Published online Sep 6, 2022. doi: 10.12998/wjcc.v10.i25.8932
Peer-review started: November 24, 2021
First decision: June 7, 2022
Revised: June 13, 2022
Accepted: July 11, 2022
Article in press: July 11, 2022
Published online: September 6, 2022
Processing time: 274 Days and 20 Hours
Alagille syndrome (ALGS) is an autosomal dominant genetic disorder caused by mutations in the JAG1 or NOTCH2 gene. It is characterized by decreased intrahepatic bile ducts associated with a variety of abnormalities in many other organ systems, such as the cardiovascular, skeletal, and urinary systems.
We report a rare case of ALGS. A 1-month-old male infant presented with su
We report a unique case of ALGS associated with TAPVC and severe xanthomas. This study has enriched the clinical manifestations of ALGS and emphasized the association between JAG1 gene and TAPVC.
Core Tip: Total anomalous pulmonary venous connection (TAPVC) and severe xanthomas are rarely reported in Alagille syndrome (ALGS) patients. These two symptoms have never appeared in the same patient at the same time. Here, we report a unique case of ALGS associated with TAPVC and severe xanthomas. This study has enriched the clinical manifestations of ALGS and emphasized the association between JAG1 gene and TAPVC.
- Citation: Zeng HS, Zhang ZH, Hu Y, Zheng GL, Wang J, Zhang JW, Guo YX. Alagille syndrome associated with total anomalous pulmonary venous connection and severe xanthomas: A case report. World J Clin Cases 2022; 10(25): 8932-8938
- URL: https://www.wjgnet.com/2307-8960/full/v10/i25/8932.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i25.8932
Alagille syndrome (ALGS, OMIM 118450) is a multiple system disorder that affects the face, eyes, liver, heart, bones and other organs[1-3]. ALGS is due to biallelic mutations in the Notch signaling pathway ligand JAG1 (JAGGED1) in 94% of patients and Notch receptors (NOTCH2) in 1%–2% of patients[4-6]. ALGS can be clinically diagnosed if three of the following features are present: Cardiac murmur, posterior embryotoxon (eye abnormalities), butterfly-like vertebrae, renal abnormalities, and characteristic faces in the presence of bile duct paucity on liver biopsy[7,8]; or at least 4 of the 5 major features if liver biopsy is not performed[9,10]. In some atypical cases, molecular confirmation of ALGS diagnosis is valuable[7].
A high percentage (97%) of ALGS patients have cardiac murmur[11,12], including branch pulmonary artery stenosis, peripheral pulmonary stenosis, tetralogy of Fallot (TOF), valvar pulmonic stenosis, atrial septal defect, ventricular septal defect, coarctation of the aorta, and similar issues[13]. To our knowledge, the association of total anomalous pulmonary venous connection (TAPVC) and ALGS has never been reported in any article, but it was reported in a conference by Sanchez-Lara et al[14].
A 20-day-old male neonate with unknown cause of jaundice since birth and TAPVC was referred to our hospital.
The patient had jaundice and TAPVC since birth, without a history of other ailments.
There was no history of past illness.
There was no family history of other genetic diseases. The father showed characteristic facial features: A prominent forehead, deep-set eyes with mild hypertelorism, pointed chin, and saddle-shaped nose with a bulbous tip.
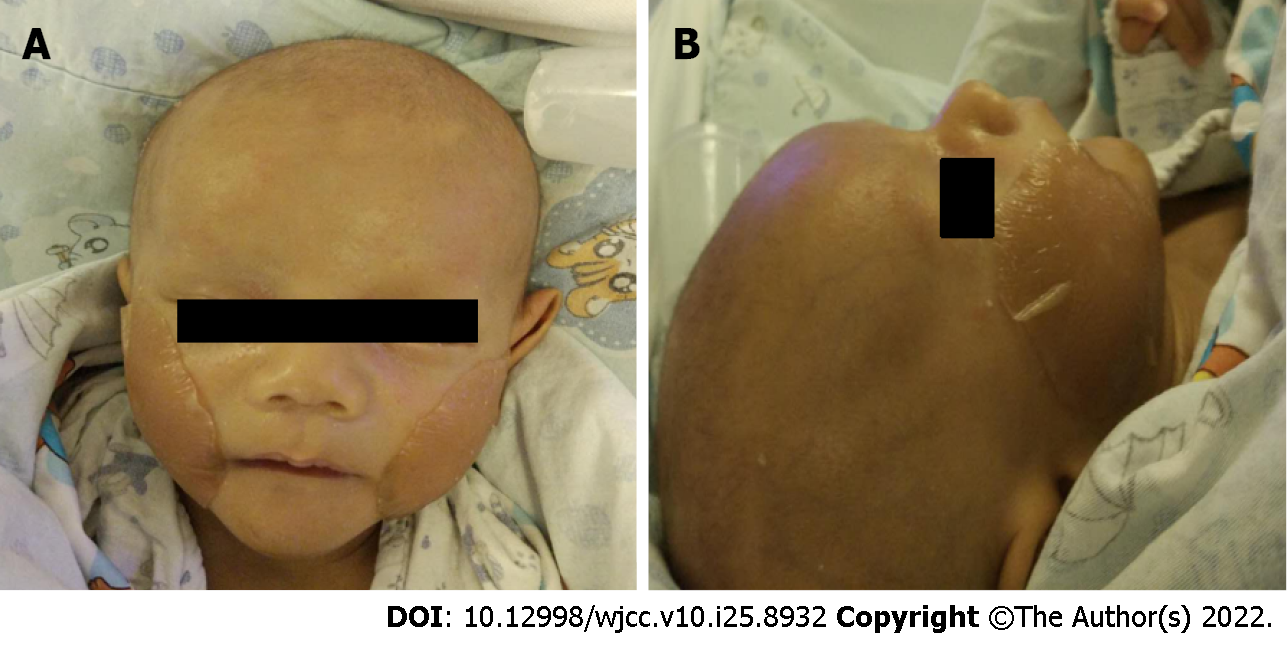
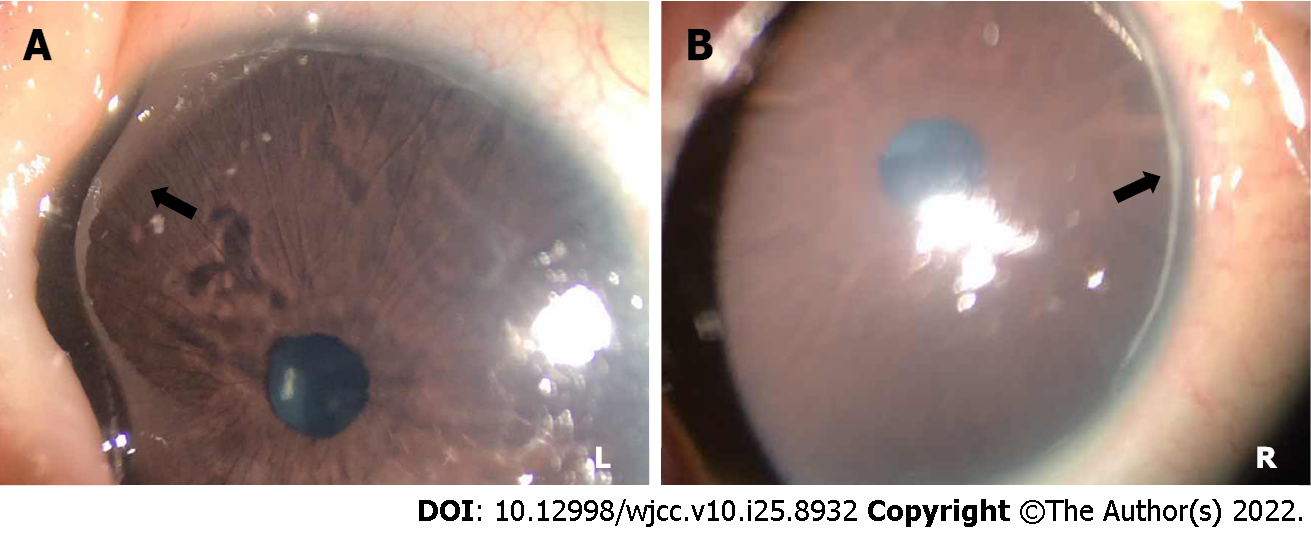
Physical examination revealed an infant weight of 3.2 kg. Jaundiced skin and sclera were observed along with a triangular appearance. The patient showed the same characteristic facial features as his father (Figure 1). He had abnormalities in both eyes (Figure 2) (posterior embryotoxon). No positive signs were found in the lungs. Abnormal sounds and murmurs were audible upon heart auscultation. The liver was palpable with a soft edge 4.0 cm below the right costal margin.
Biochemical analysis showed increased levels of serum gamma-glutamyl transpeptidase, total bilirubin (TBil), direct bilirubin (DBil), and total bile acids (TBAs), indicating cholestasis (Table 1).
| Biochemical indices | February 26, 2017 | March 5, 2017 | March 11, 2017 | March 20, 2017 | March 27, 2017 | April 3, 2017 | April 9, 2017 |
| ALT (5-40 U/L) | 107 | 198 | 266 | 527 | 256 | 324 | 444 |
| AST (5-40 U/L) | 221 | 382 | 457 | 620 | 233 | 378 | 525 |
| GGT (8-50 U/L) | 573 | 1015 | 1186 | 1065 | 1125 | 1114 | |
| ALP (20-500 U/L) | 440 | 390 | 418 | 200 | 197 | 262 | |
| TP (60.0-83.0 g/L) | 55.1 | 65.1 | 60.6 | 57.7 | 45.9 | 53.3 | 52.2 |
| Alb (35.0-55.0 g/L) | 36.5 | 40.2 | 38.2 | 38.8 | 30.4 | 34.3 | 35.5 |
| Tbil (2-19 μmol/L) | 242.4 | 241.4 | 254.0 | 469.6 | 235.4 | 295.6 | 294.5 |
| Dbil (0-6 μmol/L) | 165.5 | 163.6 | 133.1 | 245.9 | 149.0 | 138.7 | 124.4 |
| Ibil (2.56-20.9 μmol/L) | 76.9 | 77.8 | 120.9 | 223.7 | 86.4 | 156.9 | 170.1 |
| TBA (0-10 μmol/L) | 100 | 116.0 | 74.0 | 104.0 | 94.0 | 113.0 | 133.3 |
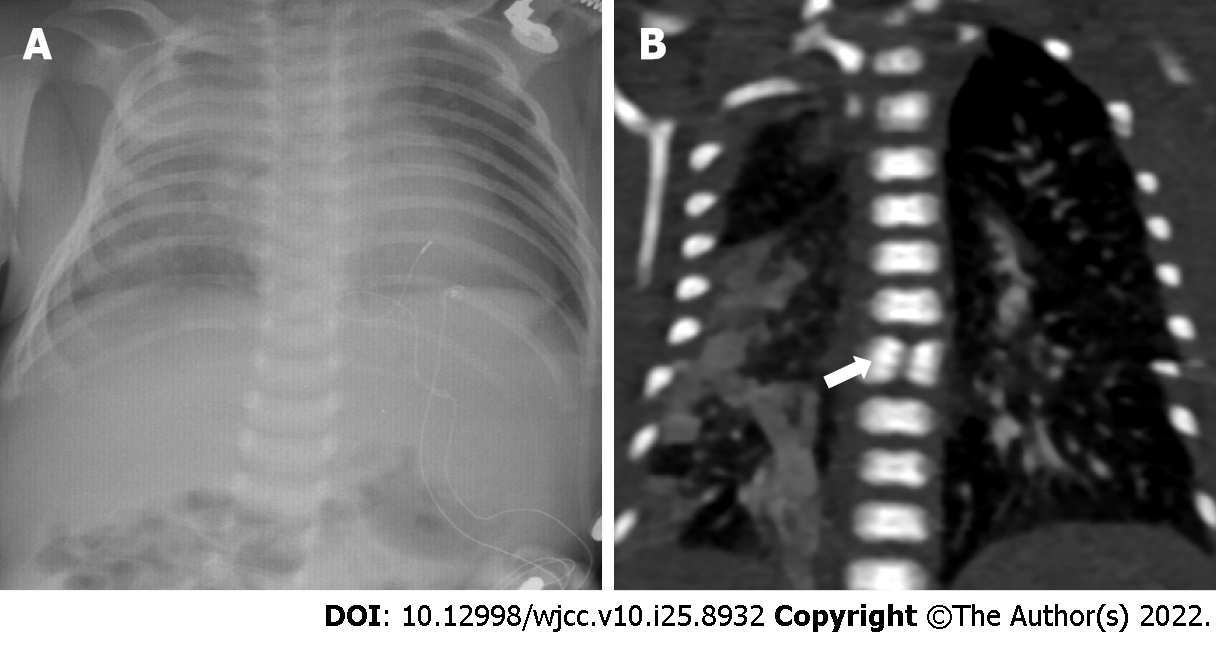
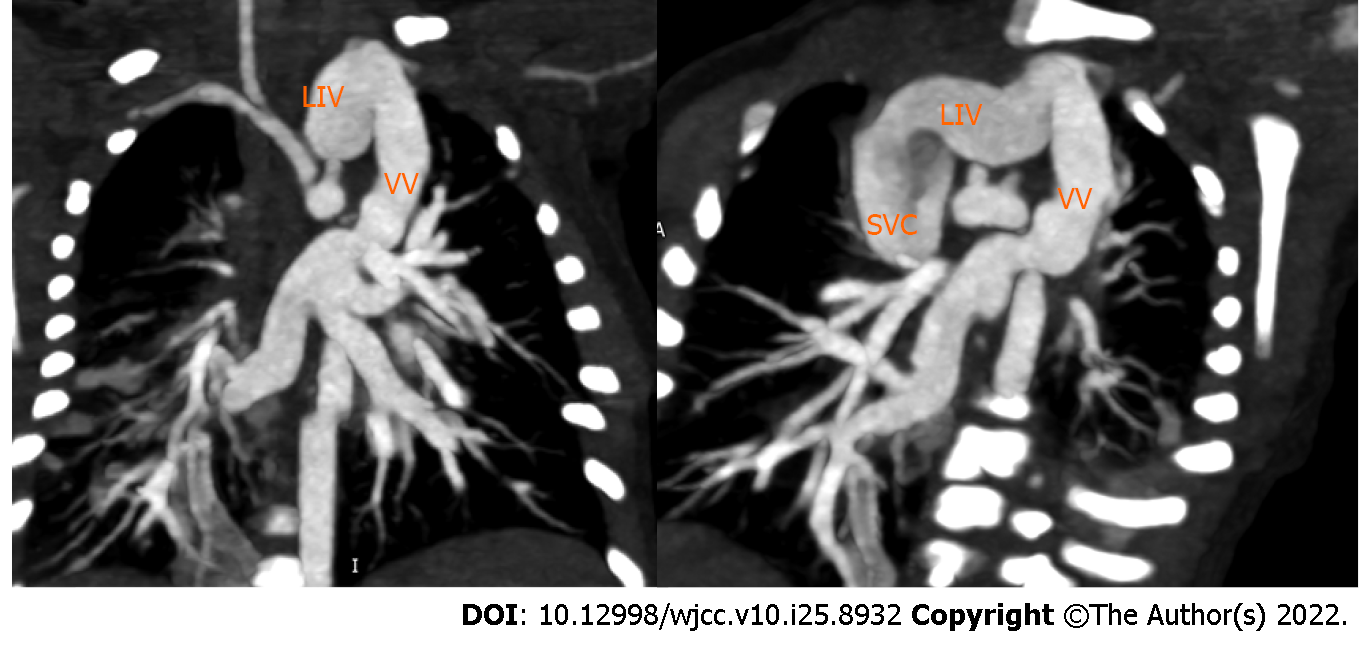
X-ray didn’t discover obvious skeletal deformities (Figure 3A), while Chest computed tomography (CT) angiography clearly shows that butterfly vertebra at the seventh thoracic vertebrae (Figure 3B). CT angiography showed that four pulmonary veins (PVs) joined together and drained into the vertical vein (VV). The VV flowed into the dilated left innominate vein (LIV), then into the superior vena cava (SVC), and finally into the right atrium (RA) (Figure 4).
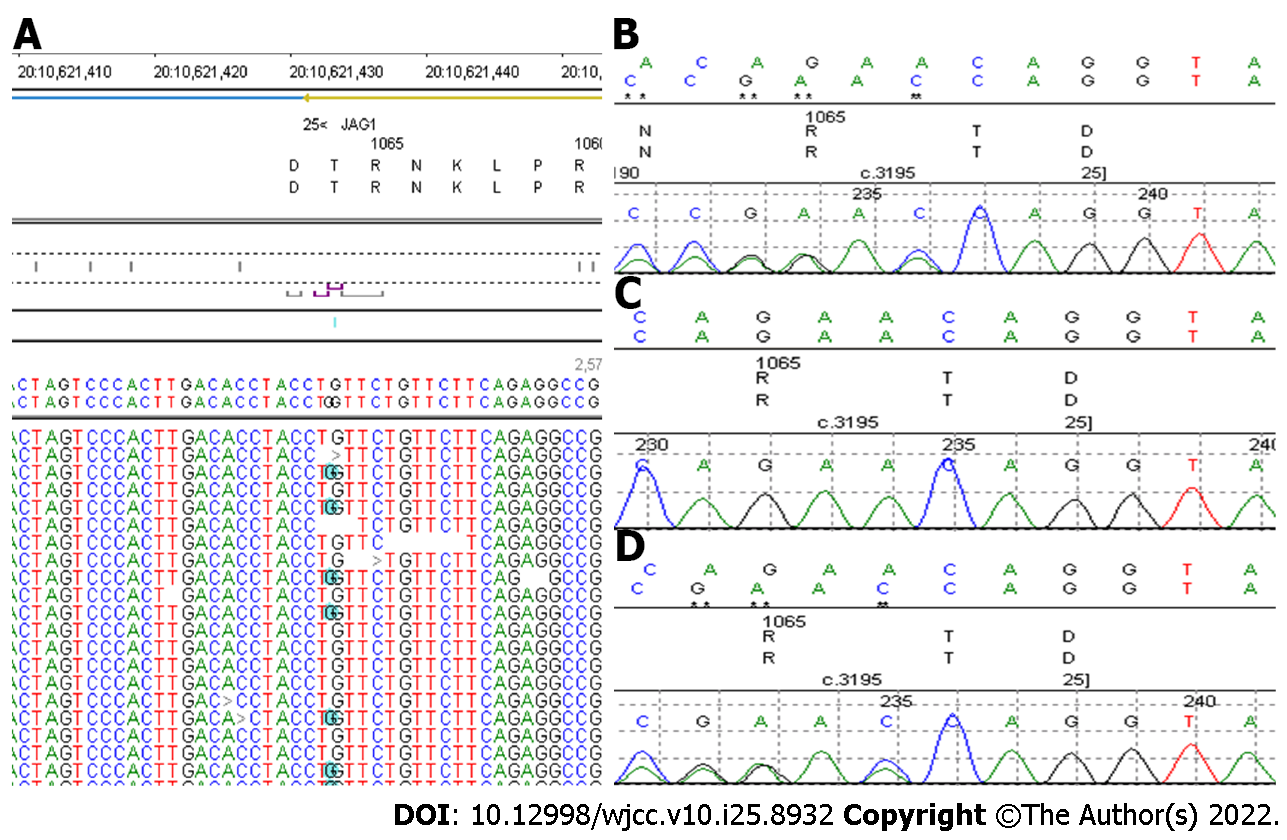
Based on these findings, ALGS was suspected and confirmed by genetic testing. A heterozygous variant (c.3197dupC) in the JAG1 gene was identified (Figure 5). This is a frame shift mutation, and it has been reported previously[10]. It is expected that the protein products encoded by this gene will be cut off prematurely, which is considered pathogenic.
Based on the clinical, imaging, and genetic findings, the final diagnosis was ALGS.
After a clear diagnosis of ALGS, the patient began ursodiol treatment, which he has been tolerating very well. The patient underwent surgical correction of his cardiac murmur at the age of 1.5 mo. He responded well to treatment and was discharged 1.5 mo later.
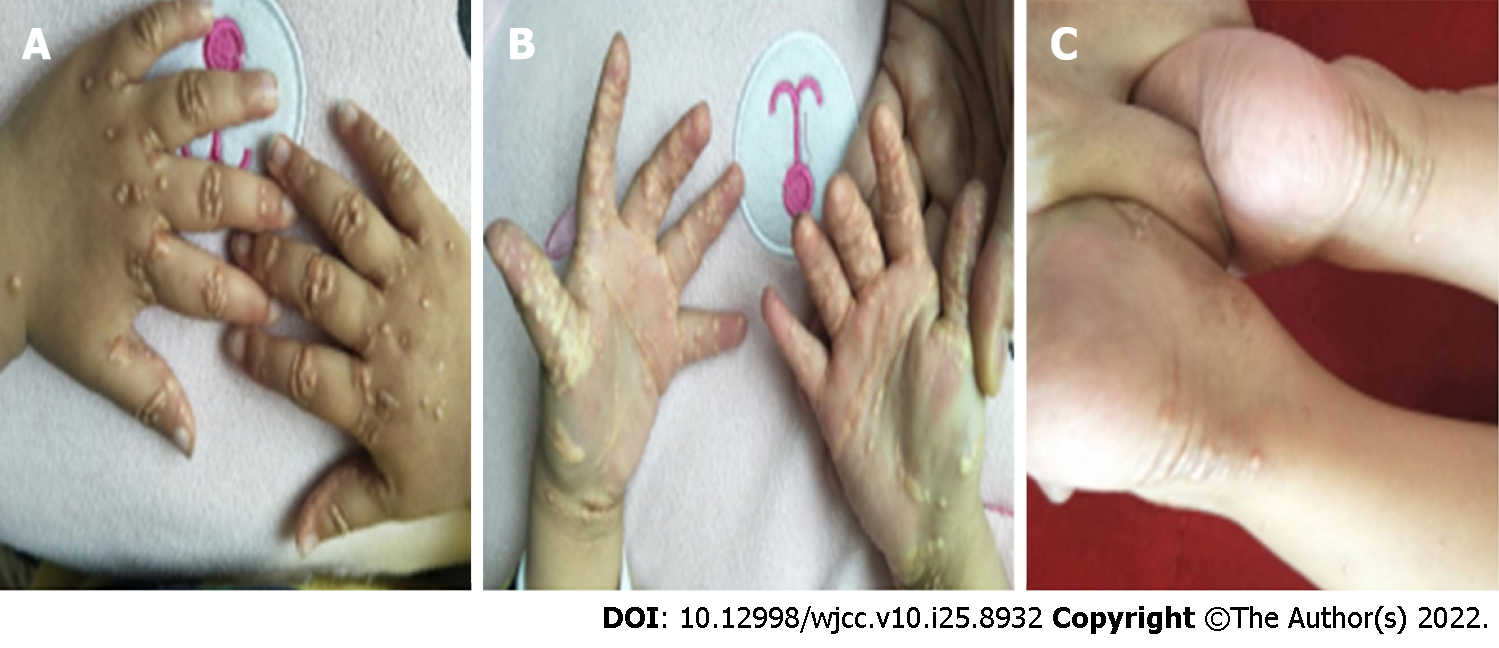
The patient did not attend regular follow-up visits at our hospital; thus, no follow-up data were obtained. He returned to our clinic due to severe xanthomas at the age of two years (Figure 6). Laboratory tests revealed increased levels of alanine aminotransferase, 339 U/L; aspartate aminotransferase, 396 U/L; alkaline phosphatase, 998 U/L; Tbil, 138.5 μmol/L; Dbil, 112.3 μmol/L; TBAs, 270.2 μmol/L; total cholesterol level, 39.9 mmol/L; and triglyceride levels, 3.79 mmol/L.
Protein Jagged-1 encoded by the JAG1 gene is one of the ligands of the Notch receptor[1]. Notch signaling pathway plays an important role in cardiovascular development[15,16]. It coordinates the morphogenesis of the cardiac chambers and valves, and regulates the formation of the cardiac outflow tract[11,12]. Therefore, malformations related to right ventricular outflow tract obstruction (RVOTO), such as stenosis at some level of the pulmonary tree and TOF, have accounted for more than 80% of cardiac murmurs in these patients[13,16]. However, a few patients have other cardiac murmurs, such as valvar pulmonic stenosis, atrial septal defect, ventricular septal defect, patent ductus arteriosus, or double-chambered RV[16].
Our patient was confirmed to have ALGS by clinical examination and genetic testing. His cardiac murmur, TAPVC, has never been reported in any article on ALGS. TAPVC is a rare cardiac murmur in which the PVs fail to return to the RA. The incidence of this rare entity is approximately 7-9 per 100000 live births or 0.7%-1.5% of all congenital heart diseases[17-20]. TAPVC is divided into four major types. Type I: Supracardiac (approximately 55%), as in this case, which is the most common type. The PVs confluence behind the left atrium, then drain into the LIV through the VV, then into the SVC or sometimes into the azygos vein, and finally into the RA[17]. Type II: Intracardiac (approximately 30%), all PVs drain directly into the RA or through the common trunk of the PVs to the coronary sinus[21,22]. Type III: Infracardiac (approximately 12%), after confluence behind the LA, the PVs pass the diaphragmatic esophageal hiatus through the VV, then flow into the portal vein or its branches[21,22]. Type IV: Mixed (approximately 3%), the PVs enter the RA through multiple channels[21,22].
JAG1 gene mutations are mainly associated with the development of RVOTO, which is a spectrum of diseases associated with the pulmonary valve, branches of the pulmonary artery, and the RV[16]. Thus, stenosis at some level of the pulmonary tree and TOF are the most common causes of cardiac murmur in ALGS patients. However, a number of other types of cardiac murmur have been discovered in ALGS patients. This indicates that JAG1 mutations have a multifaceted impact on cardiac development. TAPVC has not been reported in ALGS patients in any article; however, Sanchez-Lara et al[14] reported three ALGS patients at the 2006 ASHG Annual Meeting[14]. Therefore, at least five ALGS patients and three mutation sites have been found to be associated with TAPVC. We suspect that JAG1 is closely associated with TAPVC.
Biliary stricture is the main feature in most ALGS patients, and unusual structures can cause cholestatic liver disease. The dysfunctional liver often leads to an increase in serum total cholesterol and triglycerides. Thus, hypercholesterolemia is attributable to cholestasis and may finally lead to severe xanthomas[23]. Although some medicines have been reported to improve liver function in ALGS patients, the only way to resolve the problem is liver transplantation.
Bile duct paucity is the main characteristic feature in most cases of ALGS. Here, we report a more fatal and rarer feature, TAPVC, which requires surgical correction at an early age. We also report the unusual finding of severe xanthomas. These findings suggest that JAG1 gene may be a pathogenic gene of TAPVC. Further research should be carried out to prove this hypothesis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: De Raffele E, Italy; Moshref RH, Saudi Arabia S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Oda T, Elkahloun AG, Pike BL, Okajima K, Krantz ID, Genin A, Piccoli DA, Meltzer PS, Spinner NB, Collins FS, Chandrasekharappa SC. Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat Genet. 1997;16:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 765] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 2. | McDaniell R, Warthen DM, Sanchez-Lara PA, Pai A, Krantz ID, Piccoli DA, Spinner NB. NOTCH2 mutations cause Alagille syndrome, a heterogeneous disorder of the notch signaling pathway. Am J Hum Genet. 2006;79:169-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 502] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 3. | Tsai EA, Gilbert MA, Grochowski CM, Underkoffler LA, Meng H, Zhang X, Wang MM, Shitaye H, Hankenson KD, Piccoli D, Lin H, Kamath BM, Devoto M, Spinner NB, Loomes KM. THBS2 Is a Candidate Modifier of Liver Disease Severity in Alagille Syndrome. Cell Mol Gastroenterol Hepatol. 2016;2:663-675.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Alagille D, Odièvre M, Gautier M, Dommergues JP. Hepatic ductular hypoplasia associated with characteristic facies, vertebral malformations, retarded physical, mental, and sexual development, and cardiac murmur. J Pediatr. 1975;86:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 413] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 5. | Alagille D, Estrada A, Hadchouel M, Gautier M, Odièvre M, Dommergues JP. Syndromic paucity of interlobular bile ducts (Alagille syndrome or arteriohepatic dysplasia): review of 80 cases. J Pediatr. 1987;110:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 385] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 6. | Wang JS, Wang XH, Zhu QR, Wang ZL, Hu XQ, Zheng S. Clinical and pathological characteristics of Alagille syndrome in Chinese children. World J Pediatr. 2008;4:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Turnpenny PD, Ellard S. Alagille syndrome: pathogenesis, diagnosis and management. Eur J Hum Genet. 2012;20:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 272] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 8. | Bhatia V, Kumar P. Alagille syndrome with a previously undescribed mutation. Indian Pediatr. 2014;51:314-316. [PubMed] |
| 9. | Jesina D. Alagille Syndrome: An Overview. Neonatal Netw. 2017;36:343-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Warthen DM, Moore EC, Kamath BM, Morrissette JJ, Sanchez-Lara PA, Piccoli DA, Krantz ID, Spinner NB. Jagged1 (JAG1) mutations in Alagille syndrome: increasing the mutation detection rate. Hum Mutat. 2006;27:436-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 136] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 11. | Luxán G, D'Amato G, MacGrogan D, de la Pompa JL. Endocardial Notch Signaling in Cardiac Development and Disease. Circ Res. 2016;118:e1-e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 184] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 12. | Samsa LA, Givens C, Tzima E, Stainier DY, Qian L, Liu J. Cardiac contraction activates endocardial Notch signaling to modulate chamber maturation in zebrafish. Development. 2015;142:4080-4091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 13. | Wu KY, Treece AL, Russo PA, Wen JW. An Atypical Presentation of Alagille Syndrome. Pediatr Dev Pathol. 2018;21:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Sanchez-Lara PA, Krantz ID, Spinner NB. TAPVR: A Cardiac Defect Associated with Alagille syndrome. 2006 ASHG Annual Meeting. [cited 10 October 2021]. Available from: https://www.ashg.org/wp-content/uploads/2020/07/2006-allabstracts.pdf. |
| 15. | Penton AL, Leonard LD, Spinner NB. Notch signaling in human development and disease. Semin Cell Dev Biol. 2012;23:450-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 248] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 16. | McElhinney DB, Krantz ID, Bason L, Piccoli DA, Emerick KM, Spinner NB, Goldmuntz E. Analysis of cardiovascular phenotype and genotype-phenotype correlation in individuals with a JAG1 mutation and/or Alagille syndrome. Circulation. 2002;106:2567-2574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 184] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Kao CC, Hsieh CC, Cheng PJ, Chiang CH, Huang SY. Total Anomalous Pulmonary Venous Connection: From Embryology to a Prenatal Ultrasound Diagnostic Update. J Med Ultrasound. 2017;25:130-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Vanderlaan RD, Caldarone CA. Surgical Approaches to Total Anomalous Pulmonary Venous Connection. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2018;21:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Paladini D, Pistorio A, Wu LH, Meccariello G, Lei T, Tuo G, Donarini G, Marasini M, Xie HN. Prenatal diagnosis of total and partial anomalous pulmonary venous connection: multicenter cohort study and meta-analysis. Ultrasound Obstet Gynecol. 2018;52:24-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Fu CM, Wang JK, Lu CW, Chiu SN, Lin MT, Chen CA, Chang CI, Chen YS, Chiu IS, Wu MH. Total anomalous pulmonary venous connection: 15 years' experience of a tertiary care center in Taiwan. Pediatr Neonatol. 2012;53:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Alam T, Hamidi H, Hoshang MM. Computed tomography features of supracardiac total anomalous pulmonary venous connection in an infant. Radiol Case Rep. 2016;11:134-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Dillman JR, Yarram SG, Hernandez RJ. Imaging of pulmonary venous developmental anomalies. AJR Am J Roentgenol. 2009;192:1272-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 23. | Hannoush ZC, Puerta H, Bauer MS, Goldberg RB. New JAG1 Mutation Causing Alagille Syndrome Presenting With Severe Hypercholesterolemia: Case Report With Emphasis on Genetics and Lipid Abnormalities. J Clin Endocrinol Metab. 2017;102:350-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |