Published online Jun 26, 2022. doi: 10.12998/wjcc.v10.i18.6119
Peer-review started: November 25, 2021
First decision: January 12, 2022
Revised: January 24, 2022
Accepted: April 22, 2022
Article in press: April 22, 2022
Published online: June 26, 2022
Processing time: 203 Days and 12.2 Hours
Hepatic epithelioid hemangioendothelioma (EHE) is a rare vascular endothelial cell tumor of the liver, consisting of epithelioid and histiocyte-like vascular endothelial cells in mucus or a fibrotic matrix. Immunohistochemistry is usually positive for vascular markers, such as factor VIII-related antigen, CD31, and CD34. Hepatic EHE can have a varied clinical course; treatment includes liver transplantation, liver resection, chemotherapy, and radiation therapy.
A 46-year-old woman with abdominal discomfort and elevated serum carcinoembryonic antigen was found to have multiple low-density lesions in the liver and lung on computed tomography (CT) evaluation. An ultrasound-guided fine needle aspiration biopsy revealed a fibrous stroma with dendritic cells, containing intracellular vacuoles. Immunohistochemical staining found that the tumor cells were positive for CD34, CD31, and factor VIII-related antigen. The patient received four courses of combined chemotherapy and was followed-up for 13 years, at which time the patient was in stable condition without disease progression and a confined neoplasm, as evidenced by CT scans.
The histology and immunohistochemical characteristics of hepatic EHE are well described. Chemotherapy may be effective in patients with extrahepatic lesions.
Core Tip: The gold standard diagnosis for hepatic epithelioid hemangioendothelioma includes epithelioid and histiocyte-like vascular endothelial cells in mucus or a fibrotic matrix, and positive vascular markers. Chemotherapy may be an effective treatment; close follow-up is necessary.
- Citation: Mo WF, Tong YL. Hepatic epithelioid hemangioendothelioma after thirteen years’ follow-up: A case report and review of literature. World J Clin Cases 2022; 10(18): 6119-6127
- URL: https://www.wjgnet.com/2307-8960/full/v10/i18/6119.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i18.6119
Hepatic epithelioid hemangioendothelioma (EHE) is a rare malignant tumor of vascular origin, with an incidence of 0.1-0.2/100000[1,2]. Oral contraceptives, polyvinyl chloride, asbestos, thorotrast contrast medium, hepatic trauma, and viral hepatitis have been identified as risk factors for subsequent development of disease[3]. While laboratory findings always reveal abnormal liver function, tumor markers are always at normal levels. The patient described in this case report had a history of hepatitis A and normal liver function, but with a mildly elevated tumor marker [carcinoembryonic antigen (CEA) at 6.9 ng/mL]. The patient received four courses of chemotherapy and was found to remain in stable condition after 13 years of follow-up.
A 46-year-old woman with no significant past medical history presented at the hospital with a 1-mo history of epigastric discomfort and asthenia.
The patient had no other symptoms.
The patient had a history of acute hepatitis that had resolved without complications 20 years previously.
The patient had no personal or family history of other diseases.
Physical examination revealed no remarkable findings.
Laboratory testing on admission showed no abnormalities in markers of inflammation or abnormal liver function, or in peripheral blood panel or biochemical tests. Hepatitis B surface antigen (HBsAg), hepatitis B core antibody (HBcAb) and hepatitis C virus antibody (HCVAb) were negative. Tumor markers were in the normal ranges, except for a mildly elevated CEA (6.9 ng/mL; normal range: 0-5.0 ng/mL).
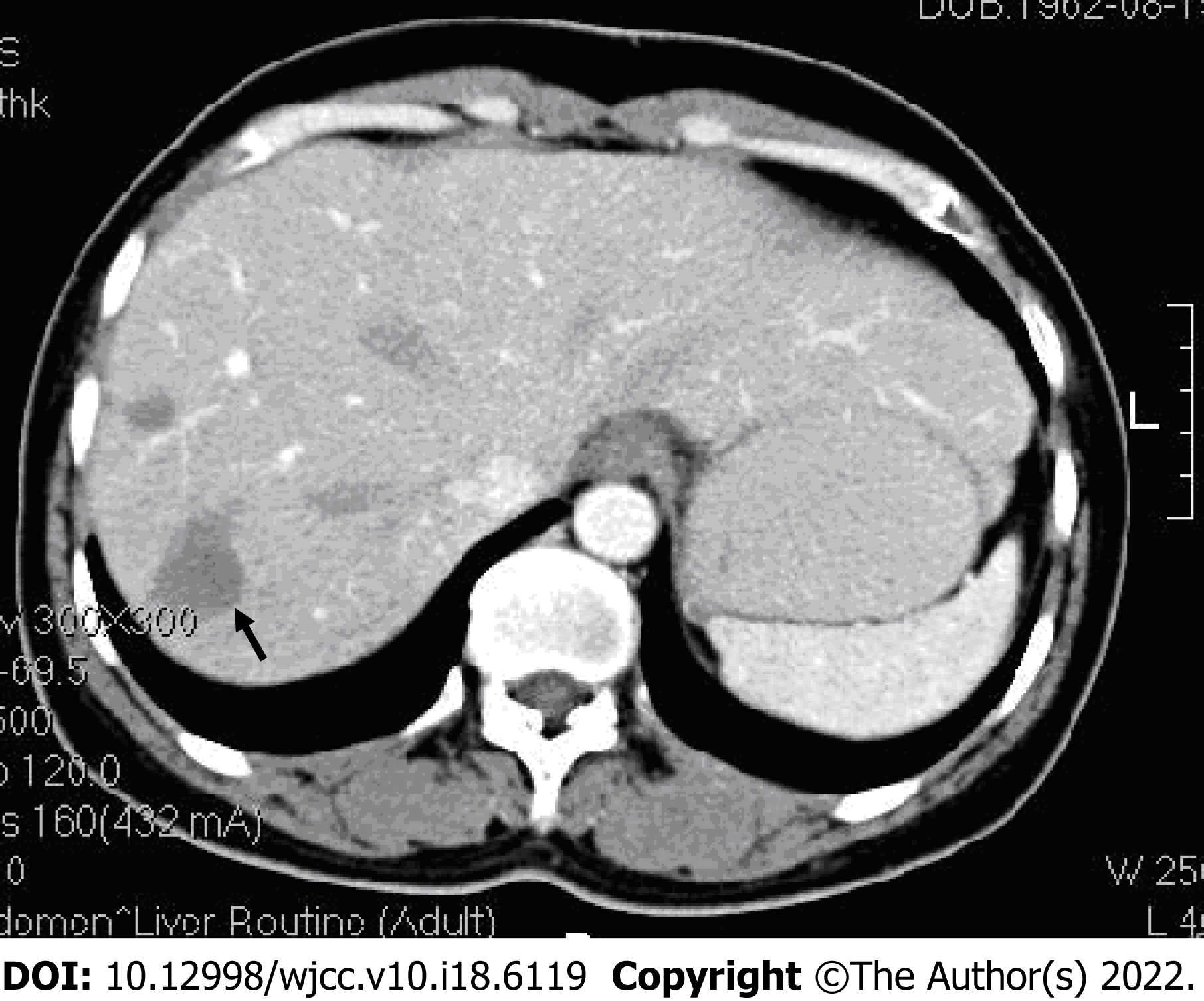
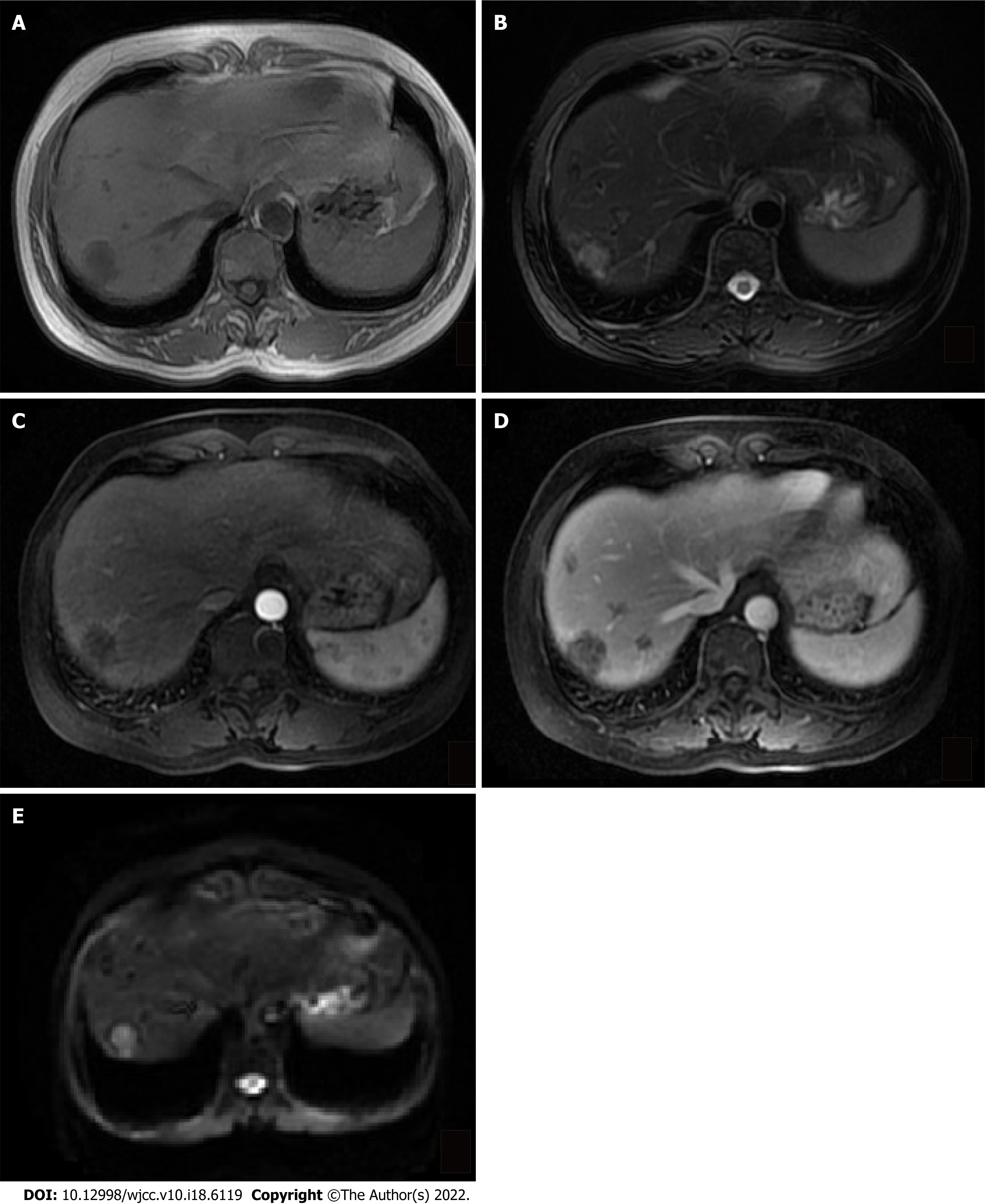
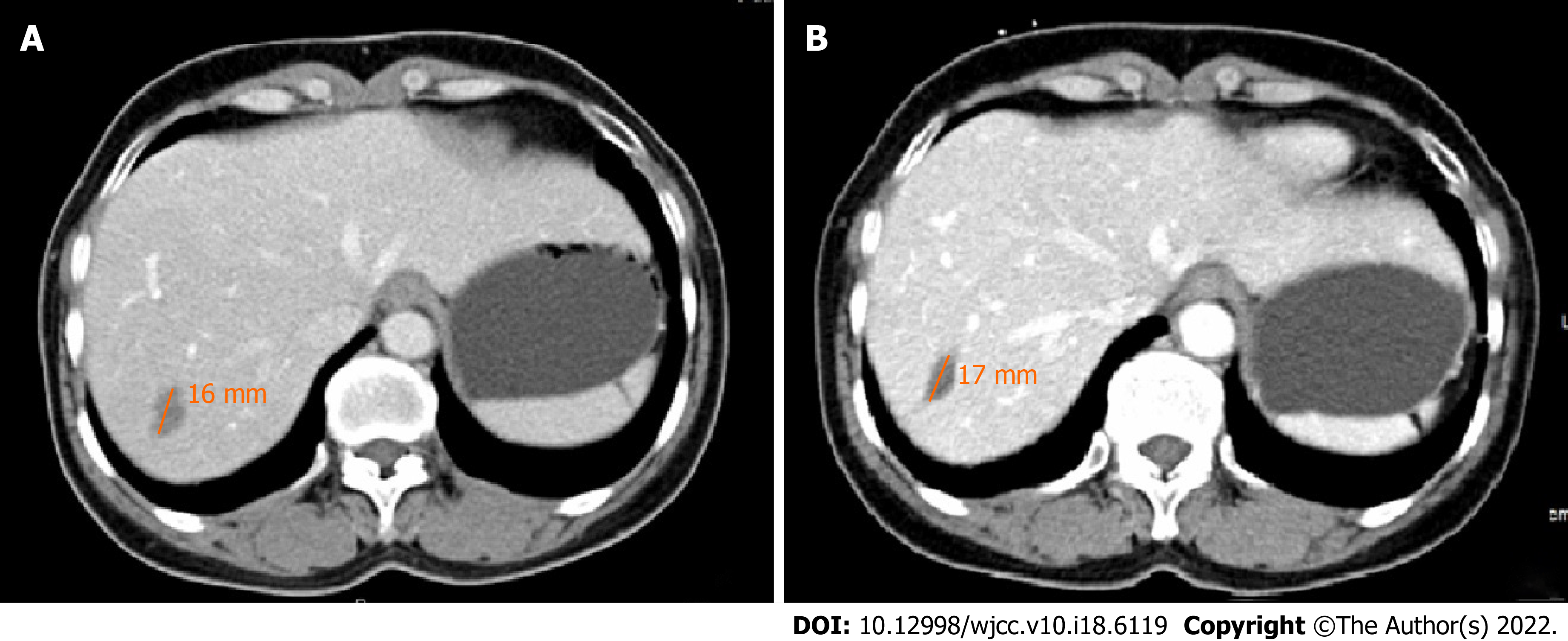
Abdominal ultrasound revealed multiple irregular hypoechoic lesions in the liver. Color doppler flow imaging showed spots of avascular reflective material. Contrast-enhanced computed tomography (CT) showed multiple low-density lesions in the right lobe of the liver. The largest was located in segment 8 and was 2.9 cm, 2.3 cm. Some lesions had mild-moderate enhancement during the arterial contrast-enhanced phase. The density was lower than the normal liver parenchyma during the portal vein and lag phase (Figure 1). Magnetic resonance (MR) T1-weighted images showed multiple low signal ovoid lesions in the right lobe of the liver that had a high signal on T2-weighted images (Figure 2). Chest X-rays yielded no remarkable findings. Ultrasound revealed enlarged bilateral lymph nodes in the neck, axilla, and groin.
Laboratory testing on admission showed no abnormalities in markers of inflammation or abnormal liver function, or in peripheral blood panel or biochemical tests. HBsAg, HBcAb and HCVAb were negative. Tumor markers were in the normal ranges, except for a mildly elevated CEA (6.9 ng/mL; normal range: 0-5.0 ng/mL).
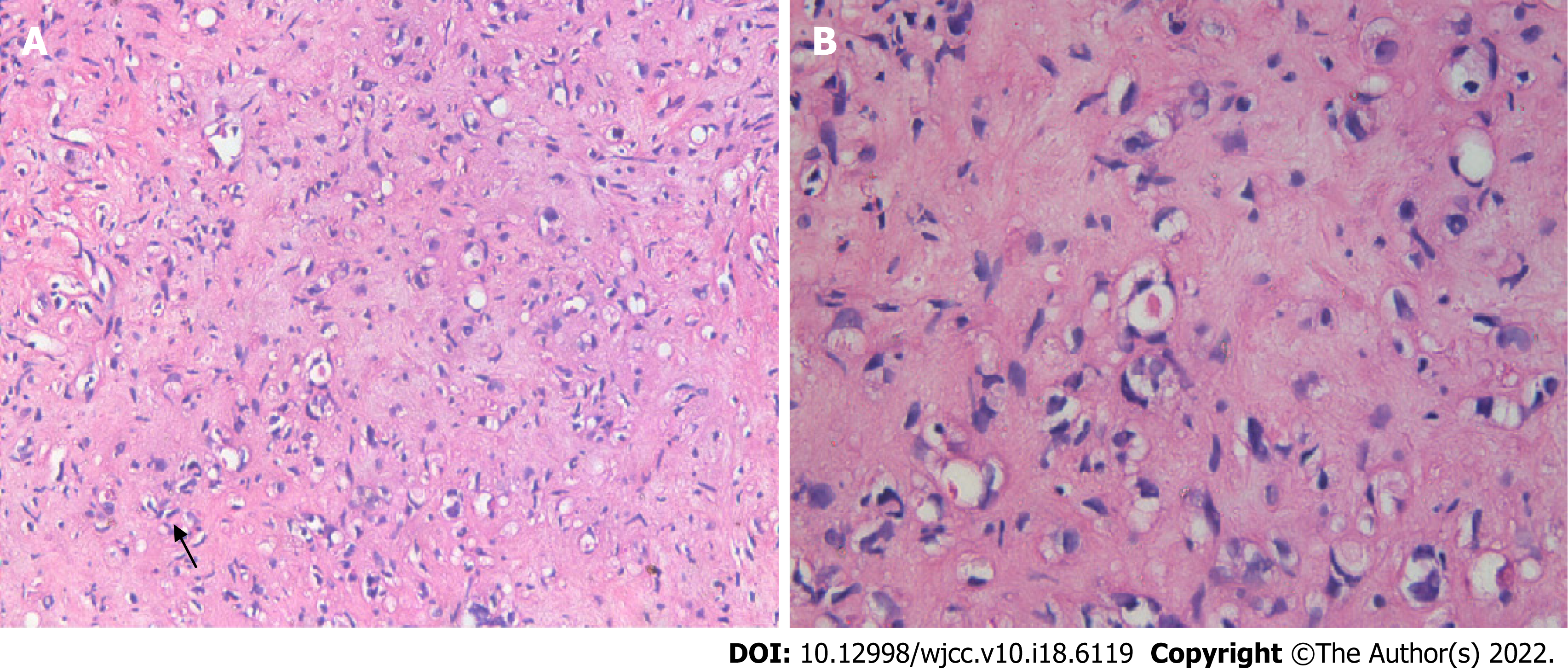
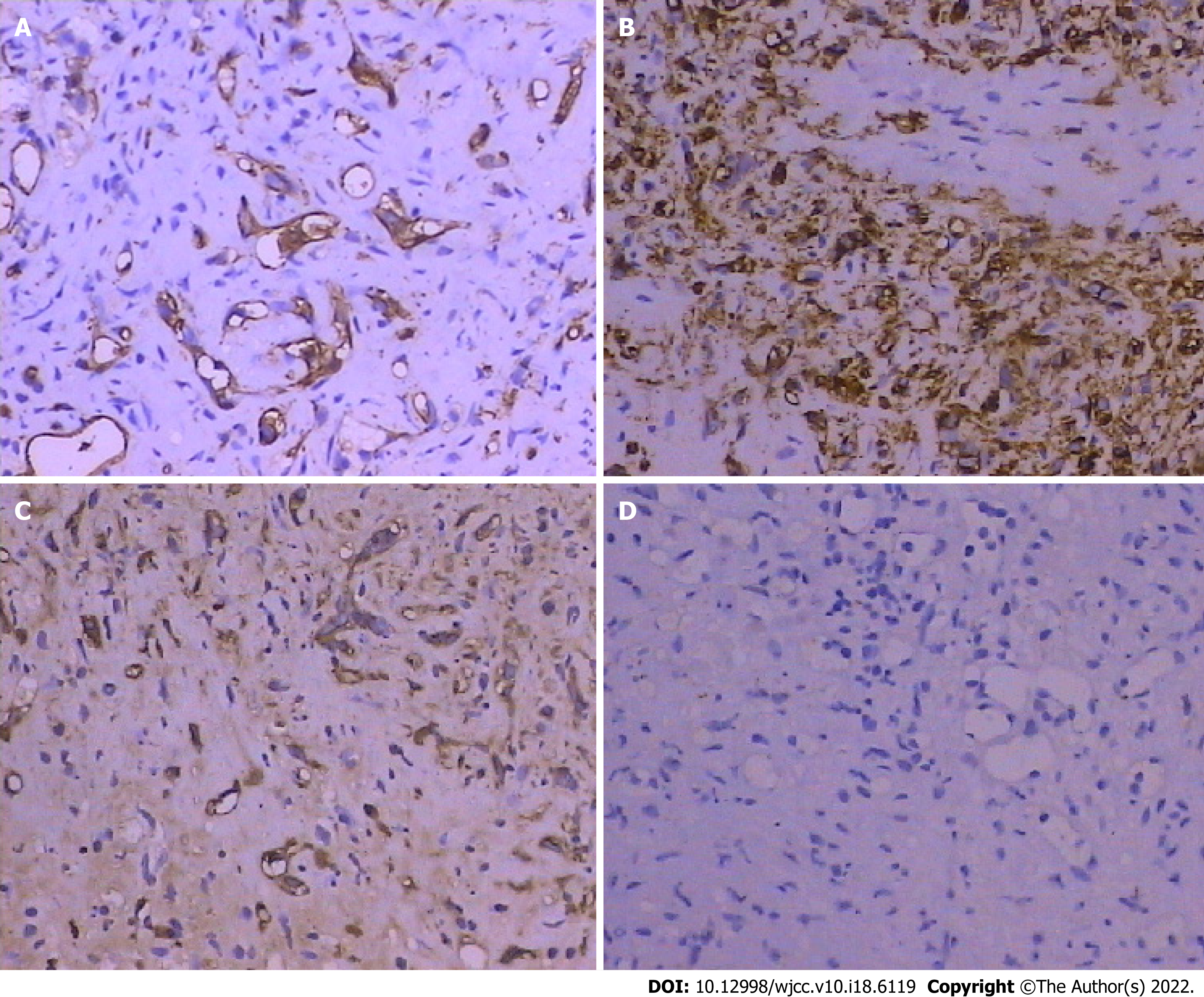
An ultrasound-guided fine needle aspiration biopsy revealed few hepatocytes and fibrous tissue with mildly heteromorphic spindle cell (dendritic cell) infiltration. The neoplastic cells were medium to large, with eosinophilic cytoplasm and vesicular nuclei having small, inconspicuous nucleoli. Signet ring cell-like structures were seen with intracytoplasmic lumina, occasionally containing red blood cells (Figure 3). Immunohistochemical staining indicated that the tumor cells were positive for CD31 (H12164PD590, EuroBioscience), CD34 (H12166F, EuroBioscience), and factor VIII-related antigen (FVIII-RAG, BH0012044, Goybio) (Figure 4A-C), while cells were negative for Pan Cytokeratin (CK+AFs-AE1/AE3+AF0-) (PD00330, Dako) (Figure 4D). Other results were lysozyme+-, P53+-/+ACY-ndash+ADs-, vimentin+-, EMA+-, CK8+ACY-ndash+ADs-, AFP+ACY-ndash+ADs-, CK18+ACY-ndash+ADs-, hepatocyte+ACY-minus+ADs-, CK20+ACY-minus+ADs-, and CD68+ACY-minus+ADs-,which weren't been shown in this article. Immunohistochemical staining results revealed evidence of+ACY-nbsp+ADs- endothelial differentiation, and consistent with hepatic epithelioid hemangioendothelioma (EHE).
During the patient’s hospital stay, she was given four cycles of combined chemotherapy with ifosfamide, cisplatin, epirubicin and recombinant human (rh) endostatin (Endostar; Simcere, Nanjing, China) injection.
After 13 years of follow-up, the patient remains in stable condition. A repeated CT scan found that the size of the lesions had not changed (Figure 5) and her liver function was normal.
Hepatic EHE is a rare tumor of vascular origin, with an incidence of 0.1-0.2/100000[1,2]. Fewer than 600 cases involving the liver are available in the literature, and it was first reported by Ishak et al[4] in 1984. Hepatic EHE is as a low-to-moderate grade tumor with a malignant potential intermediate between hemangioma and hemangiosarcoma[4]. Its metastasis rate is 27%-45% and the most common tissues of origin are the lungs (81%) and celiac lymph nodes (39%)[1]. The median age has been reported as 41.7 years, with a female predominance of 3:2[3], and the clinical manifestations are variable. The most frequent symptoms are right upper quadrant pain (48.6%), hepatomegaly (20.4%), and a constitutional syndrome with progressive liver damage and weight loss (15.6%)[3]. Some patients present with Budd-Chiari syndrome or liver failure, while others present with incidental findings[1, 5]. Laboratory findings may reveal abnormal liver function. Nearly 75% of patients have elevated alkaline phosphatase (AKP), 2.7% have elevated alpha-fetoprotein (AFP), and 18.8% have elevated serum CEA[1,3]. Our patient had good liver function, with normal AKP, AST, ALT, and AFP. Her CEA was elevated but other markers were in their normal ranges. Oral contraceptives, polyvinyl chloride, asbestos, thorotrast contrast agent, and hepatic trauma have been identified as risk factors for subsequent disease development[3], and viral hepatitis is considered as an etiology[1,4,6]. This patient had a history of viral hepatitis A, but it had resolved without complication 20 years before she presented with hepatic EHE, making a viral etiology implausible. Because of its nonspecific manifestation, the diagnosis of hepatic EHE depends mainly on radiology and histopathology.
Most lesions are peripheral, extending to the capsular margin and are frequently hypoechoic with heterogeneous internal architecture on sonography[7,8]. On CT, lesions are almost hypodense with peripheral contrast enhancement[7,9-11]. Capsular retraction adjacent to the mass is seen in fewer than 25% of patients[9,12]. On MR, T1-weighted images of lesions frequently have a low signal and T2-weighted images have heterogeneous-increased signals. Peripheral enhancement with a thin nonenhancing rim corresponding to a narrow vascular zone can be seen with arterial contrast[7, 11-13]. A lollipop sign, which is indicative of hepatic or portal veins terminating at or just within the periphery of lesions, seems to be specific for hepatic EHE[14]. The mean apparent diffusion coefficients of lesions were found to be high compared with other hepatic malignancies, which may be helpful in suggesting the diagnosis[15]. MR appears to be superior to CT, and MR with contrast may be important.
Pathologic diagnosis depends on the vascular nature of the tumor. Histologically, it is comprised of a fibrous stroma with myxohyaline areas including dendritic and epithelioid cells, often with intracellular vacuoles[1,4]. Immunohistochemical staining is positive for the expression of endothelial antigens, such as FVIII-RAG (98%), CD34 (94%), or CD31 (86%), and negative for epithelial markers[1,6]. This tumor was CD34+, vimentin+, and CD31+, and negative for epithelial markers like CK (AE1/AE3) and CK18. Podoplanin was shown to be specifically expressed in hepatic EHE (78%), and may be useful as a diagnostic marker of EHE in liver tumors[9]. Characteristic ultrastructural features include investing basal lamina, cytoplasmic intermediate filaments, Weibel–Palade bodies, and pinocytotic vesicles[4]. High cellularity, more than mitotic count, predicts an unfavorable prognosis[1,3,4]. A recent study reported that these tumors often have t(1;3) (p36.3; q25) translocations, resulting in WWTR1-CAMTA1 fusion[16]. YAP 1-TFE3 fusions have also been identified in about 10% of patients[17].
Treatment options are limited by the rarity of the tumor and currently include liver transplantation (44.8%), chemotherapy or radiotherapy (21.0%), and liver resection (9.4%), with 24.8% of patients receiving no treatment[3]. Complete liver resection should be performed if possible, but the multicentric origin of the tumor and multinodular growth make that difficult to accomplish[18]. Liver trans
Because the disease was multifocal in our patient, orthotopic liver transplantation may have been justified as a curative procedure. Unfortunately, a donor shortage and cost limitations made immediate transplantation unrealistic. Consequently, we choose to treat her with combined chemotherapy that included ifosfamide, cisplatin, epirubicin and rh-endostatin. rh-endostatin is purified in an Escherichia coli system, with an additional nine amino acid sequence of soluble protein[29]. It targets neovascular endothelial cells and has antiangiogenetic and antitumor activity. Preclinical and clinical studies showed synergistic effects of rh-endostatin and other agents that inhibit the growth of malignant tumors, with minimal toxicity[30-32]. A review by Xu et al[33] suggests that the combination of rh-endostatin with chemotherapy, radiotherapy, and biotherapy (i.e. fusion protein, or molecular-targeted therapy on cancers, etc.) may be the optimal strategy for cancer treatment[33]. Ling et al[34] reported that the antiangiogenic activity of rh-endostatin was mediated in vitro and in vivo by blocking VEGF-induced tyrosine phosphorylation of KDR/Flk-1 in endothelial cells. The vascular nature and endothelial origin of our patient’s tumor led us to choose rh-endostatin for her treatment. To date, the size of her lesions has not increased, and the patient is in stable condition with normal liver function. The patient is followed-up regularly, and liver transplantation is still recommended.
In conclusion, hepatic EHE is a rare tumor, and its atypical symptoms and varied radiographic appearance make it hard to differentiate from other tumors. Diagnosis depends on histopathology. Liver resection is the treatment of choice in patients with resectable lesions, and liver transplantation is justified as a curative procedure for multinodular disease. Donor shortage and a long waiting time, among other reasons, limit the use of liver transplantation. Chemotherapy including rh-endostatin may increase the effectiveness of hepatic EHE treatment. The focus is on its therapeutic efficacy while awaiting a suitable donor liver and for patients with extrahepatic manifestations. Further research is needed.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mohey NM, Egypt; Yang M, China S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
| 1. | Makhlouf HR, Ishak KG, Goodman ZD. Epithelioid hemangioendothelioma of the liver: a clinicopathologic study of 137 cases. Cancer. 1999;85:562-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 2. | Elleuch N, Dahmani W, Aida Ben S, Jaziri H, Aya H, Ksiaa M, Jmaa A. Hepatic epithelioid hemangioendothelioma: A misdiagnosed rare liver tumor. Presse Med. 2018;47:182-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Mehrabi A, Kashfi A, Fonouni H, Schemmer P, Schmied BM, Hallscheidt P, Schirmacher P, Weitz J, Friess H, Buchler MW, Schmidt J. Primary malignant hepatic epithelioid hemangioendothelioma: a comprehensive review of the literature with emphasis on the surgical therapy. Cancer. 2006;107:2108-2121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 308] [Article Influence: 16.2] [Reference Citation Analysis (1)] |
| 4. | Ishak KG, Sesterhenn IA, Goodman ZD, Rabin L, Stromeyer FW. Epithelioid hemangioendothelioma of the liver: a clinicopathologic and follow-up study of 32 cases. Hum Pathol. 1984;15:839-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 284] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 5. | Uchimura K, Nakamuta M, Osoegawa M, Takeaki S, Nishi H, Iwamoto H, Enjoji M, Nawata H. Hepatic epithelioid hemangioendothelioma. J Clin Gastroenterol. 2001;32:431-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Demetris AJ, Minervini M, Raikow RB, Lee RG. Hepatic epithelioid hemangioendothelioma: biological questions based on pattern of recurrence in an allograft and tumor immunophenotype. Am J Surg Pathol. 1997;21:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Lyburn ID, Torreggiani WC, Harris AC, Zwirewich CV, Buckley AR, Davis JE, Chung SW, Scudamore CH, Ho SG. Hepatic epithelioid hemangioendothelioma: sonographic, CT, and MR imaging appearances. AJR Am J Roentgenol. 2003;180:1359-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Radin DR, Craig JR, Colletti PM, Ralls PW, Halls JM. Hepatic epithelioid hemangioendothelioma. Radiology. 1988;169:145-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 60] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Amin S, Chung H, Jha R. Hepatic epithelioid hemangioendothelioma: MR imaging findings. Abdom Imaging. 2011;36:407-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Miller WJ, Dodd GD 3rd, Federle MP, Baron RL. Epithelioid hemangioendothelioma of the liver: imaging findings with pathologic correlation. AJR Am J Roentgenol. 1992;159:53-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 124] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Fulcher AS, Sterling RK. Hepatic neoplasms: computed tomography and magnetic resonance features. J Clin Gastroenterol. 2002;34:463-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Hayashi Y, Inagaki K, Hirota S, Yoshikawa T, Ikawa H. Epithelioid hemangioendothelioma with marked liver deformity and secondary Budd-Chiari syndrome: pathological and radiological correlation. Pathol Int. 1999;49:547-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Van Beers B, Roche A, Mathieu D, Menu Y, Delos M, Otte JB, Lalonde L, Pringot J. Epithelioid hemangioendothelioma of the liver: MR and CT findings. J Comput Assist Tomogr. 1992;16:420-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Alomari AI. The lollipop sign: a new cross-sectional sign of hepatic epithelioid hemangioendothelioma. Eur J Radiol. 2006;59:460-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Bruegel M, Muenzel D, Waldt S, Specht K, Rummeny EJ. Hepatic epithelioid hemangioendothelioma: findings at CT and MRI including preliminary observations at diffusion-weighted echo-planar imaging. Abdom Imaging. 2011;36:415-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Doyle LA, Fletcher CD, Hornick JL. Nuclear Expression of CAMTA1 Distinguishes Epithelioid Hemangioendothelioma From Histologic Mimics. Am J Surg Pathol. 2016;40:94-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 173] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 17. | Lotfalla MM, Folpe AL, Fritchie KJ, Greipp PT, Galliano GG, Halling KC, Mounajjed T, Torres-Mora J, Graham RP. Hepatic YAP1-TFE3 Rearranged Epithelioid Hemangioendothelioma. Case Rep Gastrointest Med. 2019;2019:7530845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Haydon E, Haydon G, Bramhall S, Mayer AD, Niel D. Hepatic epithelioid haemangioendothelioma. J R Soc Med. 2005;98:364-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Lerut JP, Orlando G, Sempoux C, Ciccarelli O, Van Beers BE, Danse E, Horsmans Y, Rahier J, Roggen F. Hepatic haemangioendothelioma in adults: excellent outcome following liver transplantation. Transpl Int. 2004;17:202-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Nissen NN, Cavazzoni E, Tran TT, Poordad FP. Emerging role of transplantation for primary liver cancers. Cancer J. 2004;10:88-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Bancel B, Patricot LM, Caillon P, Ducerf C, Pouyet M. [Hepatic epithelioid hemangioendothelioma. A case with liver transplantation. Review of the literature]. Ann Pathol. 1993;13:23-28. [PubMed] |
| 22. | Galvão FH, Bakonyi-Neto A, Machado MA, Farias AQ, Mello ES, Diz ME, Machado MC. Interferon alpha-2B and liver resection to treat multifocal hepatic epithelioid hemangioendothelioma: a relevant approach to avoid liver transplantation. Transplant Proc. 2005;37:4354-4358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Kou K, Chen YG, Zhou JP, Sun XD, Sun DW, Li SX, Lv GY. Hepatic epithelioid hemangioendothelioma: Update on diagnosis and therapy. World J Clin Cases. 2020;8:3978-3987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (3)] |
| 24. | Treska V, Daum O, Svajdler M, Liska V, Ferda J, Baxa J. Hepatic Epithelioid Hemangioendothelioma - a Rare Tumor and Diagnostic Dilemma. In Vivo. 2017;31:763-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Lau A, Malangone S, Green M, Badari A, Clarke K, Elquza E. Combination capecitabine and bevacizumab in the treatment of metastatic hepatic epithelioid hemangioendothelioma. Ther Adv Med Oncol. 2015;7:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Grenader T, Vernea F, Reinus C, Gabizon A. Malignant epithelioid hemangioendothelioma of the liver successfully treated with pegylated liposomal doxorubicin. J Clin Oncol. 2011;29:e722-e724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Lakkis Z, Kim S, Delabrousse E, Jary M, Nguyen T, Mantion G, Heyd B, Lassabe C, Borg C. Metronomic cyclophosphamide: an alternative treatment for hepatic epithelioid hemangioendothelioma. J Hepatol. 2013;58:1254-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Tan Y, Yang X, Dong C, Xiao Z, Zhang H, Wang Y. Diffuse hepatic epithelioid hemangioendothelioma with multiple splenic metastasis and delayed multifocal bone metastasis after liver transplantation on FDG PET/CT images: A case report. Medicine (Baltimore). 2018;97:e10728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Han Q, Fu Y, Zhou H, He Y, Luo Y. Contributions of Zn(II)-binding to the structural stability of endostatin. FEBS Lett. 2007;581:3027-3032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Hanna NN, Seetharam S, Mauceri HJ, Beckett MA, Jaskowiak NT, Salloum RM, Hari D, Dhanabal M, Ramchandran R, Kalluri R, Sukhatme VP, Kufe DW, Weichselbaum RR. Antitumor interaction of short-course endostatin and ionizing radiation. Cancer J. 2000;6:287-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Plum SM, Hanson AD, Volker KM, Vu HA, Sim BK, Fogler WE, Fortier AH. Synergistic activity of recombinant human endostatin in combination with adriamycin: analysis of in vitro activity on endothelial cells and in vivo tumor progression in an orthotopic murine mammary carcinoma model. Clin Cancer Res. 2003;9:4619-4626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Sun L, Ye HY, Zhang YH, Guan YS, Wu H. Epidermal growth factor receptor antibody plus recombinant human endostatin in treatment of hepatic metastases after remnant gastric cancer resection. World J Gastroenterol. 2007;13:6115-6118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in CrossRef: 13] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Xu F, Ma Q, Sha H. Optimizing drug delivery for enhancing therapeutic efficacy of recombinant human endostatin in cancer treatment. Crit Rev Ther Drug Carrier Syst. 2007;24:445-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Ling Y, Yang Y, Lu N, You QD, Wang S, Gao Y, Chen Y, Guo QL. Endostar, a novel recombinant human endostatin, exerts antiangiogenic effect via blocking VEGF-induced tyrosine phosphorylation of KDR/Flk-1 of endothelial cells. Biochem Biophys Res Commun. 2007;361:79-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 194] [Article Influence: 10.8] [Reference Citation Analysis (0)] |