Published online Sep 6, 2022. doi: 10.12998/wjcc.v10.i25.8844
Peer-review started: January 15, 2022
First decision: March 12, 2022
Revised: March 22, 2022
Accepted: July 24, 2022
Article in press: July 24, 2022
Published online: September 6, 2022
Processing time: 223 Days and 4 Hours
Preoperative evaluation of future remnant liver reserves is important for safe hepatectomy. If the remnant is small, preoperative portal vein embolization (PVE) is useful. Liver volume analysis has been the primary method of preoperative evaluation, although functional examination may be more accurate. We have used the functional evaluation liver using the indocyanine green plasma clearance rate (KICG) and 99mTc-galactosyl human serum albumin single-photon emission computed tomography (99mTc-GSA SPECT) for safe hepatectomy.
To analyze the safety of our institution’s system for evaluating the remnant liver reserve.
We retrospectively reviewed the records of 23 patients who underwent preo
All 23 patients underwent planned hepatectomies. Right hepatectomy, right trisectionectomy and left trisectionectomy were in 16, 6 and 1 cases, respectively. The mean of blood loss and operative time were 576 mL and 474 min, respectively. The increased amount of f-rem-KICG was significantly larger than that of a-rem-KICG after PVE (0.034 vs 0.012, P = 0.0273). The not marginal and marginal groups had 17 (73.9%) and 6 (26.1%) patients, respectively. The complications of Clavian-Dindo classification grade II or higher and post-hepatectomy liver failure were observed in six (26.1%) and one (grade A, 4.3%) patient, respectively. The 90-d mortality was zero. The marginal group had no significant difference in postoperative outcomes (prothrombin time/international normalised ratio, total bilirubin, complication, post-hepatectomy liver failure, hospital stay, 90-d, and mortality) compared with the not-marginal group.
Functional evaluation of the remnant liver enabled safe hepatectomy and may extend the indication for hepatectomy after PVE treatment.
Core Tip: Liver volume analysis has been a main examination; however, functional examination may be more accurate. This is a retrospective study to analyze the safety of our functional remnant liver evaluation system utilizing indocyanine green plasma clearance rate and 99mTc-galactosyl human serum albumin single-photon emission computed tomography. In this cohort, post-hepatectomy liver failure was observed in one case and 90-d mortality was zero. The system enables safe hepatectomies and extend the number of cases in which hepatectomy is indicated.
- Citation: Iwaki K, Kaihara S, Kita R, Kitamura K, Hashida H, Uryuhara K. Indocyanine green plasma clearance rate and 99mTc-galactosyl human serum albumin single-photon emission computed tomography evaluated preoperative remnant liver. World J Clin Cases 2022; 10(25): 8844-8853
- URL: https://www.wjgnet.com/2307-8960/full/v10/i25/8844.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i25.8844
Post-hepatectomy liver failure (PHLF) is one of the most severe complications of liver resection. Accurate preoperative evaluation of the future remnant liver reserve is key to preventing PHLF. Although formulas have been established to estimate liver function, only a few measures can predict resectable volume and postoperative outcomes[1]. An anatomical volume remnant indocyanine green (ICG) plasma clearance rate [(a-rem-KICG): The remnant liver anatomical volume rate × KICG)] of > 0.05 is useful criterion[2,3]. Yokoyama et al[3] reported that an a-rem-KICG < 0.05 had a strong impact on postoperative mortality. Remnant liver volume analysis, therefore, has been the primary evaluation tool.
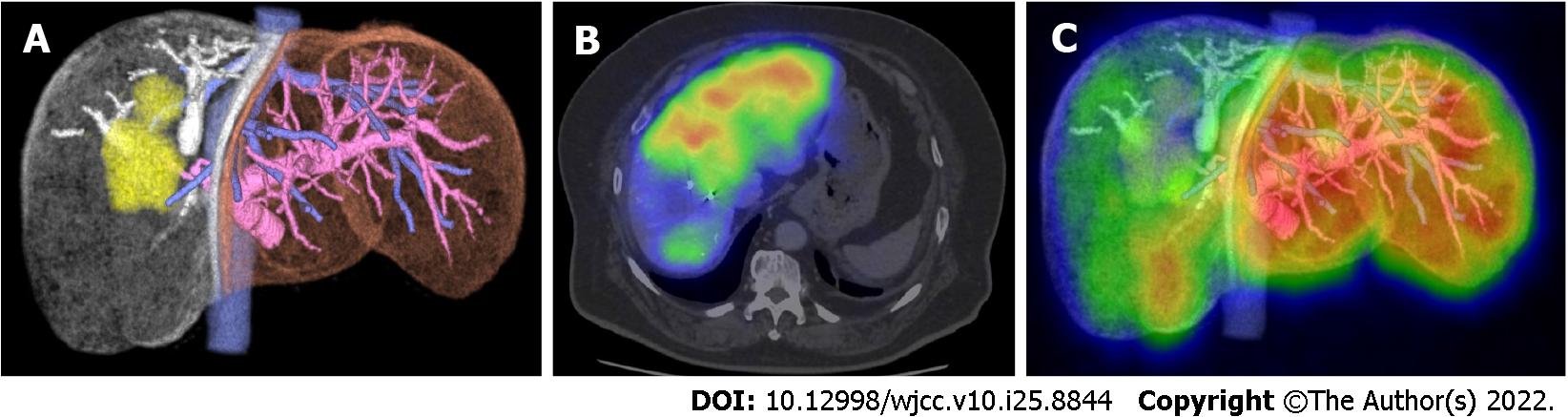
If the remnant liver volume is insufficient, portal vein embolization (PVE) is performed. PVE is an effective method for increasing the size of the remnant liver and can increase the remnant liver volume by approximately 20%. However, in 10%-20% of cases the liver became unresectable due to insufficient remnant liver reserve[2,4,5]. Therefore, an accurate evaluation of the future remnant liver reserve is needed after PVE has been performed. Anatomical liver volume does not always reflect actual liver function[6-10]. The validity of 99mTc-galactosyl human serum albumin single-photon emission computed tomography (99mTc-GSA SPECT) has been reported[11,12]. 99mTc-GSA SPECT uses 99mTc-GSA to detect binding to the asialoglycoprotein receptor in the liver. The amount of this receptor depends on the liver condition; therefore, 99mTc-GSA SPECT can be used to directly estimate functional liver volume by the uptake ratio of the remnant and whole liver[13]. Recent studies have shown that functional liver volume is a more accurate predictor of future functional liver volume than anatomical volume[6,8,10,11,14]. We speculated that 99mTc-GSA SPECT could be used to evaluate remnant liver reserve more accurately, especially for cases in which patients had undergone PVE. Since hepatectomy is the most effective treatment for various liver tumors, improving the accuracy of estimating future liver function among patients who had undergone PVE might increase the number of cases in which hepatectomy is indicated.
In our institution, we have used the remnant liver functional volume for preoperative assessment since 2004. The evaluation system used defines two kinds of remnant liver KICG: A-rem-KICG, as defined above, and functional volume remnant KICG (f-rem-KICG). The f-rem-KICG is the remnant liver functional volume rate based on 99mTc-GSA SPECT × KICG. If either of the two-remnant liver KICG values is > 0.05, hepatectomy is performed. In this study, we analyzed the validity of our remnant liver evaluation system for PVE cases.
In total, 150 patients underwent 99mTc-GSA SPECT and hepatectomy at Kobe City Medical Center General Hospital from 2004 to 2019. Within this cohort, 23 patients who underwent PVE were enrolled in this study. PHLF and postoperative complications were categorized according to the International Study Group of Liver Surgery definition and Clavian-Dindo classification system[15,16].
Informed consent was obtained, and this study was conducted in accordance with the Declaration of Helsinki following approval from the institutional review board of Kobe City Medical Center General Hospital (approval number: Zn191007).
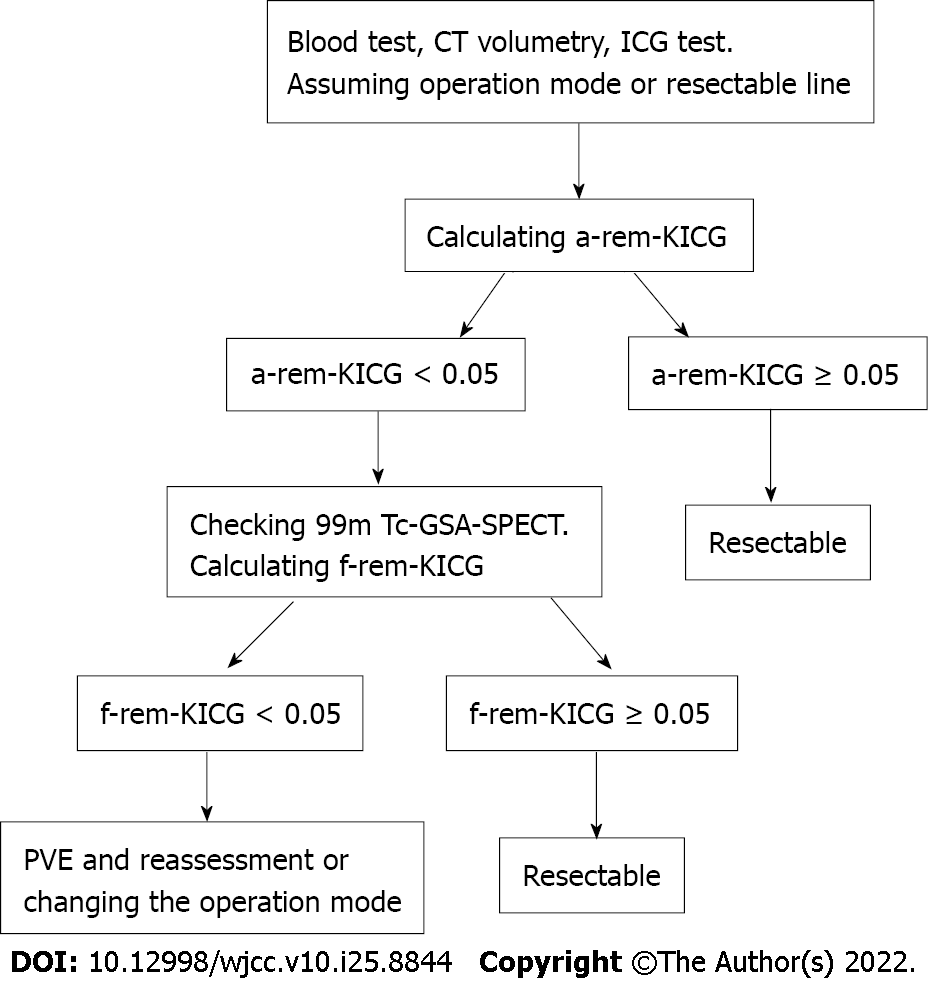
Blood tests, enhanced computed tomography, and ICG tests were performed routinely. The ICG test was performed by intravenously injecting ICG (0.5 mg/kg of body weight). Blood samples were collected at 0, 5, 10, and 15 min after injection, following which the KICG was analyzed. The a-rem-KICG was calculated using SYNAPSE VINCENT® (Fuji film, Tokyo, Japan) with the formula: Remnant liver anatomical volume rate × KICG. Then, 99mTc-GSA SPECT was performed, and the remnant liver functional volume rate was calculated using remnant liver uptake of 99mTc-GSA/whole liver uptake. Finally, the f-rem-KICG was calculated using the formula: Remnant liver functional volume rate × KICG. If either the a-rem-KICG or f-rem-KICG was > 0.05, planned liver resection was performed. Figure 1 shows how to calculate rem-KICG. If a patient presented with obstructive jaundice, endoscopic retrograde biliary drainage was performed. After confirming that the serum bilirubin level had decreased to within the normal range, an ICG test was performed. When both the a-rem-KICG and f-rem-KICG were < 0.05, PVE was performed. ICG, CT, and 99mTc-GSA SPECT examinations were conducted again 1 mo after the PVE procedure, and the post-PVE a-rem-KICG and f-rem-KICG were calculated. If either rate was > 0.05, planned liver resection was performed.
First, the perioperative factors and changes in the remnant liver KICGs after PVE were reviewed. Second, we defined the marginal group to be those patients with an a-rem-KICG of < 0.05 and a f-rem-KICG of > 0.05, and the not-marginal group to be those patients with a-rem-KICG and f-rem-KICG of > 0.05. We then compared the postoperative outcomes between the marginal and not-marginal groups to evaluate the safety of hepatectomy for the marginal group.
Continuous values are presented as mean ± SD. Statistical analyses of the data were conducted using the JMP Pro13 software (SAS Institute, Cary, NC, United States). Student’s t-test, chi-squared test, and regression analysis were used as appropriate. Statistical significance was set at P < 0.05.
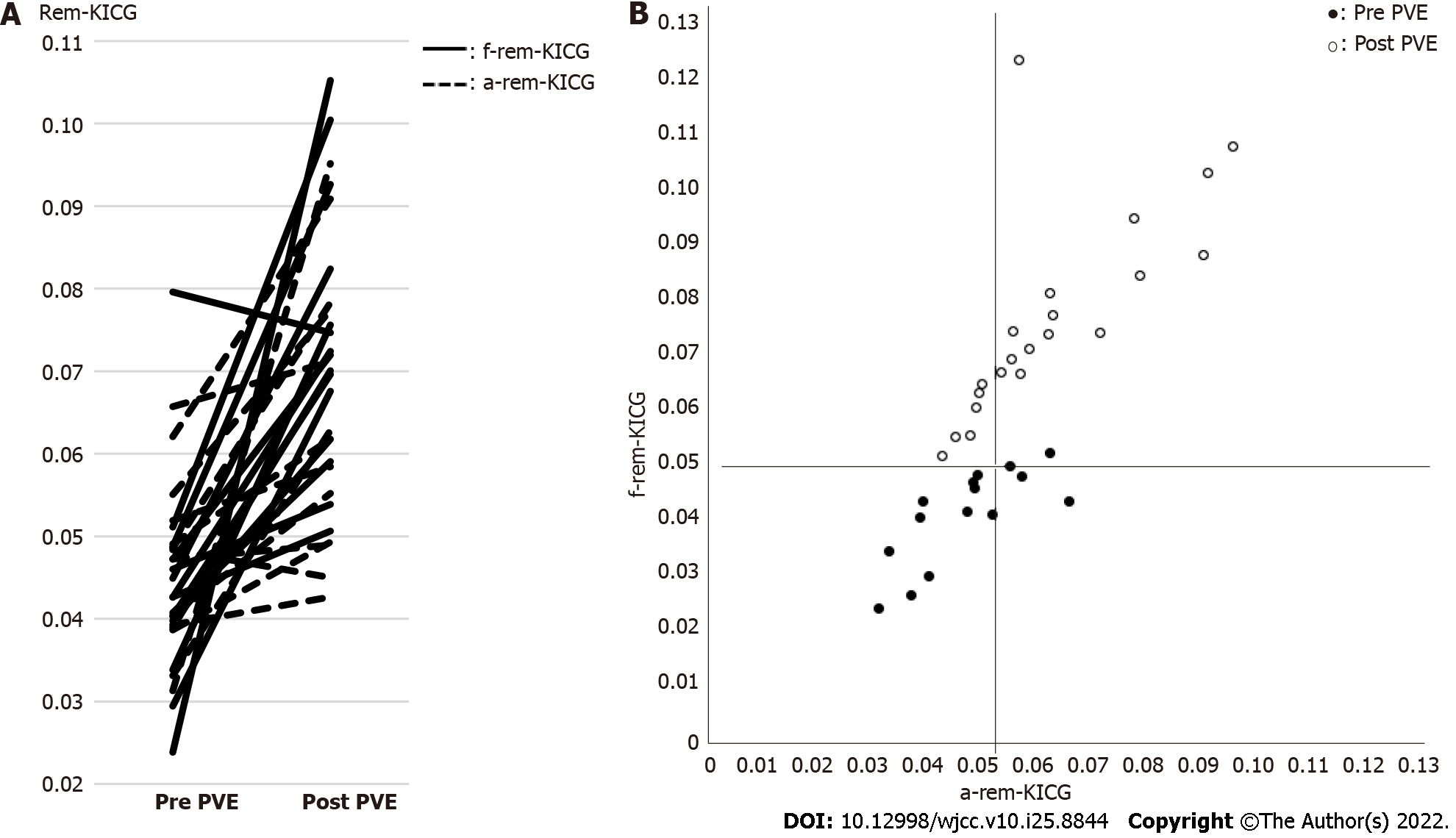
The characteristics of the 23 patients included for the analysis are shown in Table 1. Figure 2A shows the changes in the a-rem-KICG and f-rem-KICGs between pre- and post-PVE. The increase in the amount of f-rem-KICG was significantly larger than that of a-rem-KICG (0.034 vs 0.012, P = 0.0273). Figure 2B shows the scatter plots of a-rem-KICG and f-rem-KICG before and after PVE. A significant correlation was observed between a-rem-KICG and f-rem-KICG (P = 0.0002, R2 = 0.5156).
| Characteristics | Numerical value |
| Number of patients | 23 |
| Age (yr), mean ± SD | 64 ± 13.8 |
| Sex (male/female) | 18/5 |
| Desease | |
| HCC | 9 |
| Bile duct cancer | 9 |
| Liver metastasis | 4 |
| Others | 1 |
| Preoperative factor | |
| Albumin (mean ± SD) | 3.8 ± 0.5 |
| Total bilirubin (mean ± SD) | 0.7 ± 0.4 |
| PT-INR (mean ± SD) | 1.3 ± 0.2 |
| Plt (mean ± SD) | 23 ± 6 |
| Liver damage (A/B) | 20/3 |
| Child-Pugh score (5/6/7) | 16/6/1 |
| ICG-R15 (mean ± SD) | 10.6 ± 4.6 |
| KICG (mean ± SD) | 0.153 ± 0.030 |
Perioperative outcomes are shown in Table 2. The mean discrepancy between the two-remnant liver KICGs (post-PVE f-rem-KICG minus a-rem-KICG) was 0.012 (± 0.013). All 23 patients underwent planned hepatectomies. Right hepatectomy, right trisectionectomy and left trisectionectomy were in 16, 6 and 1 cases, respectively. Pancreaticoduodenectomy and bile duct reconstruction were combined in 2 and 9 cases. The mean of blood loss and operative time were 576 mL (± 426) and 474 min (± 156). Postoperative complications over grade II according to the Clavien-Dindo classification were in 6 cases (26.1%) as follows: Abdominal abscess (3 cases), refractory ascites (1 case), anastomosis leakage (1 case), and pancreatic fistula (1 case). PHLF over grade A was in 1 case (grade A, 4.3%). The 90-d mortality was zero.
| Outcome | Numerical value |
| Remnant liver KICG | |
| Pre PVE a-rem-KICG (mean ± SD) | 0.044 ± 0.010 |
| Pre PVE f-rem-KICG (mean ± SD) | 0.042 ± 0.012 |
| Post PVE a-rem-KICG (mean ± SD) | 0.062 ± 0.016 |
| Post PVE f-rem-KICG (mean ± SD) | 0.075 ± 0.017 |
| The increased amount of a-rem-KICG (mean ± SD) | 0.018 ± 0.015 |
| The increased amount of f-rem-KICG (mean ± SD) | 0.034 ± 0.022 |
| Post PVE f-rem-KICG minus a-rem-KICG (mean ± SD) | 0.012 ± 0.013 |
| Operative parameters | |
| Operative procedure | |
| Right hepatectomy | 16 |
| Right trisectionectomy | 6 |
| Left trisectionectomy | 1 |
| Combined resection | |
| Pancreaticoduodenectomy | 2 |
| Bile duct reconstruction | 9 |
| Resection volume (mL), mean ± SD | 775 ± 237 |
| Blood loss (mL), mean ± SD | 576 ± 426 |
| Operative time (min), mean ± SD | 474 ± 156 |
| Postoperative outcomes | |
| Complication (> Clavian-Dindo grade II) | 6, 26.1% |
| PHLF | 1, 4.3% |
| Hospital stay (d), mean ± SD | 23 ± 28 |
| 90-d mortality | 0 |
After PVE, the marginal group comprised six (26%) patients (Table 3). Right hemi hepatectomy, right trisectionectomy, and left trisectionectomy were performed in three, two, and one patient(s), respectively. The mean blood loss and operative time in the marginal group were 753 mL (± 220) and 586 min (± 170), respectively. Postoperative complications that exceeded grade II were observed in three patients, and all were grade IIIa: Pancreatic fistula, abdominal abscess, and ascites. The 90-d mortality for the marginal group was zero. Table 4 shows the univariate analysis of postoperative outcomes in the marginal and not-marginal groups. The postoperative maximum prothrombin time international normalized ratio, total bilirubin, complication rate, PHLF, hospital stay, and 90-d mortality were not significantly different. In particular, PHLF was not observed in the marginal group.
| Case number | Age | Diagnosis | Post-PVE a-rem-KICG | Post-PVE f-rem-KICG | Operative procedure | Operative time (min) | Blood loss (mL) | Postoperative complications | PHLF | Hospital stay (d) |
| 1 | 59 | CCA | 0.049 | 0.062 | Right trisectionectomy | 567 | 654 | None | None | 9 |
| 2 | 64 | CCA | 0.049 | 0.059 | HPD (right hemihepatectomy) | 879 | 924 | Grade IIIa, pancreatic fistula | None | 39 |
| 3 | 73 | CCA | 0.045 | 0.054 | Right trisectionectomy | 636 | 718 | Grade IIIb, abdominal abscess | None | 24 |
| 4 | 76 | HCC | 0.043 | 0.051 | Right hemi hepatectomy | 380 | 766 | None | None | 8 |
| 5 | 76 | CCA | 0.048 | 0.054 | Left trisectionectomy | 588 | 1044 | Grade IIIa, ascites | None | 128 |
| 6 | 70 | CRLM | 0.049 | 0.063 | Right hemi hepatectomy | 470 | 412 | None | None | 14 |
Liver resection is widely accepted to be the best hope for various liver cancers, although an insufficient liver reserve may result in PHLF, a severe complication. Expanding the number of cases in which hepatectomy is indicated while ensuring safe surgical treatment would therefore be beneficial. The indications for resection and operative procedures are often limited by future remnant liver reserves; however, the resectable volume is still unknown. Especially for PVE cases, preoperative evaluation of future remnant liver must be done carefully. Although PVE can increase the remnant liver volume by approximately 20%[4,5], 20% of patients are unresectable due to poor hypertrophy[2,4,5]. Remnant liver evaluation after PVE is the important turning point to cure or not.
The remnant liver anatomical volume is regarded as the most important factor in the preoperative evaluation of the remnant liver. Nagino et al[2] established the criteria for an a-rem-KICG of > 0.05, while Yokoyama et al[3] reported that an a-rem-KICG of < 0.05 is useful for predicting mortality and morbidity. This index has been adopted by many institutions. 99mTc-GSA SPECT can assess the remnant liver reserve more accurately than the conventional anatomical volume[6,9-12]. Because hepatocyte function is decreased by biliary stenosis, chronic inflammation, vascular invasion, and compression of the tumor, a discrepancy between morphological volume and actual functional volume occurs[17,18]. 99mTc-GSA SPECT can directly detect functioning cells and actual functional volume through asialoglycoprotein receptors in the liver[13]. Some studies have reported the advantage of 99mTc-GSA SPECT in detecting an improvement in remnant liver reserve after PVE[10,11]. In addition, the functional increase after PVE was greater than the morphological increase. PHLF is dependent on functional increases[6,10]. In this study, f-rem-KICG was larger than a-rem-KICG, with a mean difference of 0.012. The increase in f-rem-KICG was statistically larger than that of a-rem-KICG, which agrees with the results of recent studies. Based on these two KICG evaluations, we could perform the hepatectomies without severe PHLF occurring.
In this study, we performed hepatectomies on the six (26%) patients in the marginal group who were not indicated for hepatectomy according to the anatomical evaluation only. Their postoperative liver function was good, and PHLF was not observed in these patients. Univariate analysis of postoperative outcomes showed no significant difference in every postoperative outcome. Based on these results, f-rem-KICG may reflect a more accurate remnant liver reserve than a-rem-KICG. Previous studies have supported our results. Hayashi et al[10] reported that the functional volume assessment by 99mTc-GSA SPECT was able to detect cases from the lack of remnant liver volume group that could be operated safely.
This study has some limitations. First, severe PHLF was not observed, suggesting that the safety margin for f-rem-KICG might have been excessive. Further studies may be able to reduce the cutoff value of f-rem-KICG. Second, because no patients had PHLF, no significant relationship between PHLF and f-rem-KICG was observed; however, some studies have shown a significant relationship between PHLF and uptake of 99mTc-GSA SPECT[6,10]. Third, this was a single-center retrospective study, and the number of patients was small. Fourth, 99mTc-GSA SPECT can only be performed at a few institutions. Finally, because of the significant correlation between a-rem-KICG and f-rem-KICG found in this study, both evaluations may not need to be conducted in all patients. Checking the f-rem-KICG is recommended if a-rem-KICG < 0.05 or PVE cases. Figure 3 shows the new decision tree used in our institution for evaluating future functioning of liver remnants.
Preoperative remnant liver functional volume evaluation (f-rem-KICG > 0.05) using the ICG test and 99mTc-GSA SPECT enables safe hepatectomy for patients who have undergone PVE. This index can safely extend the number of cases in which hepatectomy is indicated.
Liver volume analysis has been the primary method of preoperative evaluation, although some studies reported that functional examination may be more accurate.
In our institution, we have used the remnant liver functional volume for preoperative assessment since 2004. We analyzed the validity of our remnant liver evaluation system.
In total, 150 patients underwent 99mTc galactosyl human serum albumin single-photon emission computed tomography and hepatectomy at our institution from 2004 to 2019. Within this cohort, 23 patients who underwent preoperative portal vein embolization (PVE) were enrolled.
First, the perioperative factors and changes in the remnant liver indocyanine green plasma clearance rate (KICG) after PVE were reviewed. Second, we defined the marginal group and the not-marginal group. We then compared the postoperative outcomes between the marginal and not-marginal groups to evaluate the safety of hepatectomy for the marginal group.
All 23 patients underwent planned hepatectomies. Right hepatectomy, right trisectionectomy, and left trisectionectomy were performed in 16, 6, and 1 case, respectively. The increased amount of remnant functional KICG was significantly larger than that of remnant anatomical KICG after PVE (0.034 vs 0.012, P = 0.0273). The not-marginal and marginal groups comprised 17 (73.9%) and 6 (26.1%) patients, respectively. The complications of Clavian-Dindo classification grade II or higher and post-hepatectomy liver failure were observed in six (26.1%) and one (grade A, 4.3%) patient, respectively. The 90-d mortality was zero. The postoperative outcomes were not significantly different between the marginal and not-marginal groups.
Functional evaluation of the remnant liver enabled safe hepatectomy and may extend the indication for hepatectomy after PVE.
We consider to increase the sample size and investigate appropriate remnant liver functional KICG cutoff values.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Cassese G, Italy; Gupta R, India; Kordzaia D, Georgia; Qiu X, China; Zheng SM, China S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Hoekstra LT, de Graaf W, Nibourg GA, Heger M, Bennink RJ, Stieger B, van Gulik TM. Physiological and biochemical basis of clinical liver function tests: a review. Ann Surg. 2013;257:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 250] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 2. | Nagino M, Kamiya J, Nishio H, Ebata T, Arai T, Nimura Y. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg. 2006;243:364-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 404] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 3. | Yokoyama Y, Nishio H, Ebata T, Igami T, Sugawara G, Nagino M. Value of indocyanine green clearance of the future liver remnant in predicting outcome after resection for biliary cancer. Br J Surg. 2010;97:1260-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 187] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 4. | Vyas S, Markar S, Partelli S, Fotheringham T, Low D, Imber C, Malago M, Kocher HM. Portal vein embolization and ligation for extended hepatectomy. Indian J Surg Oncol. 2014;5:30-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Kasai Y, Hatano E, Iguchi K, Seo S, Taura K, Yasuchika K, Mori A, Kaido T, Tanaka S, Shibata T, Uemoto S. Prediction of the remnant liver hypertrophy ratio after preoperative portal vein embolization. Eur Surg Res. 2013;51:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Hirai I, Kimura W, Fuse A, Suto K, Urayama M. Evaluation of preoperative portal embolization for safe hepatectomy, with special reference to assessment of nonembolized lobe function with 99mTc-GSA SPECT scintigraphy. Surgery. 2003;133:495-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Sugahara K, Togashi H, Takahashi K, Onodera Y, Sanjo M, Misawa K, Suzuki A, Adachi T, Ito J, Okumoto K, Hattori E, Takeda T, Watanabe H, Saito K, Saito T, Sugai Y, Kawata S. Separate analysis of asialoglycoprotein receptors in the right and left hepatic lobes using Tc-GSA SPECT. Hepatology. 2003;38:1401-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Sumiyoshi T, Shima Y, Tokorodani R, Okabayashi T, Kozuki A, Hata Y, Noda Y, Murata Y, Nakamura T, Uka K. CT/99mTc-GSA SPECT fusion images demonstrate functional differences between the liver lobes. World J Gastroenterol. 2013;19:3217-3225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Sumiyoshi T, Shima Y, Okabayashi T, Noda Y, Hata Y, Murata Y, Kozuki A, Tokumaru T, Nakamura T, Uka K. Functional discrepancy between two liver lobes after hemilobe biliary drainage in patients with jaundice and bile duct cancer: an appraisal using (99m)Tc-GSA SPECT/CT fusion imaging. Radiology. 2014;273:444-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Hayashi H, Beppu T, Okabe H, Kuroki H, Nakagawa S, Imai K, Nitta H, Chikamoto A, Ishiko T, Baba H. Functional assessment versus conventional volumetric assessment in the prediction of operative outcomes after major hepatectomy. Surgery. 2015;157:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Sumiyoshi T, Okabayashi T, Negoro Y, Hata Y, Noda Y, Sui K, Iwata J, Matsumoto M. 99mTc-GSA SPECT/CT fusion imaging for hepatectomy candidates with extremely deteriorated ICG value. Jpn J Radiol. 2018;36:537-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Mao Y, Du S, Ba J, Li F, Yang H, Lu X, Sang X, Li S, Che L, Tong J, Xu Y, Xu H, Zhao H, Chi T, Liu F, Du Y, Zhang X, Wang X, Dong J, Zhong S, Huang J, Yu Y, Wang J. Using Dynamic 99mT c-GSA SPECT/CT fusion images for hepatectomy planning and postoperative liver failure prediction. Ann Surg Oncol. 2015;22:1301-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Yamakado K, Matsumura K, Takashiba Y, Nakatsuka A, Kitano T, Ichihara T, Maeda H, Takase K, Takeda K. Binding rate constant of Tc-99m DTPA galactosyl human serum albumin measured by quantitative dynamic SPECT--clinical evaluation as a total and regional liver function test. Ann Nucl Med. 2001;15:191-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Taniguchi M, Okizaki A, Watanabe K, Imai K, Uchida K, Einama T, Shuke N, Miyokawa N, Furukawa H. Hepatic clearance measured with (99m)Tc-GSA single-photon emission computed tomography to estimate liver fibrosis. World J Gastroenterol. 2014;20:16714-16720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C, Banting S, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey JN, Greig P, Rees M, Yokoyama Y, Fan ST, Nimura Y, Figueras J, Capussotti L, Büchler MW, Weitz J. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1224] [Cited by in RCA: 1725] [Article Influence: 123.2] [Reference Citation Analysis (0)] |
| 16. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6210] [Cited by in RCA: 8618] [Article Influence: 538.6] [Reference Citation Analysis (0)] |
| 17. | Akaki S, Kanazawa S, Tsunoda M, Okumura Y, Togami I, Kuroda M, Takeda Y, Hiraki Y. Nontumorous decrease in Tc-99m GSA accumulation. Ann Nucl Med. 2000;14:477-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Imaeda T, Kanematsu M, Asada S, Seki M, Doi H, Saji S. Utility of Tc-99m GSA SPECT imaging in estimation of functional volume of liver segments in health and liver diseases. Clin Nucl Med. 1995;20:322-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |