Published online Jul 16, 2022. doi: 10.12998/wjcc.v10.i20.7178
Peer-review started: March 16, 2022
First decision: April 8, 2022
Revised: April 27, 2022
Accepted: May 27, 2022
Article in press: May 27, 2022
Published online: July 16, 2022
Processing time: 110 Days and 17.4 Hours
Phakic intraocular lens (pIOL) implantation has been commonly prescribed and is considered as a safe and effective option for correcting high myopia. However, it is associated with multiple complications.
This report describes a case of full-thickness macular hole (MH) in a patient with a history of bilateral pIOL implantation for the correction of myopia of –12.00 diopters in both eyes 7 mo ago. The MH closed after pars plana vitrectomy with internal limiting membrane removal and the best-corrected visual acuity improved to 20/40 in the left eye.
In rare cases, MH can occur following pIOL. In this present case report, we analyzed the formation process of MH following the surgery and emphasized that it is important to inform highly myopic patients about the risk of MH occurrence while being aware of the symptoms of this complication.
Core Tip: Although phakic intraocular lenses have been commonly prescribed and are safe and effective, they are still associated with multiple complications. One of the rare complications is a full-thickness macular hole occurring after phakic intraocular lens implantation. Therefore, it is important to inform highly myopic patients about the risk of MH occurrence and be attentive to the symptoms of this complication.
- Citation: Li XJ, Duan JL, Ma JX, Shang QL. Macular hole following phakic intraocular lens implantation: A case report. World J Clin Cases 2022; 10(20): 7178-7183
- URL: https://www.wjgnet.com/2307-8960/full/v10/i20/7178.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i20.7178
Phakic intraocular lens (pIOL) implantation has been commonly prescribed since the 1980s. It is accepted as a safe and effective option for correcting high myopia in patient’s ineligible for corneal refractive surgery[1]. However, complications associated with pIOL include the reduced density of central endothelial cells, development of pigmentary dispersion, cataracts and glaucoma[1]. In addition, a few cases of vitreoretinal complications such as giant retinal tears and retinal detachment have been reported[2]. We present a rare occurrence of macular hole (MH) as a complication of pIOL implantation for the treatment of high myopia.
A 29-year-old female presented with a 10-day history of impaired visual acuity (VA) and distorted vision in the left eye.
The patient had undergone pIOL implantation in the posterior chamber for the correction of myopia of -12.00 diopters in both eyes 7 mo prior to the onset of symptoms described above. PIOL implantation improved the vision of the patient whose VA was 20/20 in both eyes after the treatment.
The patient had no history of systemic illness or obvious changes in the retina or vitreous before the pIOL implantation.
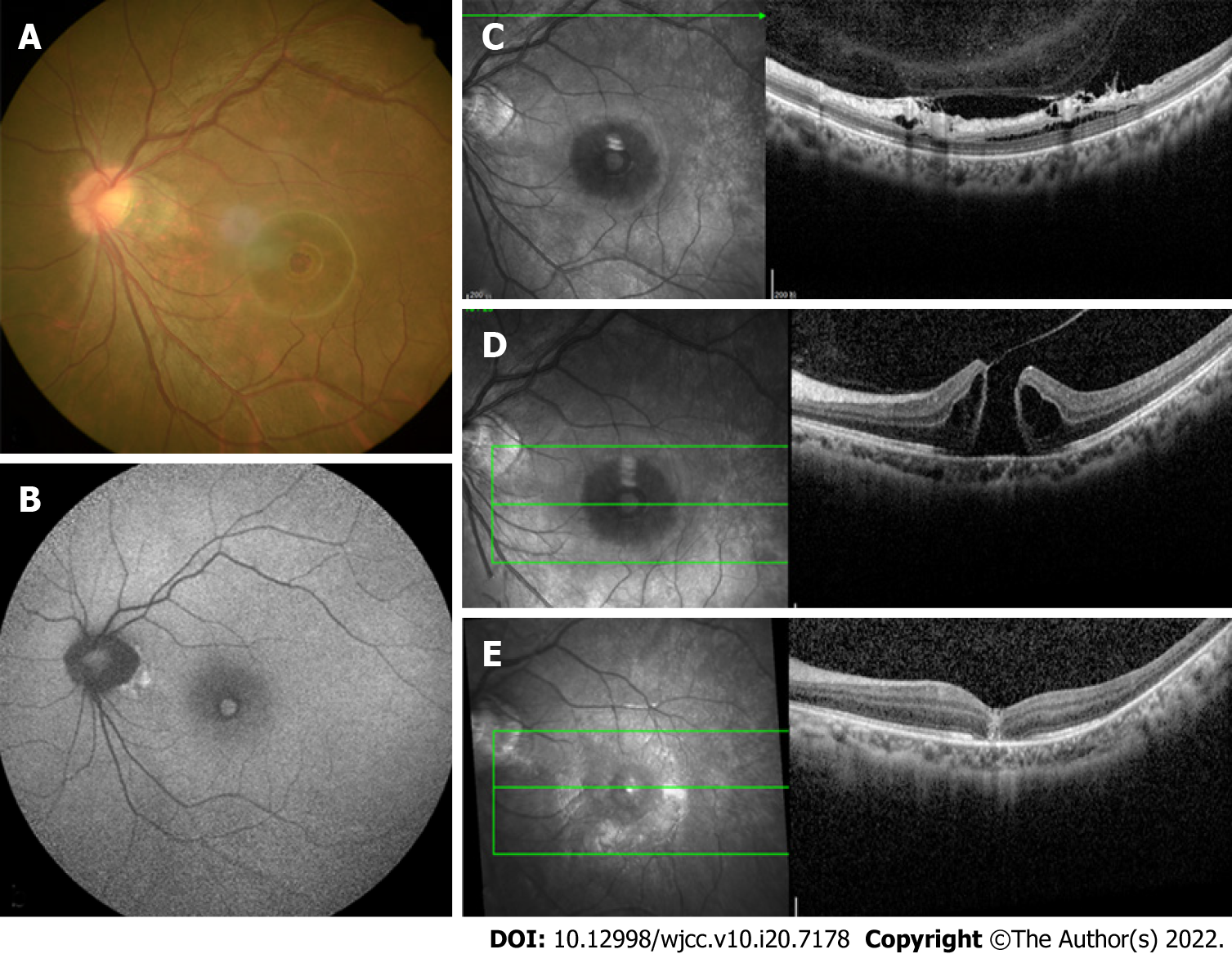
The initial examination showed that the best corrected visual acuity (BCVA) of this patient was 20/20 in the right eye and 20/100 in the left eye with normal intraocular pressure. The axial length was 27.18 mm in the right eye and 27.00 mm in the left eye. The slit-lamp examination showed that the pIOL was present in both eyes with an unremarkable anterior segment in the rest of the area. The funduscopic examination revealed a round-shaped MH in the left eye (Figure 1A) and the appearance of a tessellated fundus with a tilted disc in bilateral eyes.
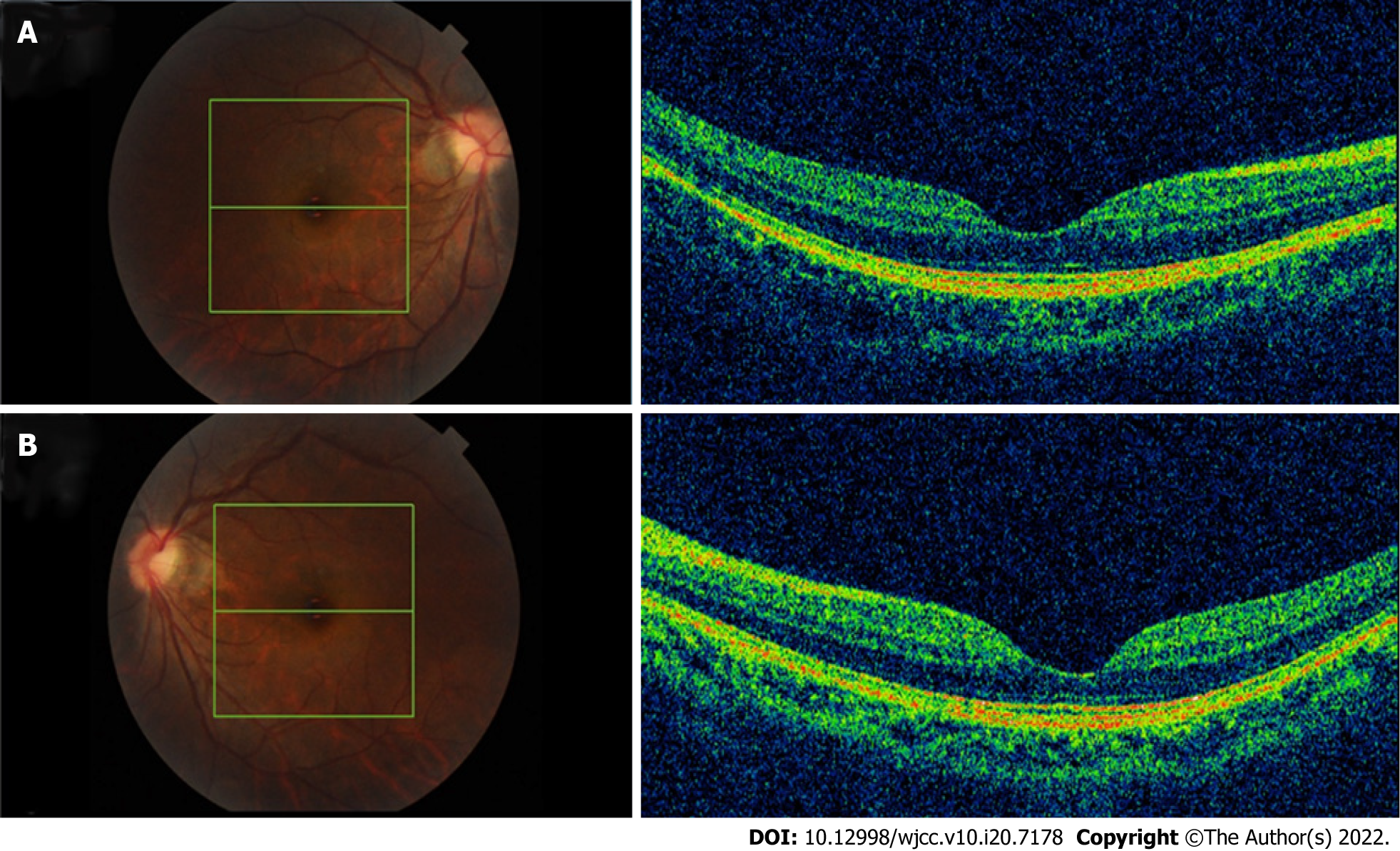
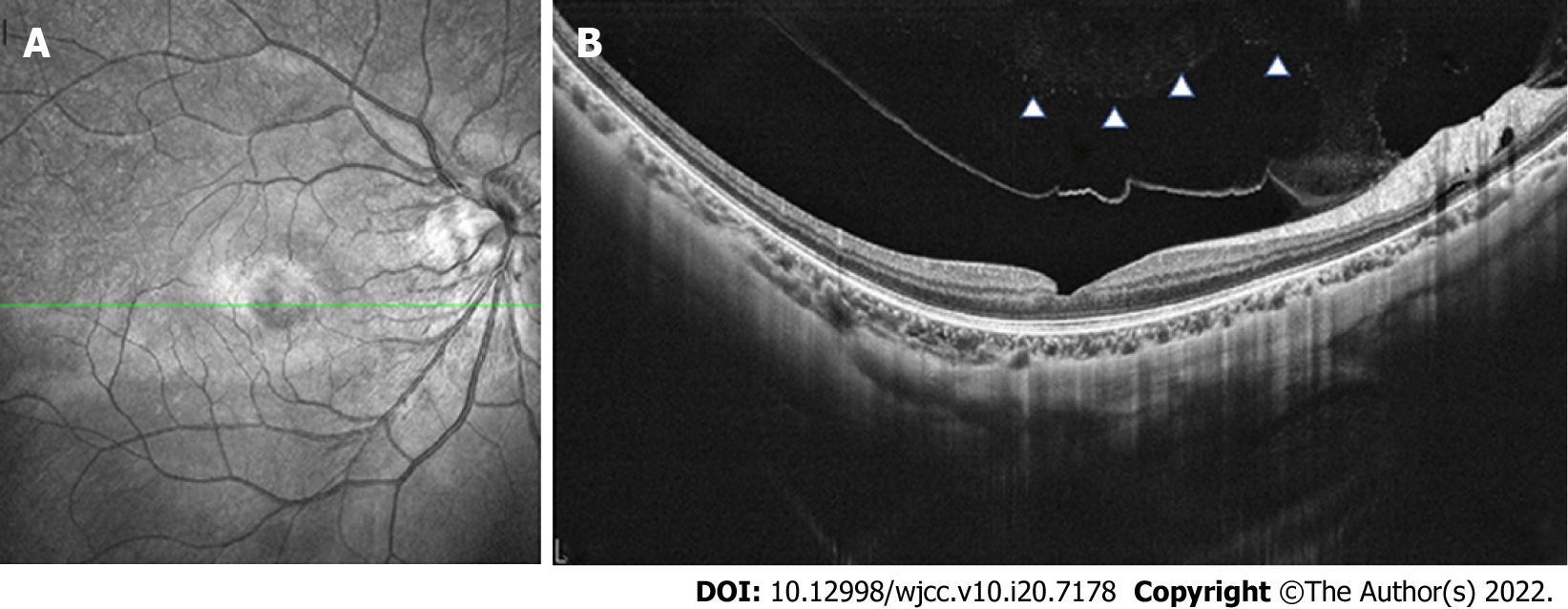
An optical coherence tomography (OCT) revealed a full-thickness MH in the left eye with surrounding subretinal fluid and perifoveal cystic changes at the rim of the hole (Figure 1D), which had a diameter of 520 mm horizontally and 482 mm vertically. In addition, vitreoretinal traction that was induced by partial posterior vitreous detachment (PVD) was documented at the fovea as well as in the area close to the superior vascular arcade, with an increased retinal thickness and retinal layer splitting (Figure 1C). It should be noted that these findings were not present in the preoperative OCT examination of both eyes (Figure 2). The fundus autofluorescence image of the left eye revealed hyper-autofluorescence surrounding the MH (Figure 1B).
Full-thickness MH following bilateral pIOL implantation.
The patient underwent 23-gauge three-port pars plana vitrectomy with triamcinolone-assisted PVD induction. Subsequently, the internal limiting membrane was removed by staining with 0.5% indocyanine green followed by gas tamponade (12% C3F8).
The MH closed at 2 wk after the operation (Figure 1E) and the BCVA had improved to 20/40.
Most MHs are idiopathic and frequently occur in the elderly and women. However, they may also be correlated to high myopia, trauma, and surgeries, including vitrectomy, pneumatic retinopexy, scleral buckling, laser-assisted in situ keratomileusis and cataract surgery[3,4]. In rare cases, MH can occur following pIOL[5,6].
To our knowledge, three cases have been described in two previous reports[5,6]. In one of these cases, the MH had a similar morphology as the traumatic MH with a rhomboid-shaped appearance. In addition, defects in the internal limiting membrane were present and a lack of subretinal fluid and cystic formation was observed. The remaining two cases had similar features as an idiopathic MH with a round-shaped appearance and edema in the perifoveal retina surrounded by subretinal fluid. The present case had characteristics similar to the latter MHs.
Previous case reports have proposed that a MH might have already existed and was misdiagnosed before pIOL implantation; otherwise, it might be a part of the natural course of high myopic change or induced by the surgery[7]. In the present case, preoperative OCT examination revealed normal morphology at the fovea in both eyes, without PVD, posterior staphyloma or foveoschisis, which differs from myopic MH. Therefore, it is likely that the PVD formation was related to the surgery and subsequently led to the occurrence of MH[8,9].
As suggested by Gass, during the PVD process, MH formation is initiated by the tangential traction of the premacular vitreous cortex at the retinal interface which then leads to foveal dehiscence that progresses from foveolar detachment to a mature full-thickness MH[10]. Highly myopic patients tend to show PVD at a younger age and the surgery might accelerate the process. Luna et al[11] have demonstrated that PVD with vitreous liquefaction was not present prior to LASIK but was documented afterwards in 24%−50% of highly myopic eyes. In recent years, in-depth research on posterior precortical vitreous pocket (PPVP) has proved that the aqueous can flow into the PPVP through the Cloquet's canal[10], therefore, we speculated that the flow of aqueous humor caused by fluctuation of intraocular pressure during the surgery was drained into the premacular space which initiated the process of PVD.
Early in the PVD process, the vitreous detaches from the perifoveal area but maintains attachment at the central fovea, the optic disc and the vascular arcade area where vitreoretinal attachments are stronger. Over time, assisted by vitreous movement, anteroposterior tractional forces at the fovea were generated leading to pathogenic vitreous traction. Mori et al[12] presented the hypothesis that dynamic traction attributable to vitreous movement associated with ocular saccades is the most important type of vitreomacular traction in MH formation. A larger PPVP in highly myopic eyes is associated with a greater level of concentrated force and a more pronounced shift in the force vector direction of the anteroposterior tensile stress[10]. The traction force varies depending on the configuration and angle of vitreous insertion into the peripheral retina[12]. Therefore, as the OCT revealed, the vitreofoveal attachment was released in the right eye (Figure 3) which led to a slight abnormality of retinal morphology. In contrast, the MH was formed in the left eye.
Furthermore, focal cellular proliferation of the vitreous face and weak adherence of the retinal pigment epithelium-photoreceptor should not be neglected. In addition, the effect of various refractive surgeries on choroidal thickness (CT) has been reported in recent years. Fang et al[13] evaluated the changes in choroid thickness after pIOL surgery and found that the CT in the foveal was significantly increased at 3 mo after the surgery and then gradually recovered. In all three cases, which had similar features as the present case, the MH formed at 4, 7, and 18 mo after the surgery. Zeng et al[14] considered that choroidal thinning might be one of the reasons for the development of MH. Therefore, the fluctuation of CT might play a role in the formation of MHs; however, the precise relationship needs to be elucidated by more clinical studies.
It remains difficult to determine the risk factors for MH formation after refractive surgery. Thus, further case-control studies are needed to address this question. Although the incidence rate of MH occurrence is low, the present case report emphasizes that it is important to inform patients about the risk of MH occurrence and be aware of the symptoms of this complication. The patients can then receive assistance promptly and efficiently after the onset of MH formation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Ophthalmology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gálvez Salazar P, Ecuador; Sheikh Hassan M, Somalia A-Editor: Zhu JQ, China S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Jiménez-Alfaro I, Benítez del Castillo JM, García-Feijoó J, Gil de Bernabé JG, Serrano de La Iglesia JM. Safety of posterior chamber phakic intraocular lenses for the correction of high myopia: anterior segment changes after posterior chamber phakic intraocular lens implantation. Ophthalmology. 2001;108:90-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 133] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 2. | Jiang T, Chang Q, Wang X, Huang X. Retinal detachment after phakic intraocular lens implantation in severe myopic eyes. Graefes Arch Clin Exp Ophthalmol. 2012;250:1725-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Arevalo JF, Mendoza AJ, Velez-Vazquez W, Rodriguez FJ, Rodriguez A, Rosales-Meneses JL, Yepez JB, Ramirez E, Dessouki A, Chan CK, Mittra RA, Ramsay RC, Garcia RA, Ruiz-Moreno JM. Full-thickness macular hole after LASIK for the correction of myopia. Ophthalmology. 2005;112:1207-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Papathanassiou M, Alonistiotis D, Petrou P, Theodossiadis P, Vergados I. Macular hole formation following phacoemulsification cataract surgery. Clin Exp Optom. 2011;94:112-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Jun JH, Kim YC, Kim KS. Macular hole after phakic intraocular lens implantation: two cases with divergent manifestations. Semin Ophthalmol. 2014;29:213-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Kumar A, Padhy SK, Dhiman R, Kumar P, Parekh T, Varshney T. Macular hole following phakic intraocular lens implantation and its management. Indian J Ophthalmol. 2019;67:1758-1760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Hayashi K, Manabe SI, Hirata A, Yoshimura K. Posterior Vitreous Detachment in Highly Myopic Patients. Invest Ophthalmol Vis Sci. 2020;61:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Kishi S, Shimizu K. Posterior precortical vitreous pocket. Arch Ophthalmol. 1990;108:979-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 118] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Itakura H, Kishi S, Li D, Akiyama H. Observation of posterior precortical vitreous pocket using swept-source optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54:3102-3107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 10. | Gass JD. Idiopathic senile macular hole. Its early stages and pathogenesis. Arch Ophthalmol. 1988;106:629-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 694] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 11. | Luna JD, Artal MN, Reviglio VE, Pelizzari M, Diaz H, Juarez CP. Vitreoretinal alterations following laser in situ keratomileusis: clinical and experimental studies. Graefes Arch Clin Exp Ophthalmol. 2001;239:416-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Mori K, Gehlbach PL, Kishi S. Posterior vitreous mobility delineated by tracking of optical coherence tomography images in eyes with idiopathic macular holes. Am J Ophthalmol. 2015;159:1132-1141.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Fang D, Li Q, Yan K, Xu S, Jiang J, Che X, Zhang Y, Qian Y, Wang Z. Retinal and Choroidal Thickness in relation to C-Reactive Protein on Swept-Source Optical Coherence Tomography. J Immunol Res. 2021;2021:6628224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Zeng J, Li J, Liu R, Chen X, Pan J, Tang S, Ding X. Choroidal thickness in both eyes of patients with unilateral idiopathic macular hole. Ophthalmology. 2012;119:2328-2333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |