Published online Jul 6, 2022. doi: 10.12998/wjcc.v10.i19.6728
Peer-review started: February 12, 2022
First decision: March 25, 2022
Revised: April 7, 2022
Accepted: April 30, 2022
Article in press: April 30 2022
Published online: July 6, 2022
Processing time: 131 Days and 23.5 Hours
Familial hypercholesterolemia (FH) is an autosomal dominant disorder that is characterized by severely increased low-density lipoprotein (LDL) cholesterol levels. At the same time, elevated LDL levels accelerated the development of coronary heart disease. Several classes of drugs are currently in use to treat FH. Proprotein convertase subtilisin/kexin type 9 inhibitor (PCSK9i) is novel one of these.
This manuscript reports a case of FH that responded modestly after treatment with PCSK9i and statin drugs. Of even more concern is that the patient frequently admitted to the hospital during a 12-year follow-up period. Subsequently, we identified a heterozygous mutation, 1448G>A (W483X) of the LDL receptor (LDLR) in this patient. The serum levels of PCSK9 (proprotein convertase subtilisin/kexin type 9) in the patient was 71.30 ± 26.66 ng/mL, which is close the average level reported in the literature. This LDLR mutation affects LDLR metabolism or structure, which may make it unsuitable for use of PCSK9i.
Our outcome demonstrates that LDLR-W483X represents a partial loss-of-function LDLR and may contribute to PCSK9i ineffective. In the meanwhile, additional measures are therefore required (particularly with gene sequencing or change the treatment plan) must be initiated as early as possible. Genetic testing for clinically challenging cases who do not respond to PCSK9i therapy is very helpful.
Core Tip: We report a male Chinese patient diagnosed with Familial hypercholesterolemia with a heterozygous mutation, 1448G>A (W483X), of the low-density lipoprotein receptor (LDLR). By reviewing the literature, we speculate that the mutation may affect LDLR metabolism or structure and may lead to that responded modestly after treatment with Proprotein convertase subtilisin/kexin type 9 inhibitor.
- Citation: Yang L, Xiao YY, Shao L, Ouyang CS, Hu Y, Li B, Lei LF, Wang H. Proprotein convertase subtilisin/kexin type 9 inhibitor non responses in an adult with a history of coronary revascularization: A case report. World J Clin Cases 2022; 10(19): 6728-6735
- URL: https://www.wjgnet.com/2307-8960/full/v10/i19/6728.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i19.6728
Coronary heart disease (CHD) is a major cause of hospitalization and mortality worldwide[1]. And elevated low-density lipoprotein (LDL) levels accelerated this process[2]. Here, we reported a patient diagnosed with familial hypercholesterolemia (FH) experienced seven percutaneous coronary interventions. It is no exaggeration to say that the patient is at a higher risk for cardiovascular events all the time. In previous reports, common causes of repeated coronary interventions are as follows: (1) Patients who do not consistently take the medicine or have missed doses; (2) Patients with clopidogrel resistance[3]; (3) Patients do not respond to hyperglycemia and hyperlipidemia drug therapy or nonattainment; and (4) Other reasons. Of these, FH is one of the most common causes of myocardial infarction[4]. The epidemiology of FH is complicated, and it is difficult to estimate the prevalence of FH in China because of underdiagnosed. Based on a population study in Arabian Gulf, the prevalence of heterozygous FH is approximately one in 300 individuals[5]. This patient had poor lipid control (Even though Proprotein convertase subtilisin/kexin type 9 inhibitor (PCSK9i) was used), which resulted in repeated coronary interventions. The purpose of this case report is to recommend lifestyle intervention and intensive lipid-lowering treatment beginning early in life to reduce the risk of coronary heart disease. This case is significant because it demonstrates the necessity of gene sequencing or switching medications.
A 43-year-old Chinese man came to our department with chest tightness for more than 4 d.
Chest tightness is located behind the sternum lasts about 2-3 min. And no other symptoms such as abdominal distension, abdominal pain, cold, and fever. According to the patient’s Medical Record files, he had no known medication allergies.
On August 4, 2009, Due to frequent chest tightness, coronary angiography was performed on the patient. The coronary angiogram revealed severe vascular calcification and stenosis in the proximal and middle left anterior descending coronary artery, the middle segment of the right coronary artery and circumflex coronary arteries. Ultimately, he chose conservative treatment.
Subsequently, he presented to our hospital on September 17, 2009, with a complaint of chest tightness for more than 3 d. After angiography, the results showed stenosis in the left anterior descending artery (60%-70%), the circumflex coronary arteries (80%-90%) and a total occlusion of the mid-right coronary artery. We implanted four stents (rapamycin-eluting stents, Lepu medical) into the stenotic segment at that time. Three months later, the patient came to the hospital again due to chest pain. Coronary angiography showed stenosis in the proximal (95%) and middle (60%) left anterior descending artery, and proximal mid-right coronary artery (60%). There was no in-stent restenosis of drug-eluting stents. At that time, two stents were implanted (proximal and middle left anterior descending coronary artery).
Four years later, the patient returned to our hospital for further treatment. During this hospital admission, angiography reveals results as follows: The stenosis in the proximal left anterior descending artery (85%), mid circumflex coronary arteries (70%) and a proximal of the right coronary artery (95%). However, there was no in-stent stenosis.
On August 25, 2013, he was admitted to our department with palpitation for 5 d. The results revealed there were no in-stent stenosis; the stenosis in the proximal-mid circumflex coronary arteries (70%) and distal portion of the right coronary artery (40%). At that time, no specific treatment was administered.
According to hospital data, the patient was admitted on December 29, 2015; July 27, 2019; and June 2021, respectively. All lesions were treated by Paclitaxel-coated balloon (2019-SeQuent Please NEO®, B. Braun; 2021-Vesselin®, Lepu medical).
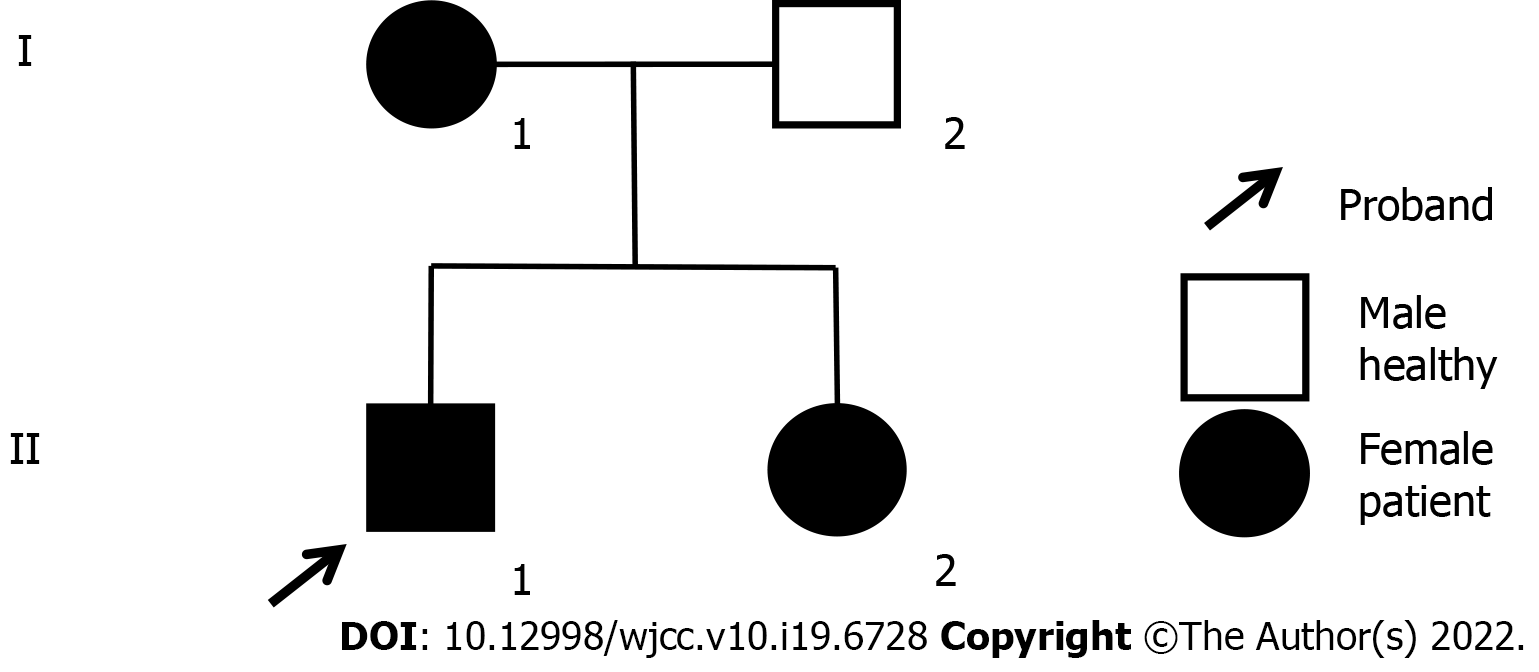
His mother and sister were also hospitalized with a diagnosis of FH. His mother had a history of sequelae of cerebral infarction for several years. In this case, we investigated the family diagnosed with FH (Figure 1). The proband was II-1(the patient), who’s average serum LDL was 222.76 mg/dL (5.77 mmol/L). A total of 3 members of this family (I-1, II-1, II-2) were diagnosed as FH.
Who weighed 72 kg and was 170 cm tall, with no changes in cardiopulmonary auscultation. All other associated signs: The varying sizes of the yellow nodules scattered throughout both upper limbs elbow joints and wrist joints. A soft mass, 45.5 mm in diameter, was palpable at the knee joints of both lower extremities. No other abnormality was found on physical examination.
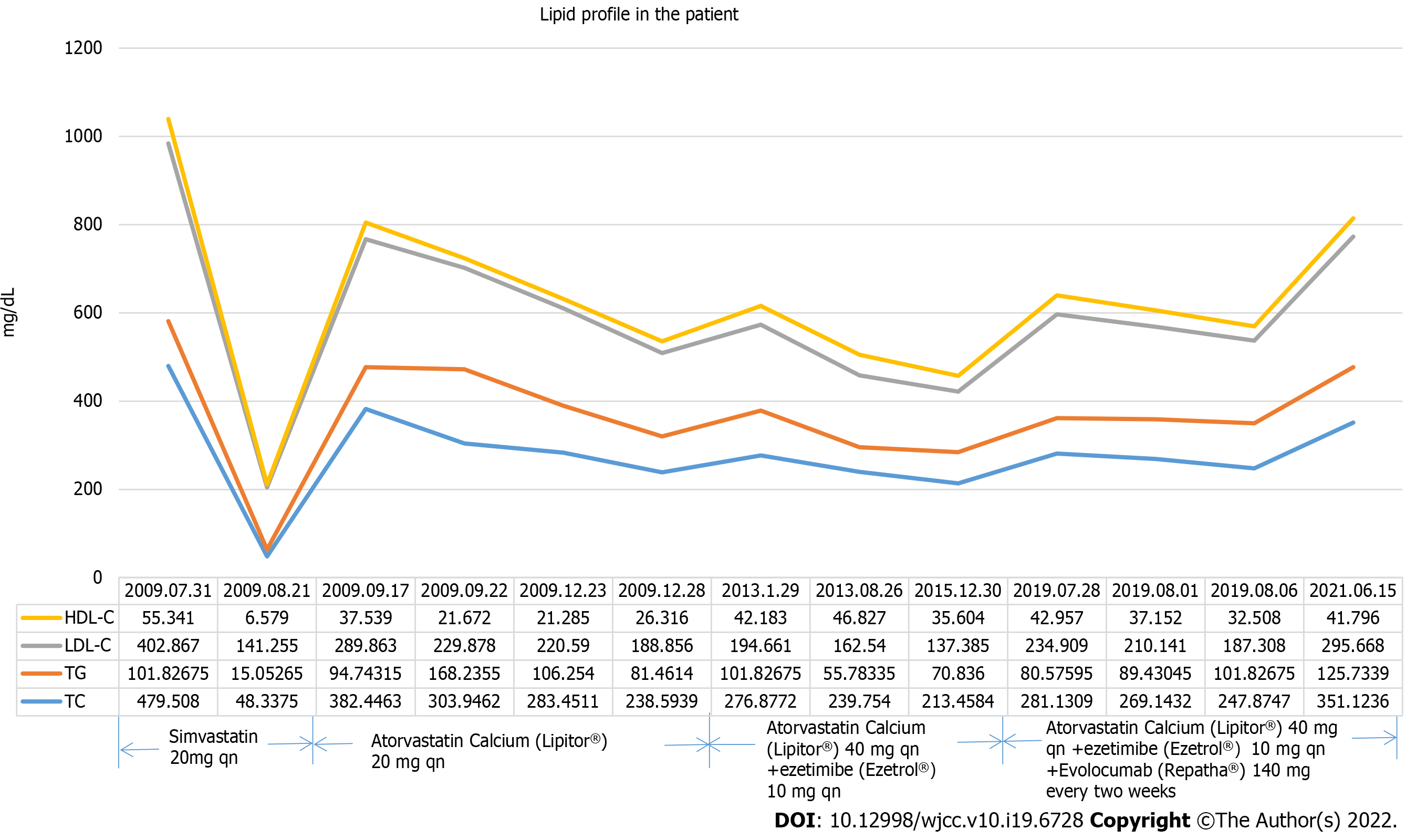
The major laboratory results of the patient are listed as follows: TG 94.8 mg/dL (1.07 mmol/L), TC 382.3 mg/dL (9.89 mmol/L), HDL 37.5mg/dL (0.97 mmol/L), LDL 289.6mg/dL (7.49 mmol/L) (Results at the first examination). Lipid profile in this patient (2009-2021) is presented in Figure 2. The lowest LDL value (grey solid line) is 141.10 mg/dL (3.65 mmol/L) (August, 2009) with the highest value 402.4 mg/dL (July 2009). The average value (222.76 mg/dL) is substandard. The results of routine blood tests (complete blood count, liver and renal function) were normal.
Chest X-ray was normal. Electrocardiogram showed sinus rhythm with a heart rate of 95 bpm, as well as incomplete left bundle branch block. Carotid ultrasound demonstrated atherosclerosis plaques bilaterally in carotid artery. LV ejection fraction was 55% with regional wall motion abnormality. B-mode ultrasound showed gallbladder stone with no notable abnormalities of the liver, pancreas or spleen.
This is a rare case that was diagnosed as definitive FH with coronary heart disease, according to the Dutch clinical lipid network (Family history-1; Clinical history-2; Physical examination-6; Investigation-5; Total 14-Definite FH>8). Criteria for the diagnosis of coronary artery disease: defined as 50% or more stenosis in one or more major epicardial vessels[6].
The physicians made recommendations, and more detailed planning (coronary artery bypass surgery) and implementation were carried out in collaboration with the patient. However, the patient refused this treatment. It was too expensive for him. The patient had received aspirin 100 mg QD, clopidogrel 75 mg QD (clopidogrel resistance have been excluded) and simvastatin 20mg QN before the admission to our hospital. After admission to our hospital, we strengthened treatment. For that, bisoprolol 2.5 mg, once a day, benazepril hydrochloride tablets 5 mg QD were prescribed. On the third admission, treatment with tirofiban 0.1μg/ (kg∙min) was added. In 2013, atorvastatin was increased from 20 mg QN to 40 mg QN and ezetimibe (10 mg, once daily), while clopidogrel 75 mg QD was changed to ticagrelor 90 mg BID. The patient did not reach the standard after 10 years of statin therapy. According to AHA guidelines, PCSK9i (140mg, twice a month, Evolocumab Injection®, Amgen Manufacturing Limited.), as a new drug, was added for the treatment (since September, 2019)[7]. The patient was put on it for 6 months. During the follow-up period of 6 mo, proper technique for injection was confirmed. However, the effectiveness was unsatisfactory.
He said “I took my medication correctly”. The patient managed conservatively with a low-fat dietary. The LDL level gradually decreased from the month (July 2019) after using PCSK9i. The value declined approximately by 41.69% when compared to the highest values, whereas it hadn’t reached the standard[8]. We also realize that future experiments may be necessary to further treatment. The patient and his mother were informed and gave their informed consent. Blood samples were drawn from the patient and his mother. A mutational analysis was performed in the present case to detect mutations in the serum sample.
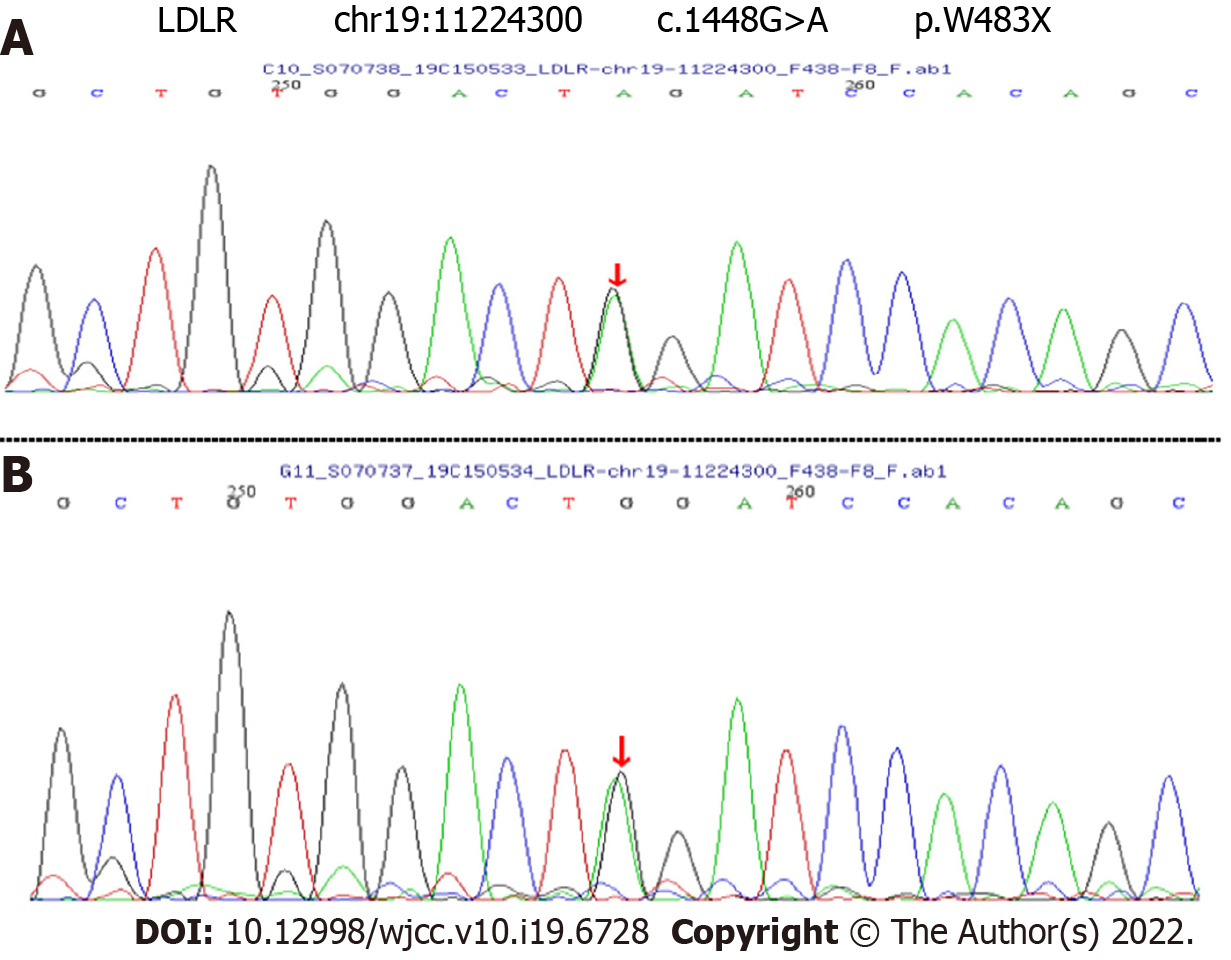
By means of high-throughput sequencing technology, two LDLR mutations and one APOB mutation were identified in the patient and his mother. There were two LDLR heterozygous mutations: (1) C.1448G>A (p.W483X); and (2) C.10700C>T (p.T3567M). The mutations described above were verified by verification of pedigree. Possible causative gene is c.1448G>A (p.W483X) according to the results (Figure 3).
Due to the mechanism of action of PCSK9i, meanwhile, we had to understand whether increased PCSK9 Level plays pivotal role during the process[9]. In this review, an algorithm to assess possible PCSK9i resistance has been proposed to classify hypo-responders and identify mechanism by Warden et al[9]. The algorithm was based on measuring plasma PCSK9 concentrations before and after treatment with a PCSK9i. In our case-report, the average serum level (the patient) of PCSK9 was 71.30 ± 26.66 ng/mL (prior to treatment). They are close the average reported in the literature (68.29 ± 28.73) ng/mL[10]. The difference was not statistically significant (P > 0.05). However, the patient was not well educated and he was not patient enough to complete all the measurements. So, plasma PCSK9 concentrations (after treatment) cannot be obtained.
Our patient is still alive now. The patient did not complain of chest tightness. The use of PCSK9i therapy was terminated due to the ineffectiveness. Further therapy (such as LDL apheresis) was discussed with the patient; however, due to the risk of complications and financial constraints, this was not started.
There is no doubt that there are indications for the use of PCSK9i among FH patients [11, 12]. In previous studies, most FH patients presented with higher LDL levels. Even if three classes of lipid-lowering drugs (statin, ezetimibe and PCSK9i) are used, it is still very difficult to achieve treatment goal for LDL-C. However, few of the studies reported ineffectiveness of the drug (PCSK9i)[13]. The patient’s average LDL value during his hospitalization was 222.763 mg/dL, that greatly exceed requirement 69.5mg/dL (the control goal)[14]. During the patient’s hospitalization, his serum LDL level decreased by approximately 15.92% compared to baseline values. Undoubtedly, LDL level control was not achieved.
Due to the mechanism of action of PCSK9i, then, we had to understand whether increased PCSK9 Level or loss-of-function mutations in LDLR plays pivotal role during the process. Once before, scholars reported on that statin have been shown to increase serum PCSK9 Level among FH patients[15]. Moreover, elevated PCSK9 Levels were an independent predictor for adverse cardiovascular outcomes in diabetic patients with stable CHD[16,17]. Based on this, the PCSK9 Level in patient who still taking statins was measured (his sister and parents declined participation). The average serum levels of PCSK9 are close the average reported in the literature [10]. That suggesting that there are some other reasons for the ineffective of using PCSK9i.
Subsequent genetic testing was performed (Figure 3). Both the patient and his mother had a heterozygous pathogenic mutation in the LDLR gene (1448G>A, W483X). The mutation was determined to be pathogenic according to the ACMG guidelines. Nonsense mutations in LDLR may result in loss of gene functions. The site of the mutation had been reported[18,19]. The heterozygosity of the loci was investigated in his mother with pedigree-based and genomic analyses. We also note that one APOB mutation was identified in the patient and his mother. However, the LDL level in FH who caused by APOB gene mutations are significantly lower than the others. We think that mutation was not crucial for the patient's phenotype. Jiang L et al[18] concluded that either LDLR binding or internalization activity of W483X was lower than the wild-type in transfected HEK-293 cells. Bioinformatics prediction and an in vitro function experiment showed that the W483X mutation is mainly retained in the ER and had serious functional defects caused by the truncated mutant protein. Therefore, we can speculate that the LDLR mutation induces lower LDLR mRNA levels in FH patients than in controls. Structural defects of the LDLR or LDLR level limit the efficacy of the drug because lacks internalization of LDL. This may explain the ineffective use of the PCSK9i in the patient. Delia Susan-Resiga.etc identified an FH patient presenting novel compound heterozygote mutations R410S and G592E of the LDL receptor (LDLR)[20]. The patient responded modestly to maximum rosuvastatin plus ezetimibe therapy, even in combination with a PCSK9i monoclonal antibody injection. The study demonstrates that LDLR-R410S of the LDL-R resulting in defective delivery of LDL to lysosomes, which is similar to that which we report here. Warden et al[9] also focus on several cases about unusual responses to PCSK9 inhibitors[21]. The authors explain why something can a fully blocked PCSK9 not exert an effect on plasma LDL-C levels. One of these reasons (mutations in LDL receptors or its ligands ApoB or ApoE that render them less susceptible to PCSK9 inhibition) was similar to our case report. In contrast, not all mutations are related to the ineffective of PCSK9i. Biochemical and cellular functional analyses suggest that some functional mutations such as D374Y result in a 10-to-25-fold increase in PCSK9 affinity for LDLR[22]. PCSK9i may be a very effective drug for these patients. In clinical treatment, however, this hypothesis remains unproved.
In any case, for patients with FH, the LDL level should be controlled and treated with early and timely diagnosis. If the outcome is poor, gene analysis should be performed in a timely manner. Based on this case, we strongly recommend that screening and treatment in young FH patients as early as possible. The mutation (W483X) may affect LDLR structure and lead to that responded modestly after treatment with PCSK9 inhibitor (PCSK9i). We are also looking forward to additional novel classes of lipid-lowering drugs
The authors would like to acknowledge the patients and their family members for taking part in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Chinese Society of Cardiology.
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Papadopoulos VP, Greece; Pradhan A, India S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Tuppo EE, Trivedi MP, Kostis JB, Daevmer J, Cabrera J, Kostis WJ; Myocardial Infarction Data Acquisition System (MIDAS 39) Study Group. The role of public health vs invasive coronary interventions in the decline of coronary heart disease mortality. Ann Epidemiol. 2021;55:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Grundy SM, Vega GL, Kesäniemi YA. Abnormalities in metabolism of low density lipoproteins associated with coronary heart disease. Acta Med Scand Suppl. 1985;701:23-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Lee K, Lee SW, Lee JW, Kim SY, Youn YJ, Ahn MS, Kim JY, Yoo BS, Yoon J, Choe KH. The significance of clopidogrel low-responsiveness on stent thrombosis and cardiac death assessed by the verifynow p(2)y(12) assay in patients with acute coronary syndrome within 6 mo after drug-eluting stent implantation. Korean Circ J. 2009;39:512-518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Li S, Zhang HW, Guo YL, Wu NQ, Zhu CG, Zhao X, Sun D, Gao XY, Gao Y, Zhang Y, Qing P, Li XL, Sun J, Liu G, Dong Q, Xu RX, Cui CJ, Li JJ. Familial hypercholesterolemia in very young myocardial infarction. Sci Rep. 2018;8:8861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Al-Rasadi K, Al-Zakwani I, Alsheikh-Ali AA, Almahmeed W, Rashed W, Ridha M, Santos RD, Zubaid M. Prevalence, management, and outcomes of familial hypercholesterolemia in patients with acute coronary syndromes in the Arabian Gulf. J Clin Lipidol. 2018;12:685-692.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Chang SM, Hakeem A, Nagueh SF. Predicting clinically unrecognized coronary artery disease: use of two- dimensional echocardiography. Cardiovasc Ultrasound. 2009;7:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Gidding SS, Champagne MA, de Ferranti SD, Defesche J, Ito MK, Knowles JW, McCrindle B, Raal F, Rader D, Santos RD, Lopes-Virella M, Watts GF, Wierzbicki AS; American Heart Association Atherosclerosis, Hypertension, and Obesity in Young Committee of Council on Cardiovascular Disease in Young, Council on Cardiovascular and Stroke Nursing, Council on Functional Genomics and Translational Biology, and Council on Lifestyle and Cardiometabolic Health. The Agenda for Familial Hypercholesterolemia: A Scientific Statement From the American Heart Association. Circulation. 2015;132:2167-2192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 519] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 8. | Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, Benetos A, Biffi A, Boavida JM, Capodanno D, Cosyns B, Crawford C, Davos CH, Desormais I, Di Angelantonio E, Franco OH, Halvorsen S, Hobbs FDR, Hollander M, Jankowska EA, Michal M, Sacco S, Sattar N, Tokgozoglu L, Tonstad S, Tsioufis KP, van Dis I, van Gelder IC, Wanner C, Williams B; ESC Scientific Document Group. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur J Prev Cardiol. 2022;29:5-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 305] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 9. | Warden BA, Fazio S, Shapiro MD. The PCSK9 revolution: Current status, controversies, and future directions. Trends Cardiovasc Med. 2020;30:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 10. | Cui Q, Ju X, Yang T, Zhang M, Tang W, Chen Q, Hu Y, Haas JV, Troutt JS, Pickard RT, Darling R, Konrad RJ, Zhou H, Cao G. Serum PCSK9 is associated with multiple metabolic factors in a large Han Chinese population. Atherosclerosis. 2010;213:632-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | Landmesser U, Chapman MJ, Farnier M, Gencer B, Gielen S, Hovingh GK, Lüscher TF, Sinning D, Tokgözoglu L, Wiklund O, Zamorano JL, Pinto FJ, Catapano AL; European Society of Cardiology (ESC); European Atherosclerosis Society (EAS). European Society of Cardiology/European Atherosclerosis Society Task Force consensus statement on proprotein convertase subtilisin/kexin type 9 inhibitors: practical guidance for use in patients at very high cardiovascular risk. Eur Heart J. 2017;38:2245-2255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Langslet G, Emery M, Wasserman SM. Evolocumab (AMG 145) for primary hypercholesterolemia. Expert Rev Cardiovasc Ther. 2015;13:477-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Rallidis LS, Liberopoulos EN, Vlachopoulos C, Skoumas I, Kolovou G, Anastasiou G, Dima I, Tousoulis D, Iliodromitis E. Very high-risk familial hypercholesterolaemia patients in real life: The remaining gap in achieving the current LDL-C targets despite the use of PCSK9 inhibitors. Atherosclerosis. 2020;309:67-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Díaz Rodríguez Á; Grupo de Trabajo de Lípidos de SEMERGEN. [Guidelines for the management of dyslipidemia]. Semergen. 2014;40 Suppl 4:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Careskey HE, Davis RA, Alborn WE, Troutt JS, Cao G, Konrad RJ. Atorvastatin increases human serum levels of proprotein convertase subtilisin/kexin type 9. J Lipid Res. 2008;49:394-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 254] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 16. | Peng J, Liu MM, Jin JL, Cao YX, Guo YL, Wu NQ, Zhu CG, Dong Q, Sun J, Xu RX, Li JJ. Association of circulating PCSK9 concentration with cardiovascular metabolic markers and outcomes in stable coronary artery disease patients with or without diabetes: a prospective, observational cohort study. Cardiovasc Diabetol. 2020;19:167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Li S, Zhang Y, Xu RX, Guo YL, Zhu CG, Wu NQ, Qing P, Liu G, Dong Q, Li JJ. Proprotein convertase subtilisin-kexin type 9 as a biomarker for the severity of coronary artery disease. Ann Med. 2015;47:386-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 18. | Jiang L, Sun LY, Pan XD, Chen PP, Tang L, Wang W, Zhao LM, Yang SW, Wang LY. Characterization of the unique Chinese W483X mutation in the low-density lipoprotein-receptor gene in young patients with homozygous familial hypercholesterolemia. J Clin Lipidol. 2016;10:538-546.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Jiang L, Sun LY, Dai YF, Yang SW, Zhang F, Wang LY. The distribution and characteristics of LDL receptor mutations in China: A systematic review. Sci Rep. 2015;5:17272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Susan-Resiga D, Girard E, Kiss RS, Essalmani R, Hamelin J, Asselin MC, Awan Z, Butkinaree C, Fleury A, Soldera A, Dory YL, Baass A, Seidah NG. The Proprotein Convertase Subtilisin/Kexin Type 9-resistant R410S Low Density Lipoprotein Receptor Mutation: A NOVEL MECHANISM CAUSING FAMILIAL HYPERCHOLESTEROLEMIA. J Biol Chem. 2017;292:1573-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Warden BA, Miles JR, Oleaga C, Ganda OP, Duell PB, Purnell JQ, Shapiro MD, Fazio S. Unusual responses to PCSK9 inhibitors in a clinical cohort utilizing a structured follow-up protocol. Am J Prev Cardiol. 2020;1:100012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Cunningham D, Danley DE, Geoghegan KF, Griffor MC, Hawkins JL, Subashi TA, Varghese AH, Ammirati MJ, Culp JS, Hoth LR, Mansour MN, McGrath KM, Seddon AP, Shenolikar S, Stutzman-Engwall KJ, Warren LC, Xia D, Qiu X. Structural and biophysical studies of PCSK9 and its mutants linked to familial hypercholesterolemia. Nat Struct Mol Biol. 2007;14:413-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 365] [Article Influence: 20.3] [Reference Citation Analysis (0)] |