Published online Aug 26, 2022. doi: 10.12998/wjcc.v10.i24.8535
Peer-review started: March 23, 2022
First decision: May 30, 2022
Revised: June 9, 2022
Accepted: July 16, 2022
Article in press: July 16, 2022
Published online: August 26, 2022
Processing time: 145 Days and 13.1 Hours
Post-transarterial chemoembolization (TACE) liver failure occurs frequently in hepatocellular carcinoma (HCC) patients. The identification of predictors for post-TACE liver failure is of great importance for clinical decision-making in this population.
To investigate the occurrence rate and predictive factors of post-TACE liver failure in this retrospective study to provide clues for decision-making regarding TACE procedures in HCC patients.
The clinical records of HCC patients treated with TACE therapy were reviewed. Baseline clinical characteristics and laboratory parameters of these patients were extracted. Logistic models were used to identify candidates to predict post-TACE liver failure.
A total of 199 HCC patients were enrolled in this study, and 70 patients (35.2%) developed post-TACE liver failure. Univariate and multivariate logistic models indicated that microspheres plus gelatin embolization and main tumor size > 5 cm were risk predictors for post-TACE liver failure [odds ratio (OR): 4.4, 95% confidence interval (CI): 1.2-16.3, P = 0.027; OR: 2.3, 95%CI: 1.05-5.3, P = 0.039, respectively]. Conversely, HCC patients who underwent tumor resection surgery before the TACE procedure had a lower risk for post-TACE liver failure (OR: 0.4, 95%CI: 0.2-0.95, P = 0.039).
Microspheres plus gelatin embolization and main tumor size might be risk factors for post-TACE liver failure in HCC patients, while prior tumor resection could be a favorable factor reducing the risk of post-TACE liver failure.
Core Tip: Post-transarterial chemoembolization (TACE) liver failure occurs frequently in hepatocellular carcinoma (HCC) patients. Unfortunately, the incidence and risk factors for post-TACE liver failure are inconsistent worldwide. This study addressed the occurrence rate and potential risk factors for post-TACE liver failure according to a single-center retrospective report. The results of this study should attract the attention of relevant medical practitioners and provide predictive clues for the precise interventional treatment of HCC patients.
- Citation: Yuan M, Chen TY, Chen XR, Lu YF, Shi J, Zhang WS, Ye C, Tang BZ, Yang ZG. Identification of predictive factors for post-transarterial chemoembolization liver failure in hepatocellular carcinoma patients: A retrospective study. World J Clin Cases 2022; 10(24): 8535-8546
- URL: https://www.wjgnet.com/2307-8960/full/v10/i24/8535.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i24.8535
Hepatocellular carcinoma (HCC) is predicted to be one of the most lethal cancers worldwide[1,2]. According to the Surveillance, Epidemiology, and End Results (SEER) registration agency project research, the incidence of HCC will continue to increase by 2030[1]. In addition, the annual mortality rate associated with liver cancer has increased significantly in the past two decades[1], and the survival of HCC patients with intermediate-advanced tumor stages has progressively decreased[1,3,4]. Currently, transarterial chemoembolization (TACE) is recommended as a first-line treatment strategy for patients with unresectable HCC[1,5]. Although the benefits of the TACE procedure have been demonstrated[1,6], there is still a lack of reliable evidence showing that TACE has a clear superiority over bland embolization. Even worse, the incidence of severe adverse events significantly increased after TACE[7]. Thus, post-TACE liver failure that most commonly causes death after TACE should not be ignored[5].
Post-TACE liver failure is one of the most lethal complications in HCC patients. In South Korea, 12% to 15% of patients treated with TACE developed acute liver failure within 14 d[8,9]. A prospective study in Hong Kong showed that the incidence of liver failure after TACE was approximately 20%[10]. In India, the incidence of post-TACE liver failure was 23.8% to 28.8% in HCC patients[11,12]. In a randomized trial in Europe, the results illustrated that approximately 60% of patients had liver failure after TACE more than once[13]. According to the results of the meta-analysis, 7.5% (range 0-48.6%) of HCC patients developed liver failure after TACE. The mortality rate associated with TACE treatment is 2.4% (0-9.5%), which is mainly due to liver failure after TACE[9]. In the first year after TACE treatment, more than 90% of post-TACE liver failure cases died. Therefore, liver failure after TACE is an independent risk factor for the lower survival rate of liver cancer patients[8]. Hence, identifying the risk factors that can predict the occurrence of post-TACE liver failure in HCC patients is of great importance[8].
This retrospective study aimed to assess the potential clinical characteristics and laboratory parameters that could be predictors for post-TACE liver failure, in the hope that our findings might be helpful for the early detection and early intervention of post-TACE liver failure in HCC patients.
The study protocol was reviewed and approved by the Ethics Committee, Shanghai Public Health Clinical Center, Fudan University (approval No. 2021-S062-01). Written informed consent was waived for this retrospective study.
The diagnosis of HCC was determined by pathology or according to radiological standards, according to the "Guidelines for the Diagnosis and Treatment of Primary Liver Cancer in China (2019 edition)”. Two imaging approaches, including computed tomography (CT) or magnetic resonance imaging (MRI) to show arterial enhancement quality or an imaging study (CT or MRI) showing arterial enhancement quality and alpha-fetoprotein (AFP) level greater than 400 ng/mL were used[14]. HCC patients undergoing TACE as part of standard therapy between January 2019 and May 2020 in Shanghai Public Health Clinical Center, Fudan University, were included. The inclusion criteria were age ≥ 18 years and Child-Turcotte-Pugh (CTP) stage A and B. HCC patients who were pregnant or CTP stage C or had uncontrolled encephalopathy, underlying kidney failure, acute coronary syndromes or valvular heart diseases were excluded from our study.
The China Liver Cancer Staging was used to determine the necessity for the TACE procedure[14]. All HCC patients fasted overnight. The femoral artery was catheterized with a 5F sheath under local anesthesia. A thorough angiographic examination depicting the anatomy of the hepatic artery, tumor blush, feeding arteries, and arteriovenous shunts was performed. Contrast-enhanced CT or MRI and indirect portography were performed during angiography to ensure stable flow in the portal vein. A microcatheter for the injection of chemotherapeutic drugs and embolic agents was placed selectively in the segment arteries or superselectively in the tumor supplying arteries, which feed the HCC lesions. Two types of microspheres, 300-500 μm and 500-700 μm, were used. Combined embolization of microspheres and gelatin sponge particles was applied for patients with larger tumor sizes (diameter > 5 cm). The volume of lipiodol ranged from 4 to 30 mL, pirarubicin ranged from 0 to 50 mg, and lobaplatin ranged from 0 to 200 mg. After confirming the correct position of the catheter tip, the chemotherapeutic and embolic agents were infused under radiographic guidance. To control the correct administration of drugs and the occlusion of tumor vessels with flow stasis, a final angiography was performed. TACE combined with radiofrequency ablation (RFA) treatment were applied for HCC patients with a single nodule > 3 cm and ≤ 5 cm or those with 2-3 nodules ≤ 3 cm.
As in previous reports[8,10,11], post-TACE liver failure in our study was modified and defined as the presence of any of the following conditions within one week after TACE: Increase in total bilirubin ≥ 17.1 μmol/L, increase in prothrombin time ≥ 3 s, new onset hepatic encephalopathy, and increase in ascites.
All patients underwent blood examinations including routine blood tests, liver and kidney function tests, coagulation function tests, serum tumor markers, HBsAg, HBeAg, hepatitis B virus (HBV) DNA and anti-hepatitis C virus (HCV) antibody, prior to the procedure. HCV RNA was measured if a positive anti-HCV antibody was detected. Post-TACE liver function tests and coagulation function tests were conducted every 3 d within the first week after TACE. The serum samples were collected, transported and tested following the standard operating procedures of the Department of Medical Laboratory, Shanghai Public Health Clinical Center.
The assessment of post-TACE liver failure was performed at 7 d or earlier. Abdominal CT/MRI and chest CT were also performed prior to TACE procedures to assess the clinicopathological characteristics including main tumor size, tumor number, cirrhosis status, metastasis, portal vein tumor thrombus, vascular invasion, ascites and pleural effusion. Other medical information, including disease history and treatment history, was also collected. All TACE procedures were assessed separately.
Based on the variable types, Student's t test and chi-square test were used to analyze the differences in variables between groups. The parameters related to the results were evaluated by univariate and multivariate logistic regression. The results are reported as odds ratios (OR) with 95% confidence intervals (CI). Parameters significantly associated with the outcomes in the multivariate logistic model were included in the risk prediction model by nomogram with the “rms” package in the R software program. A calibration plot was presented to evaluate the performance of the nomogram, which was also established in the “rms” package in the R program. The area under the receiver operating characteristic curve (AUROC) was computed to assess the prediction efficiency of the latent predictors. Stata software version 16.0 (Stata Corp LLC, Texas, United States) was used. A two-sided P < 0.05 was considered significant.
In total, 199 HCC patients who received TACE therapy were included in this study, and 70 patients (35.2%) developed post-TACE liver failure. As summarized in Table 1, more patients in the post-TACE liver failure group received microspheres plus gelatin embolization than in the nonpost-TACE liver failure group (24.3% vs 3.9%, P < 0.001, Table 1). The frequency of patients with a main tumor size ≥ 5 cm was significantly higher in the post-TACE liver failure group than in the nonpost-TACE liver failure group (58.6% vs 40.3%, P = 0.014, Table 1). More patients received combination therapy with RFA and had a resection surgery treatment history in the nonpost-TACE liver failure group than in the post-TACE liver failure group (P = 0.025 and P = 0.032, respectively, Table 1). Patients who developed post-TACE liver failure had significantly higher hematocrit, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBiL) and direct bilirubin (DBiL) levels than those without post-TACE liver failure (all P < 0.05, Table 1). However, patients who developed post-TACE liver failure had significantly lower serum cystatin C and creatinine levels and higher estimated glomerular filtration rate (eGFR) levels than those without post-TACE liver failure (all P < 0.05, Table 1). The other baseline clinical characteristics and laboratory parameters were not significantly distributed between these two groups (all P > 0.05, Table 1).
| Variables | Total (n = 199) | None post-TACE liver failure (n = 129) | Post-TACE liver failure (n = 70) | P value |
| Age, yr, mean ± SD | 59.4 ± 10.9 | 59.7 ± 11.1 | 58.9 ± 10.7 | 0.59 |
| Male, n (%) | 157 (78.9) | 105 (81.4) | 52 (74.3) | 0.24 |
| Metastasis, n (%) | ||||
| Renal/adrenal | 5 (2.5) | 5 (3.9) | 0 (0) | 0.095 |
| Abdomen/pelvis | 6 (3.0) | 4 (3.1) | 2 (2.9) | 0.92 |
| Lymphnode | 5 (2.5) | 3 (2.3) | 2 (2.9) | 0.82 |
| Bone | 8 (4.0) | 7 (5.4) | 1 (1.4) | 0.17 |
| Lung | 10 (5.0) | 5 (3.9) | 5 (7.1) | 0.31 |
| Intrahepatic | 11 (5.5) | 5 (3.9) | 6 (8.6) | 0.17 |
| Pleural effusion, n (%) | 21 (10.6) | 15 (11.6) | 6 (8.6) | 0.50 |
| Ascites, n (%) | 59 (29.6) | 38 (29.5) | 21 (30.0) | 0.94 |
| PVTT, n (%) | 56 (28.1) | 33 (25.6) | 23 (32.9) | 0.28 |
| Vascular invasion, n (%) | 16 (8.0) | 13 (10.1) | 3 (4.3) | 0.15 |
| Tumor number, n (%) | 0.81 | |||
| 1 | 71 (37.8) | 44 (36.1) | 27 (40.9) | |
| 2 | 27 (14.4) | 18 (14.8) | 9 (13.6) | |
| ≥ 3 | 90 (47.9) | 60 (49.2) | 30 (45.5) | |
| Times of TACE prior to inclusion, median (IQR) | 1 (0, 3) | 1 (0, 2) | 1 (0, 3) | 0.82 |
| Embolization, n (%) | ||||
| Microspheres | 55 (27.6) | 33 (25.6) | 22 (31.4) | 0.378 |
| Gelatin | 20 (10.1) | 12 (9.3) | 8 (11.4) | 0.634 |
| Microspheres plus gelatin | 22 (11.1) | 5 (3.9) | 17 (24.3) | < 0.001 |
| Lipiodol, mL, median (IQR) | 10 (5, 10) | 8 (5, 10) | 10 (6, 10) | 0.17 |
| Pirarubicin, mg, n (%) | 0.68 | |||
| 0 | 55 (27.6) | 37 (28.7) | 18 (25.7) | |
| 10 | 36 (18.1) | 25 (19.4) | 11 (15.7) | |
| 20 | 99 (49.7) | 63 (48.8) | 36 (51.4) | |
| 30 | 4 (2.0) | 2 (1.6) | 2 (2.9) | |
| 40 | 4 (2.0) | 2 (1.6) | 2 (2.9) | |
| 50 | 1 (0.5) | 0 (0) | 1 (1.4) | |
| Lobaplatin, mg, n (%) | 0.46 | |||
| 0 | 45 (22.6) | 28 (21.7) | 17 (24.3) | |
| 25 | 1 (0.5) | 1 (0.8) | 0 (0) | |
| 50 | 152 (76.4) | 100 (77.5) | 52 (74.3) | |
| 200 | 1 (0.5) | 0 (0) | 1 (1.4) | |
| Combination with RFA, n (%) | 22 (11.1) | 19 (14.7) | 3 (4.3) | 0.025 |
| Main tumor size ≥ 5cm, n (%) | 93 (46.7) | 52 (40.3) | 41 (58.6) | 0.014 |
| CNLC, n (%) | 0.071 | |||
| I | 93 (46.7) | 66 (51.2) | 27 (38.6) | |
| II | 50 (25.1) | 26 (20.2) | 24 (34.3) | |
| III | 39 (19.6) | 28 (21.7) | 11 (15.7) | |
| NA | 17 (8.5) | 9 (7.0) | 8 (11.4) | |
| Cirrhosis, n (%) | 185 (93.0) | 121 (93.8) | 64 (91.4) | 0.53 |
| CTP score, median (IQR) | 5 (5, 6) | 5 (5, 6) | 6 (5, 6) | 0.438 |
| MELD score, median (IQR) | 29.6 (27.3, 32.2) | 29.7 (27.4, 32.3) | 29.5 (27.1, 32.0) | 0.463 |
| Treatment history, n (%) | ||||
| Sorafenib | 21 (10.6) | 14 (10.9) | 7 (10.0) | 0.85 |
| Resection | 49 (24.6) | 38 (29.5) | 11 (15.7) | 0.032 |
| Radiology | 72 (36.2) | 45 (34.9) | 27 (38.6) | 0.61 |
| Hypertension, n (%) | 43 (21.6) | 27 (20.9) | 16 (22.9) | 0.75 |
| Diabetes, n (%) | 29 (14.6) | 23 (17.8) | 6 (8.6) | 0.077 |
| Blood routine tests, median (IQR) | ||||
| WBC, 103/mm3 | 4.7 (3.5, 6.0) | 4.6 (3.4, 6.1) | 5.0 (3.8, 5.9) | 0.41 |
| RBC, 104/mm3 | 4.1 (3.6, 4.6) | 4.1 (3.6, 4.5) | 4.3 (3.7, 4.6) | 0.13 |
| Hemoglobin, g/L | 127 (112, 141) | 124 (109, 140.5) | 132.5 (116, 144) | 0.069 |
| Hematocrit, % | 37.0 (6.3) | 36.3 (6.6) | 38.3 (5.7) | 0.032 |
| PLT, 103/mm3 | 123.5 (80, 163) | 127 (82.5, 167) | 115 (75, 160) | 0.42 |
| Neutrophils, 103/mm3 | 2.9 (2.0, 3.9) | 2.9 (1.9, 3.8) | 2.9 (2.0, 3.9) | 0.53 |
| Lymphocytes, 103/mm3 | 1.1 (0.8, 1.4) | 1.1 (0.7, 1.4) | 1.1 (0.8, 1.4) | 0.44 |
| Monocytes, 103/mm3 | 0.4 (0.3, 0.6) | 0.4 (0.3, 0.5) | 0.4 (0.3, 0.6) | 0.61 |
| Hypersensitive CRP, mg/L | 3.0 (0.7, 23.5) | 3.6 (0.7, 23.5) | 2.6 (0.6, 23.7) | 0.77 |
| Liver functions, median (IQR) | ||||
| ALT, U/L | 29 (20, 44) | 26 (19, 38) | 37 (25, 54) | 0.002 |
| AST, U/L | 38 (26, 61) | 32 (25, 53) | 45.5 (32, 69) | < 0.001 |
| GGT, U/L | 78 (41, 175) | 63 (40, 156) | 115 (54, 210) | 0.057 |
| AKP, U/L | 137 (97, 197) | 129 (94, 192) | 154.5 (115, 207) | 0.068 |
| TBiL, μmol/L | 19 (13.1, 28.7) | 17.3 (12.2, 25.2) | 21.9 (15.6, 30.9) | 0.010 |
| DBiL, μmol/L | 8.5 (5.9, 12.7) | 7.9 (5.4, 11.5) | 9.9 (6.5, 15.6) | 0.007 |
| TBA, μmol/L | 17.7 (8.2, 40.5) | 15 (7.8, 32.8) | 22.1 (8.5, 44.9) | 0.091 |
| Albumin, g/L | 37.8 (33.7, 42.1) | 37.7 (33.7, 42.2) | 38.2 (34.4, 41.7) | 0.93 |
| Cholinesterase, U/L | 5224 (3698, 6826) | 5056 (3745, 6814) | 5297.5 (3493, 6917) | 0.73 |
| Kidney functions, median (IQR) | ||||
| Serum cystatin C, mg/L | 0.97 (0.8, 1.13) | 0.99 (0.8, 1.19) | 0.89 (0.77, 1.04) | 0.021 |
| Urea, mmol/L | 4.8 (4.0, 5.9) | 5.0 (4.0, 6.17) | 4.47 (4, 5.7) | 0.28 |
| Creatinine, μmol/L | 62.4 (52.7, 74.5) | 64.5 (54.0, 81.2) | 59.7 (51, 68) | 0.025 |
| eGFR, mL/(min∙1.73 m2) | 116.1 (93.6, 137.9) | 111.6 (87.8, 135.7) | 127 (104.8, 140.3) | 0.039 |
| Serum ammonia, median (IQR) | 45 (34, 60) | 43.5 (34, 57) | 48 (35, 62) | 0.27 |
| Coagulation function tests, median (IQR) | ||||
| PTA, % | 84 (73.5, 93) | 83 (73, 92) | 86 (74, 95) | 0.34 |
| Prothrombin time, s | 14.4 (13.8, 15.5) | 14.5 (13.8, 15.5) | 14.3 (13.5, 15.4) | 0.40 |
| INR | 1.11 (1.04, 1.22) | 1.12 (1.05, 1.22) | 1.1 (1.03, 1.21) | 0.31 |
| APTT, s | 39.3 (36.4, 42.1) | 38.9 (36.7, 42) | 39.4 (36.3, 42.9) | 1.00 |
| TT, s | 17.7 (17, 18.7) | 17.6 (16.9, 18.6) | 17.7 (17.2, 18.8) | 0.34 |
| Fibrinogen, g/L | 2.9 (2.4, 3.7) | 2.9 (2.4, 3.7) | 3.0 (2.4, 3.7) | 0.99 |
| Serum tumor markers, median (IQR) | ||||
| CA125, U/L | 21.8 (12.3, 59.1) | 21.8 (12.3, 62.8) | 22.3 (12.9, 54.3) | 0.86 |
| CA153, U/L | 12.9 (9.7, 18.6) | 12.4 (9.0, 18.5) | 13.7 (10.7, 18.7) | 0.40 |
| CA199, U/L | 19 (10.8, 38.5) | 18.5 (10.7, 34.6) | 21.1 (12.5, 41.4) | 0.42 |
| AFP, ng/mL | 26.8 (5.2, 745.1) | 20.3 (5.3, 639.1) | 58.2 (5, 1210) | 0.46 |
| CEA, ng/mL | 2.7 (1.9, 4.2) | 3.1 (2.0, 5.1) | 2.5 (1.8, 3.8) | 0.082 |
Univariate logistic analysis revealed that microspheres plus gelatin embolization, RFA combination, main tumor size, resection history, diabetes, hematocrit, ALT, AST, gamma-glutamyl transferase, TBiL, DBiL, total bile acid, serum cystatin C, creatinine and eGFR levels were all potential predictors for post-TACE liver failure (all P < 0.1, Table 2). When these variables were included in the multivariate model, microspheres plus gelatin embolization and main tumor size > 5 cm were risk factors for the occurrence of post-TACE liver failure (OR: 4.4, 95%CI: 1.2-16.3, P = 0.027 and OR: 2.3, 95%CI: 1.05-5.3, P = 0.039, respectively, Table 2). Conversely, HCC patients who underwent tumor resection surgery before the TACE procedure had a lower risk for post-TACE liver failure (OR: 0.4, 95%CI: 0.2-0.95, P = 0.039, Table 2).
| Variables | Univariate | P value | Multivariate | P value | ||
| OR | 95%CI | OR | 95%CI | |||
| Microsphere plus gelatin embolization, yes vs no | 8.0 | 2.8-22.7 | < 0.001 | 4.4 | 1.2-16.3 | 0.027 |
| Combination with RFA, yes vs no | 0.3 | 0.1-0.9 | 0.035 | 0.3 | 0.1-1.2 | 0.094 |
| Main tumor size ≥ 5 cm, yes vs no | 2.1 | 1.2-3.8 | 0.014 | 2.3 | 1.05-5.3 | 0.039 |
| Resection history, yes vs no | 0.4 | 0.2-0.9 | 0.034 | 0.4 | 0.2-0.95 | 0.039 |
| Diabetes, yes vs no | 0.4 | 0.2-1.1 | 0.084 | 0.3 | 0.1-1.03 | 0.056 |
| Hematocrit, per increase 1% | 1.1 | 1.0-1.1 | 0.034 | 1.1 | 0.997-1.1 | 0.06 |
| ALT, per increase 1 U/L | 1.01 | 1.0-1.03 | 0.026 | 1.01 | 0.99-1.03 | 0.262 |
| AST, per increase 1 U/L | 1.0 | 1.0-1.01 | 0.074 | 0.99 | 0.98-1.004 | 0.177 |
| GGT, per increase 1 U/L | 1.0 | 1.0-1.0 | 0.08 | 1.0 | 1.0-1.0 | 0.499 |
| TBiL, μmol/L | ||||||
| Normal | Reference | - | 1.0 | Reference | - | 1.0 |
| 1-2 ULN | 1.8 | 1.0-3.5 | 0.065 | 1.8 | 0.8-4.2 | 0.163 |
| ≥ 2 ULN | 2.3 | 1.0-5.3 | 0.062 | 2.1 | 0.6-7.5 | 0.269 |
| DBiL, per increase 1 μmol/L | 1.8 | 1.0-3.2 | 0.054 | 0.99 | 0.96-1.02 | 0.718 |
| TBA ≥ 17.7μmol/L, yes vs no | 1.8 | 1.0-3.3 | 0.044 | 1.6 | 0.7-3.5 | 0.263 |
| Serum cystatin C, per increase 1 mg/L | 0.4 | 0.1-1.0 | 0.041 | 0.6 | 0.1-3.6 | 0.584 |
| Creatinine, per increase 1 μmol/L | 0.98 | 0.97-1.0 | 0.048 | 0.99 | 0.95-1.02 | 0.499 |
| eGFR, per increase 1 ml/(min∙1.73 m2) | 1.01 | 1.0-1.02 | 0.033 | 1.0 | 0.98-1.02 | 0.81 |
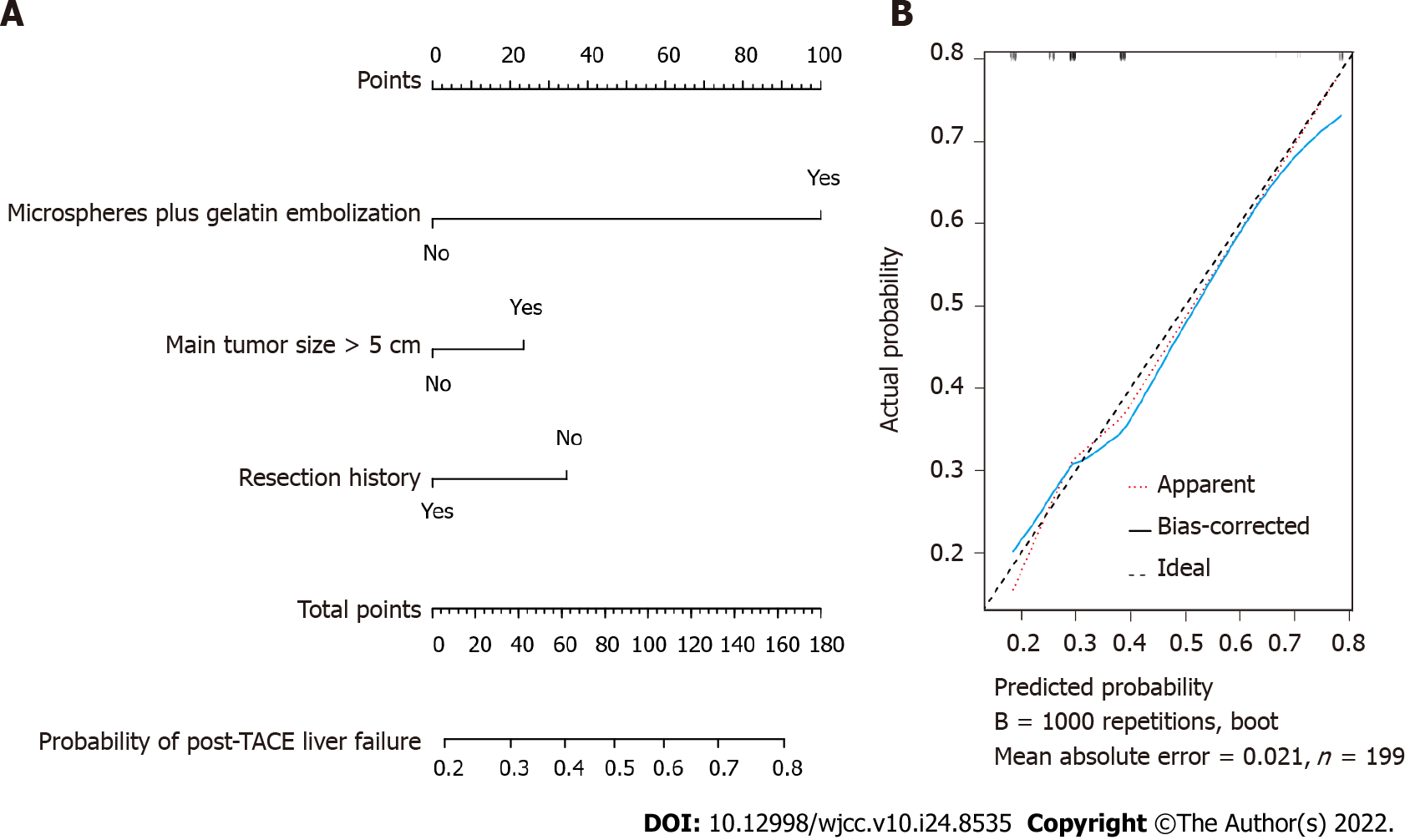
Based on the multivariate logistic analysis, we included microspheres plus gelatin embolization, main tumor size and liver tumor resection history as predictors to establish a nomogram model, which is shown in Figure 1A. The calibration curve of the nomogram model with internal bootstrapping was calculated and is presented in Figure 1B.
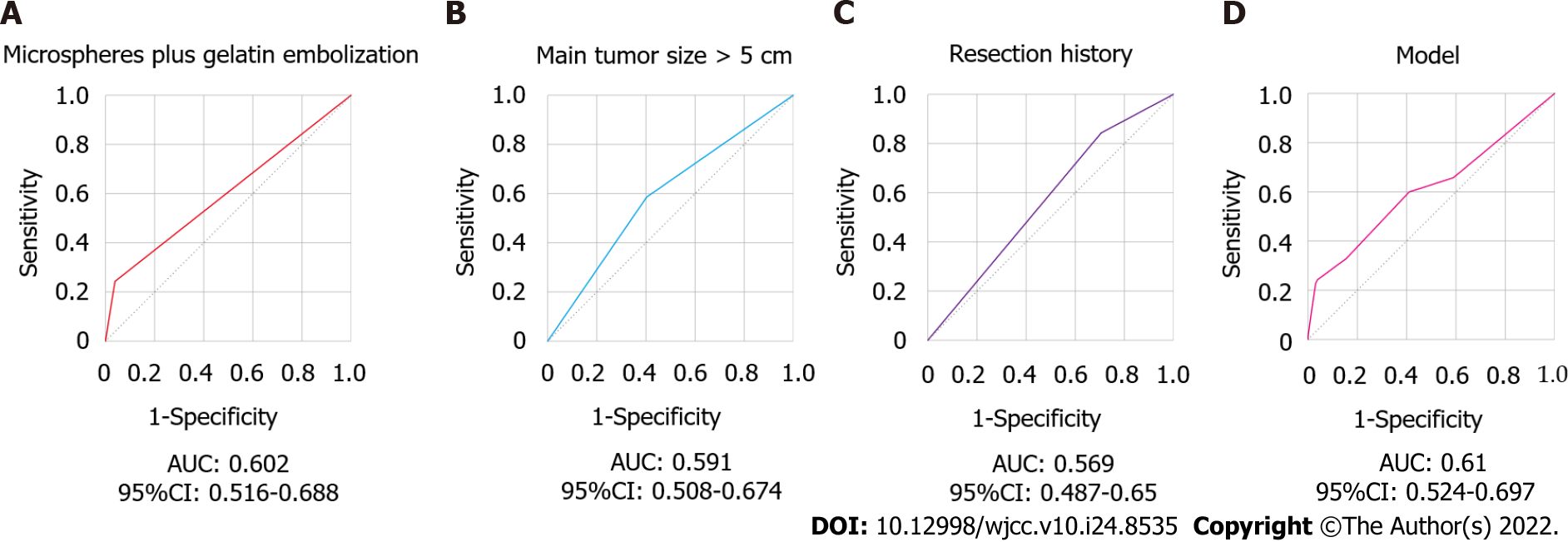
ROC analysis was also performed to evaluate the predictive ability of the indicators. As shown in Figure 2, the AUROCs of microspheres plus gelatin embolization, main tumor size, liver tumor resection history, and the nomogram model were 0.602, 0.591, 0.569 and 0.61, respectively (Figure 2). Unfortunately, all the AUROCs were less than 0.7, leading to an unsatisfactory discrimination ability of these parameters for screening post-TACE liver failure in HCC patients.
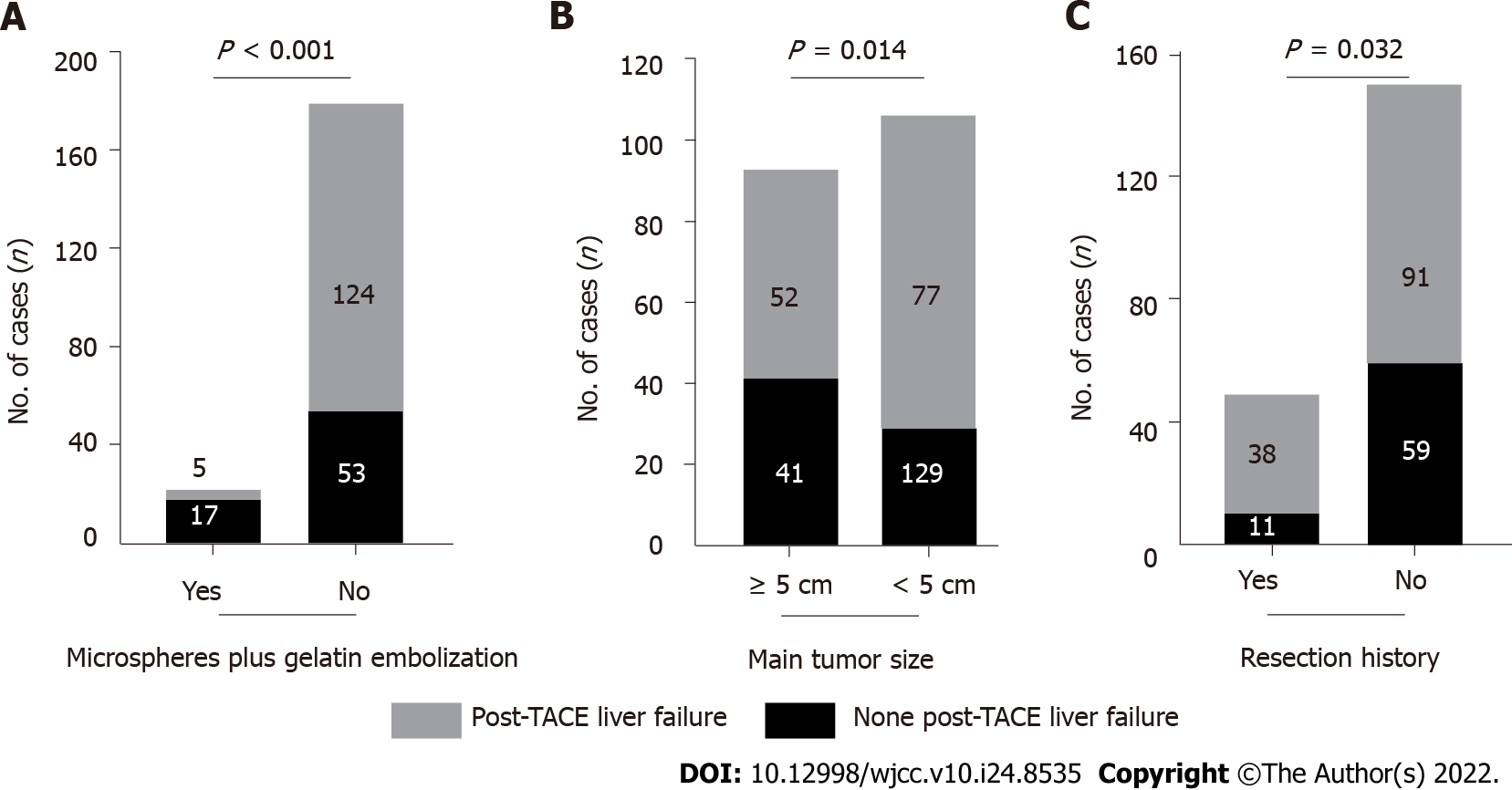
HCC patients who received microspheres plus gelatin embolization developed post-TACE liver failure more frequently than those without microspheres combined with gelatin embolization (17/22, 77.3% vs 53/177, 29.9%, P < 0.001, Figure 3A). Patients with a main tumor size ≥ 5 cm experienced post-TACE liver failure significantly more frequently than those with a main tumor size < 5 cm (41/93, 44.1% vs 29/106, 27.4%; P = 0.014, Figure 3B). The incidence of post-TACE liver failure was significantly lower in HCC patients with a tumor resection history than in those without a liver tumor surgery history (11/49, 22.4% vs 39.3%, P = 0.032, Figure 3C).
The greatest concerns for TACE procedures are toxicity and safety issues. Post-TACE liver failure is one of the most severe complications and can lead to significant morbidity and mortality[15]. According to previous reports[8-11,13], post-TACE liver failure occurs frequently in HCC patients. In our study, post-TACE liver failure occurred in approximately 35% of HCC patients, regardless of whether they were TACE naïve patients or patients who received TACE treatment several times. To avoid the risk of post-TACE liver failure, early detection and prediction are of great importance in addressing TACE approaches.
This study indicated that microspheres plus gelatin embolization beyond chemotherapy drugs might contribute to a higher risk of post-TACE liver failure. Microsphere embolization is recommended because their controllable size distribution and spherical shape may improve and strengthen the embolization effect[16]. The microsphere embolization strategy for vascular catheter calibration can maximize tumor necrosis[17]. Gelatin embolization also demonstrated beneficial clinical outcomes in the treatment of HCC[18]. However, TACE includes hepatic artery infusion che
In line with a previous report, TACE in patients with tumors larger than 5 cm predicts postprocedure liver failure[23]. Tumor size is a vital parameter that should be considered in the selection of multidisciplinary treatment strategies[24]. Multiple studies have demonstrated that a larger tumor size correlates with worse survival in HCC patients who receive TACE treatment[25-27]. There is consensus that a larger tumor size increases treatment difficulties, aggravates complications and deteriorates outcomes in HCC patients. Current evidence indicates that TACE plus sorafenib significantly improves progression-free survival over TACE alone in patients with unresectable HCC[28]. According to these findings, it is essential to avoid combined embolization with microspheres and gelatin during the TACE procedure in unresected HCC patients with tumors larger than 5 cm in size. Unfortunately, no alternative solution has been envisaged to treat these large lesions in the current report.
The current guidelines recommend that surgical therapies, including resection and transplantation are the first-line choice for early-stage HCC patients[14,29]. This study revealed that patients who received liver tumor resection had a lower risk for post-TACE liver failure when they received TACE therapy in the late stages. Undoubtably, the hepatic functional reserve of patients who received liver tumor resection was relatively better than that of HCC patients who had not undergone tumor resection. Inadequate hepatic functional reserve is one of the determining factors for post-hepatectomy liver failure[30,31]. Hepatic functional reserve has been proven to be associated with treatment selection, tumor recurrence and survival in patients with advanced HCC[32,33]. In clinical practice, preoperative assessment of hepatic functional reserve is of great importance for prevention of post-TACE liver failure, regardless of liver tumor resection history. Previous reports indicate that poor hepatic functional reserve, high-dose chemotherapy drug infusion, portal vein thrombosis, ascites, gastrointestinal bleeding, elevated AFP, and history of multiple embolization operations are risk factors for predicting post-TACE liver failure[8,10,11,13,15,21,34]. In addition, several models, including the CTP score, Barcelona Clinic Liver Cancer staging, Mode for End-stage Liver Disease score, and the Hepatoma Arterial-embolisation Prognostic score, were helpful for avoiding the post chemoembolization toxicity and treatment decision-making[3,35-37]. Considering the various definitions of post-TACE liver failure in these studies and our analysis, future research is still urgently needed to investigate candidates that could predict the occurrence of post-TACE liver failure.
This study has some limitations. The primary limitation is that this study had a relatively short follow up period, leading to no observations of recovery or irreversible post-TACE liver failure. Second, the retrospective design with a relatively small sample size in a single center might reduce its representativeness, and there was no calculation or justification of the sample size selected in this study. Third, the assessment of ascites increases in the definition of post-TACE liver failure brought subjectivity, and potential biases such as information bias and selection bias existed in this retrospective study. Fourth, the predictive performance of the risk factors and nomogram model for post-TACE liver failure is not promising enough for clinical application. However, our results indicate that post-TACE liver failure commonly occurred, especially in HCC patients who received microspheres plus gelatin embolization therapy, and those with larger main tumor sizes. A good hepatic functional reserve should be favorable for the occurrence of post-TACE liver failure.
Post-TACE liver failure occurs frequently in HCC patients. However, there is no uniform definition of post-TACE liver failure. The incidence of post-TACE liver failure varies from 0 to 60% globally. This retrospective study concluded that microspheres plus gelation embolization and large tumor size might be involved in the increased risk of post-TACE liver failure. Cautious preprocedure investigation of the risks and benefits of TACE therapy in HCC patients with large tumors is suggested. Embolization approaches should also be evaluated to avoid the risk of post-TACE liver failure. In addition, HCC patients who had undergone liver tumor resection had a lower risk for post-TACE liver failure when they received TACE therapy in the late stages. Moreover, early detection and prediction of post-TACE liver failure by monitoring the hepatic functional reserve should be addressed. Conclusively, it is essential to avoid combined embolization with microspheres and gelatin during the TACE procedure in unresected HCC patients with large tumor sizes.
Post-transarterial chemoembolization (TACE) liver failure occurs frequently in hepatocellular carcinoma (HCC) patients received TACE procedure.
Identification of risk factors for post-TACE liver failure is important for TACE treatment decision-making.
The aim of this retrospective study was to assess the occurrence rate and predictive factors of post-TACE liver failure in HCC patients.
Baseline characteristics and laboratory parameters of HCC patients received TACE therapy were assessed.
A total of 35.2% (70/199) HCC patients occurred post-TACE liver failure after TACE therapy. Logistic models indicated that microspheres plus gelatin embolization and main tumor size > 5 cm were risk predictors for the occurrence of post-TACE liver failure. Conversely, HCC patients who underwent tumor resection surgery before the TACE procedure had a lower risk for post-TACE liver failure.
Microspheres plus gelatin embolization and main tumor size might be risk factors for the occurrence of post-TACE liver failure in HCC patients, while tumor resection history could be a favorable factor for post-TACE liver failure.
Pre-TACE assessment including embolization strategy, tumor size, and hepatic functional reserve is of great importance for avoiding post-TACE liver failure. More studies need to be done to confirm these findings.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Adhoute X, France; Borzio M, Italy; Kumar R, India S-Editor: Wang DM L-Editor: A P-Editor: Wang DM
| 1. | Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1628] [Cited by in RCA: 1644] [Article Influence: 205.5] [Reference Citation Analysis (0)] |
| 2. | Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2066] [Cited by in RCA: 3173] [Article Influence: 528.8] [Reference Citation Analysis (37)] |
| 3. | Giannini EG, Moscatelli A, Pellegatta G, Vitale A, Farinati F, Ciccarese F, Piscaglia F, Rapaccini GL, Di Marco M, Caturelli E, Zoli M, Borzio F, Cabibbo G, Felder M, Sacco R, Morisco F, Missale G, Foschi FG, Gasbarrini A, Baroni GS, Virdone R, Masotto A, Trevisani F; Italian Liver Cancer (ITA. LI.CA) Group; Italian Liver Cancer ITA LI CA Group. Application of the Intermediate-Stage Subclassification to Patients With Untreated Hepatocellular Carcinoma. Am J Gastroenterol. 2016;111:70-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Yang Z, Zhuang L, Szatmary P, Wen L, Sun H, Lu Y, Xu Q, Chen X. Upregulation of heat shock proteins (HSPA12A, HSP90B1, HSPA4, HSPA5 and HSPA6) in tumour tissues is associated with poor outcomes from HBV-related early-stage hepatocellular carcinoma. Int J Med Sci. 2015;12:256-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 134] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 5. | Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology. 2016;64:106-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 521] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 6. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3029] [Article Influence: 432.7] [Reference Citation Analysis (3)] |
| 7. | Facciorusso A, Bellanti F, Villani R, Salvatore V, Muscatiello N, Piscaglia F, Vendemiale G, Serviddio G. Transarterial chemoembolization vs bland embolization in hepatocellular carcinoma: A meta-analysis of randomized trials. United European Gastroenterol J. 2017;5:511-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 8. | Hsin IF, Hsu CY, Huang HC, Huang YH, Lin HC, Lee RC, Chiang JH, Lee FY, Huo TI, Lee SD. Liver failure after transarterial chemoembolization for patients with hepatocellular carcinoma and ascites: incidence, risk factors, and prognostic prediction. J Clin Gastroenterol. 2011;45:556-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Marelli L, Stigliano R, Triantos C, Senzolo M, Cholongitas E, Davies N, Tibballs J, Meyer T, Patch DW, Burroughs AK. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? Cardiovasc Intervent Radiol. 2007;30:6-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 618] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 10. | Chan AO, Yuen MF, Hui CK, Tso WK, Lai CL. A prospective study regarding the complications of transcatheter intraarterial lipiodol chemoembolization in patients with hepatocellular carcinoma. Cancer. 2002;94:1747-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 183] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Shalimar, Jain S, Gamanagatti SR, Kedia S, Thakur B, Nayak B, Kaur H, Gunjan D, Paul SB, Acharya SK. Role of Indocyanine Green in Predicting Post-Transarterial Chemoembolization Liver Failure in Hepatocellular Carcinoma. J Clin Exp Hepatol. 2018;8:28-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Khisti R, Patidar Y, Garg L, Mukund A, Thomas SS, Sarin SK. Correlation of baseline Portal pressure (hepatic venous pressure gradient) and Indocyanine Green Clearance Test With Post-transarterial Chemoembolization Acute Hepatic Failure. J Clin Exp Hepatol. 2019;9:447-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Groupe d'Etude et de Traitement du Carcinome Hépatocellulaire. A comparison of lipiodol chemoembolization and conservative treatment for unresectable hepatocellular carcinoma. N Engl J Med. 1995;332:1256-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 596] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 14. | Department of Medical Administration, National Health and Health Commission of the People's Republic of China. [Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)]. Zhonghua Gan Zang Bing Za Zhi. 2020;28:112-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 65] [Reference Citation Analysis (0)] |
| 15. | Min YW, Kim J, Kim S, Sung YK, Lee JH, Gwak GY, Paik YH, Choi MS, Koh KC, Paik SW, Yoo BC. Risk factors and a predictive model for acute hepatic failure after transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. Liver Int. 2013;33:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Hu J, Albadawi H, Chong BW, Deipolyi AR, Sheth RA, Khademhosseini A, Oklu R. Advances in Biomaterials and Technologies for Vascular Embolization. Adv Mater. 2019;31:e1901071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 151] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 17. | Alvarez MM, Aizenberg J, Analoui M, Andrews AM, Bisker G, Boyden ES, Kamm RD, Karp JM, Mooney DJ, Oklu R, Peer D, Stolzoff M, Strano MS, Trujillo-de Santiago G, Webster TJ, Weiss PS, Khademhosseini A. Emerging Trends in Micro- and Nanoscale Technologies in Medicine: From Basic Discoveries to Translation. ACS Nano. 2017;11:5195-5214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Wáng YX, De Baere T, Idée JM, Ballet S. Transcatheter embolization therapy in liver cancer: an update of clinical evidences. Chin J Cancer Res. 2015;27:96-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 64] [Reference Citation Analysis (0)] |
| 19. | Buijs M, Vossen JA, Frangakis C, Hong K, Georgiades CS, Chen Y, Liapi E, Geschwind JF. Nonresectable hepatocellular carcinoma: long-term toxicity in patients treated with transarterial chemoembolization--single-center experience. Radiology. 2008;249:346-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Cui DC, Lu WL, Sa EA, Gu MJ, Lu XJ, Fan TY. Poly(acrylic acid) microspheres loaded with lidocaine: preparation and characterization for arterial embolization. Int J Pharm. 2012;436:527-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Katsushima S, Inokuma T, Oi H, Okamura J, Higashi T, Takeuchi R, Hidaka A, Shigeno C, Iida Y, Konishi J. Acute hepatic failure following transcatheter arterial embolization for the treatment of hepatocellular carcinoma. Digestion. 1997;58:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Tian L, Lu L, Feng J, Melancon MP. Radiopaque nano and polymeric materials for atherosclerosis imaging, embolization and other catheterization procedures. Acta Pharm Sin B. 2018;8:360-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Siriwardana RC, Niriella MA, Dassanayake AS, Liyanage CA, Upasena A, Sirigampala C, de Silva HJ. Factors affecting post-embolization fever and liver failure after trans-arterial chemo-embolization in a cohort without background infective hepatitis- a prospective analysis. BMC Gastroenterol. 2015;15:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | O'Leary C, Mahler M, Soulen MC. Liver-directed therapy for hepatocellular carcinoma. Chin Clin Oncol. 2021;10:8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Liu L, Zhang Q, Geng J, Li S, Zhao S, Zhang X, Hu J, Feng D. Comparison of radiofrequency ablation combined with sorafenib or sorafenib alone in patients with ECOG performance score 1: identifying optimal candidates. Ann Transl Med. 2020;8:583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Nam JY, Choe AR, Sinn DH, Lee JH, Kim HY, Yu SJ, Kim YJ, Yoon JH, Lee JM, Chung JW, Choi SY, Lee JK, Baek SY, Lee HA, Kim TH, Yoo K. A differential risk assessment and decision model for Transarterial chemoembolization in hepatocellular carcinoma based on hepatic function. BMC Cancer. 2020;20:504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | Zhao S, Zhang X, Wang M, Tan K, Dou W, Fan Q, Li H, Du X, Liu L. Identifying optimal candidates for liver resection or transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma. Ann Transl Med. 2020;8:586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, Tsumura H, Kuzuya T, Isoda N, Yasui K, Aino H, Ido A, Kawabe N, Nakao K, Wada Y, Yokosuka O, Yoshimura K, Okusaka T, Furuse J, Kokudo N, Okita K, Johnson PJ, Arai Y; TACTICS study group. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69:1492-1501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 503] [Article Influence: 100.6] [Reference Citation Analysis (0)] |
| 29. | Hester CA, Yopp AC. Surgical Therapies in Hepatocellular Carcinoma. 2019 Aug 6. In: Hepatocellular Carcinoma: Translational Precision Medicine Approaches [Internet]. Cham (CH): Humana Press; 2019–. [PubMed] |
| 30. | Lin WH, Li K. [Recent advances in preoperative assessment of hepatic functional reserve for hepatectomy]. Zhonghua Wai Ke Za Zhi. 2021;59:392-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 31. | Li M, Wang J, Song J, Shen F, Song L, Ni X, Suo T, Liu H, Zhong M. Preoperative ICG Test to Predict Posthepatectomy Liver Failure and Postoperative Outcomes in Hilar Cholangiocarcinoma. Biomed Res Int. 2021;2021:8298737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Ochi H, Hiraoka A, Hirooka M, Koizumi Y, Amano M, Azemoto N, Watanabe T, Yoshida O, Tokumoto Y, Mashiba T, Yokota T, Abe M, Michitaka K, Hiasa Y, Joko K. Direct-acting antivirals improve survival and recurrence rates after treatment of hepatocellular carcinoma within the Milan criteria. J Gastroenterol. 2021;56:90-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 33. | Uchikawa S, Kawaoka T, Ando Y, Yamaoka K, Kosaka Y, Suehiro Y, Fujii Y, Morio K, Nakahara T, Murakami E, Tsuge M, Hiramatsu A, Imamura M, Takahashi S, Chayama K, Aikata H. Trends in Hepatic Functional Reserve of Patients with Hepatocellular Carcinoma Treated with Tyrosine Kinase Inhibitors. Oncology. 2020;98:727-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Jeon SH, Park KS, Kim YH, Shin YS, Kang MK, Jang BK, Chung WJ, Cho KB, Hwang JS. [Incidence and risk factors of acute hepatic failure after transcatheter arterial chemoembolization for hepatocellular carcinoma]. Korean J Gastroenterol. 2007;50:176-182. [PubMed] |
| 35. | Kadalayil L, Benini R, Pallan L, O'Beirne J, Marelli L, Yu D, Hackshaw A, Fox R, Johnson P, Burroughs AK, Palmer DH, Meyer T. A simple prognostic scoring system for patients receiving transarterial embolisation for hepatocellular cancer. Ann Oncol. 2013;24:2565-2570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 280] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 36. | Vogeler M, Mohr I, Pfeiffenberger J, Sprengel SD, Klauss M, Teufel A, Chang DH, Springfeld C, Longerich T, Merle U, Mehrabi A, Weiss KH, Mieth M. Applicability of scoring systems predicting outcome of transarterial chemoembolization for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2020;146:1033-1050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 2610] [Article Influence: 870.0] [Reference Citation Analysis (59)] |