Published online Jul 6, 2022. doi: 10.12998/wjcc.v10.i19.6349
Peer-review started: January 13, 2022
First decision: March 8, 2022
Revised: March 11, 2022
Accepted: May 8, 2022
Article in press: May 8, 2022
Published online: July 6, 2022
Processing time: 162 Days and 4.7 Hours
Helicobacter pylori (H. pylori) infection is highly prevalent in East Asia. The overall seroprevalence rate of H. pylori infection is 44.2% in China, 37.6%-43.2% in Japan, and 51.0% in South Korea. H. pylori can cause peptic ulcer disease and gastric cancer. East Asian countries have high rates of gastric cancer (age-standardized incidence rate: 20-30 per 100000). The Kyoto global consensus report emphasized that H. pylori gastritis should be considered the main cause for the development of gastric cancer. H. pylori treatment guidelines in China, Japan, and South Korea have recently been revised according to data from each of those countries. However, emerging antibiotic resistance is an important barrier to H. pylori eradication. The recommended H. pylori treatment regimens differ among those three East Asian countries. In this review, recent guidelines and up-to-date research on H. pylori treatment regimens from China, Japan, and South Korea are discussed.
Core Tip: Since 2000, the standard triple regimen containing clarithromycin (CAM) has been used as a legacy therapy to eradicate Helicobacter pylori (H. pylori). Resistance to CAM by H. pylori has increased to > 15% in East Asia. First-line eradication rates below 80% are strongly associated with CAM-resistant H. pylori strain emergence. H. pylori treatment guidelines in China, Japan, and South Korea were revised according to new data. In China, adding bismuth to H. pylori regimens was recommended as an empirical first-line treatment. In Japan, H. pylori treatment success increased when the potassium-competitive acid blocker (P-CAB) was introduced. In South Korea, tailored H. pylori eradication based on molecular testing for CAM resistance is used as the first-line treatment option. Dual therapy involving frequent administration of high-dose amoxicillin has shown good efficacy for H. pylori eradication in clinical trials. Furthermore, P-CABs, with their rapid and strong acid-suppressing activity, may contribute to successful H. pylori treatment in future.
- Citation: Cho JH, Jin SY. Current guidelines for Helicobacter pylori treatment in East Asia 2022: Differences among China, Japan, and South Korea. World J Clin Cases 2022; 10(19): 6349-6359
- URL: https://www.wjgnet.com/2307-8960/full/v10/i19/6349.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i19.6349
Helicobacter pylori (H. pylori) can cause peptic ulcer disease and gastric cancer[1]. In H. pylori–infected stomachs, gastric atrophy and intestinal metaplasia are linked to gastric cancer development[2]. H. pylori is present in more than half of the world’s population[3]. The overall seroprevalence rate of H. pylori infection is 44.2% in China, 37.6%-43.2% in Japan, and 51.0% in South Korea[4-6]. Gastric cancer is the fourth most common cause of cancer-related mortality worldwide[7]. Almost half of the incident cases and deaths occur in East Asia. In 2019, the age-standardized incidence rate of gastric cancer per 100000 was 30.64 in China, 28.29 in Japan, and 28.67 in South Korea[8].
The Kyoto Global Consensus recommends H. pylori treatment to prevent gastric cancer in countries with a high incidence thereof[9]. In Japan, eradication therapy for all H. pylori-positive subjects has been covered by the national insurance system since 2013[10]. In China, H. pylori treatment was strongly recommended for preventing primary gastric cancer in a recent consensus report[11]. In 2018, the South Korean government insurance system started to cover eradication therapy for H. pylori gastritis. However, primary antibiotic resistance of H. pylori has increased in East Asia, so obtaining successful therapeutic outcomes using antibiotic regimens is challenging[12]. To prevent primary gastric cancer, H. pylori should be successfully eradicated. Based on a comparison of recent guidelines, this review focuses on the current status of H. pylori treatment in China, Japan, and South Korea in terms of H. pylori resistance to antibiotics, recommended H. pylori treatment regimens, and up-to-date results of H. pylori therapy in the three countries.
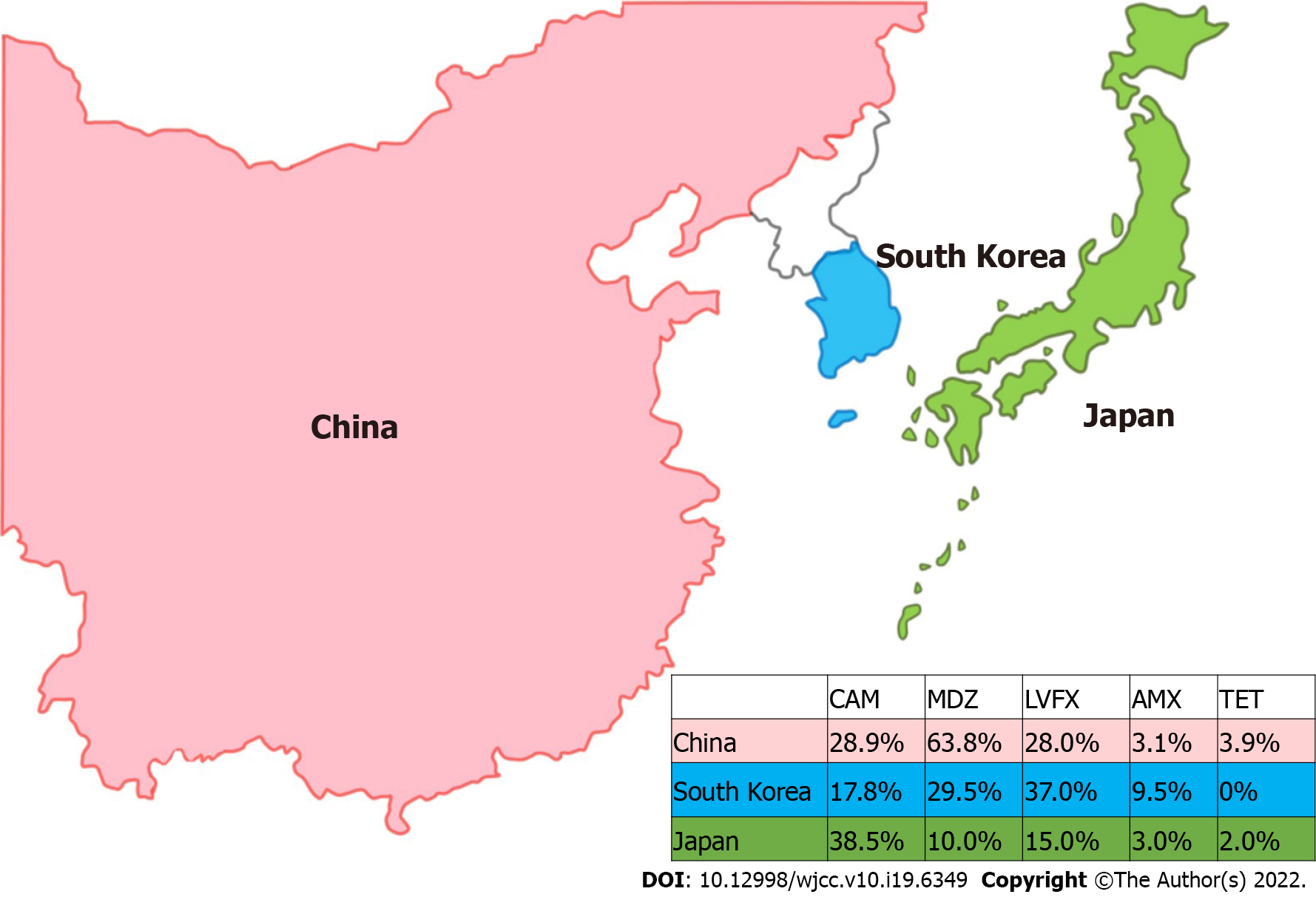
Since 2000, the standard triple regimen containing clarithromycin (CAM) has been used as a legacy therapy to eradicate H. pylori[13]. Macrolides have been widely used to treat other infectious diseases. Accordingly, the number of CAM-resistant H. pylori strains has increased rapidly in many countries over the past decade[14]. In East Asia, CAM resistance of H. pylori has increased by > 15%. Therefore, first-line H. pylori eradication rates using triple therapy have decreased to < 80%[15]. H. pylori resistance rates to metronidazole (MDZ) and fluoroquinolone are also high in East Asia (Figure 1). In China, primary resistance of H. pylori to CAM, MDZ, and levofloxacin (LVFX) is high and has increased over time (28.9%, 63.8%, and 28.0%, respectively)[16]. These patterns of H. pylori resistance are similar to those in South Korea. A recent nationwide study reported H. pylori resistance rates to CAM, MDZ, and LVFX of 17.8%, 29.5%, and 37.0%, respectively[17]. In Japan, the CAM resistance rate has increased gradually from 7% in 2000 to 38.5% in 2014[18]. The resistance rate of H. pylori to LVFX is relatively high at approximately 15%[19]. In Japan, MDZ resistance is lower (< 10%) than in other Asian countries. Low MDZ resistance against H. pylori is thought to be correlated with antibiotic consumption in the community. The use of MDZ is strictly regulated in Japan, where it has been approved for the treatment of only selected diseases, such as trichomoniasis[20]. In contrast, the H. pylori resistance rates to amoxicillin (AMX) and tetracycline (TET) are equally low among the three countries (3.1% and 3.9% in China, 3.0% and 2.0% in Japan, and 9.5% and 0% in South Korea, respectively)[21].
Some differences in H. pylori treatment regimens exist among the guidelines of China, Japan, and South Korea. Bismuth-based H. pylori regimens are strongly recommended in China. In Japan, potassium-competitive acid blockers (P-CABs) are widely used to eradicate H. pylori. The current South Korean guidelines are similar to those of Western countries. Notably, molecular testing to detect CAM resistance is recommended as the initial treatment option.
According to the Fifth Chinese Consensus for H. pylori Management of 2016, seven H. pylori therapies containing bismuth salt are recommended as empirical regimens[11]. The various antibiotic combinations include two of the following six antimicrobial agents: AMX, CAM, MDZ, TET, LVFX, and furazolidone (FZD). The recommended eradication regimens for H. pylori include AMX and CAM, AMX and MDZ, AMX and LVFX, AMX and FZD, AMX and TET, MDZ and TET, and FZD and TET (Table 1). The standard dose of a proton pump inhibitor (PPI) and 220 mg of bismuth are prescribed twice daily with the two antibiotics. Bismuth has long been used to treat peptic ulcer disease, dyspepsia, parasite infections, and infectious diarrhea[22]. The antibacterial effects of bismuth include inhibition of protein and cell wall synthesis in H. pylori. The main role of bismuth is to increase the eradication rate by 30%-40% in resistant H. pylori strains[23].
| Country | Regimen | Drugs | Duration (d) | |
| China | 1 | PPI bid + bismuth 220 mg bid + AMX 1000 mg bid + CAM 500 mg bid | 14 | |
| 2 | PPI bid + bismuth 220 mg bid + AMX 1000 mg bid + MDZ 400 mg tid or qid | 14 | ||
| 3 | PPI bid + bismuth 220 mg bid + AMX 1000 mg bid + LVFX 500 mg qd or 200 mg bid | 14 | ||
| 4 | PPI bid + bismuth 220 mg bid + AMX 1000 mg bid + FZD 100 mg bid or tid | 14 | ||
| 5 | PPI bid + bismuth 220 mg bid + AMX 1000 mg bid + TET 500 mg tid or qid | 14 | ||
| 6 | PPI bid + bismuth 220 mg bid + MDZ 400 mg tid or qid + TET 500 mg tid or qid | 14 | ||
| 7 | PPI bid + bismuth 220 mg bid + FZD 100 mg bid + TET 500 mg tid or qid | 14 | ||
| Japan | First-line | PPI or vonoprazan 20 mg bid + AMX 750 mg bid + CAM 200 mg bid | 7 | |
| Second-line | PPI bid + AMX 750 mg bid + MDZ 250 mg bid | 7 | ||
| Third-line | PPI bid + AMX 750 mg or MDZ 250 mg bid + STFX 100 mg bid | 7 | ||
| South Korea | First-line | Standard triple therapy | PPI bid + AMX 1000 mg bid + CAM 500 mg bid | 14 |
| Sequential therapy | PPI bid + AMX 1000 mg bid (5 d), then CAM 500 mg bid + MDZ 500 mg bid (5 d) | 10 | ||
| Concomitant therapy | PPI bid + AMX 1000 mg bid + CAM 500 mg bid + MDZ 500 mg bid | 10 | ||
| Tailored therapy | Standard triple therapy (CAM-sensitive) or classic BQT (CAM-resistant) | 7-14 | ||
| Second-line | Classic BQT | PPI bid + bismuth 120 mg qid + MDZ 500 mg tid + TET 500 mg qid | 10-14 | |
| Third-line | - | PPI bid + AMX 1000 mg bid + LVFX 500 mg qd or 250 mg bid | 10-14 | |
Zhang et al[24] reported on a 14-d modified bismuth quadruple therapy (BQT) containing CAM or MDZ that was effective against H. pylori in a region with high resistance to CAM (26.5%) and MDZ (45.5%). CAM- and MDZ-containing regimens displayed high eradication rates of 88.8% and 88.9% in the intention-to-treat (ITT) analysis, and 94.9% and 96.9% in the per-protocol (PP) analysis, respectively. In an MDZ-containing regimen, 1 g of AMX twice daily was used as a substitute for 500 mg of TET four times daily. Notably, a modified BQT containing AMX and MDZ was demonstrated to be effective (> 90%) in MDZ-resistant H. pylori strains. A high dosage (1500 mg/d) of MDZ for 14 d can overcome H. pylori resistance[25]. Chen et al[26] performed a comparative study between the classic BQT and a modified one containing AMX and MDZ as a rescue H. pylori therapy. In comparison with the classic BQT, the modified BQT achieved a similar eradication rate (87.2%-95.3% vs 88.5%-93.7%). Adverse events occurred less frequently with the modified than the classic BQT regimen (34.0% vs 51.9%, P = 0.001).
FZD is a nitrofuran antibiotic effective against gram-negative and -positive bacteria[27]. Qiao et al[28] reported that an FZD-based BQT showed a similar first-line H. pylori eradication rate in PP analysis to a CAM-based BQT (95.8% vs 93.4%). The resistance rates of H. pylori to AMX, TET, and FZD remain low in China at < 5%[29]. Accordingly, a modified BQT containing FZD/AMX and FZD/TET achieved a > 90% H. pylori eradication rate in patients who did not respond to the previous treatment[30]. Compared to the classic BQT, two FZD-containing regimens resulted in less frequent adverse events. The H. pylori eradication efficacy of regimens employing AMX and TET may not be inferior to other regimens[31]. However, TET and FZD have limited availability[32].
Interestingly, the recommended H. pylori therapies were not categorized into first- and second-line regimens. This strategy is markedly different from Japanese and South Korean guidelines, in which the first- and second-line regimens are divided. Any of the seven regimens can be prescribed to patients to eradicate H. pylori. After failed initial therapy, a second-line regimen is selected from among the remaining regimens. Among the antibiotic combinations, the LVFX-containing regimen is not recommended as an initial treatment due to a high resistance rate[33]. It can be considered an alternative to rescue therapy in combination with bismuth salt. AMX, TET, and FZD can be reused after treatment failure because these drugs rarely produce secondary resistance. Repeat MDZ prescription requires an optimized dose (at least 1500-1600 mg/d). In contrast, reuse of CAM and LVFX should be avoided after failed eradication.
Since H. pylori treatment for chronic gastritis was approved by the Japanese national health insurance system in 2013, prescriptions for eradication therapy have markedly increased. Approximately 8.5 million H. pylori-positive patients received eradication regimens from 2013 to 2019[34]. The revised 2016 guidelines were similar to previous ones[35]. As a first-line regimen, standard triple therapy was still recommended to eradicate H. pylori (Table 1). The AMX and CAM dosages were lower than those of China and South Korea. The recommended AMX and CAM doses are 750 and 200 mg twice daily, respectively. The 200 mg dose of CAM twice daily has similar efficacy to 400 mg twice daily, and no significant difference was found between 7- and 14-d treatment durations. However, adverse drug events, such as dysgeusia, occurred more frequently during the 14-d therapy.
In Japan, the successful eradication rates for all types of H. pylori therapy decreased to < 80% in 2014. Since the P-CAB was launched in February 2015, the eradication rates of triple therapy, including AMX and CAM with P-CAB, have been significantly higher than those with conventional PPIs[36]. The novel P-CAB vonoprazan (VPZ) exerts a rapid and sustained suppressive effect on gastric acid for optimal H. pylori treatment[37]. The proportion of regimens including VPZ has increased rapidly, and is now 80%[34]. As first-line treatment, standard triple therapy containing 20 mg VPZ twice daily resulted in higher H. pylori eradication rates than those of a PPI-based regimen (86.4%-91.2% vs 71.7%-79.4%)[38,39]. There was no significant difference between the VPZ- and PPI-based triple regimens for eradicating CAM-susceptible H. pylori strains. H. pylori eradication rates were 87.3% and 76.5% (P = 0.21) in the ITT analysis, and 88.9% and 86.7% (P = 0.77) in the PP analysis, respectively[40]. However, VPZ was superior to the PPI-based triple regimen for CAM-resistant H. pylori strains (73.2%-87.5% vs 40.0%-53.8%)[41].
CAM resistance is a strong contributor to H. pylori eradication failure after first-line treatment[42]. Therefore, MDZ-based triple therapy with PPI/VPZ is recommended as the second-line eradication therapy. MDZ (250 mg) is prescribed twice daily for 7 d. The MDZ-based H. pylori treatment results in a higher eradication rate (> 90%) than CAM-based triple therapy because of the low MDZ resistance in Japan[43,44]. In a meta-analysis by Shinozaki et al[45], VPZ was more efficacious for second-line H. pylori eradication compared to conventional PPIs. Seven-day VPZ, AMX, and MDZ triple therapy can be a strong candidate as an empirical H. pylori regimen. Sitafloxacin (STFX)-based triple therapy has been recommended as a third-line therapy in combination with AMX or MDZ[46]. STFX is a new quinolone antibacterial agent expected to be efficacious due to its low minimum inhibitory concentration (MIC) for H. pylori, even for LVFX-resistant strains[47]. STFX has a good H. pylori eradication effect in combination with AMX or MDZ, even for third-line treatment[48].
First-line H. pylori therapies consist of three empirical regimens and one tailored eradication regimen (Table 1)[49]. One of the following empirical regimens can be chosen: standard triple therapy for 14 d, non-BQT for 10 d, or classic BQT for 10-14 d. Standard triple therapy consists of a standard PPI dose, 1000 mg of AMX, and 500 mg of CAM, twice daily for 14 d. The eradication rate of 14-d standard triple therapy is superior to those of the 7- and 10-d therapeutic regimens. The pooled eradication rate of 14-d therapy was 78.1%, which is unacceptable for successful H. pylori eradication.
Non-BQT has been divided into concomitant and sequential therapy. A PPI, 500 mg of CAM, 1 g of AMX, and 500 mg of MDZ are prescribed twice daily for 10 d for the concomitant therapy. The sequential therapy consists of a PPI and 1 g of AMX twice daily for 5 d, followed by the PPI, 500 mg of CAM, and 500 mg of MDZ twice daily for 5 d. Concomitant and sequential therapy resulted in good H. pylori eradication rates of 94.2% and 91.7% in a modified ITT analysis, and 95.6% and 91.4% in the PP analysis, respectively[50]. Sequential therapy was superior to 7- and 10-d standard triple therapy as a first-line treatment[51]. However, the role of sequential therapy in H. pylori eradication is diminishing according to Western guidelines[52]. Lee et al[53] reported a higher eradication rate for concomitant than sequential therapy (94.4% vs 84.4%, P = 0.018). A recent nationwide study demonstrated that the H. pylori eradication rate of concomitant therapy is significantly higher than that of sequential therapy (91.8% vs 86.1%, P < 0.001)[54]. Antibiotic overuse and the emergence of multidrug-resistant H. pylori may be problematic for concomitant therapy[55].
As the last of the empirical first-line therapies, the classic BQT (standard dose PPI twice a day, MDZ 500 mg three times a day, TET 500 mg, and bismuth 120 mg four times a day) is administered for 10-14 d. However, the guidelines report high rates of adverse drug events. The relatively high rates of adverse events and high pill burden are problematic[56]. According to the South Korean nationwide registry database, the prescription rate of the classic BQT as a first-line therapy is currently low (2.63%)[57]. Thus, the classic BQT can be considered as a rescue therapy for other regimens.
Tailored H. pylori eradication shows promise for achieving more successful outcomes before treatment, compared to conventional therapies[58]. Antimicrobial susceptibility testing using a culture method has been recommended for effective first-line H. pylori eradication in regions with a high rate of antibiotic resistance[14]. However, susceptibility-guided therapy is time-consuming and requires specific expertise in clinical practice[59]. As an alternative, molecular methods for detecting CAM resistance are available for tailored H. pylori eradication in South Korea[60]. H. pylori resistance against CAM is mediated by point mutations in 23S ribosomal RNA[61]. The treatment regimen is selected based on the presence of the A2142G and A2143G point mutations that cause CAM resistance[62]. H. pylori-infected subjects without point mutations are treated with the 7-d standard triple regimen. Lee et al[63] demonstrated that the 7-d triple therapy was as effective as the 14-d therapy in patients without a point mutation. The classic BQT is recommended when A2142G and/or A2143G point mutations are detected. In contrast, MDZ-based triple therapy has an unacceptably low eradication rate in CAM-resistant H. pylori strains[64]. Tailored H. pylori eradication using molecular testing was more efficacious as a first-line treatment than standard CAM-based triple therapy (97.0% vs 81.8%)[65]. A recent study showed that the H. pylori eradication rate was similar between tailored eradication using DPO-PCR and the classic BQT (96.0% vs 95.7%, P = 0.9)[66]. Adverse drug events occurred less frequently with tailored eradication than BQT (12.0% vs 43.7%, P < 0.001).
H. pylori is an infectious disease; as such, the general principles regarding the treatment of infectious diseases, such as antimicrobial stewardship, are relevant to H. pylori[67]. Accordingly, recent research has focused on the optimal drug regimen in terms of dose, treatment duration, and minimal adverse events[68]. AMX, a beta-lactam antibiotic, has a half-life of approximately one hour, exhibiting time-dependent killing[69]. Therefore, frequent dosing has clinical advantages which help to maintain the plasma concentration higher than the MIC. The time above the MIC can reach 24 hours if 500 mg of AMX is dosed four times a day[70]. Along with efficacious antibiotics, sufficient and continuous acid suppression is required for successful eradication[71]. When H. pylori enters a replicative state between pH 6 and 8, the pathogen becomes highly susceptible to antibiotics, such as AMX. Recently, P-CABs have been found to increase the intragastric pH to 6 or more, which improves antibiotic stability and bioavailability[72].
The primary and secondary H. pylori resistance rates to AMX are low. In 1989, PPI-AMX dual therapy (DT) was first used to eradicate H. pylori[73]. However, the dosage of AMX, dosing intervals, and duration of therapy differed among previous investigators. Thereafter, a satisfactory H. pylori eradication using DT has not been achieved consistently[74]. DT has good H. pylori eradication efficacy, and is gaining increasing attention worldwide. In 2015, Yang et al[75] introduced a modified 14-d DT by increasing the dosage and frequency of administration (second-generation PPI and 750 mg of AMX four times daily). In a meta-analysis by Gao et al[76], DT and other commonly used regimens achieved similar efficacies in the ITT analysis (83.2% vs 85.3%, P = 0.87) and PP analysis (87.5% vs 90.1%, P = 0.33). In the DT group, drug-related adverse events occurred less frequently compared to the current mainstream therapy recommended by guidelines (12.9% vs 28.0%, P < 0.001).
In a meta-analysis, Li et al[77] included randomized controlled studies in which both a PPI and AMX were administered four times daily. As a first-line H. pylori treatment, administering high-dose DT resulted in a higher eradication rate than other regimens in ITT analysis (89.8% vs 84.2%, P = 0.04) and PP analysis (92.9% vs 88.3%, P = 0.06). Zou et al[78] reported that 14-d DT had a higher H. pylori eradication rate than 10-d DT in ITT (89.7% vs 78.4%, P = 0.039) and PP (92.9% vs 80.0%, P = 0.014) analyses. The dosage and treatment duration of AMX were recommended as 3 g/d for 14 d to optimize the DT, respectively. As a first-line treatment, high-dose, high-frequency DT was effective and safe for treating H. pylori infections in elderly patients and those with multiple comorbidities[79]. Successful H. pylori eradication was achieved in 90.9% of patients, and adverse events (11.1%) were mainly mild.
P-CABs are highly active drugs targeting H+, K+-ATPase in the gastric acid secretion of parietal cells. The mechanism of action is different from that of PPIs. Conventional PPIs require 3-5 d to achieve maximal and steady-state gastric acid inhibition, whereas P-CABs increase the intragastric pH to nearly 7 within four hours[80].
As an alternative to a PPI, VPZ was efficacious when combined with DT. Furuta et al[81] compared the first-line H. pylori eradication rate of VA-DT (20 mg of VPZ twice daily and 500 mg of AMX three times daily for 7 d) and CAM-based triple therapy using VPZ. The eradication rates using the VPZ-based dual and triple therapies were 92.9% and 91.9% (P = 0.728) in the ITT analysis and 94.4% and 92.7% (P = 0.715) in the PP analysis, respectively. VA-DT showed a comparable H. pylori eradication rate without the need for CAM. In a randomized trial by Suzuki et al[82], the eradication rate of VA-DT (20 mg of VPZ and 750 mg of AMX twice daily for 7 d) was similar to that for VPZ-based triple therapy (84.5% vs 89.2%, P = 0.203 in the ITT analysis; 87.1% vs 90.2%, P = 0.372 in the PP analysis). In the subgroup analysis, the eradication rates of VA-DT in CAM-resistant H. pylori strains were significantly higher than those of VPZ-based triple therapy (92.3% vs 76.2%, P = 0.048). When CAM-resistant H. pylori infection was treated with VPZ and AMX, CAM was not beneficial. Thus, extending the treatment duration of VA-DT to 14 d may be a promising alternative H. pylori treatment.
At present, the availability of specific drugs and reagents differs among China, Japan, and South Korea. Bismuth is not licensed for use in Japan, whereas classic and modified BQTs are recommended in China and South Korea. Since 2015, VPZ was introduced to eradicate H. pylori in Japan. In contrast, few studies have focused on VPZ outside of Japan. In South Korea, molecular tests for CAM-resistant H. pylori are commercially available. In Japan, a low rate of MDZ resistance results from the limited use of MDZ by the national health insurance system. Unlike in the other two countries, MDZ has not been approved as a component of first-line H. pylori regimens in Japan. Therefore, the strategy for the treatment of H. pylori infection might be selected based on the antibiotic resistance rate and medical policy in each country.
In China, adding bismuth to all H. pylori regimens is recommended as the empirical first-line treatment. Clinical trials with DT involving frequent administration of high-dose AMX have been widely performed in the Chinese population. In Japan, H. pylori treatment success has increased since VPZ was launched. Furthermore, VPZ may play an important role in DT by optimizing the intragastric environment for AMX action. PPIs may be replaced by VPZ due to its rapid and strong acid suppression. In South Korea, tailored eradication can be used as a first-line H. pylori treatment option based on the presence of a point mutation. The advantages of the H. pylori regimens used in China, Japan, and South Korea need to be combined in future research.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: The Korean Society of Gastroenterology.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Herawati F, Indonesia; Megraud F, France S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Crowe SE. Helicobacter pylori Infection. N Engl J Med. 2019;380:1158-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 273] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 2. | Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014;345:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 571] [Article Influence: 51.9] [Reference Citation Analysis (1)] |
| 3. | Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu JCY, Chan FKL, Sung JJY, Kaplan GG, Ng SC. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017;153:420-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 2051] [Article Influence: 256.4] [Reference Citation Analysis (0)] |
| 4. | Ren S, Cai P, Liu Y, Wang T, Zhang Y, Li Q, Gu Y, Wei L, Yan C, Jin G. Prevalence of Helicobacter pylori infection in China: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2021 Dec 3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 143] [Article Influence: 47.7] [Reference Citation Analysis (36)] |
| 5. | Ueda J, Gosho M, Inui Y, Matsuda T, Sakakibara M, Mabe K, Nakajima S, Shimoyama T, Yasuda M, Kawai T, Murakami K, Kamada T, Mizuno M, Kikuchi S, Lin Y, Kato M. Prevalence of Helicobacter pylori infection by birth year and geographic area in Japan. Helicobacter. 2014;19:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Lee JH, Choi KD, Jung HY, Baik GH, Park JK, Kim SS, Kim BW, Hong SJ, Lim H, Shin CM, Lee SH, Jeon SW, Kim JH, Choi CW, Jung HK, Choi SC, Cho JW, Lee WS, Na SY, Sung JK, Song KH, Chung JW, Yun SC; Korean College of Helicobacter and Upper Gastrointestinal Research. Seroprevalence of Helicobacter pylori in Korea: A multicenter, nationwide study conducted in 2015 and 2016. Helicobacter. 2018;23:e12463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 7. | International Agency for Research on Cancer. Globocan 2020. Cancer today. Available from: https://gco.iarc.fr/today/home. |
| 8. | Yang X, Zhang T, Zhang H, Sang S, Chen H, Zuo X. Temporal trend of gastric cancer burden along with its risk factors in China from 1990 to 2019, and projections until 2030: comparison with Japan, South Korea, and Mongolia. Biomark Res. 2021;9:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (1)] |
| 9. | Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, Haruma K, Asaka M, Uemura N, Malfertheiner P; faculty members of Kyoto Global Consensus Conference. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353-1367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1322] [Cited by in RCA: 1185] [Article Influence: 118.5] [Reference Citation Analysis (0)] |
| 10. | Asaka M, Kato M, Sakamoto N. Roadmap to eliminate gastric cancer with Helicobacter pylori eradication and consecutive surveillance in Japan. J Gastroenterol. 2014;49:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 11. | Liu WZ, Xie Y, Lu H, Cheng H, Zeng ZR, Zhou LY, Chen Y, Wang JB, Du YQ, Lu NH; Chinese Society of Gastroenterology, Chinese Study Group on Helicobacter pylori and Peptic Ulcer. Fifth Chinese National Consensus Report on the management of Helicobacter pylori infection. Helicobacter. 2018;23:e12475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 331] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 12. | Thung I, Aramin H, Vavinskaya V, Gupta S, Park JY, Crowe SE, Valasek MA. Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther. 2016;43:514-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 410] [Cited by in RCA: 553] [Article Influence: 61.4] [Reference Citation Analysis (2)] |
| 13. | Papastergiou V, Georgopoulos SD, Karatapanis S. Treatment of Helicobacter pylori infection: Past, present and future. World J Gastrointest Pathophysiol. 2014;5:392-399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Shiotani A, Roy P, Lu H, Graham DY. Helicobacter pylori diagnosis and therapy in the era of antimicrobial stewardship. Therap Adv Gastroenterol. 2021;14:17562848211064080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 15. | Huang CC, Tsai KW, Tsai TJ, Hsu PI. Update on the first-line treatment for Helicobacter pylori infection - a continuing challenge from an old enemy. Biomark Res. 2017;5:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Hu Y, Zhu Y, Lu NH. Primary Antibiotic Resistance of Helicobacter pylori in China. Dig Dis Sci. 2017;62:1146-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (2)] |
| 17. | Lee JH, Ahn JY, Choi KD, Jung HY, Kim JM, Baik GH, Kim BW, Park JC, Jung HK, Cho SJ, Shin CM, Choi YJ, Lee SH, Kim JH, Lee WS, Sung JK, Chung JW, Cheung DY, Lee H, Min YW, Kim JJ, Kim SY; Korean College of Helicobacter; Upper Gastrointestinal Research. Nationwide antibiotic resistance mapping of Helicobacter pylori in Korea: A prospective multicenter study. Helicobacter. 2019;24:e12592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 18. | Okamura T, Suga T, Nagaya T, Arakura N, Matsumoto T, Nakayama Y, Tanaka E. Antimicrobial resistance and characteristics of eradication therapy of Helicobacter pylori in Japan: a multi-generational comparison. Helicobacter. 2014;19:214-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Suzuki H, Mori H. World trends for H. pylori eradication therapy and gastric cancer prevention strategy by H. pylori test-and-treat. J Gastroenterol. 2018;53:354-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 20. | Murata M, Sugimoto M, Mizuno H, Kanno T, Satoh K. Clarithromycin Versus Metronidazole in First-Line Helicobacter Pylori Triple Eradication Therapy Based on Resistance to Antimicrobial Agents: Meta-Analysis. J Clin Med. 2020;9:543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Kuo YT, Liou JM, El-Omar EM, Wu JY, Leow AHR, Goh KL, Das R, Lu H, Lin JT, Tu YK, Yamaoka Y, Wu MS; Asian Pacific Alliance on Helicobacter and Microbiota. Primary antibiotic resistance in Helicobacter pylori in the Asia-Pacific region: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2:707-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 233] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 22. | Lu H, Zhang W, Graham DY. Bismuth-containing quadruple therapy for Helicobacter pylori: lessons from China. Eur J Gastroenterol Hepatol. 2013;25:1134-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Hu Y, Zhu Y, Lu NH. Recent progress in Helicobacter pylori treatment. Chin Med J (Engl). 2020;133:335-343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 24. | Zhang W, Chen Q, Liang X, Liu W, Xiao S, Graham DY, Lu H. Bismuth, lansoprazole, amoxicillin and metronidazole or clarithromycin as first-line Helicobacter pylori therapy. Gut. 2015;64:1715-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 25. | Graham DY, Dore MP, Lu H. Understanding treatment guidelines with bismuth and non-bismuth quadruple Helicobacter pylori eradication therapies. Expert Rev Anti Infect Ther. 2018;16:679-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 26. | Chen Q, Zhang W, Fu Q, Liang X, Liu W, Xiao S, Lu H. Rescue Therapy for Helicobacter pylori Eradication: A Randomized Non-Inferiority Trial of Amoxicillin or Tetracycline in Bismuth Quadruple Therapy. Am J Gastroenterol. 2016;111:1736-1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 27. | Graham DY, Lu H. Furazolidone in Helicobacter pylori therapy: misunderstood and often unfairly maligned drug told in a story of French bread. Saudi J Gastroenterol. 2012;18:1-2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Qiao C, Li Y, Liu J, Ji C, Qu J, Hu J, Ji R, Wan M, Lin B, Lin M, Qi Q, Zuo X. Clarithromycin versus furazolidone for naïve Helicobacter pylori infected patients in a high clarithromycin resistance area. J Gastroenterol Hepatol. 2021;36:2383-2388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Liu DS, Wang YH, Zhu ZH, Zhang SH, Zhu X, Wan JH, Lu NH, Xie Y. Characteristics of Helicobacter pylori antibiotic resistance: data from four different populations. Antimicrob Resist Infect Control. 2019;8:192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Liang X, Xu X, Zheng Q, Zhang W, Sun Q, Liu W, Xiao S, Lu H. Efficacy of bismuth-containing quadruple therapies for clarithromycin-, metronidazole-, and fluoroquinolone-resistant Helicobacter pylori infections in a prospective study. Clin Gastroenterol Hepatol. 2013;11:802-807.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 31. | Lv ZF, Wang FC, Zheng HL, Wang B, Xie Y, Zhou XJ, Lv NH. Meta-analysis: is combination of tetracycline and amoxicillin suitable for Helicobacter pylori infection? World J Gastroenterol. 2015;21:2522-2533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Zhang J, Han C, Lu WQ, Wang N, Wu SR, Wang YX, Ma JP, Wang JH, Hao C, Yuan DH, Liu N, Shi YQ. A randomized, multicenter and noninferiority study of amoxicillin plus berberine vs tetracycline plus furazolidone in quadruple therapy for Helicobacter pylori rescue treatment. J Dig Dis. 2020;21:256-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Lee YC, Dore MP, Graham DY. Diagnosis and Treatment of Helicobacter pylori Infection. Annu Rev Med. 2022;73:183-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 87] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 34. | Deguchi H, Uda A, Murakami K. Current Status of Helicobacter pylori Diagnosis and Eradication Therapy in Japan Using a Nationwide Database. Digestion. 2020;101:441-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Kato M, Ota H, Okuda M, Kikuchi S, Satoh K, Shimoyama T, Suzuki H, Handa O, Furuta T, Mabe K, Murakami K, Sugiyama T, Uemura N, Takahashi S. Guidelines for the management of Helicobacter pylori infection in Japan: 2016 Revised Edition. Helicobacter. 2019;24:e12597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 212] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 36. | Kiyotoki S, Nishikawa J, Sakaida I. Efficacy of Vonoprazan for Helicobacter pylori Eradication. Intern Med. 2020;59:153-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 37. | Akazawa Y, Fukuda D, Fukuda Y. Vonoprazan-based therapy for Helicobacter pylori eradication: experience and clinical evidence. Therap Adv Gastroenterol. 2016;9:845-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 38. | Suzuki S, Gotoda T, Kusano C, Iwatsuka K, Moriyama M. The Efficacy and Tolerability of a Triple Therapy Containing a Potassium-Competitive Acid Blocker Compared With a 7-Day PPI-Based Low-Dose Clarithromycin Triple Therapy. Am J Gastroenterol. 2016;111:949-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 39. | Sue S, Kuwashima H, Iwata Y, Oka H, Arima I, Fukuchi T, Sanga K, Inokuchi Y, Ishii Y, Kanno M, Terada M, Amano H, Naito M, Iwase S, Okazaki H, Komatsu K, Kokawa A, Kawana I, Morimoto M, Saito T, Kunishi Y, Ikeda A, Takahashi D, Miwa H, Sasaki T, Tamura T, Kondo M, Shibata W, Maeda S. The Superiority of Vonoprazan-based First-line Triple Therapy with Clarithromycin: A Prospective Multi-center Cohort Study on Helicobacter pylori Eradication. Intern Med. 2017;56:1277-1285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 40. | Sue S, Ogushi M, Arima I, Kuwashima H, Nakao S, Naito M, Komatsu K, Kaneko H, Tamura T, Sasaki T, Kondo M, Shibata W, Maeda S. Vonoprazan- vs proton-pump inhibitor-based first-line 7-day triple therapy for clarithromycin-susceptible Helicobacter pylori: A multicenter, prospective, randomized trial. Helicobacter. 2018;23:e12456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 41. | Li M, Oshima T, Horikawa T, Tozawa K, Tomita T, Fukui H, Watari J, Miwa H. Systematic review with meta-analysis: Vonoprazan, a potent acid blocker, is superior to proton-pump inhibitors for eradication of clarithromycin-resistant strains of Helicobacter pylori. Helicobacter. 2018;23:e12495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 42. | Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of Antibiotic Resistance in Helicobacter pylori: A Systematic Review and Meta-analysis in World Health Organization Regions. Gastroenterology. 2018;155:1372-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 617] [Cited by in RCA: 823] [Article Influence: 117.6] [Reference Citation Analysis (0)] |
| 43. | Nishizawa T, Maekawa T, Watanabe N, Harada N, Hosoda Y, Yoshinaga M, Yoshio T, Ohta H, Inoue S, Toyokawa T, Yamashita H, Saito H, Kuwai T, Katayama S, Masuda E, Miyabayashi H, Kimura T, Nishizawa Y, Takahashi M, Suzuki H. Clarithromycin Versus Metronidazole as First-line Helicobacter pylori Eradication: A Multicenter, Prospective, Randomized Controlled Study in Japan. J Clin Gastroenterol. 2015;49:468-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 44. | Adachi T, Matsui S, Watanabe T, Okamoto K, Okamoto A, Kono M, Yamada M, Nagai T, Komeda Y, Minaga K, Kamata K, Yamao K, Takenaka M, Asakuma Y, Sakurai T, Nishida N, Kashida H, Kudo M. Comparative Study of Clarithromycin- versus Metronidazole-Based Triple Therapy as First-Line Eradication for Helicobacter pylori. Oncology. 2017;93 Suppl 1:15-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 45. | Shinozaki S, Kobayashi Y, Osawa H, Sakamoto H, Hayashi Y, Lefor AK, Yamamoto H. Effectiveness and Safety of Vonoprazan versus Proton Pump Inhibitors for Second-Line Helicobacter pylori Eradication Therapy: Systematic Review and Meta-Analysis. Digestion. 2021;102:319-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 46. | Murakami K, Furuta T, Ando T, Nakajima T, Inui Y, Oshima T, Tomita T, Mabe K, Sasaki M, Suganuma T, Nomura H, Satoh K, Hori S, Inoue S, Tomokane T, Kudo M, Inaba T, Take S, Ohkusa T, Yamamoto S, Mizuno S, Kamoshida T, Amagai K, Iwamoto J, Miwa J, Kodama M, Okimoto T, Kato M, Asaka M; Japan GAST Study Group. Multi-center randomized controlled study to establish the standard third-line regimen for Helicobacter pylori eradication in Japan. J Gastroenterol. 2013;48:1128-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 47. | Sugimoto M, Sahara S, Ichikawa H, Kagami T, Uotani T, Furuta T. High Helicobacter pylori cure rate with sitafloxacin-based triple therapy. Aliment Pharmacol Ther. 2015;42:477-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 48. | Nishizawa T, Munkjargal M, Ebinuma H, Toyoshima O, Suzuki H. Sitafloxacin for Third-Line Helicobacter pylori Eradication: A Systematic Review. J Clin Med. 2021;10:2722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 49. | Jung HK, Kang SJ, Lee YC, Yang HJ, Park SY, Shin CM, Kim SE, Lim HC, Kim JH, Nam SY, Shin WG, Park JM, Choi IJ, Kim JG, Choi M; Korean College of Helicobacter and Upper Gastrointestinal Research. Evidence-Based Guidelines for the Treatment of Helicobacter pylori Infection in Korea 2020. Gut Liver. 2021;15:168-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 90] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 50. | Park SM, Kim JS, Kim BW, Ji JS, Choi H. Randomized clinical trial comparing 10- or 14-day sequential therapy and 10- or 14-day concomitant therapy for the first line empirical treatment of Helicobacter pylori infection. J Gastroenterol Hepatol. 2017;32:589-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 51. | Kim BJ, Lee H, Lee YC, Jeon SW, Kim GH, Kim HS, Sung JK, Lee DH, Kim HU, Park MI, Choi IJ, Yoon SM, Kim SW, Baik GH, Lee JY, Kim JI, Kim SG, Kim J, Lee J, Kim JG, Kim JJ; Korean College of Helicobacter Upper Gastrointestinal Research. Ten-Day Concomitant, 10-Day Sequential, and 7-Day Triple Therapy as First-Line Treatment for Helicobacter pylori Infection: A Nationwide Randomized Trial in Korea. Gut Liver. 2019;13:531-540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 52. | Fallone CA, Moss SF, Malfertheiner P. Reconciliation of Recent Helicobacter pylori Treatment Guidelines in a Time of Increasing Resistance to Antibiotics. Gastroenterology. 2019;157:44-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 201] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 53. | Lee HJ, Kim JI, Lee JS, Jun EJ, Oh JH, Cheung DY, Chung WC, Kim BW, Kim SS. Concomitant therapy achieved the best eradication rate for Helicobacter pylori among various treatment strategies. World J Gastroenterol. 2015;21:351-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 54. | Lee BE, Kim JS, Kim BW, Kim JH, Kim JI, Chung JW, Jeon SW, Lee JH, Kim N, Lee JY, Seo SY, Park SY, Kim SE, Joo MK, Song HJ, Kim KB, Bang CS, Kim HJ. Consistency of Helicobacter pylori eradication rates of first-line concomitant and sequential therapies in Korea: A nationwide multicenter retrospective study for the last 10 years. Helicobacter. 2021;26:e12780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 55. | White B, Winte M, DeSipio J, Phadtare S. Clinical Factors Implicated in Antibiotic Resistance in Helicobacter pylori Patients. Microorganisms. 2022;10:322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 56. | Graham DY, Lee SY. How to Effectively Use Bismuth Quadruple Therapy: The Good, the Bad, and the Ugly. Gastroenterol Clin North Am. 2015;44:537-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 57. | Kim BJ, Yang CH, Song HJ, Jeon SW, Kim GH, Kim HS, Kim TH, Shim KN, Chung IK, Park MI, Choi IJ, Kim JH, Kim BW, Baik GH, Han SW, Seo HE, Jung WT, Hwan Oh J, Kim SG, Lee JH, Park SK, Park BJ, Yang BR, Lee J, Kim JG. Online registry for nationwide database of Helicobacter pylori eradication in Korea: Correlation of antibiotic use density with eradication success. Helicobacter. 2019;24:e12646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 58. | Chen H, Dang Y, Zhou X, Liu B, Liu S, Zhang G. Tailored Therapy Versus Empiric Chosen Treatment for Helicobacter pylori Eradication: A Meta-Analysis. Medicine (Baltimore). 2016;95:e2750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 59. | Gisbert JP. Empirical or susceptibility-guided treatment for Helicobacter pylori infection? Therap Adv Gastroenterol. 2020;13:1756284820968736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 60. | Nishizawa T, Suzuki H. Mechanisms of Helicobacter pylori antibiotic resistance and molecular testing. Front Mol Biosci. 2014;1:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 61. | Kim SY, Park JM, Lim CH, Lee HA, Shin GY, Choe Y, Cho YK, Choi MG. Types of 23S Ribosomal RNA Point Mutations and Therapeutic Outcomes for Helicobacter pylori. Gut Liver. 2021;15:528-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 62. | Cho JH, Jin SY. Optimized diagnosis of Helicobacter pylori and tailored eradication therapy for preventing gastric cancer: a proposal for SHAKE strategy. Expert Rev Gastroenterol Hepatol. 2020;14:553-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 63. | Lee JW, Kim SJ, Choi CW, Kim HJ, Kang DH, Kim HW, Park SB, Nam HS, Ryu DG. Seven-day triple therapy is sufficient to eradicate infection caused by Helicobacter pylori without 23S rRNA point mutation. Medicine (Baltimore). 2021;100:e26133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 64. | Seo SI, Do BJ, Kang JG, Kim HS, Jang MK, Kim HY, Shin WG. Helicobacter pylori Eradication According to Sequencing-Based 23S Ribosomal RNA Point Mutation Associated with Clarithromycin Resistance. J Clin Med. 2019;9:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 65. | Kim JL, Cho SJ, Chung SJ, Lee A, Choi J, Chung H, Kim SG. Empiric Versus Clarithromycin Resistance-Guided Therapy for Helicobacter pylori Based on Polymerase Chain Reaction Results in Patients With Gastric Neoplasms or Gastric Mucosa-Associated Lymphoid Tissue Lymphoma: A Randomized Controlled Trial. Clin Transl Gastroenterol. 2020;11:e00194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 66. | Choi YI, Chung JW, Park DK, Kim KO, Kwon KA, Kim YJ, Seo JY. Tailored eradication vs empirical bismuth-containing quadruple therapy for first-line Helicobacter pylori eradication: A comparative, open trial. World J Gastroenterol. 2019;25:6743-6751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 67. | Dyar OJ, Huttner B, Schouten J, Pulcini C; ESGAP (ESCMID Study Group for Antimicrobial stewardshiP). What is antimicrobial stewardship? Clin Microbiol Infect. 2017;23:793-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 468] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 68. | Graham DY, Dore MP. Helicobacter pylori therapy: a paradigm shift. Expert Rev Anti Infect Ther. 2016;14:577-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 69. | Marcus EA, Inatomi N, Nagami GT, Sachs G, Scott DR. The effects of varying acidity on Helicobacter pylori growth and the bactericidal efficacy of ampicillin. Aliment Pharmacol Ther. 2012;36:972-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 70. | Furuta T, Graham DY. Pharmacologic aspects of eradication therapy for Helicobacter pylori Infection. Gastroenterol Clin North Am. 2010;39:465-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 71. | Takahashi N, Take Y. Tegoprazan, a Novel Potassium-Competitive Acid Blocker to Control Gastric Acid Secretion and Motility. J Pharmacol Exp Ther. 2018;364:275-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 72. | Oshima T, Miwa H. Potent Potassium-competitive Acid Blockers: A New Era for the Treatment of Acid-related Diseases. J Neurogastroenterol Motil. 2018;24:334-344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 73. | Yun J, Wu Z, Qi G, Han T, Zhang D. The high-dose amoxicillin-proton pump inhibitor dual therapy in eradication of Helicobacter pylori infection. Expert Rev Gastroenterol Hepatol. 2021;15:149-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 74. | Graham DY, Lu H, Shiotani A. Failure of optimized dual proton pump inhibitor amoxicillin therapy: What now? Saudi J Gastroenterol. 2017;23:265-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 75. | Yang JC, Lin CJ, Wang HL, Chen JD, Kao JY, Shun CT, Lu CW, Lin BR, Shieh MJ, Chang MC, Chang YT, Wei SC, Lin LC, Yeh WC, Kuo JS, Tung CC, Leong YL, Wang TH, Wong JM. High-dose dual therapy is superior to standard first-line or rescue therapy for Helicobacter pylori infection. Clin Gastroenterol Hepatol. 2015;13:895-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 76. | Gao CP, Zhang D, Zhang T, Wang JX, Han SX, Graham DY, Lu H. PPI-amoxicillin dual therapy for Helicobacter pylori infection: An update based on a systematic review and meta-analysis. Helicobacter. 2020;25:e12692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (1)] |
| 77. | Li C, Shi Y, Suo B, Tian X, Zhou L, Song Z. PPI-amoxicillin dual therapy four times daily is superior to guidelines recommended regimens in the Helicobacter pylori eradication therapy within Asia: A systematic review and meta-analysis. Helicobacter. 2021;26:e12816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 78. | Zou PY, Hu J, Zhao JT, Zhao Z, Mei H, Yang J, Zhu YJ, Zhang Y, Lan CH. 10-Day and 14-day high-dose dual therapy for the treatment of Helicobacter pylori: A propensity score matching analysis. Helicobacter. 2021;26:e12833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 79. | Gao W, Ye H, Deng X, Wang C, Xu Y, Li Y, Zhang X, Cheng H. Rabeprazole-amoxicillin dual therapy as first-line treatment for H pylori eradication in special patients: A retrospective, real-life study. Helicobacter. 2020;25:e12717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (2)] |
| 80. | Miftahussurur M, Pratama Putra B, Yamaoka Y. The Potential Benefits of Vonoprazan as Helicobacter pylori Infection Therapy. Pharmaceuticals (Basel). 2020;13:276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 81. | Furuta T, Yamade M, Kagami T, Uotani T, Suzuki T, Higuchi T, Tani S, Hamaya Y, Iwaizumi M, Miyajima H, Umemura K, Osawa S, Sugimoto K. Dual Therapy with Vonoprazan and Amoxicillin Is as Effective as Triple Therapy with Vonoprazan, Amoxicillin and Clarithromycin for Eradication of Helicobacter pylori. Digestion. 2020;101:743-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 82. | Suzuki S, Gotoda T, Kusano C, Ikehara H, Ichijima R, Ohyauchi M, Ito H, Kawamura M, Ogata Y, Ohtaka M, Nakahara M, Kawabe K. Seven-day vonoprazan and low-dose amoxicillin dual therapy as first-line Helicobacter pylori treatment: a multicentre randomised trial in Japan. Gut. 2020;69:1019-1026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 173] [Article Influence: 34.6] [Reference Citation Analysis (0)] |