Published online Aug 6, 2022. doi: 10.12998/wjcc.v10.i22.7728
Peer-review started: January 6, 2022
First decision: February 8, 2022
Revised: March 7, 2022
Accepted: June 13, 2022
Article in press: June 13, 2022
Published online: August 6, 2022
Processing time: 196 Days and 16.8 Hours
Radiation therapy, especially the development of linear accelerators, plays a key role in cancer management. The fast-rotating coplanar O-ring Halcyon Linac has demonstrated many advantages. The previous literature has mainly focused on the machine parameters and plan quality of Halcyon, with a lack of relevant research on its clinical application.
To evaluate the clinical performance of the O-ring Halcyon treatment system in a real-world application setting.
Data from sixty-one patients who were treated with the Halcyon system throughout the entire radiotherapy process in Peking Union Medical College Hospital between August 2019 and September 2020 were retrospectively reviewed. We evaluated the target tumour response to radiotherapy and irradiation toxicity from 1 to 3 mo after treatment. Dosimetric verification of Halcyon plans was performed using a quality assurance procedure, including portal dosimetry, ArcCHECK and point dose measurements for verification of the system delivery accuracy.
Of the 61 patients in the five groups, 16, 12, 7 and 26 patients had complete response, partial response, progressive disease and stable disease, respectively. No increase in the irradiated target tumour volume was observed when separately evaluating the local response. Regarding irradiation toxicity, no radiation-induced deaths were observed. Thirty-eight percent (23/61 patients) had no radiation toxicity after radiotherapy, 56% (34/61 patients) experienced radiation toxicity that resolved after treatment, and 6% (4/61 patients) had irreversible adverse reactions. The average gamma passing rates with a 2% dose difference and 2-mm distance to agreement for IMRT/VMAT/SRT plans were ArcCHECK at 96.4% and portal dosimetry at 96.7%, respectively. All of the validated clinical plans were within 3% for point dose measurements, and Halcyon’s ArcCHECK demonstrated a high pass rate of 99.1% ± 1.1% for clinical gamma passing criteria of 3%/3 mm.
The O-ring Halcyon Linac could achieve a better therapeutic effect on the target volume by providing accurate treatment delivery plans with tolerable irradiation toxicity.
Core Tip: The fast-rotating coplanar O-ring Halcyon Linac has demonstrated many advantages in radiation therapy. Unlike previous studies, which focused more on the machine parameters and quality control aspects of the O-ring Halcyon Linac, our institution evaluated Halcyon more from the perspective of practical clinical applications concerning radiotherapy effects and irradiation toxicity. The O-ring Halcyon Linac can generate desired treatment plans that meet clinically accepted constraints, pass routine patient-specific quality assurance for delivery accuracy verification, and present acceptable radiation toxicity under prospective yield.
- Citation: Wang GY, Zhu QZ, Zhu HL, Jiang LJ, Zhao N, Liu ZK, Zhang FQ. Clinical performance evaluation of O-Ring Halcyon Linac: A real-world study. World J Clin Cases 2022; 10(22): 7728-7737
- URL: https://www.wjgnet.com/2307-8960/full/v10/i22/7728.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i22.7728
With the development and advancement of precision radiotherapy and intelligent radiotherapy, the requirements of radiotherapy equipment are also increasing. Rapid technology evolution and updated radiotherapy equipment can better protect organs at risk (OARs) and deliver highly accurate treatment to the target tissue[1,2]. A commercially available, fast-rotating coplanar O-ring linear accelerator (Linac) Halcyon treatment platform was launched by Varian Medical Systems (Palo Alto, CA, United States) in China in 2019. This machine is equipped with a single-energy six-megavolt (6 MV) fattening filter-free (FFF) beam with a dual-layer staggered 1 cm-wide Multi-Leaf Collimator (MLC) and compulsive image guide, which can achieve higher dose rates, reduce the out-of-field dose, and de
In terms of the plan quality and machine parameters of Halcyon, previous studies have focused more on comparisons with C-arm Linac[5,8,9]. In contrast to C-arm Truebeam Linac (Varian Medical Systems, Palo Alto, CA, United States), Halcyon has the highest achievable maximal dose rate of 800 MU/min, with two times faster leaf speed (5 cm/s), four times faster collimator rotation (2.5 RPM), and four times faster gantry speed (4 RPM)[10]. In addition, the Halcyon system supports automatic couch shifting to replace manual isocentre shifting and faster image-guided procedures, which can compensate for the time needed, further improving daily treatment delivery accuracy, as well as patient compliance and safety. These factors explain why C-arm Truebeam Linac has a higher maximum available dose rate setting (1400 MU/min) than Halcyon Linac (800 MU/min), but the overall treatment time for Truebeam is no longer than that for Halcyon.
The Halcyon system theoretically improves the quality of radiotherapy planning, improves the positioning accuracy, shortens the treatment time, and has potential radiobiology advantages, but what does it look like in practice? Halcyon version 2.0 was implemented in our institution and the modulation resolution of MLC was 0.5 cm. Initial acceptance testing and commissioning data confirmed that the machine met the manufacturer specifications described above. After using this machine for a certain period, our institution has certain clinical experience and research foundations for its use. This study therefore intends to retrospectively analyse patients treated with the Halcyon Linac at our institution and evaluate the effectiveness, safety, and quality assurance of Halcyon products in clinical application to provide a reference and suggestions for oncologists using Halcyon equipment.
We retrospectively obtained data from sixty-one patients who were treated with the Halcyon system throughout the entire radiotherapy process at the Department of Radiation Oncology, Peking Union Medical College Hospital, between August 2019 and September 2020. According to treatment area, the identified patients were divided into five groups, including the head and neck group, chest group, abdomen group, pelvic group, and spine and bone group. The inclusion criteria were as follows: full use of the Halcyon system throughout the entire radiotherapy process; completion of the radiotherapy plan; a clear and evaluable target volume; and complete patient medical records, radiotherapy data and follow-up information. Patients with the following clinical scenarios were excluded: other types of Linac systems used during irradiation of the target volume; failure to complete the radiotherapy plan for various reasons; loss to follow-up or a lack of patient clinical data; and no evaluation of the lesion. Demographic and clinical information, including sex, race, age, clinical diagnosis, pathological type, radiotherapy plan scheduling, course timeline, treatment progress, target volume, OARs, radiotherapy positioning, dose, and concurrent therapy, were retrieved from electronic medical records and Linac systems. At the same time, imaging evaluation data from before and after treatment and equipment operation records, such as machine failure records and maintenance records, were consulted. This study was reviewed and approved by the Institutional Review Board of Peking Union Medical College Hospital (No. S-K1883).
Radiotherapy was administered to patients according to the pathological characteristics of the lesion, the patient’s physical status and willingness, and the doctor’s preference. Radiotherapy was performed using a 6-MV X-ray Halcyon linear accelerator and intensity modulated radiotherapy (IMRT), volumetric modulated arc therapy (VMAT) or stereotactic radiotherapy (SRT) modalities. All of the patients met the indications for radiotherapy. All of the patients were scanned by a Philips Brilliance Big Bore CT scanner to obtain CT-based simulation images, and the images were transmitted to an Eclipse15.5 treatment planning system (Varian, United States). The doctors drew the target volumes and OARs, the physicists designed the plan, and the therapists operated the equipment. CBCT examination was performed before every treatment, and then radiotherapy was completed with the Halcyon Linac.
The imaging data of patients from 1 to 3 mo after treatment with the Halcyon Linac were reviewed and compared with imaging data before treatment to evaluate the target tumour response after radiation treatment. Tumour response was evaluated according to the Response Evaluation Criteria in Solid Tumours (RECIST), except for bone metastasis. Bone tumour response was assessed using criteria developed by the M.D. Anderson Cancer Center (MDA). Systemic progression, such as distant metastasis, was recorded. All of the patients were followed up for 1 to 3 mo after radiotherapy by outpatient, inpatient or telephone visits to evaluate them for irradiation toxicity. Toxicities, such as acute skin reactions, myelosuppression, mucosal reactions, radiation pneumonia or gastrointestinal disorders, were evaluated using the Common Terminology Criteria for Adverse Events Version 4.0 (CTCAE 4.0).
Dosimetric verification of Halcyon plans was performed using quality assurance procedures such as portal dosimetry, ArcCHECK and point dose measurements to verify the system delivery accuracy[11]. The treatment delivery accuracy was evaluated by delivering a plan in quality assurance measurement mode to the Linac via an on-board electronic portal imaging device (EPID) imager and recording the gamma analysis pass rates via portal dosimetry. For portal dosimetry, gamma evaluation criteria of 2%/2 mm with a 10% low dose threshold were used. A cube solid water phantom with multiple water-equivalent plastic blocks and spacers was used to verify the dose distributions for the clinical plans[12], and the measured point doses were compared to point doses calculated at the same location. Then, percent differences were reported. ArcCHECK (SunNuclear, FL, United States) used 3%/3 mm and 2%/2 mm gamma evaluation criteria with low dose thresholds of 5% and 10%, respectively, to compare planar doses.
Between August 2019 and September 2020, a total of 61 patients who completed radiotherapy by Halcyon were enrolled. There were 12 patients in the head and neck group, 13 in the chest group, 10 in the abdomen group, 14 in the pelvic group, and 12 in the spine and bone group. Among them, cervical cancer was the most common cancer type (18%; 11 patients). One patient was treated with SRT, 21 patients with IMRT, and 39 patients with VMAT. Regarding the irradiated site, 56% of patients were treated for a primary tumour, 1% for recurrence in situ postoperatively, and 43% for metastasis. Table 1 summarizes the clinical characteristics of the enrolled patients.
| Characteristics | Number (n) | Percent (%) |
| Median age (yr) | 59 | |
| Age range (yr) | 26-82 | |
| Sex | ||
| Male | 33 | 54 |
| Female | 28 | 46 |
| Treatment area | ||
| Head and neck | 12 | 20 |
| Chest | 13 | 21 |
| Abdomen | 10 | 16 |
| Pelvic | 14 | 23 |
| Spine and bone | 12 | 20 |
| Treatment type | ||
| Primary tumour | 34 | 56 |
| Recurrence in situ | 1 | 1 |
| Metastasis | 26 | 43 |
| Radiation technology | ||
| IMRT | 21 | 35 |
| VMAT | 39 | 64 |
| SRT | 1 | 1 |
| Concurrent therapy | ||
| Chemotherapy | 15 | |
| Other1 | 15 | |
| No | 31 | |
| Radiation toxicities | ||
| No | 23 | 38 |
| Cured after treatment | 34 | 56 |
| Yes | 4 | 6 |
By comparing the imaging data of patients before and 1-3 mo after treatment and the results of other auxiliary examination methods, the changes in the tumour size of the irradiated site before and after treatment were evaluated. The detailed response evaluations and the time intervals for evaluation are shown in Table 2. The irradiated lesions of most patients were evaluated for nearly 1 mo after radiotherapy. The most effective response, reported complete response (CR), was in the pelvic group, with nine cases of cervical cancer. Seven patients experienced distant metastasis within 1 to 3 mo after the completion of radiotherapy, indicating progressive disease (PD). In the abdomen and spine and bone groups, the results showed that the majority of patients had stable disease.
| Group | The time interval for evaluation (n) | Response evaluation (n) | |||||
| 1 mo | 2 mo | 3 mo | CR | PR | PD | SD | |
| Head and neck | 6 | 4 | 2 | 5 | 3 | 1 | 3 |
| Chest | 7 | 6 | 0 | 0 | 4 | 3 | 6 |
| Abdomen | 6 | 4 | 0 | 0 | 2 | 0 | 8 |
| Pelvic | 13 | 1 | 0 | 11 | 2 | 0 | 1 |
| Spine and bone | 5 | 6 | 1 | 0 | 1 | 3 | 8 |
| Total | 37 | 21 | 3 | 16 | 12 | 7 | 26 |
All of the patients completed the prescription dose of radiotherapy. Regarding toxicity, no radiation-induced deaths were observed. According to the outpatient, inpatient or telephone follow-up records, none of the patients felt discomfort during radiotherapy. Thirty-eight percent (23/61 patients) had no radiation toxicity after radiotherapy, 56% (34/61 patients) had radiation toxicities that resolved after treatment, and 6% (4/61 patients) had irreversible adverse reactions. The most common adverse effect was a haematological reaction (57%; 35/61 patients). Among the patients experiencing haematological reactions, 26 patients had grade 1-2 myelosuppression, but no patients had grade 4 myelosuppression during follow-up. In the head and neck group, the radiation toxicities observed after subsequent treatment were hypogeusia (2 patients), oral ulceration (3 patients), dysphagia (2 patients), and increased and sticky pharyngeal secretion (1 patient), which resolved after treatment. In the chest group, the radiation toxicities that resolved after treatment included radiation pneumonitis, radiodermatitis, cutaneous pigmentation, chest and back pain (one case of each). There were few adverse reactions other than myelosuppression, urinary tract reactions and gastrointestinal tract reactions in the abdomen, pelvic, spine and bone groups. Regarding long-term complications, two patients from the head and neck group had xerostomia, one patient with brain metastases receiving SRT had hypomnesia, and one patient with lung cancer developed radiation pulmonary fibrosis.
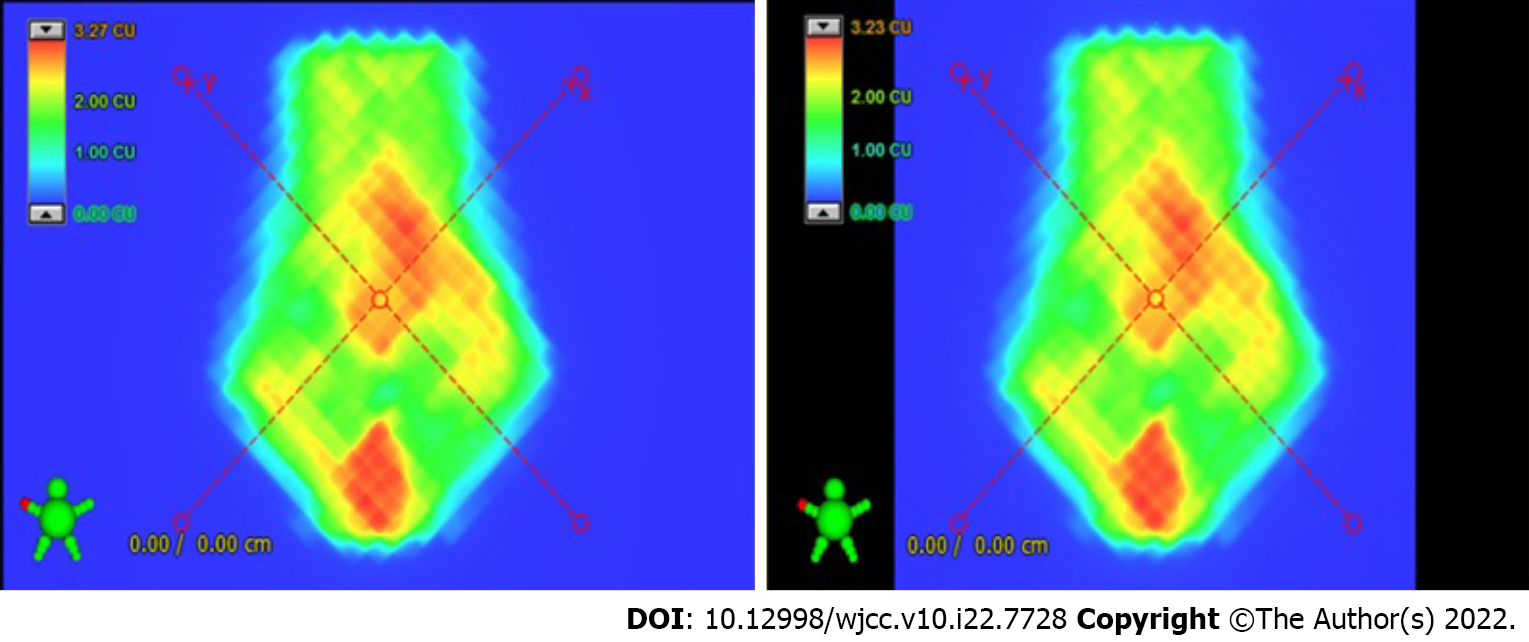
Table 3 shows the mean values of the treatment delivery parameter (and range) differences, including point dose measurements, ArcCHECK (2 mm/2%), ArcCHECK (3 mm/3%) and portal dosimetry. A total of 61 plans were generated in the Eclipse15.5 treatment planning system, and we performed 29, 20, 23, and 16 dosimetric verifications of the Halcyon plans for the above treatment delivery parameters. All of the ArcCHECK results were greater than 95% with 3 mm/3% gamma criteria, and only two portal dosimetry (88.6% and 89.7%) results were less than the 10% low dose threshold. The results of point dose measurements were all controlled at 3%. Figure 1 shows an example of the predicted dose compared with the detected dose.
| Dosimetric verification | Point dose measurements (%) | ArcCHECK (2%/2 mm) (%) | ArcCHECK (3%/3 mm) (%) | Portal dosimetry (2%/2 mm) (%) |
| Head and neck | 0.26 ± 1.1 (-0.22-1.92) | 95.5 ± 1.8 (93.2-97.7) | 99.4 ± 0.5 (98.8-100) | 97.8 ± 2.4 (93.3-100) |
| Chest | 1 ± 0.6 (0.45-2.18) | 97.63 ± 1.1 (96.1-98.4) | 99.2 ± 0.8 (97.9-100) | 89.7 |
| Abdomen | 1.11 ± 0.7 (0.23-2.01) | 97.1 ± 2.0 (95.5-99.3) | 98.8 ± 1.5 (96.5-99.7) | 96 |
| Pelvic | -0.15 ± 0.7 (-0.99-1.38) | 96.0 ± 2.2 (94.2-99.2) | 99 ± 1.4 (96.3-100) | 98.4 ± 2.4 (98.1-98.6) |
| Spine and bone | 0.2 ± 1.8 (-1.8-2.72) | 96.5 ± 1.8 (93.6-97.8) | 99.2 ± 0.9 (97.9-100) | 93.2 ± 6.4 (88.6-97.7) |
| Total | 0.42 ± 1 (-1.8-2.72) | 96.4 ± 1.8 (93.3-99.3) | 99.1 ± 1.1 (96.3-100) | 96.7 ± 3.4 (88.6-100) |
We explored the effectiveness, safety, and quality assurance of Halcyon in clinical application between June 2015 and July 2018 by analysing 61 patients subdivided into five groups. Our results showed that O-ring Halcyon Linac could achieve a better therapeutic effect on the target volume by providing accurate treatment delivery plans with tolerable toxicity of irradiation. For clinics that use Halcyon for treatment delivery, administering radiotherapy with this system is feasible and safe.
According to previous studies, the Halcyon treatment platform showed good performance for radiotherapy modalities. An early study by Cozzi et al[13] reported that Halcyon could deliver radiotherapy to conventionally fractionated breast, head and neck, and high-risk prostate tissue quickly and effectively with plans of similarly high clinical quality when compared to the C-arm Linac. Pokhrel et al[14] reported an analysis of stereotactic body radiation therapy (SBRT) treatment of abdominal and pelvic oligometastatic lymph nodes with single-isocentre VMAT using Halcyon. They showed that acceptable plan quality and effective treatment delivery could be achieved for SBRT using the Halcyon Linac. These studies demonstrate that the Halcyon platform can generate treatment plans that meet clinically accepted constraints and pass routine patient-specific quality assurance testing for delivery accuracy verification. Compared with these previous studies, which mostly reported product per
Assessment of solid tumour response, except for in the spine and bone group, was performed using criteria developed by RECIST, version 1.1[15]. Four patients were evaluated as having PD due to distant metastasis, but no increase in the irradiated target tumour volume was observed when separately evaluating the local response. This finding demonstrated the effectiveness of Halcyon for the local control of cancer. In the previous literature, there have been few evaluations of the efficacy of a specific machine in the field of radiotherapy. Early disease-control outcomes in patients treated with Halcyon were comparable to published reports with no recurrences in the radiation field, although with a relatively short median follow-up[16,17]. Gupta et al[18] found 13.56% local (with or without distant metastasis) first recurrence in neoadjuvant chemotherapy followed by concomitant chemoradiation for cervical cancer. The small cohort of cervical cancer patients in our abdomen group all showed CR, demonstrating a good start to long-term survival. Most of the patients with PD were in the chest group (3/4 patients). Three patients (two with small cell lung cancer and one with oesophageal squamous carcinoma) in the chest group with PD were closely related to the strong biologic invasiveness of these two tumours and the tendency for distant metastasis[19,20]. This finding serves as a reminder that radiotherapy, as a topical treatment for cancer patients, is not a replacement for systemic treatment. In the efficacy evaluation of irradiation response, the patients with cervical cancer achieved the most CR among the enrolled patients (9/16 patients), with a significant advantage compared with other diseases. This finding is closely related to China having made great progress in cervical cancer treatment, with a nearly five percent increase in five-year overall survival compared to that in the United States[21]. Our institution has conducted in-depth basic and clinical research in the field of radiotherapy for cervical cancer and established a model of precise radiotherapy for cervical cancer[22-24].
Bone is one of the most common sites of metastasis, and external beam radiotherapy is an important treatment modality that plays a key role in controlling lesion progression[25,26]. For the evaluation of bone tumour response, we did not use the International Union Against Cancer (UICC) or WHO criteria, which define bone tumour response by plain radiography and skeletal scintigraphy[27,28], or the RECIST criteria, which regard bone metastases as unmeasurable lesions[15]. In our study, we referred to a revised set of response criteria for bone metastases proposed by the MDA[29], which presents a practical approach for the diagnosis and assessment of bone metastasis. For all twelve patients in the spine and bone group, the target volumes were bone metastases, and three patients had PD because of distant metastasis. When we evaluated the bone response to radiotherapy, there was fill-in or sclerosis of lytic lesions, normalization of osteoblastic lesions, no increase in the size of any existing measurable lesions in the irradiated sites, and other similar imaging findings, regarded as no local lesion progression following the MDA criteria.
In terms of safety, this study examined outpatient and inpatient records and performed telephone follow-up. The results showed that acute toxicities were well tolerated in all patients, and no patients felt discomfort during radiotherapy. Most patients had radiation toxicities related to haematological reactions, but their symptoms subsided over time. Myelosuppression was closely related to the irradiation site, and the main reason for the occurrence of myelosuppression in most patients is likely the administration of concurrent chemotherapy or other therapies, which definitely exacerbate haematological toxicity[30,31]. Although we made great efforts in the planning design and machine performance, late toxic reactions are inevitable due to the physics of radiation and the proximity to OARs[32,33]. Among the patients with irreversible adverse reactions, most patients experienced xerostomia as a long-term side effect (50%; 2/4 patients), which was closely related to the inevitable damage to the parotid gland caused by the physical characteristics of the radiation dose reduction and tumour location during radiotherapy for head and neck tumour patients[34].
To obtain a better radiotherapy effect and achieve uniform coverage while maintaining safe doses to the target volume, steep dose gradients must be achieved with precise dose delivery. Quality assurance, especially for dosimetric verification, is required to ensure accurate plan delivery. According to previous studies, Halcyon has demonstrated great quality assurance results. Pokhrel et al[35] described the plan quality, treatment delivery efficacy and accuracy of SBRT treatments using the O-ring Halcyon Linac via VMAT. Petroccia et al[10] reported that Halcyon could potentially reduce the dose to OARs while simultaneously increasing the dose delivered to the tumour. We also performed some dosimetric verification of Halcyon plans, and the results were within the acceptable range, except for two portal dosimetry (88.6% and 89.7%) results. We redesigned the treatment plan, performed dosimetric verification again for these two patients, and treated them after the verification results passed the set low dose threshold. In addition, Halcyon’s ArcCHECK and portal dosimetry demonstrated high gamma passing rates greater than an average of 95% with criteria of 2%/2 mm and 3%/3 mm. All of the validated clinical plans were within 3% for point dose measurements. These quality assurance measurements verified that accurate delivery could be achieved with Halcyon.
Some of the limitations of this study are as follows. First, the incidence of radiation toxicities might have been underestimated because of the retrospective nature of the study, most of the patients being outpatients, and the short-term telephone follow-up, which might not illustrate the full picture. Second, as a retrospective, single-centre study, selection bias might exist. Nonetheless, our sample size was sufficiently large when compared to analogous studies. Third, unlike previous research on machine features and parameters, this study was a descriptive study that focused more on Halcyon products in clinical treatment applications; thus, we did not include controls.
In summary, we evaluated the clinical performance of the Halcyon treatment system in a real-world application setting by analysing patients who received Halcyon Linac throughout the entire radiotherapy process. The results of this study indicate that the Halcyon platform can generate treatment plans that meet clinically accepted constraints, can pass routine patient-specific quality assurance evaluations for delivery accuracy verification, and has acceptable radiation toxicities under prospective yield.
Radiation therapy is commonly used in cancer management. Halcyon, a novel 6MV-flattening-filter-free O-ring linear accelerator (6X-FFF ORL), was designed to deliver treatment with greater speed than a traditional C-arm Linac, demonstrating great advantages.
The development of linear accelerators has played a key role in cancer management. Halcyon, as a new accelerator with many breakthrough innovations, is worthy of further exploration for clinical application. Previous studies have mainly focused on the machine parameters and plan quality of Halcyon, while relevant research on its clinical application has been lacking.
To evaluate the clinical performance of the O-ring Halcyon treatment system in a real-world application setting and share our clinical experience with 6X-FFF ORL radiation therapy for cancer management.
Patients who were treated with the Halcyon system throughout the entire radiotherapy process were retrospectively reviewed. We evaluated the Halcyon from three aspects: effects of radiotherapy, irradiation toxicity and quality assurance. Dosimetric verification of Halcyon plans was performed using quality assurance procedures such as portal dosimetry, ArcCHECK and point dose measurements to verify the system delivery accuracy.
Of the 61 patients in the five groups, no increase in the irradiated target tumour volume was observed when separately evaluating local response. Regarding irradiation toxicity, thirty-eight percent (23/61 patients) had no radiation toxicity after radiotherapy, 56% (34/61 patients) experienced radiation toxicity that resolved after treatment, and 6% (4/61 patients) had irreversible adverse reactions. All of the validated clinical plans were within 3% for point dose measurements, and the average gamma passing rates with a 2% dose difference and 2-mm distance to agreement for IMRT/VMAT/SRT plans were ArcCHECK at 96.4% and portal dosimetry at 96.7%, respectively.
We showed that the Halcyon platform can generate treatment plans that meet clinically accepted constraints and pass routine patient-specific quality assurance for delivery accuracy verification. For clinics that choose Halcyon as the treatment delivery option, administering VMAT and IMRT is feasible and safe.
Further follow-up is needed to assess late toxicity and long-term outcomes.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yoshioka K, Japan; Zhang Y, China A-Editor: Liu X, China S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Witt JS, Rosenberg SA, Bassetti MF. MRI-guided adaptive radiotherapy for liver tumours: visualising the future. Lancet Oncol. 2020;21:e74-e82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 2. | Yang WC, Hsu FM, Yang PC. Precision radiotherapy for non-small cell lung cancer. J Biomed Sci. 2020;27:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 3. | Scorsetti M, Alongi F, Castiglioni S, Clivio A, Fogliata A, Lobefalo F, Mancosu P, Navarria P, Palumbo V, Pellegrini C, Pentimalli S, Reggiori G, Ascolese AM, Roggio A, Arcangeli S, Tozzi A, Vanetti E, Cozzi L. Feasibility and early clinical assessment of flattening filter free (FFF) based stereotactic body radiotherapy (SBRT) treatments. Radiat Oncol. 2011;6:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 4. | Fogliata A, Cayez R, Garcia R, Khamphan C, Reggiori G, Scorsetti M, Cozzi L. Technical Note: Flattening filter free beam from Halcyon linac: Evaluation of the profile parameters for quality assurance. Med Phys. 2020;47:3669-3674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Wang R, Du Y, Yao K, Liu Z, Wang H, Yue H, Zhang Y, Wu H. Halcyon clinical performance evaluation: A log file-based study in comparison with a C-arm Linac. Phys Med. 2020;71:14-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (2)] |
| 6. | Jarema T, Aland T. Using the iterative kV CBCT reconstruction on the Varian Halcyon linear accelerator for radiation therapy planning for pelvis patients. Phys Med. 2019;68:112-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | Cai B, Laugeman E, Mazur TR, Park JC, Henke LE, Kim H, Hugo GD, Mutic S, Li H. Characterization of a prototype rapid kilovoltage x-ray image guidance system designed for a ring shape radiation therapy unit. Med Phys. 2019;46:1355-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 8. | Huang Y, Du Y, Li C, Wang H, Zu Z, Feng Z, Zhou S, Wu H, Zhang Y. Pediatric cone beam CT on Varian Halcyon and TrueBeam radiotherapy systems: radiation dose and positioning accuracy evaluations. J Radiol Prot. 2019;39:739-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Michiels S, Poels K, Crijns W, Delombaerde L, De Roover R, Vanstraelen B, Haustermans K, Nuyts S, Depuydt T. Volumetric modulated arc therapy of head-and-neck cancer on a fast-rotating O-ring linac: Plan quality and delivery time comparison with a C-arm linac. Radiother Oncol. 2018;128:479-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 10. | Petroccia HM, Malajovich I, Barsky AR, Ghiam AF, Jones J, Wang C, Zou W, Teo BK, Dong L, Metz JM, Li T. Spine SBRT With Halcyon™: Plan Quality, Modulation Complexity, Delivery Accuracy, and Speed. Front Oncol. 2019;9:319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Laugeman E, Heermann A, Hilliard J, Watts M, Roberson M, Morris R, Goddu S, Sethi A, Zoberi I, Kim H, Mutic S, Hugo G, Cai B. Comprehensive validation of halcyon 2.0 plans and the implementation of patient specific QA with multiple detector platforms. J Appl Clin Med Phys. 2020;21:39-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Low DA, Gerber RL, Mutic S, Purdy JA. Phantoms for IMRT dose distribution measurement and treatment verification. Int J Radiat Oncol Biol Phys. 1998;40:1231-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 64] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 13. | Cozzi L, Fogliata A, Thompson S, Franzese C, Franceschini D, de Rose F, Tomatis S, Scorsetti M. Critical Appraisal of the Treatment Planning Performance of Volumetric Modulated Arc Therapy by Means of a Dual Layer Stacked Multileaf Collimator for Head and Neck, Breast, and Prostate. Technol Cancer Res Treat. 2018;17:1533033818803882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 14. | Pokhrel D, Webster A, Stephen J, St Clair W. SBRT treatment of abdominal and pelvic oligometastatic lymph nodes using ring-mounted Halcyon Linac. J Appl Clin Med Phys. 2021;22:162-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 21630] [Article Influence: 1351.9] [Reference Citation Analysis (1)] |
| 16. | Chopra S, Gupta S, Kannan S, Dora T, Engineer R, Mangaj A, Maheshwari A, Shylasree TS, Ghosh J, Paul SN, Phurailatpam R, Charnalia M, Alone M, Swamidas J, Mahantshetty U, Deodhar K, Kerkar R, Shrivastava SK. Late Toxicity After Adjuvant Conventional Radiation Versus Image-Guided Intensity-Modulated Radiotherapy for Cervical Cancer (PARCER): A Randomized Controlled Trial. J Clin Oncol. 2021;39:3682-3692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 105] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 17. | de Boer SM, Powell ME, Mileshkin L, Katsaros D, Bessette P, Haie-Meder C, Ottevanger PB, Ledermann JA, Khaw P, D'Amico R, Fyles A, Baron MH, Jürgenliemk-Schulz IM, Kitchener HC, Nijman HW, Wilson G, Brooks S, Gribaudo S, Provencher D, Hanzen C, Kruitwagen RF, Smit VTHBM, Singh N, Do V, Lissoni A, Nout RA, Feeney A, Verhoeven-Adema KW, Putter H, Creutzberg CL; PORTEC Study Group. Adjuvant chemoradiotherapy vs radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019;20:1273-1285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 314] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 18. | Gupta S, Maheshwari A, Parab P, Mahantshetty U, Hawaldar R, Sastri Chopra S, Kerkar R, Engineer R, Tongaonkar H, Ghosh J, Gulia S, Kumar N, Shylasree TS, Gawade R, Kembhavi Y, Gaikar M, Menon S, Thakur M, Shrivastava S, Badwe R. Neoadjuvant Chemotherapy Followed by Radical Surgery Versus Concomitant Chemotherapy and Radiotherapy in Patients With Stage IB2, IIA, or IIB Squamous Cervical Cancer: A Randomized Controlled Trial. J Clin Oncol. 2018;36:1548-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 283] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 19. | Rossi G, Bertero L, Marchiò C, Papotti M. Molecular alterations of neuroendocrine tumours of the lung. Histopathology. 2018;72:142-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Ohashi S, Miyamoto S, Kikuchi O, Goto T, Amanuma Y, Muto M. Recent Advances From Basic and Clinical Studies of Esophageal Squamous Cell Carcinoma. Gastroenterology. 2015;149:1700-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 413] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 21. | Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ, Estève J, Ogunbiyi OJ, Azevedo E Silva G, Chen WQ, Eser S, Engholm G, Stiller CA, Monnereau A, Woods RR, Visser O, Lim GH, Aitken J, Weir HK, Coleman MP; CONCORD Working Group. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2711] [Cited by in RCA: 3429] [Article Influence: 489.9] [Reference Citation Analysis (1)] |
| 22. | Wang W, Zhang F, Hu K, Hou X. Image-guided, intensity-modulated radiation therapy in definitive radiotherapy for 1433 patients with cervical cancer. Gynecol Oncol. 2018;151:444-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 23. | Liu X, Meng Q, Wang W, Zhou Z, Zhang F, Hu K. Predictors of Distant Metastasis in Patients with Cervical Cancer Treated with Definitive Radiotherapy. J Cancer. 2019;10:3967-3974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Zhou Z, Zhao J, Hu K, Hou X, Sun X, Pan X, Wang X, Li N, Yang Z, Zhang F, Zhou Q, Zhan L. Single High-Dose Radiation Enhances Dendritic Cell Homing and T Cell Priming by Promoting Reactive Oxygen Species-Induced Cytoskeletal Reorganization. Int J Radiat Oncol Biol Phys. 2021;109:95-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Coleman RE, Croucher PI, Padhani AR, Clézardin P, Chow E, Fallon M, Guise T, Colangeli S, Capanna R, Costa L. Bone metastases. Nat Rev Dis Primers. 2020;6:83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 315] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 26. | Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s-6249s. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1481] [Cited by in RCA: 1640] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 27. | Hayward JL, Carbone PP, Rubens RD, Heuson JC, Kumaoka S, Segaloff A. Assessment of response to therapy in advanced breast cancer (an amendment). Br J Cancer. 1978;38:201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 29. | Hamaoka T, Madewell JE, Podoloff DA, Hortobagyi GN, Ueno NT. Bone imaging in metastatic breast cancer. J Clin Oncol. 2004;22:2942-2953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 400] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 30. | Bauml JM, Vinnakota R, Anna Park YH, Bates SE, Fojo T, Aggarwal C, Limaye S, Damjanov N, Di Stefano J, Ciunci C, Genden EM, Wisnivesky JP, Ferrandino R, Mamtani R, Langer CJ, Cohen RB, Sigel K. Cisplatin Every 3 Weeks Versus Weekly With Definitive Concurrent Radiotherapy for Squamous Cell Carcinoma of the Head and Neck. J Natl Cancer Inst. 2019;111:490-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 31. | Wu Q, Zhu C, Zhang S, Zhou Y, Zhong Y. Hematological Toxicities of Concurrent Chemoradiotherapies in Head and Neck Cancers: Comparison Among Cisplatin, Nedaplatin, Lobaplatin, and Nimotuzumab. Front Oncol. 2021;11:762366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Brunt AM, Wheatley D, Yarnold J, Somaiah N, Kelly S, Harnett A, Coles C, Goodman A, Bahl A, Churn M, Zotova R, Sydenham M, Griffin CL, Morden JP, Bliss JM; FAST-Forward Trial Management Group. Acute skin toxicity associated with a 1-week schedule of whole breast radiotherapy compared with a standard 3-week regimen delivered in the UK FAST-Forward Trial. Radiother Oncol. 2016;120:114-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 164] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 33. | Wang X, Palaskas NL, Yusuf SW, Abe JI, Lopez-Mattei J, Banchs J, Gladish GW, Lee P, Liao Z, Deswal A, Lin SH. Incidence and Onset of Severe Cardiac Events After Radiotherapy for Esophageal Cancer. J Thorac Oncol. 2020;15:1682-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 34. | Tribius S, Haladyn S, Hanken H, Busch CJ, Krüll A, Petersen C, Bergelt C. Parotid sparing and quality of life in long-term survivors of locally advanced head and neck cancer after intensity-modulated radiation therapy. Strahlenther Onkol. 2021;197:219-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Pokhrel D, Tackett T, Stephen J, Visak J, Amin-Zimmerman F, McGregor A, Strup SE, St Clair W. Prostate SBRT using O-Ring Halcyon Linac - Plan quality, delivery efficiency, and accuracy. J Appl Clin Med Phys. 2021;22:68-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |