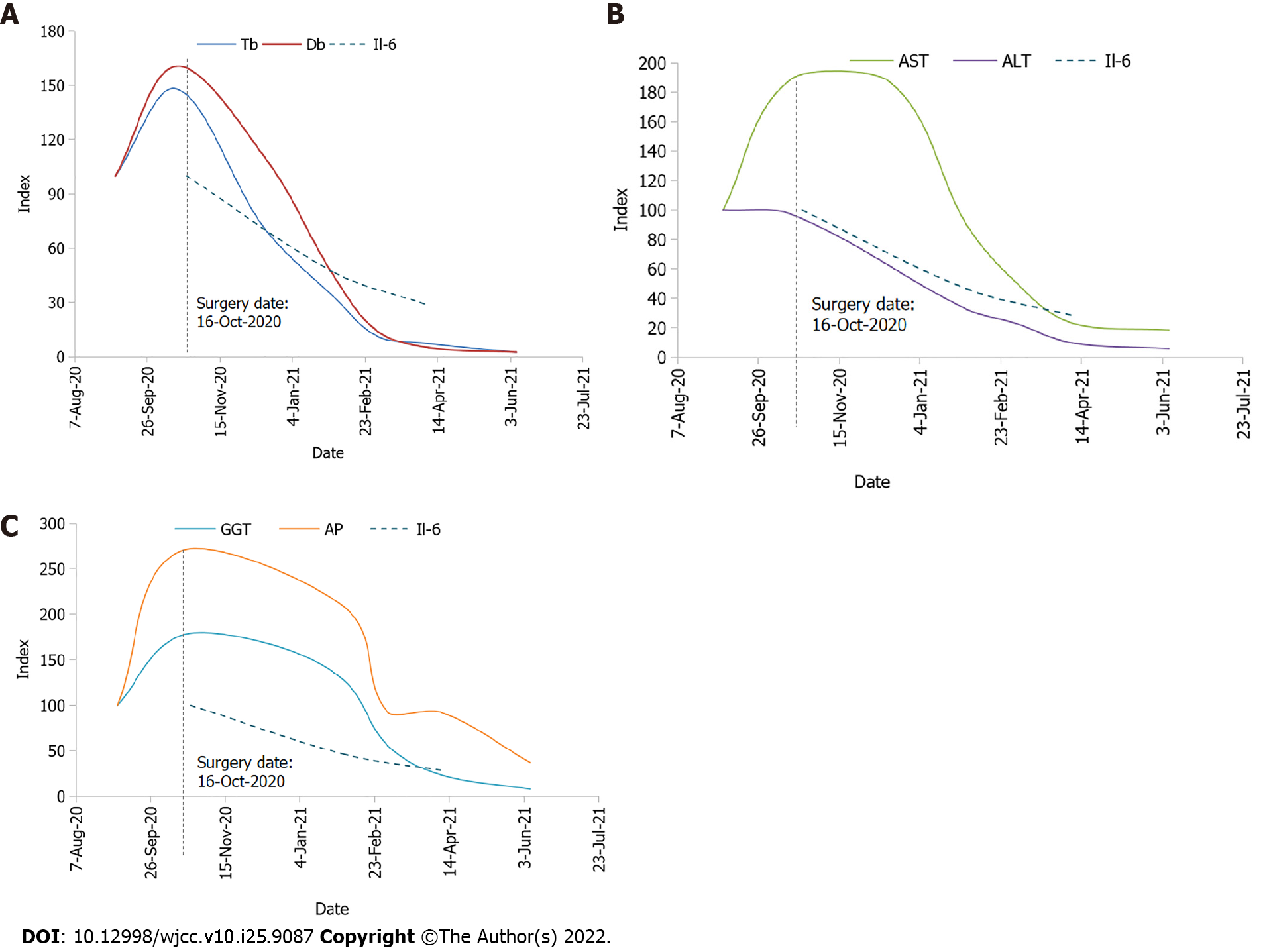
In the preoperative workup, IL-6 was 24.12 ng/mL (normal level: < 7.00). On October 16, 2020, an open right nephroureterectomy was performed. On the 3rd postoperative day, the patient developed bleeding from the drainage, which required a revision surgery and hemostasis of small venous vessels that had grown to the liver. Histology indicated a clear cell, G2 type RCC, with a diameter of 3 cm and tumor thrombus in the lumen of the vena cava inferior, staged as pT3bNxMx G2 LVI (Figure 2). The perioperative and postoperative laboratory findings are summarized in Table 2 and expressed graphically in Figure 3.
| Parameters | October 16, 2020 (operation) | October 18, 2020 | October 20, 2020 | October 23, 2020 | December 16, 2020 | February 2, 2021 | April 8, 2021 | June 7, 2021 | October 5, 2021 |
| Tbil/Dbil in µmol/L | > 428/> 257 | 401/> 257 | 317/242 | > 428/> 257 | 204/176 | 94/70 | 21/8 | 7/4 | 7/2 |
| AST in U/L | 333 | 236 | 256 | 374 | 234 | 112 | 29 | 23 | 18 |
| ALT in U/L | 304 | 219 | 225 | 342 | 73 | 22 | 13 | 18 | |
| GGT in U/L | > 999 | > 999 | 927 | > 999 | 944 | 724 | 133 | 44 | 52 |
| AP in U/L | 819 | 494 | 426 | 1219 | 635 | 281 | 113 | ||
| Creat in µmol/L | 106 | 126 | 107 | 129 | 158 | ||||
| Alb in g/L | 35 | 28 | 28 | 34 | 34 | 39 | 42 | ||
| INR | 1.19 | 1.03 | 1.12 | ||||||
| IL-6 < 7.00 in ng/mL | 24.1 | 11.18 | 6.18 | ||||||
| Procalcitonin < 0.046, in ng/mL | 0.675 | 0.44 |
The Oncology Committee recommended adjuvant radiotherapy to be initiated when findings of liver function tests reached normal range.
The patient was followed up until November 30, 2020, when he was re-admitted to our Gastroenterology Clinic due to persistence of the jaundice and intensive pruritus despite ongoing treatment with hepatoprotectors, antiacids, and antihypertensive therapy. On routine abdominal ultrasound study, the imaging showed no dynamics of the liver or portal blood flow. An additional finding worth mentioning was a mild decrease in cholinesterase and a drop in IL-6 level. Supportive therapy was continued, and antifungal and anaerobe prophylactics were added.
The patient was discharged when slight clinical and laboratory improvement was achieved, and outpatient hepatoprotective and antioxidant therapies were ordered. On January 20, 2021, restaging was conducted via positron emission tomography (PET)-CT and provided no convincing data of residual tumor tissue, locoregional relapse, or distant dissemination related to the oncological process. The current performance status of the patient is 1, and PET-CT monitoring has shown no indications of local relapse or distant dissemination.
RCC is related to various paraneoplastic syndromes classified as endocrine and non-endocrine[7]. RCC represents 2% to 4% of all newly diagnosed cancers in the developed countries on an annual basis, making it the most commonly encountered primary renal malignant tumor[8]. According to data from the American Cancer Society in 2015, the 5-year survival rates for localized tumor are high. Controversially, metastases are commonly found. In fact, during the primary diagnostic work up, 20% of the patients present with metastatic spread. Eventually 20% to 40% of all patients will develop RCC metastases after radical nephrectomy[9,10]. The liver is amongst the most common metastatic site, leading to a significant decrease in the 5-year survival rate (to 20%)[11].
Despite the vast knowledge of carcinogenesis and metastasis spread of most of the malignant diseases, paraneoplastic syndromes remain poorly understood. Due to the multifaceted clinical presentation, their diagnosis remain difficult. Cholestasis can be observed in various malignancies such as the liver, pancreas, gall bladder, and bile ducts. It can be due to an obstruction of the biliary tree or liver metastases. In rare cases, cholestasis is a paraneoplastic manifestation-Stauffer syndrome; classically seen in RCC, it has also been described in prostate, bronchial, and urinary bladder malignancies. It is mainly presented with elevated cholestatic liver function tests, thrombocytosis, coagulation impairment and hepatosplenomegaly, in the absence of hepatic metastasis. The most common laboratory findings are elevated AP (90% of cases), hyperbilirubinemia (15%), and elevated transaminases (21%)[12].
The process is probably caused by IL-6, a proinflammatory cytokine produced by cancer cells, since impaired laboratory results normalize after anti-IL-6 monoclonal antibody therapy. Clinical symptoms and laboratory abnormalities often persist during active malignancy but improve with nephrectomy[13].
The reported frequencies of Stauffer syndrome range between 3% and 20% of RCC cases[7]. Elevated IL-6 is often present, and associations between the levels of IL-6, AP, C-reactive protein (CRP) and haptoglobin have been reported in the event of RCC[14,15].
IL-6, in particular, has been proposed to play a major role in the pathophysiology of this syndrome[2,12,16]. Bhangoo et al[15] proposed a mechanism in which the proinflammatory activity of IL-6 causes impairment of biliary outflow via elevation of CRP and haptoglobin, and inhibition of the hepatobiliary transporter gene expression.
Recently, a more uncommon variant of the syndrome that initially presents with jaundice (icteric cholestasis) was described. According to Chavarriaga et al[17], there have been 11 cases of paraneoplastic cholestatic jaundice syndrome, including their case, reported in the literature.
In 1997, Dourakis et al[2] described 2 cases: of a 65-year-old woman and a 48-year-old male. They both were presented with jaundice and urinary hyperpigmentation and were diagnosed with renal carcinoma. After nephrectomy the conditions in both cases improved. These were the first described cases of icteric Stauffer syndrome.
In 2005, Giannakos et al[18] reported a similar case. A 73-year-old male presented with pruritus, painless jaundice, hyperpigmented urine and enlarged liver. The patient was diagnosed with clear cell RCC and underwent radical nephrectomy, after which normalization of abnormalities was observed. None of the above cases had evidence of metastatic disease.
According to a recent review from Elseidy et al[19] (from 2022), diagnostic criteria for Stauffer syndrome are currently lacking. A stepwise diagnostic plan was proposed that includes liver function tests, abdominal ultrasound, MRCP and contrast-enhanced CT of the abdomen and pelvis. Multidisciplinary team meeting is recommended by the authors to take place before treatment initiation. This diagnostic plan is similar to the one that was applied by our team for the case described herein.
Patients with obstructive jaundice have increased operative mortality rates, increased risk of infections, disseminated intravascular coagulation, gastrointestinal bleeding, delayed wound healing, wound dehiscence, etc[20]. This case report clearly demonstrates successful operative treatment despite higher perioperative risk. The strength of this study is in the multidisciplinary approach to making the decision for surgery of the patient with established liver failure. A limitation of the study is the lack of the baseline examination of IL-6 levels, as one of the explanations of dynamic changes in IL-6 values may be the invasive procedure itself. Another limitation is the fact that there was a positive result for anti-HEV IgG without any data for a past viral infection. However, during the hospital stay, active infection from HEV was excluded. In cases of absent diagnostic criteria, the case is solved as a diagnosis of exclusion.
Stauffer syndrome and the icteric form (the rarest variant) should be considered as a possibility of an underlying neoplastic process in cases of unexplained liver impairment. Reversibility of the liver damage after successful treatment of the underlying malignancy should not be underestimated. The opinion of our team is that in order to quickly reach a diagnosis, clinicians of various related specific fields should be aware of the condition. Despite the rarity of the syndrome, further investigation for improvement of the diagnosis and treatment should be initialized.
The authors are thankful to the Department of Urology, University Hospital of Oncology, Sofia, Bulgaria for performing the surgical treatment and postsurgical care of this patient, and to Prof. Radina Ivanova for the histology image.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: Bulgarian Association of Ultrasound in Medicine; Bulgarian Society of Gastroenterology.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Bulgaria
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Bezabih YS; Chen C, China S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Stauffer H. Nephrogenous hepatosplenomegaly. Gastroenterolog. 1961;40:694. |
| 2. | Dourakis SP, Sinani C, Deutsch M, Dimitriadou E, Hadziyannis SJ. Cholestatic jaundice as a paraneoplastic manifestation of renal cell carcinoma. Eur J Gastroenterol Hepatol. 1997;9:311-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Boxer RJ, Waisman J, Lieber MM, Mampaso FM, Skinner DG. Non-metastatic hepatic dysfunction associated with renal carcinoma. J Urol. 1978;119:468-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 34] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Sacco E, Pinto F, Sasso F, Racioppi M, Gulino G, Volpe A, Bassi P. Paraneoplastic syndromes in patients with urological malignancies. Urol Int. 2009;83:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Sharara AI, Panella TJ, Fitz JG. Paraneoplastic hepatopathy associated with soft tissue sarcoma. Gastroenterology. 1992;103:330-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Agha RA, Franchi T, Sohrabi C, Mathew G, Kerwan A; SCARE Group. The SCARE 2020 Guideline: Updating Consensus Surgical CAse REport (SCARE) Guidelines. Int J Surg. 2020;84:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4265] [Cited by in RCA: 4714] [Article Influence: 942.8] [Reference Citation Analysis (0)] |
| 7. | Gold PJ, Fefer A, Thompson JA. Paraneoplastic manifestations of renal cell carcinoma. Semin Urol Oncol. 1996;14:216-222. [PubMed] |
| 8. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21370] [Article Influence: 2137.0] [Reference Citation Analysis (3)] |
| 9. | McKay RR, Kroeger N, Xie W, Lee JL, Knox JJ, Bjarnason GA, MacKenzie MJ, Wood L, Srinivas S, Vaishampayan UN, Rha SY, Pal SK, Donskov F, Tantravahi SK, Rini BI, Heng DY, Choueiri TK. Impact of bone and liver metastases on patients with renal cell carcinoma treated with targeted therapy. Eur Urol. 2014;65:577-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 203] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 10. | Leibovich BC, Blute ML, Cheville JC, Lohse CM, Frank I, Kwon ED, Weaver AL, Parker AS, Zincke H. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer. 2003;97:1663-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 606] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 11. | Bianchi M, Sun M, Jeldres C, Shariat SF, Trinh QD, Briganti A, Tian Z, Schmitges J, Graefen M, Perrotte P, Menon M, Montorsi F, Karakiewicz PI. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol. 2012;23:973-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 480] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 12. | Sharma N, Darr U, Darr A, Sood G. Stauffer Syndrome: A Comprehensive Review of the Icteric Variant of the Syndrome. Cureus. 2019;11:e6032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Blay JY, Rossi JF, Wijdenes J, Menetrier-Caux C, Schemann S, Négrier S, Philip T, Favrot M. Role of interleukin-6 in the paraneoplastic inflammatory syndrome associated with renal-cell carcinoma. Int J Cancer. 1997;72:424-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Jangouk P, Hashash JG. An unusual cause of painless jaundice. Renal cell carcinoma (Stauffer syndrome). Gastroenterology. 2014;146:913, 1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Bhangoo MS, Cheng B, Botta GP, Thorson P, Kosty MP. Reversible intrahepatic cholestasis in metastatic prostate cancer: An uncommon paraneoplastic syndrome. Mol Clin Oncol. 2018;8:609-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Fontes-Sousa M, Magalhães H, da Silva FC, Maurício MJ. Stauffer's syndrome: A comprehensive review and proposed updated diagnostic criteria. Urol Oncol. 2018;36:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Chavarriaga J, Fakih N, Cataño J, Villaquiran C, Rodriguez S, Patino G. Stauffer syndrome, clinical implications and knowledge gaps, does size matter? BMC Urol. 2020;20:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Giannakos G, Papanicolaou X, Trafalis D, Michaelidis I, Margaritis G, Christofilakis C. Stauffer's syndrome variant associated with renal cell carcinoma. Int J Urol. 2005;12:757-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Elseidy SA, Awad AK, Mandal D, Elbadawy MA, Iqbal A. Stauffer syndrome: a comprehensive review of the disease and diagnostic plan proposal. Egypt J Intern Med. 2022;34(1):39. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Pitiakoudis M, Mimidis K, Tsaroucha AK, Papadopoulos V, Karayiannakis A, Simopoulos C. Predictive value of risk factors in patients with obstructive jaundice. J Int Med Res. 2004;32:633-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |