Published online Jul 16, 2022. doi: 10.12998/wjcc.v10.i20.6915
Peer-review started: September 6, 2021
First decision: November 7, 2021
Revised: November 11, 2021
Accepted: April 21, 2022
Article in press: April 21, 2022
Published online: July 16, 2022
Processing time: 301 Days and 18.8 Hours
The use of endoscopic submucosal dissection (ESD) for treating early signet ring cell carcinoma (SRC) is controversial due to the risk of lymph node metastasis.
To carry out a meta-analysis to evaluate ESD for therapeutic efficacy and safety in early signet ring cell gastric cancer.
The PubMed, Web of Science, Cochrane Library, and EMBASE databases were used to search for relevant studies evaluating the therapeutic efficacy and safety of ESD in SRC. The rates of recurrence, complete resection, incomplete resection, curative resection, en bloc resection, and adverse events were extracted and analyzed. The methodological quality of the enrolled studies was assessed using the Newcastle-Ottawa Scale. Publication bias was evaluated by the Egger’s test. Institutional review board approval and written consent were not needed for this report.
This meta-analysis enrolled seven studies with 653 participants undergoing ESD treatment for early SRC. The overall recurrence rate was 0.010 [95% confidence interval (CI): 0.000-0.040, Z = 1.422, P = 0.155]. The total lymph
ESD constitutes a promising therapeutic approach for early undifferentiated SRC gastric cancer. However, further improvements are required for increasing its treatment efficacy and reducing adverse outcomes.
Core Tip: Endoscopic submucosal dissection (ESD) is widely used as a curative treatment for early gastric cancer (EGC), whereas its efficacy and safety remain unclear. Totally 653 participants were included in this meta-analysis assessing the therapeutic efficacy and safety of ESD in EGC. Based on the data collected and presented, we conclude that ESD is a promising treatment option for undifferentiated signet ring cell carcinoma EGC. Large, long-term trials are warranted to confirm the current results.
- Citation: Weng CY, Sun SP, Cai C, Xu JL, Lv B. Endoscopic submucosal dissection for early signet ring cell gastric cancer: A systematic review and meta-analysis. World J Clin Cases 2022; 10(20): 6915-6926
- URL: https://www.wjgnet.com/2307-8960/full/v10/i20/6915.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i20.6915
Endoscopic resection procedures, e.g., endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), are broadly utilized to cure early gastric cancer (EGC) with a dismal odds of lymph node metastasis[1]. ESD has many advantages over EMR, including accurate histopathological assessment of resection margins, a reduced recurrence rate, and the possibility of curative resection[2]. In addition, ESD results in decreased morbidity and mortality and increased quality of life in comparison with surgery in EGC with undifferentiated (UD) histology[2]. As a result, ESD is currently considered an effective and widely used treatment for EGC.
ESD is mostly indicated for differentiated gastric cancer due to elevated risk of lymph node metastasis in UD EGC. Notably, however, UD EGC has been recently treated by ESD under specific settings, with favorable results.
UD EGC is utilized for lowly differentiated adenocarcinoma or signet ring cell carcinoma (SRC), despite the lack of specific criteria in the WHO classification[3]. Multiple reports have shown that SRC has improved prognosis and decreased lymph node metastasis rate in comparison with other UD EGC types[4,5]. Additionally, SRC shows a better outcome compared with poorly differentiated tubular adenocarcinoma (an EGC) upon endoscopic therapy[6]. Consequently, SRC may be an indication for ESD. However, ESD for treating SRC EGC remains debatable. Thus, we carried out a meta-analysis for assessing the clinical outcomes of ESD in SRC EGC patients with UD lesions.
PubMed, EMBASE, the Web of Science, and the Cochrane Library were searched using common keywords related to ESD for SRC EGC with UD-type histology (from inception to March 2021). Medical Subject Headings (MeSH) terminology was used because all four databases allow searches using the MeSH terminology. The keywords used included “gastric cancer”, “endoscopic submucosal dissection”, “ESD”, “signet ring cell carcinoma”, or “undifferentiated” using Boolean operators. Only publications on human subjects were searched, and the bibliographies of relevant articles were also reviewed to identify additional studies. The language of publication was not restricted.
Due to a lack of randomized-controlled studies relevant to this topic, we included non-randomized studies meeting the following criteria: (1) Designed to evaluate ESD for SRC EGC with UT histology in the target or control group; and (2) Included at least one outcome (complete resection rate, curative resection rate, en bloc resection rate, recurrence rate, or procedure-related adverse event rate) that enabled an evaluation of feasibility of ESD for SRC EGC with UD-type histology. The exclusion criteria were as follows: (1) Incomplete data; (2) Review article; and (3) Abstract only (study not published as full-text article).
Two of the authors independently evaluated the eligibility of all studies retrieved from the databases based on the predetermined selection criteria. The abstracts of all identified studies were reviewed to exclude irrelevant articles. Full text reviews were performed to determine whether the inclusion criteria were satisfied by the remaining studies. Disagreements between the two evaluators were resolved by discussion or consultation with a third author.
The methodological quality of the enrolled studies was assessed using the Newcastle-Ottawa Scale. This tool comprises three parameters: The selection of the study population, the comparability of the groups, and the ascertainment of the exposure or outcome. Each parameter consists of subcategorized questions: Selection (n = 4), comparability (n = 1) and exposure or outcome (n = 3)[7,8]. The stars awarded for each item allow for a rapid visual assessment of the methodological quality of the studies. A study could be awarded a maximum of nine stars, indicating the highest quality. Two of the authors independently evaluated the methodological quality of all studies, and disagreements between the two evaluators were resolved by discussion or consultation with a third author.
Two of the authors independently extracted the outcomes of all studies, and disagreements between the two evaluators were resolved by discussion or consultation with a third author. The primary outcome was recurrence rate. The secondary outcomes were as follows: (1) En bloc resection rate, i.e., the proportion of cancers removed as a single piece without fragmentation; (2) Complete resection rate, i.e., the proportion of cancers with no neoplastic components at the lateral or vertical margins on microscopic analysis, and no lymphovascular invasion; (3) Curative resection rate, i.e., the proportion of cancers with 20 mm or less intramucosal tumor without ulceration, neoplastic components at the lateral or vertical margins, or lymphovascular invasion; and (4) ESD adverse event rate, i.e., the proportion of cancers whose treatment resulted in procedure-related gastric hemorrhage or perforation.
Pooled effect size with 95% confidence interval (CI) was used to describe the ratio of clinical outcomes (recurrence rate, en bloc resection rate, complete resection rate, etc.) after ESD in SRC EGC patients. Statistical heterogeneity was assessed by the I2 statistic and Cochran's Q test. Then, pooled estimates were obtained using the fixed-effects (Mantel and Haenszel; I2 ≤ 50%, P > 0.1) or random-effects (M-H heterology; I2 > 50%, P ≤ 0.1) model[9]. In addition, sensitivity analysis was applied to evaluate whether the meta-analysis results were stable and reliable. Publication bias was tested by the Begg's test. All analyses were carried out through the application of the commands metan, metaninf, and metabias in STATA 15.1 (StataCorp).
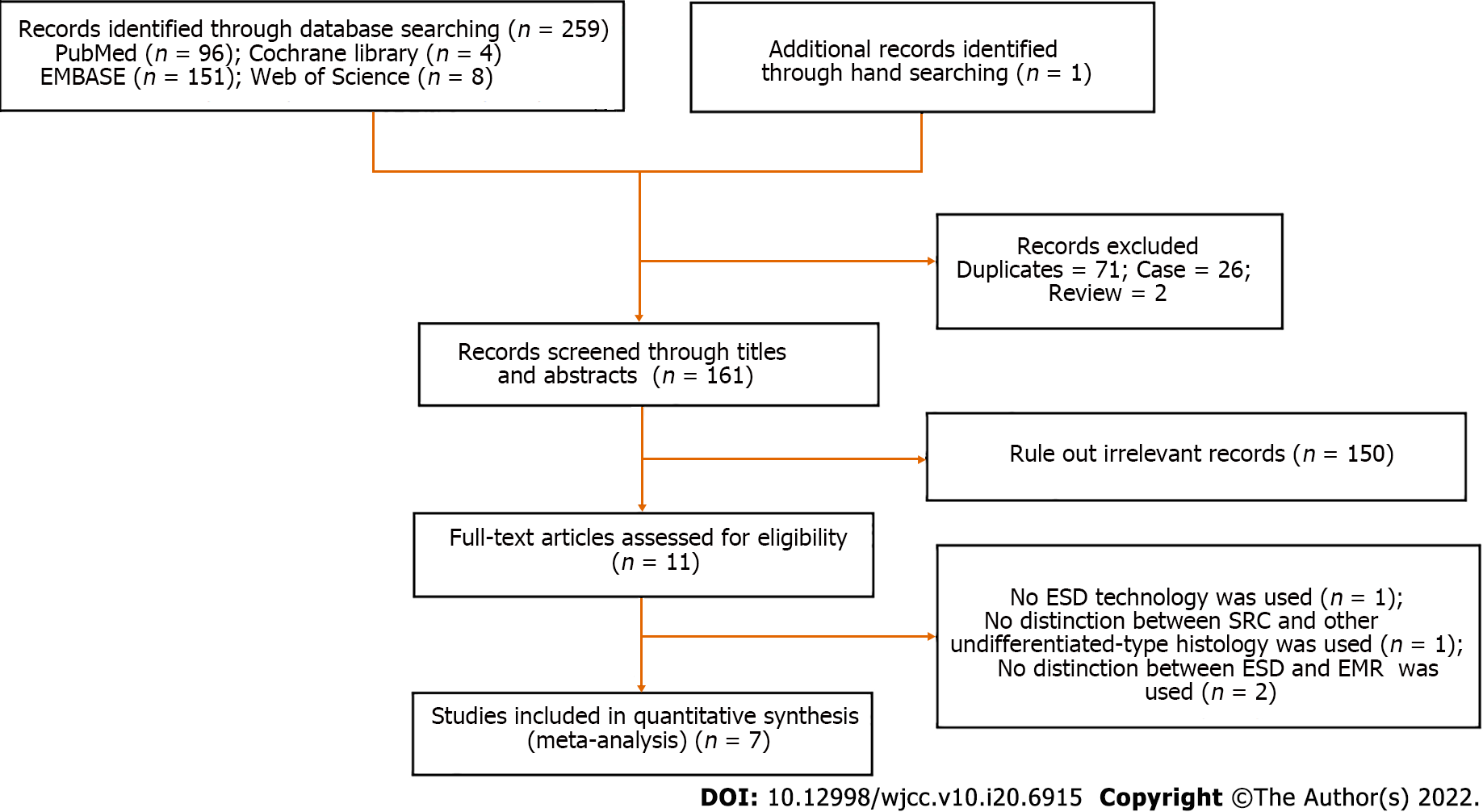
Figure 1 depicts the study selection procedure. Totally 260 reports were retrieved from the four core databases, and the bibliographies of relevant studies were further screened for potential studies of interest. Totally 71 duplicate reports, 2 review articles, 26 case reports, and an additional 150 reports were excluded based on title and abstract. After full text review of the remaining 11 reports, four were further excluded for no ESD technology used (n = 1), no distinction between SRC and other UD-type histology (n = 1), and no distinction between ESD and EMR (n = 2). Therefore, seven non-randomized trials were meta-analyzed.
The seven included reports, involving five from South Korea[10-14] and two from Japan[15,16] investigated 653 cases of UD SRC EGC. The enrolled trials are described in detail in Tables 1 and 2. The analyzed reports were published from 2010 to 2020. The totality of trials were carried out in Asian countries, including six and one in South Korea and Japan, respectively. Six English reports and one Korean report were selected. Four studies reported en bloc resection rates and five reported complete resection and incomplete resection rates. Curative resection rates were reported in two studies. Resection margins were lymphovascular invasion in six studies, and lateral and vertical margin invasion in five (Table 2). Procedure-related adverse events included hemorrhage and perforation in three studies (Table 2).
| Ref. | Location (language) | Total patients | Age (yr) | Gender (male/female) | Size; mm | Tumor location | Gross type; n | Ulceration; n |
| Kang et al[10], 2010 | South Korea (English) | 30 | 55.4 ± 11.3 | 14/16 | 13.6 ± 5.4 | Upper: 0; Middle: 7; Lower: 23 | Elevated and flat: 8; Depressed: 19; Mixed: 3 | Positive: 7 |
| Choi et al[11], 2013 | South Korea (Korean) | 27 | 50.9 ± 11.2 | 14/13 | ≤ 10: 8; >10, ≤ 20: 20 | Upper: 2; Middle: 13; Lower: 13 | Elevated: 5; Flat: 13; Depressed: 10 | NA |
| Kim et al[12], 2014 | South Korea (English) | 126 | 55 (range: 28–85) | 70/56 | < 10: 43; ≥ 10, < 20: 43; ≥ 20: 40 | Upper: 9; Middle: 66; Lower: 55 | Elevated: 10; Flat: 33; Depressed: 20; Mixed: 63 | NA |
| Jeon et al[13], 2018 | South Korea (English) | 36 | 63.6 ± 11.1 | 14/22 | ≤ 20: 32; > 20: 4 | Upper: 1; Middle: 14; Lower: 21 | Elevated: 5; Flat: 8; Depressed: 23 | Positive: 0 |
| Horiuchi et al[15], 2018 | Japan (English) | 129 | 54.6 ± 11.0 | 73/56 | > 20: 2 | Upper: 2; Middle: 13; Lower: 16 | Elevated and flat: 8; Depressed: 19; Mixed: 7 | Positive: 0 |
| Kwak et al[14], 2018 | South Korea (English) | 176 | 52.2 ± 10.8 | 153/178 | NA | NA | Elevated: 39; Flat/Depressed: 292 | Positive: 56 |
| Ahn et al[16], 2020 | Japan (English) | 129 | 53.6 ± 12.1 | 52/77 | Median (IQR): 12 (10–15) | Upper: 6; Middle: 65; Lower: 58 | Elevated: 13; Flat: 13/Depressed: 116 | NA |
| Ref. | Location (language) | Total patients | Depth of invasion, n (%) | Marginal residual tumor | Resection method, n (%) | Result of resection, n (%) | Procedure-related adverse events |
| Kang et al[10], 2010 | South Korea (English) | 30 | Mucosa: 25 (83.3); SM: 5 (16.7) | Vi(+): 1 (3.3) | NA | Cr: 16 (53.3); Ir: 14 (46.7) | NA |
| Li(+): 5 (16.7) | |||||||
| Lmi(+): 9 (30) | |||||||
| Vmi(+): 4 (13.3) | |||||||
| Choi et al[11], 2013 | South Korea (Korean) | 27 | Mucosa: 25 (89.3); SM: 3 (10.7) | Li(+): 1 (3.6) | Ebr: 26 (92.9); Pr: 2 (7.1) | Cr: 25 (89.3); Ir: 3 (10.7) | NA |
| Lmi(+): 1 (3.6) | |||||||
| Vmi(+): 1 (3.6) | |||||||
| Kim et al[12], 2014 | South Korea (English) | 126 | Mucosa: 108 (85.6); SM1: ≤ 500 μm: 4 (3.2); SM2: > 500 μm: 14 (11.2) | Li(+): 6 (4.8) | Ebr: 117 (92.9); Pr: 2 (7.1) | Cr: 81 (64.3); Ir: 45 (35.7) | Gh: 3 (2.4); Gp: 3 (2.4) |
| Lmi(+): 24 (19.0) | |||||||
| Vmi(+): 3 (2.4) | |||||||
| Bmi(+): 3 (2.4) | |||||||
| Jeon et al[13], 2018 | South Korea (English) | 36 | Mucosa: 30 (83.3); SM: 6 (16.7) | Li(+): 1 (2.8) | Ebr: 36 (100.0); Cr: 24 (66.7) | Cr: 28 (77.8); Ir: 8 (22.2) | Gh: 3 (8.3); Gp: 0 |
| Lmi(+): 7 (19.4) | |||||||
| Vmi(+): 0 | |||||||
| Horiuchi et al[15], 2018 | Japan (English) | 129 | Mucosa: 125 (96.9); SM1: ≤ 500 μm: 3 (2.3); SM2: > 500 μm: 1 (0.8) | Li(+): 0 | Ebr: 129 (100); Cr: 121 (93.8) | NA | Gh: 2 (4.8); Gp: 0 |
| Hmi(+): 2 (1.6) | |||||||
| Vmi(+): 0 | |||||||
| Vi(+): 0 | |||||||
| Kwak et al[14], 2018 | South Korea (English) | 176 | NA | Li(+): 10 (11.0%) | Cr: (48.3); size criterion = 1.5 cm, 54.9%; size criterion = 1.0 cm, 63.3%; size criterion = 0.6 cm, 63.6% | NA | NA |
| Ahn et al[16], 2020 | Japan (English) | 129 | Lamina propria: 95 (73.6) | NA | NA | NA | NA |
| Muscularis: 87 (39.9) |
As for methodological quality, there were averagely 7.86 stars awarded, including 7, 8 and 9 in two, four, and one study, respectively (Table 3). Most trials had high quality; therefore, sensitivity analysis according to methodological quality was not carried out.
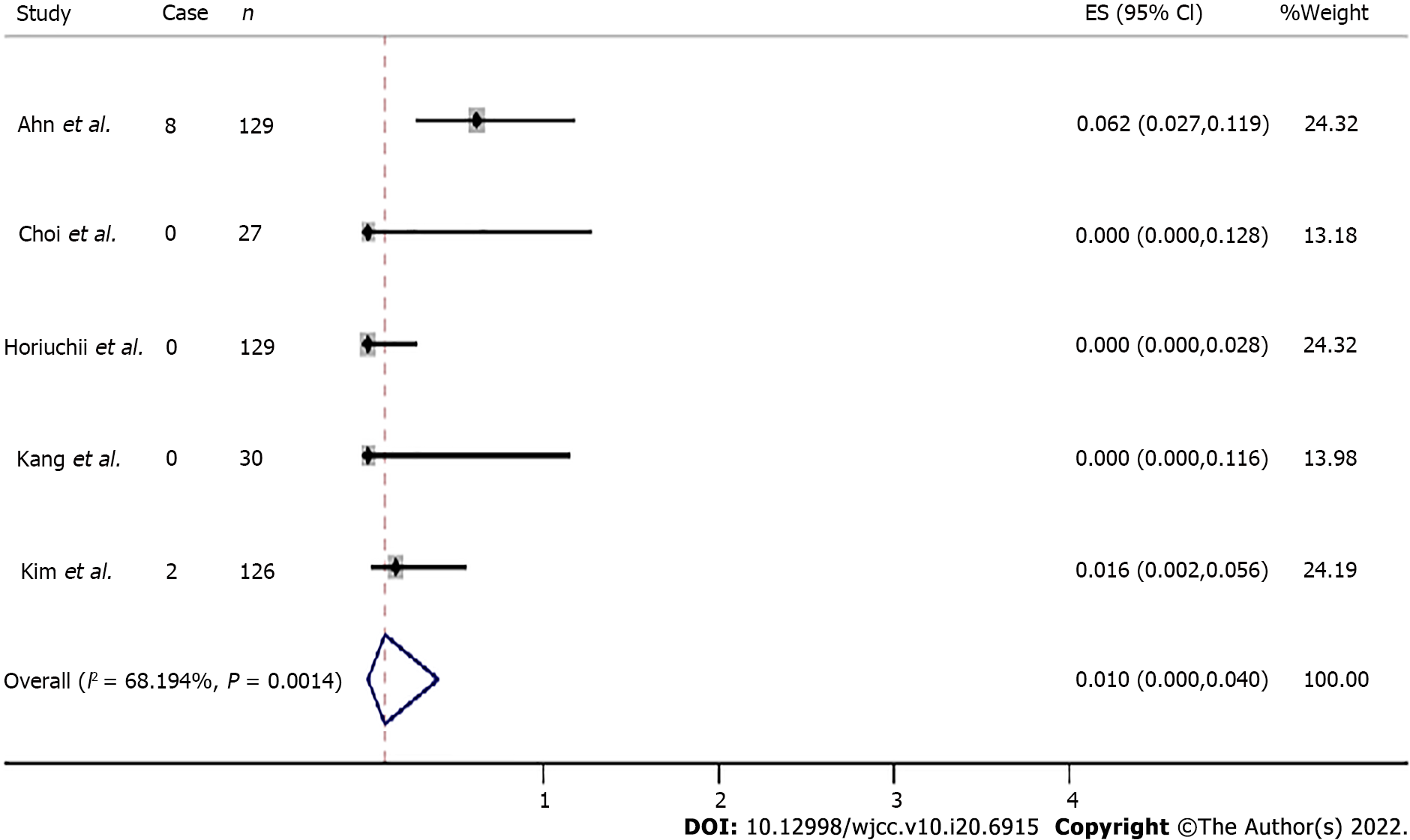
Recurrence rates were available for five studies. During the 16 to 75.6 mo of median follow-up in the 441 included cases, recurrence was found in ten cases after ESD for SRC EGC. The overall recurrence rate was 0.010 (95%CI: 0.000-0.040, Z = 1.422, P = 0.155) (Figure 2).
Invasive depth: Depth of invasion post-ESD for SRC EGC was available in all six articles assessing a total of 477 patients. There were 442 patients with deep invasion into the mucosal layer, and 36 with deep invasion into the submucosal layer. The total mucosal and submucosal invasion rates ranged from 83.3% to 100% and 0% to 16.7%, respectively.
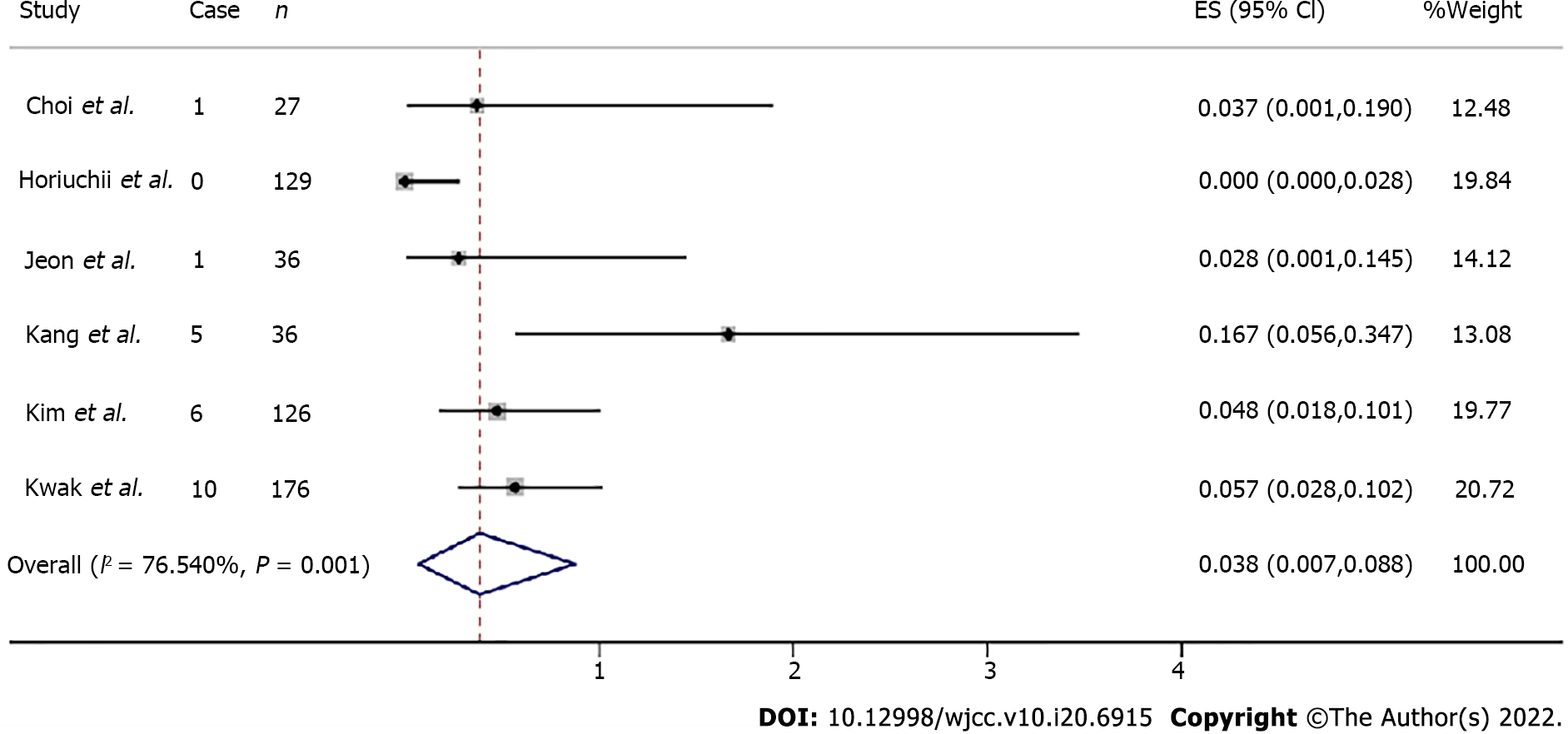
Lymphovascular invasion and resection margin invasion: Lymphovascular invasion was examined in six articles with a total of 524 patients. Four articles including 119 individuals assessed lateral margin invasion and five including 348 individuals evaluated vertical margin invasion. The total lymp
The overall efficacy of ESD for UD EGC was assessed by recurrence and complete, incomplete, en bloc, and curative resection rates. En bloc resection was considered for resection carried out in one piece. In case lesions were removed in many segments, the diverse samples underwent reconstruction to the fullest extent possible. Curative resection was considered in case of intramucosal UD EGC lesions ≤ 20 mm in diameter with no signs of ulceration, no horizontal or vertical margin, and no lymphovascular invasion based on the JGCA gastric cancer treatment guidelines[17].
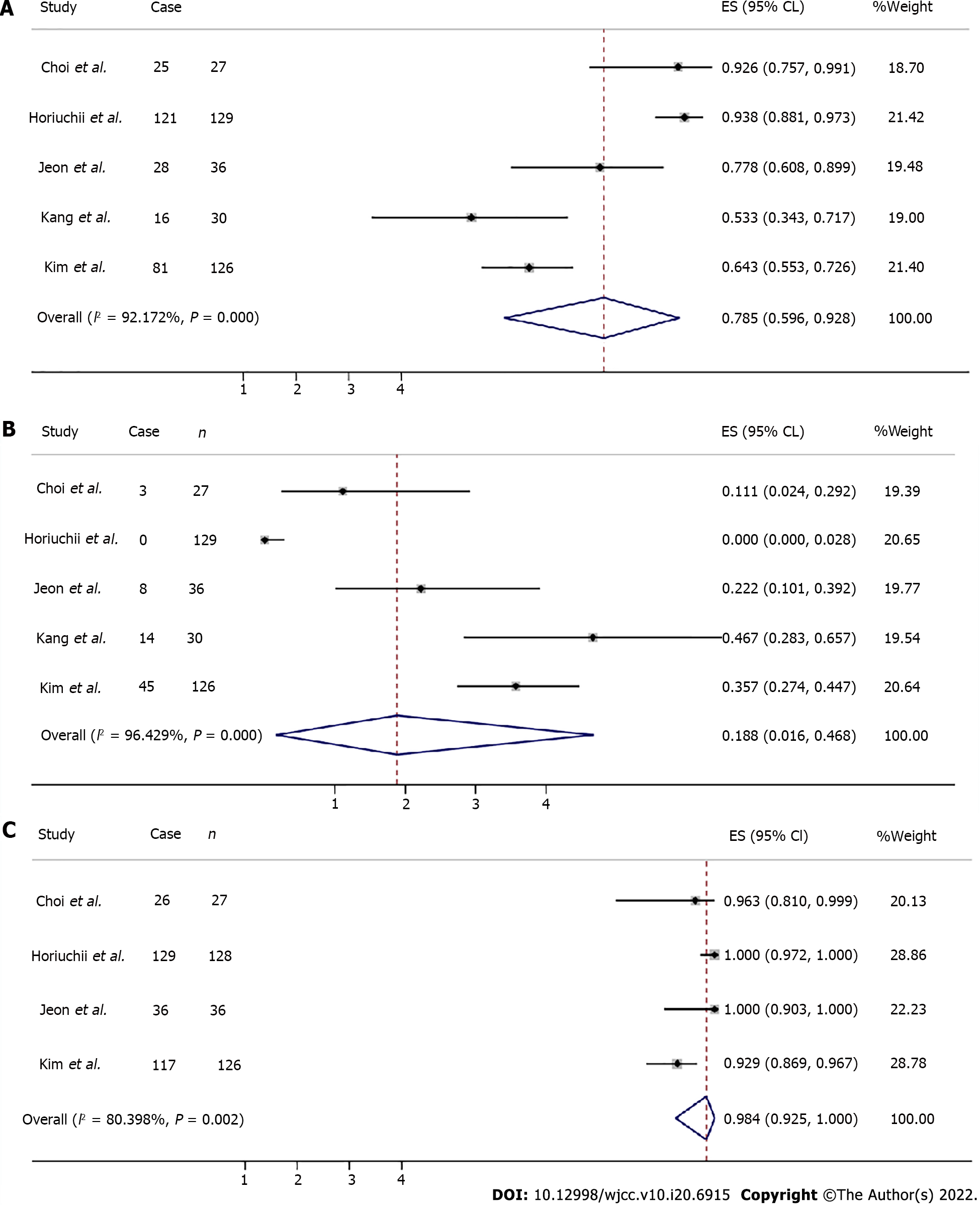
Complete resection data were available in five articles for a total of 348 patients, as well as incomplete resection. The total complete and incomplete resection rates were estimated at 0.785 (95%CI: 0.596-0.928, Z = 9.789, P = 0.000) and 0.188 (95%CI: 0.016-0.468, Z = 2.531, P = 0.011), respectively (Figure 4A and B).
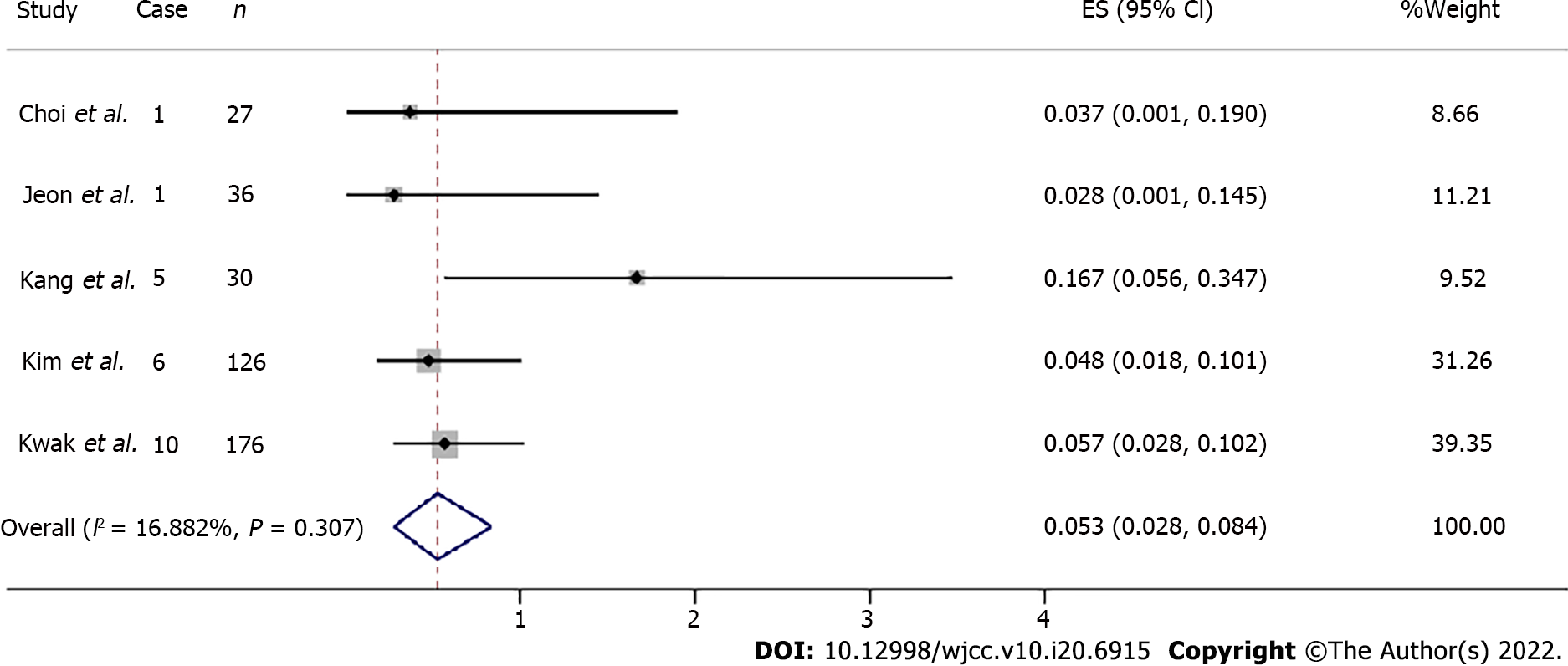
The total en bloc resection rate was estimated at 0.984 (95%CI: 0.925-1.000, Z = 19.463, P = 0.000) (Figure 4C). Total curative resection rates were available in three articles assessing a total of 341 patients, ranging from 46.7% to 93.8%.
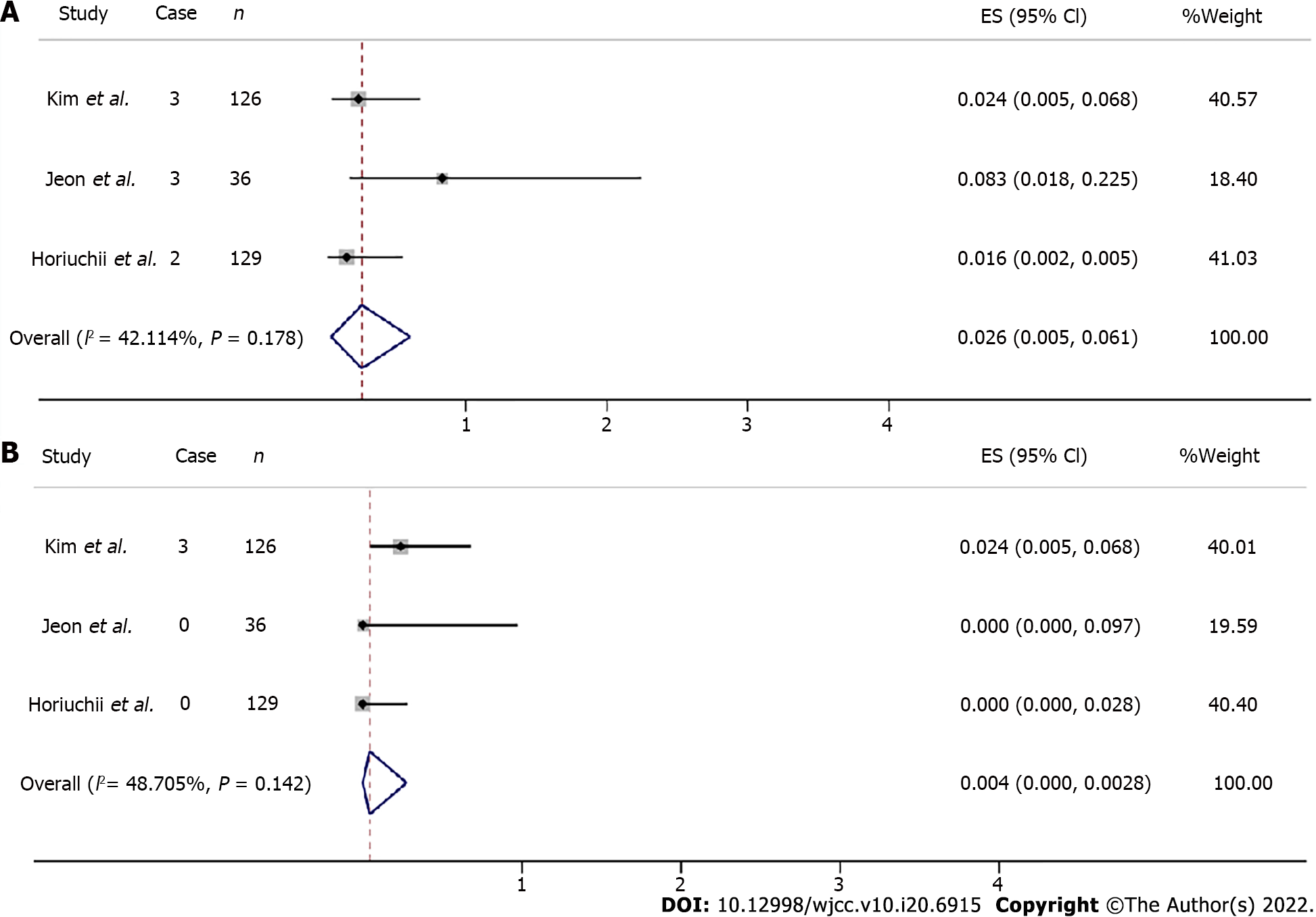
The overall safety of ESD for UD SRC EGC was assessed for procedure-associated adverse events, with sub-analysis based on gastric hemorrhage and perforation. The overall procedure-associated gastric hemorrhage rate was estimated at 0.026 (95%CI: 0.005-0.061, Z = 3.006 P = 0.003) (Figure 5A). Three articles including 291 individuals assessed gastric perforation and three patients showed occurrence. The total procedure-related perforation rate was estimated at 0.004 (95%CI: 0.000-0.028, Z = 0.938, P = 0.348) (Figure 5B).
The one-study-removed meta-analysis both highlighted influential study for the lymphovascular invasion (Figure 6) and curative resection (removed before vs after, I2 = 76.54% vs I2 = 16.882%, I2 = 97.745% vs I2 = 0.0000%, respectively)[15]. As for en bloc resection, Horiuchi et al[15] also showed the biggest effect size (en bloc resection rate: 129/129).
In studies assessing recurrence rates, the Egger’s regression test showed an intercept of -0.269295 (95%CI: -1.642078-1.103488, P = 0.577).
In studies reporting invasive depth rates, the Egger’s regression test showed that the depth of invasion into the mucosal layer had an intercept of -0.8693172 (95%CI: -3.136281-1.397646, P = 0.347); the SM group had an intercept of 1.008564 (95%CI: -1.170687-3.187815, P = 0.268).
For the studies of lymphovascular invasion rate, the Egger’s regression test showed that the intercept was 0.3516007 (95%CI: -0.9736407-1.676842, P = 0.502). For the studies of vertical margin invasion and lateral margin invasion, the Egger’s regression test showed that the intercepts were 0.5103315 (95%CI: -0.8703667-1.89103, P = 0.324) and -0.1883809 (95%CI: -4.773827-4.397065, P = 0.876), respectively.
In studies reporting en bloc, complete, incomplete, and curative resections, the Egger’s regression test revealed intercepts of 0.1672684 (95%CI: -2.680985-3.015522, P = 0.824), -0.5344952 (95%CI: -7.413003 to -6.344013, P = 0.821), 1.027296 (95%CI: -6.759482-8.814074, P = 0.703), and 1.635003 (95%CI: -96.74514-100.0151, P = 0.868), respectively.
Overall, publication bias was not detected in the analysis of total EGC lesions.
In the current meta-analysis, ESD was shown to be a promising therapeutic approach for UD SRC EGC. The total recurrence rate was estimated at 2.27%. The recurrence rate of SRC EGC after ESD should be validated by long-term follow-up. The total mucosal and SM invasion rates were estimated at 92.2% and 7.8%, respectively. The overall en bloc, overall complete, and incomplete resection rates were 98%, 78.5%, and 18.8%, respectively. However, treatment outcomes were not fully satisfactory. A total curative resection rate of 72.1% was obtained. As for procedure-associated adverse events, gastric hemorrhage and perforation rates for UD SRC EGC were 2.6% and 0.04%, respectively.
Gastric SRC represents a type of poorly cohesive carcinoma (WHO classification) that exhibits distinct biologic behaviors compared to other UD EGC[5]. Its particular ring appearance reflects a mucin-rich cytoplasm and crescent-shaped nucleus. On the basis of the Japanese Classification System, gastric SRC cases are considered UD[18]. Contrasting other gastric adenocarcinoma cases, signet ring cells show no intercellular adhesion because of downregulated E-cadherin, reflecting cell-to-cell adhesion[19]. E-cadherin suppression results in the migration to and invasion of surrounding tissues[20]. Thus, SRC patients have a poor prognosis, and surgery represents a treatment of choice. However, the rate of lymph node metastasis was reduced in early SRC EGC (5.3%–7.6%) compared with tumors of other UD histologies (14.7%–17.8%)[21-24] but comparable to differentiated EGC (8.2%–9.8%)[25,26], suggesting that early SRCs are possible candidates for minimally invasive surgery. Therefore, SRC EGC may be more indicated for endoscopic therapy compared with other UD EGCs.
In EGC patients, ESD yielded good long-term outcome, and this approach is increasingly utilized for many diseases[27]. The extended indications for ESD consider three discrete criteria utilized for EGC types I to III[28]. The long-term outcomes of ESD for UD EGC cases meeting these expanded indication criteria were similar to those of surgery for this malignancy.
However, multiple factors hinder ESD application for SRC EGC. For instance, lesion size and margins are not accurately determined. A report examining endoscopic therapy in SRC EGC revealed that tumor sizes are underestimated by 30.2%[12]. However, patient prognosis in SRC EGC has been evaluated on the basis of pathological results instead of endoscopic findings[29,30]. In endoscopic therapy, endoscopists should consider that the actual tumor may have a greater size than that reflected by endoscopy-obtained values, and treating EGC tumors above 2 cm endoscopically may be risky. Therefore, the low complete resection rate is a serious problem. Curative resection rates of 36.4%-65.2% in UD tumors have been reported, and SRC commonly involves lateral margin[31-33]. Another problem is that lymph node dissection is impossible with endoscopic treatment. Reports assessing patient outcome after endoscopic therapy for EGC tumors with no or poor differentiation, e.g., SRC, revealed no distant metastasis and limited lymph node recurrence[31]. In a Japanese trial, EGC recurrence upon surgery showed no association with tumor histology. However, in some cases, SRCs in EGC may spread to distant lymph nodes and organs[34,35]. Endoscopic therapy of SRCs in EGC could be increasingly applied only after overcoming the above issues.
This study is the first meta-analysis of the therapeutic outcomes of ESD for SRC EGC. A strength of the present analysis is the comprehensive literature search, with no language limitations, despite the lack of Western trials. In addition, possible modifiers were examined whenever possible, and data robustness was confirmed by sensitivity analysis.
However, this study also had multiple limitations. First, the included trials had considerable methodological heterogeneity, with potential impact on effect size estimates. The most notable modifiers were outcome heterogeneity and the inconsistent implementation of indications. The published outcomes for en bloc, curative, and complete resection rates varied and were not consistent among the enrolled studies. Second, only retrospective trials were included, indicating a potential effect of selection bias on treatment outcome in ESD. In addition, the histological properties were not divided according to pure SRC or mixed SRC. Furthermore, the country was another significant modifier. The one-study-removed meta-analysis showed the highlighted influential study for lymphovascular invasion and curative resection. The above limitations can result in outcome heterogeneity and publication bias. Since no related prospective/randomized trials have been performed, large, well-organized, long-term follow-up trials are warranted for elucidating ESD feasibility in UD SRC EGC.
ESD is a promising treatment modality for SRC EGC. Nevertheless, the current findings should be cautiously interpreted due to study heterogeneity. Inconsistent implementation of indications, short follow-up, and various nations cause such heterogeneity. Future reports assessing frequent primary outcomes in large and long-term trials are warranted to determine ESD feasibility in SRC EGC.
Endoscopic submucosal dissection (ESD) for the treatment of early signet ring cell carcinoma (SRC) is controversial.
SRC may represent an indication for ESD. Nevertheless, ESD for SRC early gastric cancer (EGC) remains debatable. Therefore, a meta-analysis was carried out for assessing the clinical outcomes of ESD for undifferentiated (UD) SRC EGC cases.
This work aimed to meta-analyzed reports evaluating the therapeutic efficacy and safety of ESD in early SRC gastric cancer.
The PubMed, Web of Science, Cochrane Library, and EMBASE databases were searched for relevant reports evaluating the efficacy and safety of ESD for treating SRC.
The total lymphovascular invasion and en bloc resection rates were 3.8% and 98.4%, respectively. The total complete and incomplete resection rates were estimated at 78.5% and 18.8%, respectively. The total procedure-associated gastric hemorrhage and perforation rates were 2.6% and 0.4%, respectively. The curative resection, vertical margin invasion, and lateral margin invasion rates were 72.1%, 2.3%, and 34.45%, respectively.
ESD represents a promising therapeutic approach for UD SRC EGC. Further improvements are required to increase treatment efficacy and reduce adverse outcomes.
ESD as a treatment tool constitutes a critical step in improving daily clinical practice associated with SRC EGC. Future trials with a larger sample size and longer follow-up duration are warranted to evaluate the long-term efficacy and safety of ESD treatment for SRC EGC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Kawabata H, Japan; Yoshida A, Japan S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Cai YX
| 1. | Hanada Y, Choi AY, Hwang JH, Draganov PV, Khanna L, Sethi A, Bartel MJ, Goel N, Abe S, De Latour RA, Park K, Melis M, Newman E, Hatzaras I, Reddy SS, Farma JM, Liu X, Schlachterman A, Kresak J, Trapp G, Ansari N, Schrope B, Lee JY, Dhall D, Lo S, Jamil LH, Burch M, Gaddam S, Gong Y, Del Portillo A, Tomizawa Y, Truong CD, Brewer Gutierrez OI, Montgomery E, Johnston FM, Duncan M, Canto M, Ahuja N, Lennon AM, Ngamruengphong S. Low Frequency of Lymph Node Metastases in Patients in the United States With Early-stage Gastric Cancers That Fulfill Japanese Endoscopic Resection Criteria. Clin Gastroenterol Hepatol. 2019;17:1763-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Guo HM, Zhang XQ, Chen M, Huang SL, Zou XP. Endoscopic submucosal dissection vs endoscopic mucosal resection for superficial esophageal cancer. World J Gastroenterol. 2014;20:5540-5547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 122] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (3)] |
| 3. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2428] [Article Influence: 485.6] [Reference Citation Analysis (3)] |
| 4. | Chiu CT, Kuo CJ, Yeh TS, Hsu JT, Liu KH, Yeh CN, Hwang TL, Jan YY, Lin CJ. Early signet ring cell gastric cancer. Dig Dis Sci. 2011;56:1749-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Kunisaki C, Shimada H, Nomura M, Matsuda G, Otsuka Y, Akiyama H. Therapeutic strategy for signet ring cell carcinoma of the stomach. Br J Surg. 2004;91:1319-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 95] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077-3079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 814] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 7. | Deeks JJ, Dinnes J, D'Amico R, Sowden AJ, Sakarovitch C, Song F, Petticrew M, Altman DG; International Stroke Trial Collaborative Group; European Carotid Surgery Trial Collaborative Group. Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7:iii-iix, 1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1450] [Cited by in RCA: 1692] [Article Influence: 76.9] [Reference Citation Analysis (0)] |
| 8. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 12634] [Article Influence: 842.3] [Reference Citation Analysis (0)] |
| 9. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11206] [Cited by in RCA: 11027] [Article Influence: 689.2] [Reference Citation Analysis (0)] |
| 10. | Kang HY, Kim SG, Kim JS, Jung HC, Song IS. Clinical outcomes of endoscopic submucosal dissection for undifferentiated early gastric cancer. Surg Endosc. 2010;24:509-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Choi MH, Hong SJ, Han JP, Song JY, Kim DY, Seo SW, Ha JS, Lee YN, Ko BM, Lee MS. [Therapeutic outcomes of endoscopic submucosal dissection in undifferentiated-type early gastric cancer]. Korean J Gastroenterol. 2013;61:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Kim MN, Kim HK, Shim CN, Lee HJ, Lee H, Park JC, Shin SK, Lee SK, Lee YC. Tumour size is related to the curability of signet ring cell early gastric cancer with endoscopic submucosal dissection: a retrospective single centre study. Dig Liver Dis. 2014;46:898-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Jeon HK, Lee SJ, Kim GH, Park DY, Lee BE, Song GA. Endoscopic submucosal dissection for undifferentiated-type early gastric cancer: short- and long-term outcomes. Surg Endosc. 2018;32:1963-1970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Kwak DS, Min YW, Lee JH, Kang SH, Jang SH, Lee H, Min BH, Kim JJ, Kim KM, Sohn TS, Kim S. Outcomes of Endoscopic Submucosal Dissection for Early Gastric Cancer with Undifferentiated-Type Histology: A Clinical Simulation Using a Non-Selected Surgical Cohort. Gut Liver. 2018;12:263-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Horiuchi Y, Fujisaki J, Yamamoto N, Ishizuka N, Omae M, Ishiyama A, Yoshio T, Hirasawa T, Yamamoto Y, Nagahama M, Takahashi H, Tsuchida T. Mixed poorly differentiated adenocarcinoma in undifferentiated-type early gastric cancer predicts endoscopic noncurative resection. Gastric Cancer. 2018;21:689-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Ahn JY, Kim YI, Shin WG, Yang HJ, Nam SY, Min BH, Jang JY, Lim JH, Kim J-, Lee WS, Lee BE, Joo MK, Park JM, Lee HL, Gweon TG, Park MI, Choi J, Tae CH, Kim YW, Park B, Choi IIJ. Comparison between endoscopic submucosal resection and surgery for the curative resection of undifferentiated-type early gastric cancer within expanded indications: a nationwide multi-center study. Gastric Cancer. 2021;24:731-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1575] [Cited by in RCA: 1914] [Article Influence: 239.3] [Reference Citation Analysis (1)] |
| 18. | Sugano H, Nakamura K, Kato Y. Pathological studies of human gastric cancer. Acta Pathol Jpn. 1982;32 Suppl 2:329-347. [PubMed] |
| 19. | Humar B, Blair V, Charlton A, More H, Martin I, Guilford P. E-cadherin deficiency initiates gastric signet-ring cell carcinoma in mice and man. Cancer Res. 2009;69:2050-2056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 20. | Furue M. Epithelial tumor, invasion and stroma. Ann Dermatol. 2011;23:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Hyung WJ, Noh SH, Lee JH, Huh JJ, Lah KH, Choi SH, Min JS. Early gastric carcinoma with signet ring cell histology. Cancer. 2002;94:78-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 139] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Imamura T, Komatsu S, Ichikawa D, Kawaguchi T, Kosuga T, Okamoto K, Konishi H, Shiozaki A, Fujiwara H, Otsuji E. Early signet ring cell carcinoma of the stomach is related to favorable prognosis and low incidence of lymph node metastasis. J Surg Oncol. 2016;114:607-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Kang SH, Kim JS, Moon HS, Lee ES, Kim SH, Sung JK, Lee BS, Jeong HY. Signet ring cell carcinoma of early gastric cancer, is endoscopic treatment really risky? Medicine (Baltimore). 2017;96:e7532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Chen J, Cai R, Ren G, Zhao J, Li H, Guo C, He W, Wu X, Zhang W. Differences in clinicopathological characteristics and computed tomography findings between signet ring cell carcinoma and nonsignet ring cell carcinoma in early and advanced gastric cancer. Cancer Med. 2018;7:1160-1169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Guo CG, Zhao DB, Liu Q, Zhou ZX, Zhao P, Wang GQ, Cai JQ. Risk Factors for Lymph Node Metastasis in Early Gastric Cancer with Signet Ring Cell Carcinoma. J Gastrointest Surg. 2015;19:1958-1965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Ha TK, An JY, Youn HK, Noh JH, Sohn TS, Kim S. Indication for endoscopic mucosal resection in early signet ring cell gastric cancer. Ann Surg Oncol. 2008;15:508-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 27. | Choi IJ, Lee JH, Kim YI, Kim CG, Cho SJ, Lee JY, Ryu KW, Nam BH, Kook MC, Kim YW. Long-term outcome comparison of endoscopic resection and surgery in early gastric cancer meeting the absolute indication for endoscopic resection. Gastrointest Endosc. 2015;81:333-41.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 28. | Yao K, Uedo N, Kamada T, Hirasawa T, Nagahama T, Yoshinaga S, Oka M, Inoue K, Mabe K, Yao T, Yoshida M, Miyashiro I, Fujimoto K, Tajiri H. Guidelines for endoscopic diagnosis of early gastric cancer. Dig Endosc. 2020;32:663-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 131] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 29. | Pernot S, Voron T, Perkins G, Lagorce-Pages C, Berger A, Taieb J. Signet-ring cell carcinoma of the stomach: Impact on prognosis and specific therapeutic challenge. World J Gastroenterol. 2015;21:11428-11438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 178] [Cited by in RCA: 228] [Article Influence: 22.8] [Reference Citation Analysis (3)] |
| 30. | Lee SH, Jee SR, Kim JH, Seol SY. Intramucosal gastric cancer: the rate of lymph node metastasis in signet ring cell carcinoma is as low as that in well-differentiated adenocarcinoma. Eur J Gastroenterol Hepatol. 2015;27:170-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Han JP, Hong SJ, Kim HK. Long-term outcomes of early gastric cancer diagnosed as mixed adenocarcinoma after endoscopic submucosal dissection. J Gastroenterol Hepatol. 2015;30:316-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Abe S, Oda I, Suzuki H, Nonaka S, Yoshinaga S, Odagaki T, Taniguchi H, Kushima R, Saito Y. Short- and long-term outcomes of endoscopic submucosal dissection for undifferentiated early gastric cancer. Endoscopy. 2013;45:703-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 33. | Bang CS, Park JM, Baik GH, Park JJ, Joo MK, Jang JY, Jeon SW, Choi SC, Sung JK, Cho KB. Therapeutic Outcomes of Endoscopic Resection of Early Gastric Cancer with Undifferentiated-Type Histology: A Korean ESD Registry Database Analysis. Clin Endosc. 2017;50:569-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 34. | Kobayashi M, Araki K, Matsuura K, Kawai S, Moriki T. Early gastric cancer giving rise to bone and brain metastases--a review of the Japanese literature. Hepatogastroenterology. 2002;49:1751-1754. [PubMed] |
| 35. | Kang SH, Kim JI, Moon HS, Kang HM, Kim SH, Seong JK, Lee BS, Jeong HY, Song KS, Noh SM, Shin KS, Cho JS. Overt bone marrow metastasis from early gastric cancer. Endoscopy. 2008;40 Suppl 2:E34-E35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |