Published online Aug 6, 2022. doi: 10.12998/wjcc.v10.i22.7890
Peer-review started: August 10, 2021
First decision: October 20, 2021
Revised: October 29, 2021
Accepted: June 24, 2022
Article in press: June 24, 2022
Published online: August 6, 2022
Processing time: 345 Days and 19.7 Hours
Leukemic hematopoietic cells acquire enhanced self-renewal capacity and impaired differentiation. The emergence of symptomatic leukemia also requires the acquisition of a clonal proliferative advantage. Untreated leukemia patients usually experience an aggressive process. However, spontaneous remission occasionally occurs in patients with acute myeloid leukemia (AML), most frequently after recovery from a febrile episode, and this is generally attributed to the triggering of antineoplastic immunity. There may be another explanation for the spontaneous remission as implicated in this paper.
A 63-year-old Chinese man presented with high fever, abdominal pain and urticaria-like skin lesions. He was diagnosed with AML-M4 with t(8;21) (q22;q22)/RUNX1-RUNX1T1 based on morphological, immunological, cytogenetic and molecular analyses. He had a complex chromosome rea-rrangement of 48,XY,t(8;21)(q22;q22),+13,+13[9]/49,idem,+mar[9]/49,idem,+8[2]. He also had a mutated tyrosine kinase domain in fms-like tyrosine kinase 3 gene. He was treated with antibiotics and glucocorticoids for gastrointestinal infection and urticaria-like skin lesions. The infection and skin lesions were quickly resolved. Unexpectedly, he achieved hematological remission along with resolution of the febrile episode, gastrointestinal symptoms and skin lesions. Notably, after relapse, repeating these treatments resulted in a return to hematological remission. Unfortunately, he demonstrated strong resistance to antibiotic and glucocorticoid treatment after the second relapse and died of sepsis from bacterial infection with multidrug resistance. The main clinical feature of this patient was that symptomatic AML emerged with flaring of the gut inflammatory disorder and it subsided after resolution of the inflammation. Learning from the present case raises the possibility that in a subgroup of AML patients, the proliferative advantage of leukemia cells may critically require the presence of inflammatory stresses.
Inflammatory stresses, most likely arising from gastrointestinal infection, may sustain the growth and survival advantage of leukemic cells.
Core Tip: Untreated leukemia patients usually experience an aggressive process. However, spontaneous remission occasionally occurs in a small number of patients with acute myeloid leukemia. Here, we report an acute myeloid leukemia (AML) patient with t(8;21) translocation who achieved recapitulated spontaneous remissions after antibiotic and dexamethasone treatments for febrile episodes and skin lesions. These antibiotic and dexamethasone treatment-induced spontaneous remissions indicated that inflammatory stresses, most likely arising from gastrointestinal infection, sustained the growth and survival advantage of the leukemia cells. Inflammation-sustained proliferation may represent a specific subgroup of AML.
- Citation: Sun XY, Yang XD, Yang XQ, Ju B, Xiu NN, Xu J, Zhao XC. Antibiotic and glucocorticoid-induced recapitulated hematological remission in acute myeloid leukemia: A case report and review of literature. World J Clin Cases 2022; 10(22): 7890-7898
- URL: https://www.wjgnet.com/2307-8960/full/v10/i22/7890.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i22.7890
Acute myeloid leukemia (AML) is a highly heterogeneous group of malignant hematological diseases caused by somatic mutations in multipotential hematopoietic cells. Leukemic hematopoietic cells acquire enhanced self-renewal capacity and impaired differentiation. The emergence of symptomatic leukemia not only requires the acquisition of enhanced self-renewal capacity but also critically requires the acquisition of clonal growth and survival advantages. It is the growth and survival advantages that lead to the accumulation and infiltration of transformed hematopoietic cells in the bone marrow, taking up the hematopoietic pool, inhibiting normal hematopoiesis and ultimately resulting in a reduced capacity to produce mature blood cells[1-4].
Chemotherapy is currently the main initial treatment for AML, the aim of which is to reduce the number of leukemia cells and to achieve complete hematological remission. Untreated AML patients usually experience an aggressive process[1]. However, spontaneous remission occasionally occurs in a small number of AML patients, which frequently follows a febrile episode and is generally attributed to the overproduction of proinflammatory cytokines and the activation of antineoplastic activities[5]. This spontaneous remission could occur not only in patients with fused genes in recurrent chromosome rearrangements and other cytogenetic abnormalities but also in patients with mutated genes in recurrent molecular abnormalities and other transcription factors. Here, we report an AML patient with the recurrent chromosome rearrangement t(8;21)(q22;q22)/RUNX1-RUNX1T1 who achieved unex
Abdominal pain and fever for 3 d and pruritic skin lesions for 2 d.
A 63-year-old Chinese man presented with abdominal pain and fever for 3 d in the absence of headache, chest pain, dyspnea, cough and sputum. The highest body temperature was 39.7 °C. Oral administration of antibiotics could not resolve the febrile episode or gastrointestinal symptoms. Urticaria-like pruritic skin lesions occurred 2 d before, and treatment with astemizole could partially relieve the pruritus but could not completely resolve the skin lesions. Within the last month, his performance status exacerbated, with gradually aggravated fatigue, dizziness and palpitation.
The patient had no history of diseases in the hematological or other systems.
No family history of hematological diseases, autoimmune diseases or malignant diseases was recorded.
His height was 1.71 m, body weight 74.5 kg. His body temperature was 38.3 °C, breathing rate 21 bp per minute, heart rate 92 bp per minute, and blood pressure 17.6/10.4 Kpa (132/78 mmHg). Upon physical examination, prominent signs were panabdominal tenderness and urticaria-like skin lesions. Conspicuous mucocutaneous hemorrhage and jaundice were not found. No significant signs in the nervous system, respiratory system, cardiovascular system, urogenital system or skeletal musculature system were identified.
Routine laboratory examinations: On admission, complete blood count (CBC) revealed the following results: White blood cells (WBCs), 19.13 × 109/L; absolute neutrophil count (ANC), 4.55 × 109/L; absolute monocyte count (AMC), 8.88 × 109/L; red blood cells (RBCs), 2.38 × 1012/L; hemoglobin level (Hb), 80 g/L; platelets (Plts), 32 × 109/L; absolute reticulocyte count (Ret), 5.61 × 109/L; and C-reactive protein (CRP), 142.7 mg/L. The coagulation profile and the urine examination did not show any abnormalities. Fecal examination revealed the presence of increased pyocytes. Biochemical analysis found elevated serum levels of lactate dehydrogenase (2834 IU/L), hydroxybutyric dehydrogenase (2394 IU/L) and β2-microglobulin (47.3 mg/L) in the absence of abnormalities in liver and renal functions. Pathogenic culture of his blood was sterile. Serological tests for hepatitis A, B, and C virus and human immunodeficiency virus were negative. Biomarkers of neoplasms were also negative.
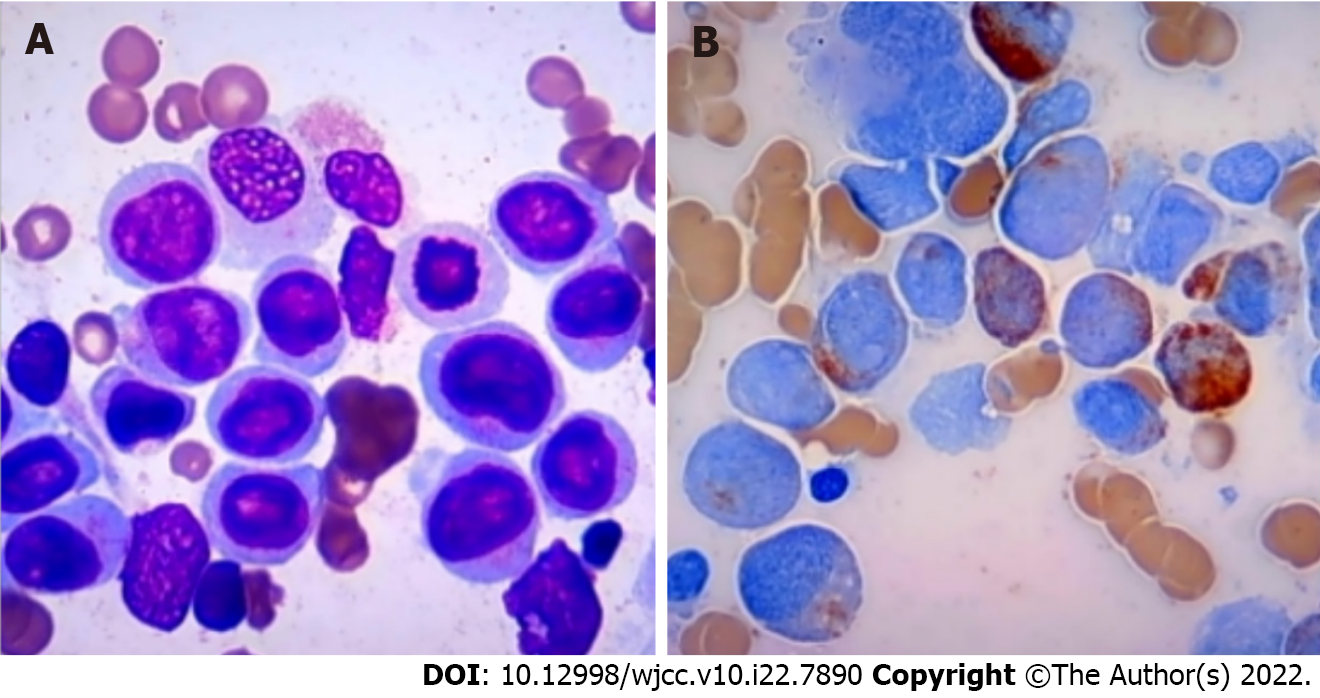
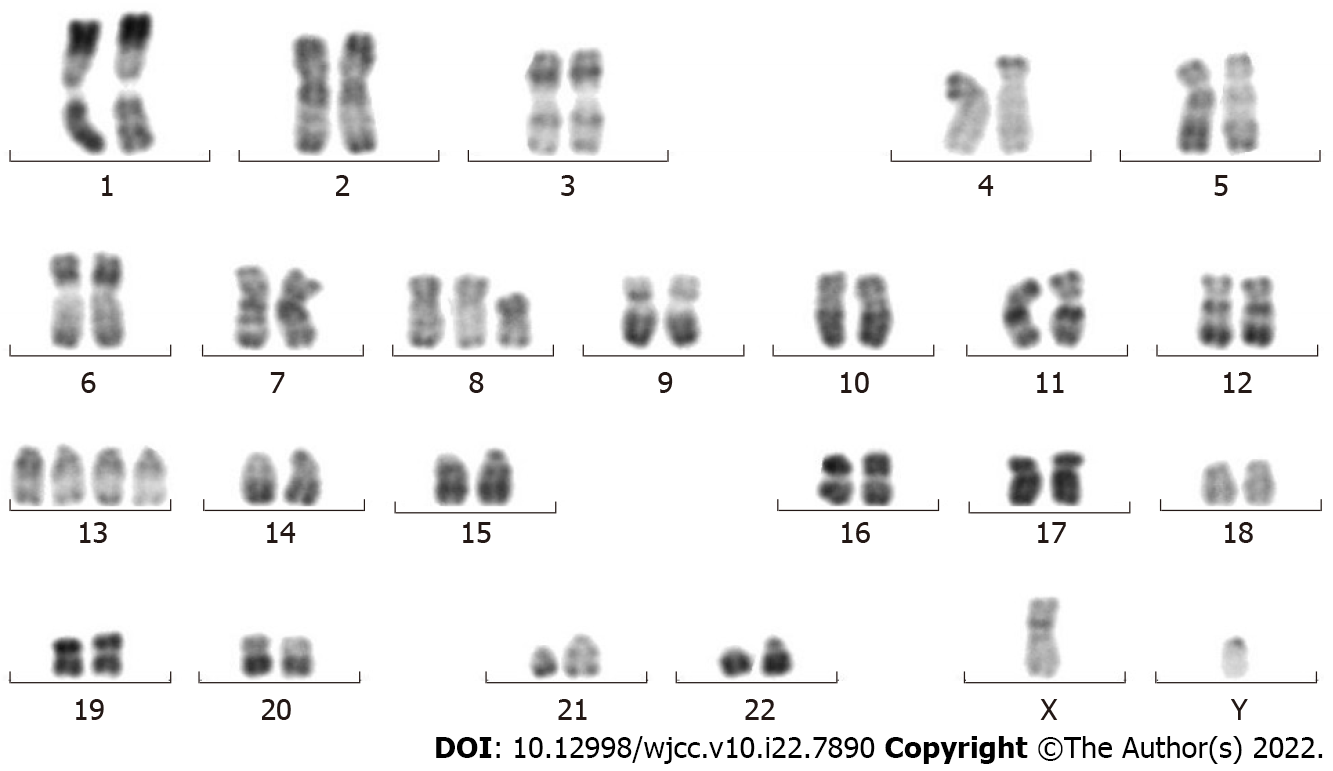
Morphological, immunophenotyping, cytogenetic and molecular biological analysis of leukemic hematopoietic cells: Morphological evaluation of the bone marrow smears showed a heavily hypercellular bone marrow, with substantially increased percentages of monoblasts (accounting for 44.5% of the total nucleated hematopoietic cells) and premonocytes (24.5%). Morphological evaluation of the blood smears showed a highly increased number of WBCs, with substantially increased percentages of premonocytes (accounting for 44% of the total nucleated cells) and monocytes (46%) (Figure 1). Two groups of abnormal myeloid precursors were detected in the bone marrow samples by flow cytometric immunophenotyping analysis. One group (accounting for 32.53% of the total nucleated cells) expressed CD13, CD33, CD14, CD11b, CD36, CD56, CD64, CD123 and human leukocyte antigen-DR (HLA-DR); another group (accounting for 48.95% of the total nucleated cells) expressed CD34, CD117, CD38, HLA-DR, CD13, CD33, CD11b, CD56 and CD123. Cytogenetic analysis by culturing the bone marrow cells reported a karyotype of 48,XY,t(8;21)(q22;q22),+13,+13[9]/49,idem,+mar[9]/49, idem,+8[2] (Figure 2). Molecular biological analysis revealed the presence of a fused AML1–ETO gene and a mutated tyrosine kinase domain in fms-like tyrosine kinase 3 (FLT3-TKD) gene.
No positive findings were observed in the chest computed tomography (CT) images. However, abdominal CT imaging revealed striking bowel wall thickening in the small and large intestines, abnormally gas-filled small intestine, and paper-like dilation of the small intestines and sigmoid colon with perienteric hypervascular fat proliferation, together with the symptoms and signs of the gastrointestinal tract indicating the presence of gut inflammatory lesions.
He was made a definitive diagnosis of AML-M4 with the recurrent chromosome arrangement of t(8;21)(q22;q22)/RUNX1-RUNX1T1[1,5].
Because of the presence of obvious gastrointestinal infection and his poor performance status, cytostatic therapies were deferred. He was treated with piperacillin-tazobactam and etimicin for his febrile disease and with dexamethasone for his urticaria-like skin lesions. He was also prescribed an oral administration of polyglycol electrolyte solution (1500 mL daily for 2 d) followed by rifaximin (200 mg, four times daily) and berberine (0.3 g, three times daily) in an attempt to quickly eliminate the pathogens and their metabolites from the intestines.
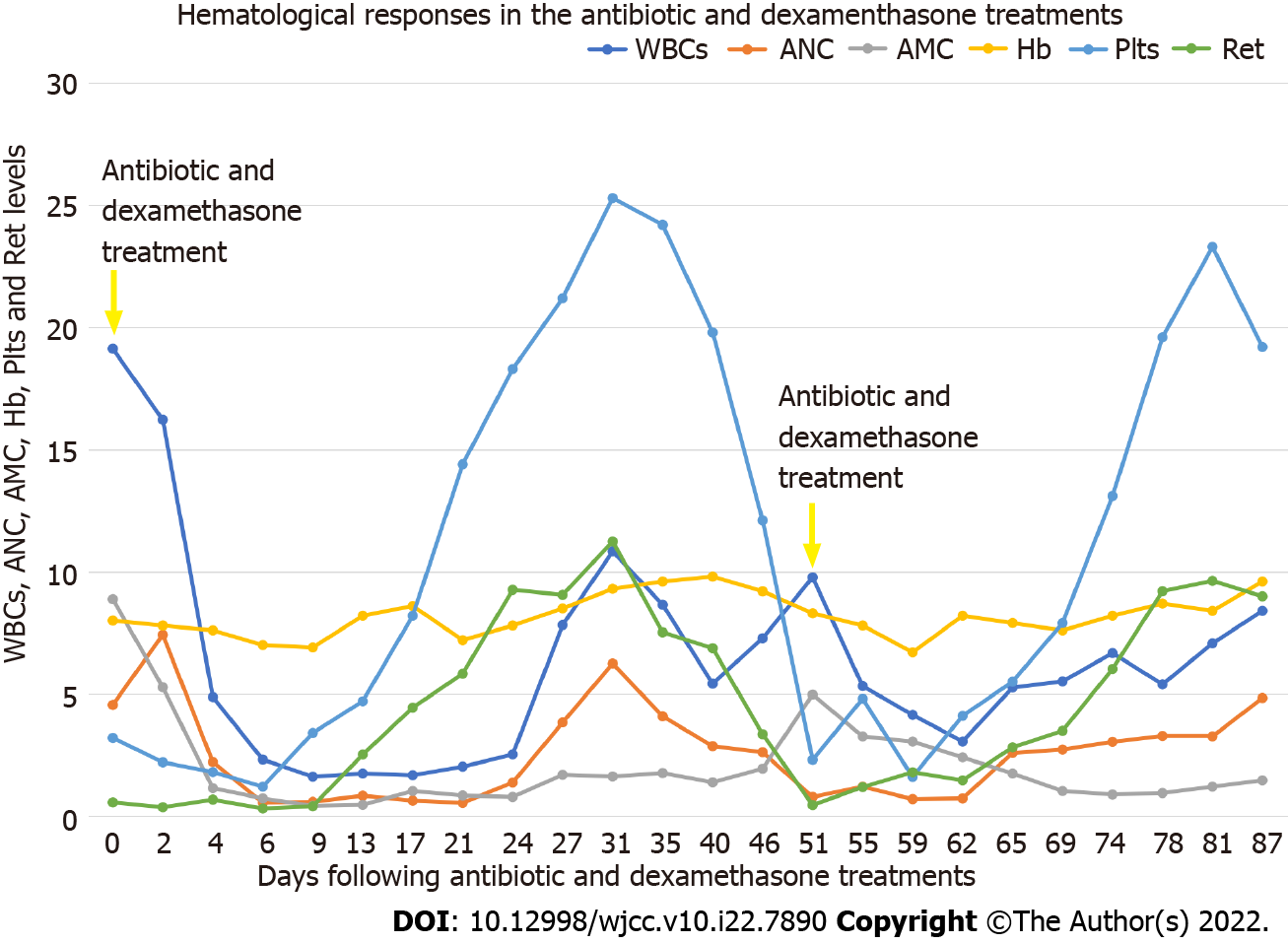
The febrile episode, gastrointestinal symptoms and urticaria-like skin lesions quickly resolved after antibiotic and glucocorticoid treatment. Unexpectedly, his hematological parameters gradually improved. Along with a decline in the AMC and CRP, the ANC, Plts and Ret rapidly increased, and the RBCs and Hb steadily increased. On day 31, CBC showed the following results: WBCs, 10.83 × 109/L; ANC, 6.24 × 109/L; AMC, 1.62 × 109/L; RBCs, 2.74 × 1012/L; Hb, 93 g/L; Plts, 253 × 109/L; and Ret, 112.45 × 109/L. When the blood smears were examined, there were no evident morphological abnormalities in the blood cells except for the left shift in neutrophils. The significantly improved hematological parameters and the absence of leukemia cells on blood smears indicated clearance of the leukemia cells from the peripheral blood and an achievement of clinical hematological remission. Because he declined chemotherapy and hypomethylation therapy, he was discharged from our center.
He maintained a good performance status for approximately three weeks since he was discharged from our center. On day 51, he was sent to our center with identical symptoms as when he was first hospitalized. The CBC results and the morphological evaluation of the blood smears confirmed disease recurrence. Because of the history of the achievement of a hematological response to antibiotic and glucocorticoid treatment and because of the existence of an obvious gastrointestinal infection, he was tentatively treated with the same modality as when he was first hospitalized. As we anticipated, repeating the treatment resulted in a second clinical and hematological remission.
He refused chemotherapy and hypomethylation therapy again, and he was discharged. During the follow-up, he experienced a second relapse on day 105 with the same symptoms, but this time, he demonstrated strong resistance to antibiotic and glucocorticoid treatment and eventually died of an overwhelming infection at another hospital. Pathogenic culture of his blood samples reported a positive result for Acinetobacter baumannii infection with multidrug resistance.
Hematological examinations of WBCs, ANC, AMC, Hb, Plt and Ret levels during the treatments in our center are outlined in Figure 3.
In the present case, the presence of increased percentages of blasts and CD34+ progenitors, the identification of the chromosome rearrangement of t(8;21)(q22;q22) and the fused AML1–ETO gene fulfilled the diagnostic criteria for AML with the recurrent chromosome rearrangement of t(8;21)(q22;q22)/ RUNX1-RUNX1T1[1,4]. On admission, he presented with the major complaints of high fever, overt gastrointestinal symptoms and urticaria-like skin lesions. In this setting, chemotherapy was deferred. He was prescribed antibiotics to treat the febrile episode, dexamethasone to treat urticaria-like skin lesions and a gut-cleansing preparation to remove gastrointestinal pathogens and their metabolites. His gastrointestinal infection and skin lesions were quickly resolved. Along with the resolution of the gastrointestinal infection and the skin lesions, his hematological profile significantly improved. The disappearance of the leukemia cells from his blood smears suggested an achievement of clinical hematological remission, although bone marrow aspiration was not performed at that time.
Because he declined chemotherapy and hypomethylation therapy, we had the opportunity to observe the recapitulated treatment response after disease relapse. The relapse-remission regularity was that symptomatic AML emerged with flaring of the gastrointestinal infection, and symptomatic AML subsided after resolution of the gastrointestinal infection by antibiotic and glucocorticoid treatments. These recapitulated treatment responses indicated that hematological remission was induced by antibiotic and glucocorticoid treatments. This raises the possibility that the clonal growth and survival advantage of the leukemia cells were sustained by the inflammatory stresses, probably derived from the gut inflammatory condition. With effective treatment of the gut inflammatory condition, the leukemia cells lost their proliferative advantage, and normal hematopoiesis was restored.
AML is highly heterogeneous in clinical presentation and treatment responses, which results from the high diversity of impaired genes, not only driving genes in the transformation of hematopoietic progenitors and in the acquisition of proliferative advantage but also nondriving genes affecting the clinical and biological activities of transformed leukemia cells. To date, hundreds of genes have been found to be associated with leukemia pathogenesis, each of which has a distinctive impact on disease development, progression and treatment responses[1-4]. The natural history of AML is generally aggressive, leading to death usually within weeks to months after the emergence of symptomatic disease in the absence of specific treatments[1,4]. However, spontaneous remission occasionally occurs in a small number of AML patients[5].
Although spontaneous remission is a rare event, more than 100 adult AML cases have been recorded. Spontaneous remission was reported in AML patients with various recurrent cytogenetic abnormalities, such as t(8;21)(q22;q22)/RUNX1-RUNX1T1[6-9], t(15;17)(q31;q22)/PML-RAR-α[10], t(v;11q23)/KMT2A rearrangement[11-13], inv(16)(p13;q22) or t(16;16)(p13;q22)/CBFB-MYH11[14,15] and t(8;16)(p11;p13)/MOZ-CBP[16]. Spontaneous remission was also reported in AML patients with a normal karyotype and other cytogenetic abnormalities, with +8 being the most frequently observed cytogenetic abnormality[17-21]. Spontaneous remission has been reported in AML patients with recurrent gene mutations such as nucleophosmin 1 and RUNX1[22-24], with gene mutations in epigenetic modulation such as Ten-Eleven Translocation-2, BCOR, isocitrate dehydrogenase 1 and 2; splicing factors such serine/arginine-rich splicing factor 1, U2AF1 and pre-mRNA processing factor 8; and cell growth receptors and their signaling pathway components such as FLT3-ITD, BRAF, NRAS, KRAS and neurofibromatosis type 1 (NF1)[22-26]. Spontaneous remission even occurs in relapsed AML patients many years after allogeneic hematopoietic stem cell transplantation[13,27]. These AML patients encompassed M0-M6 subtypes with monocyte differentiation accounting for approximately half of the reported cases[6,10,11,14,16]. Patient bone marrow may be either hypercellular or hypocellular, and WBCs may be either elevated or reduced, with reduced WBCs occurring in a large proportion of reported cases.
In the majority of reported cases, the emergence of AML was concomitant with the flaring of an infectious episode, and spontaneous remission occurred after recovery from the infectious disease by treatment with antibiotics, corticosteroids, recombinant human granulocyte colony stimulating factor (rH-GSF) and/or surgical drainage. Infections range from localized infections[5,6,9,15] to fulminant sepsis[5,6,28-30]. Several extrapolations have been proposed to explain the occurrence of spontaneous remission in AML: (1) Overproduced inflammatory cytokines suppress the proliferation and promote the apoptosis of leukemia cells[31-33]; (2) Restored or acquired cellular and innate immune responses target leukemia cells[11,34]; (3) Restored or acquired humoral immune response targets leukemia cells[8,35]; (4) Acquired graft-versus-leukemia effects suppress the proliferation of leukemia cells[13,21,27]; (5) Glucocorticoids promote the apoptosis of leukemia cells[9]; and (6) Granulocyte CSF promotes the differentiation of leukemia cells[7,10,17]. However, these mechanisms do not legitimately explain the features of spontaneous remissions in our present case. This raises the possibility that an inflammation-sustained proliferative advantage of leukemia cells promotes the emergence of symptomatic disease, which may be the best explanation for these antibiotic and glucocorticoid treatment-induced hematological remissions. Symptomatic AML emerged when the inflammatory stresses flared, and the symptomatic AML subsided after the inflammatory stresses had been resolved by effective treatments. In other reported cases, spontaneous remissions occurred frequently after recovery from a febrile episode in response to diverse treatments rather than during the flaring of the infectious episode, also indicating an inflammation-sustained proliferative advantage, at least in a fraction of the reported cases.
It is generally accepted that constitutionally activated growth factor receptor signaling pathways are responsible for the growth and survival advantage of leukemic stem cells. Activated growth factor receptors and their signaling pathway components, such as the formation of fused genes involving ABL, FGFR1 and platelet-derived growth factor receptor and mutated genes involving FLT3, KIT, interleukin-3R, RAS, CBL, PTPN11 and NF1, result in autonomous proliferation[1-4]. In some AML patients, activation of certain mutated genes may not be autonomous but instead ligand-dependent, resembling mutated genes in the B-cell receptor signaling pathway during lymphoma pathogenesis in which the antigen-dependent growth and survival advantages have been well described[36-39]. In this setting, mutated genes in growth factor receptor signaling pathways may play a tonic role in intensifying proliferative signaling after ligands bind to their receptor, thereby acquiring growth and survival advantages. While clonal B cells proliferate in response to antigens binding to B-cell receptors[36-39], myeloid hematopoietic progenitors proliferate in response to ligands binding to pattern recognition receptors, cytokine receptors and colony-stimulating factor receptors[40-42]. Inflammatory cytokines and colony-stimulating factors could directly promote the growth and survival of leukemia cells[43-47]. In our present case, the FLT3-TKD mutation was identified, which might be responsible for the proliferative advantage in inflammatory conditions.
This study has several limitations. First, the diagnosis of spontaneous remission was dependent on hematological improvements and the disappearance of leukemia cells from blood smears, lacking morphological evaluation of bone marrow smears and cytogenetic and molecular monitoring. Second, the exact ligands responsible for the proliferative advantage were not identified. Therefore, additional studies are merited to confirm the extrapolation.
The recapitulated hematological remissions provide strong evidence for the treatment responses being induced by antibiotic and glucocorticoid treatments. AML is a highly heterogeneous hematological malignancy. In our present case, removing the underlying infection could induce a transient hematological remission, suggesting that the growth and survival advantage in this subgroup of leukemia cells may be sustained by inflammation. The ligands may be infection-related components such as microbes or their metabolites, inflammatory cytokines or colony-stimulating factors produced in response to infection. This phenomenon warrants further investigation and may aid in investigating AML pathogenesis and in improving therapeutic outcomes in this subgroup of AML patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Musoni L, Morocco; Son TQ, Viet Nam S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Liesveld JL, Lichtman MA. Acute myelogenous leukemia. In: Kaushansky K, Prchal JT, Press OW, Lichtman MA, Levi M, Burns LJ, Caligiuri MA, editors. Williams Hematology, 9th ed. New York: McGraw-Hill, 2016: 1373-1436. |
| 2. | Kishtagari A, Levine RL. The Role of Somatic Mutations in Acute Myeloid Leukemia Pathogenesis. Cold Spring Harb Perspect Med. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Higgins A, Shah MV. Genetic and Genomic Landscape of Secondary and Therapy-Related Acute Myeloid Leukemia. Genes (Basel). 2020;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5219] [Cited by in RCA: 6746] [Article Influence: 749.6] [Reference Citation Analysis (0)] |
| 5. | Rashidi A, Fisher SI. Spontaneous remission of acute myeloid leukemia. Leuk Lymphoma. 2015;56:1727-1734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Trof RJ, Beishuizen A, Wondergem MJ, Strack van Schijndel RJ. Spontaneous remission of acute myeloid leukaemia after recovery from sepsis. Neth J Med. 2007;65:259-262. [PubMed] |
| 7. | Zomas A, Stefanoudaki-Sofianatou K, Fisfis M, Anagnostopoulos NI. Dose dependent long-term in vivo remission of AML1/ETO positive acute myeloid leukemia with G-CSF. Hematology. 2004;9:107-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Mitterbauer M, Fritzer-Szekeres M, Mitterbauer G, Simonitsch I, Knöbl P, Rintelen C, Schwarzinger I, Haas OA, Silberbauer K, Frey K, Bibus B, Pabinger I, Radaszkiewicz T, Lechner K, Jaeger U. Spontaneous remission of acute myeloid leukemia after infection and blood transfusion associated with hypergammaglobulinaemia. Ann Hematol. 1996;73:189-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Shimohakamada Y, Shinohara K, Fukuda N. Remission of acute myeloblastic leukemia after severe pneumonia treated with high-dose methylprednisolone. Int J Hematol. 2001;74:173-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Yamasaki Y, Izumi Y, Sawada H, Fujita K. Probable in vivo induction of differentiation by recombinant human granulocyte colony stimulating factor (rhG-CSF) in acute promyelocytic leukaemia (APL). Br J Haematol. 1991;78:579-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Müller CI, Trepel M, Kunzmann R, Lais A, Engelhardt R, Lübbert M. Hematologic and molecular spontaneous remission following sepsis in acute monoblastic leukemia with translocation (9;11): a case report and review of the literature. Eur J Haematol. 2004;73:62-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Piccaluga PP, Martinelli G, Malagola M, Rondoni M, Bianchini M, Visani G, Baccarani M. Complete remission in acute myeloid leukemia with granulocyte-colony stimulating factor without chemotherapy. Report of cytogenetic remission of a t(9;11)(p22q23) positive AML patient and review of literature. Haematologica. 2003;88:ECR28. [PubMed] |
| 13. | Hudecek M, Bartsch K, Jäkel N, Heyn S, Pfannes R, Al-Ali HK, Cross M, Pönisch W, Gerecke U, Edelmann J, Ittel T, Niederwieser D. Spontaneous remission of acute myeloid leukemia relapse after hematopoietic cell transplantation in a high-risk patient with 11q23/MLL abnormality. Acta Haematol. 2008;119:111-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Beinart G, Jones D, Abruzzo LV, Ravandi F. Spontaneous Hematologic and Cytogenetic Remission in a Case of Acute Myelogenous Leukemia with Inversion 16. Clin Leuk. 2007;1:243-246. [DOI] [Full Text] |
| 15. | Jehn UW, Mempel MA. Spontaneous remission of acute myeloid leukemia. A report of a case and brief review of the literature. Blut. 1986;52:165-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Hoshino T, Taki T, Takada S, Hatsumi N, Sakura T. Spontaneous remission of adult acute myeloid leukemia with t(8;16)(p11;p13)/MOZ-CBP fusion. Leuk Lymphoma. 2018;59:253-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Camera A, Volpicelli M, Villa MR, Risitano AM, Rossi M, Rotoli B. Complete remission induced by high dose erythropoietin and granulocyte colony stimulating factor in acute erythroleukemia (AML-M6 with maturation). Haematologica. 2002;87:1225-1227. [PubMed] |
| 18. | Suyama T, Hasebe K. Spontaneous remission of acute monocytic leukemia with trisomy 8 and trisomy 18. J Clin Exp Hematop. 2019;59:96-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Daccache A, Kizhakekuttu T, Siebert J, Veeder M. Hematologic and cytogenetic spontaneous remission in acute monocytic leukemia (FAB M5b) with trisomy 8. J Clin Oncol. 2007;25:344-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Teng CJ, Yang CF, Gau JP, Liu JH, Hong YC, Liu CY, Yu YB, Hsiao LT, Wang WS, Tzeng CH. Spontaneous remission in acute myelogenous leukemia evidenced by cytogenetic changes. Ann Hematol. 2011;90:981-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Ifrah N, James JM, Viguie F, Marie JP, Zittoun R. Spontaneous remission in adult acute leukemia. Cancer. 1985;56:1187-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Camus V, Etancelin P, Jardin F, Lenain P, Contentin N, Daliphard S, Buchonnet G, Lemasle E, Lanic H, Leprêtre S, Penther D, Dubois S, Tilly H, Bastard C, Stamatoullas A. Spontaneous remission in three cases of AML M5 with NPM1 mutation. Clin Case Rep. 2015;3:955-959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Vachhani P, Mendler JH, Evans A, Deeb G, Starostik P, Wallace PK, Wang ES. Spontaneous Remission in an Older Patient with Relapsed FLT3 ITD Mutant AML. Case Rep Hematol. 2016;2016:1259759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Helbig D, Quesada AE, Xiao W, Roshal M, Tallman MS, Knorr DA. Spontaneous Remission in a Patient With Acute Myeloid Leukemia Leading to Undetectable Minimal Residual Disease. J Hematol. 2020;9:18-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Khalife-Hachem S, Pegliasco J, Saada V, Hernandez E, Camara-Clayette V, Cotteret S, Benabdelali R, de Botton S, Marzac C, Micol JB. Spontaneous molecular response of IDH2 acute myeloid leukemia. Ann Hematol. 2020;99:353-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Bradley T, Zuquello RA, Aguirre LE, Mackrides N, Chapman J, Cimmino L, Thomassen A, Watts J. Spontaneous remission of acute myeloid leukemia with NF1 alteration. Leuk Res Rep. 2020;13:100204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Rautenberg C, Kaivers J, Germing U, Haas R, Schroeder T, Kobbe G. Spontaneous remission in a patient with very late relapse of acute myeloid leukemia 17 years after allogeneic blood stem cell transplantation. Eur J Haematol. 2019;103:131-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Mozafari R, Moeinian M, Asadollahi-Amin A. Spontaneous Complete Remission in a Patient with Acute Myeloid Leukemia and Severe Sepsis. Case Rep Hematol. 2017;2017:9593750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Al-Tawfiq JA, Al-Khatti AA. Spontaneous remission of acute monocytic leukemia after infection with Clostridium septicum. Int J Lab Hematol. 2007;29:386-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Fassas A, Sakellari I, Anagnostopoulos A, Saloum R. Spontaneous remission of acute myeloid leukemia in a patient with concurrent Pneumocystis carinii pneumonia. Nouv Rev Fr Hematol. 1991;33:363-364. [PubMed] |
| 31. | Musto P, D'Arena G, Melillo L, Cascavilla N, La Sala A, Ladogana S, Carotenuto M. Spontaneous remission in acute myeloid leukaemia: a role for endogenous production of tumour necrosis factor and interleukin-2? Br J Haematol. 1994;87:879-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Jimemez C, Ribera JM, Abad E, Pintos G, Milla F, Junca J, Feliu E. Increased serum tumour necrosis factor during transient remission in acute leukaemia. Lancet. 1993;341:1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Ankerst J, Fäldt R, Nilsson PG, Flodgren P, Sjögren HO. Complete remission in a patient with acute myelogenous leukemia treated with leukocyte alpha-interferon and cimetidine. Cancer Immunol Immunother. 1984;17:69-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Müller-Schmah C, Solari L, Weis R, Pfeifer D, Scheibenbogen C, Trepel M, May AM, Engelhardt R, Lübbert M. Immune response as a possible mechanism of long-lasting disease control in spontaneous remission of MLL/AF9-positive acute myeloid leukemia. Ann Hematol. 2012;91:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Jindal N, Nampoothiri R, Rajpal S, Sreedharanunni S, Varma N, Malhotra P. Does Development of Plasmacytosis have a Role in Spontaneous Remission of Acute Myeloid Leukemia? Indian J Hematol Blood Transfus. 2021;37:167-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Thurner L, Hartmann S, Neumann F, Hoth M, Stilgenbauer S, Küppers R, Preuss KD, Bewarder M. Role of Specific B-Cell Receptor Antigens in Lymphomagenesis. Front Oncol. 2020;10:604685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Marcucci F, Mele A. Hepatitis viruses and non-Hodgkin lymphoma: epidemiology, mechanisms of tumorigenesis, and therapeutic opportunities. Blood. 2011;117:1792-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 182] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 38. | Mueller A, O'rourke J, Chu P, Chu A, Dixon MF, Bouley DM, Lee A, Falkow S. The role of antigenic drive and tumor-infiltrating accessory cells in the pathogenesis of helicobacter-induced mucosa-associated lymphoid tissue lymphoma. Am J Pathol. 2005;167:797-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 39. | Hoogeboom R, van Kessel KP, Hochstenbach F, Wormhoudt TA, Reinten RJ, Wagner K, Kater AP, Guikema JE, Bende RJ, van Noesel CJ. A mutated B cell chronic lymphocytic leukemia subset that recognizes and responds to fungi. J Exp Med. 2013;210:59-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 40. | Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, Takatsu K, Kincade PW. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 684] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 41. | Chiba Y, Mizoguchi I, Hasegawa H, Ohashi M, Orii N, Nagai T, Sugahara M, Miyamoto Y, Xu M, Owaki T, Yoshimoto T. Regulation of myelopoiesis by proinflammatory cytokines in infectious diseases. Cell Mol Life Sci. 2018;75:1363-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 42. | Mitroulis I, Kalafati L, Bornhäuser M, Hajishengallis G, Chavakis T. Regulation of the Bone Marrow Niche by Inflammation. Front Immunol. 2020;11:1540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 43. | Carey A, Edwards DK 5th, Eide CA, Newell L, Traer E, Medeiros BC, Pollyea DA, Deininger MW, Collins RH, Tyner JW, Druker BJ, Bagby GC, McWeeney SK, Agarwal A. Identification of Interleukin-1 by Functional Screening as a Key Mediator of Cellular Expansion and Disease Progression in Acute Myeloid Leukemia. Cell Rep. 2017;18:3204-3218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 211] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 44. | Suzuki T, Morio T, Tohda S, Nagata K, Yamashita Y, Imai Y, Aoki N, Hirashima K, Nara N. Effects of interleukin-6 and granulocyte colony-stimulating factor on the proliferation of leukemic blast progenitors from acute myeloblastic leukemia patients. Jpn J Cancer Res. 1990;81:979-986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Sung PJ, Sugita M, Koblish H, Perl AE, Carroll M. Hematopoietic cytokines mediate resistance to targeted therapy in FLT3-ITD acute myeloid leukemia. Blood Adv. 2019;3:1061-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 46. | Aref S, Azmy E, El Ghannam D, Haroun M, Ibrahim L, Sabry M. Clinical value of CD25/CD123 co-expression in acute myeloid leukemia patients. Cancer Biomark. 2020;29:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Testa U, Riccioni R, Militi S, Coccia E, Stellacci E, Samoggia P, Latagliata R, Mariani G, Rossini A, Battistini A, Lo-Coco F, Peschle C. Elevated expression of IL-3Ralpha in acute myelogenous leukemia is associated with enhanced blast proliferation, increased cellularity, and poor prognosis. Blood. 2002;100:2980-2988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 257] [Article Influence: 11.2] [Reference Citation Analysis (0)] |