Published online Jun 16, 2022. doi: 10.12998/wjcc.v10.i17.5916
Peer-review started: January 22, 2022
First decision: March 16, 2022
Revised: March 21, 2022
Accepted: April 9, 2022
Article in press: April 9, 2022
Published online: June 16, 2022
Processing time: 137 Days and 18.3 Hours
Tyrosine kinase inhibitors (TKI) have been the standard first-line therapy for advanced non-small cell lung cancer (NSCLC) of epidermal growth factor receptor (EGFR) sensitive mutations. Uncommon EGFR mutations are increasingly reported with the development of next-generation sequencing. However, their sensitivity to TKIs is variable with limited clinical evidence.
Here, we report a patient with the rare delE709_T710insD mutation, who showed the favorable efficacy of dacomitinib and achieved a partial response with a progression-free survival of 7.0 mo.
To our knowledge, this is the first report displaying the clinical efficacy of dacomitinib for patients with delE709_T710insD, which may help to provide alternatives in non-classical variant NSCLC patients. Further studies are warranted to make the optimal choice of EGFR-TKI for rare mutations.
Core Tip: DelE709_T710insD is an extremely rare complex in-frame deletion mutation in exon 18 and accounts for only 0.11% of epidermal growth factor receptor mutations. The development of next-generation sequencing enabled the more identification of rare variants. Our case is the first report describing the clinical efficacy of dacomitinib for delE709_T710insD and achieved a progression-free survival of 7.0 mo. More patients with the rare variants may benefit from dacomitinib targeted therapy based on our study.
- Citation: Xu F, Xia ML, Pan HY, Pan JW, Shen YH. Response to dacomitinib in advanced non-small-cell lung cancer harboring the rare delE709_T710insD mutation: A case report. World J Clin Cases 2022; 10(17): 5916-5922
- URL: https://www.wjgnet.com/2307-8960/full/v10/i17/5916.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i17.5916
Among non-small-cell lung cancer (NSCLC) patients with epidermal growth factor receptor (EGFR) mutations, the most common mutations are exon 19 deletions and exon 21 L858R point mutations, accounting for 80%-90% of all EGFR mutations[1]. With the development of next-generation sequencing (NGS), more rare or atypical mutations, such as EGFR exon 20 and exon 18, have been identified, but their responses to TKIs have been variable and less investigated.
Mutations in EGFR exon 18, including point mutations and deletion-insertion mutations, were observed in approximately 4% of patients with EGFR mutations[2]. DelE709_T710insD is a rare complex in-frame deletion mutation in exon 18 and accounts for only 0.11% of EGFR mutations (33/31015) according to the Catalog of Somatic Mutations in Cancer (COSMIC) v.94 database[3]. Evidence regarding its response to available EGFR-TKIs is limited.
Here, we present a patient with advanced lung adenocarcinoma harboring the rare EGFR delE709_T710insD mutation who responded well to the second-generation EGFR TKI dacomitinib.
A 56-year-old female patient presented with right chest discomfort for 3 mo.
Chest computed tomography (CT) revealed a 1.9 cm × 2.1 cm mass in the anterior segment of the right upper lobe and multiple nodules in the bilateral lungs, accompanied by right pleural effusion. Moreover, the right hilar, mediastinal, and paratracheal lymph nodes (LNs) were found to be enlarged.
The patient had no history of any other diseases.
The patient was free of any known congenital disease.
The right supraclavicular painless lymph node was palpated in the size of a soybean.
The laboratory test data revealed that the serum carcinoembryonic antigen level was 279.6 ng/mL.
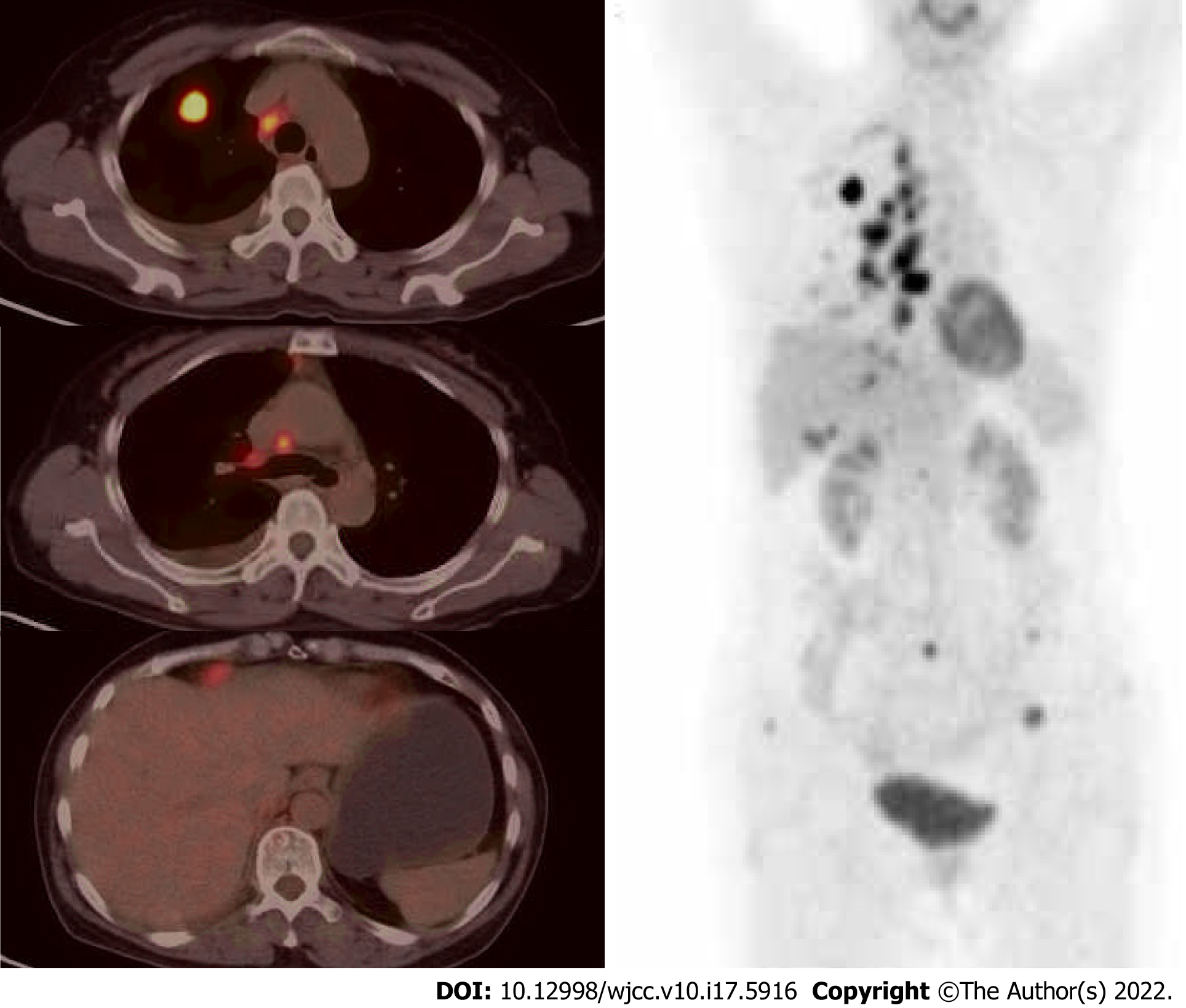
A positron emission tomography (PET) scan showed increased fluorodeoxyglucose (FDG) uptake in the right upper lobe mass, multiple pulmonary and subpleural nodules, and right supraclavicular, mediastinal, and right hilar lymph nodes. PET also indicated hypermetabolic nodules with low density in segment 6 of the liver and anterolateral area of the liver capsule, along with multiple bone destruction changes and high FDG uptake in T7 and T8 vertebral bodies and appendages, L5 spinous processes, and bilateral iliac bones (Figure 1). Magnetic resonance imaging of the brain was negative.
She subsequently underwent ultrasound-guided needle biopsy of the right supraclavicular lymph node and right closed thoracic drainage. Endobronchial ultrasound-transbronchial needle aspiration (EBUS-TBNA) was performed on LN 7 and 11R. Cancer cells were found both in the pleural effusion and clavicular lymph nodes. Pathological results of LN 11R were identified pulmonary adenocarcinoma, with P40 (-), CK7 (+), TTF-1 (+), Napsin A (+), CK5/6 (-), ALK Ventana (-), ALK-Negative (-) through immunohistochemistry (IHC). Genetic testing was performed on cell block samples from pleural effusion by polymerase chain reaction (PCR). Routine molecular genetic testing, including mutation of EGFR, KRAS, NRAS, BRAF, HER2, MET, and PIK3CA, and fusion of ALK, RET, and ROS1, were all negative. Supplementary material listed all gene and mutation sites of the PCR diagnostic kits.
Based on this, the patient was identified as "driver gene-negative" right lung adenocarcinoma, cT1cN3M1c (TNM 8th Edition), stage IVB.
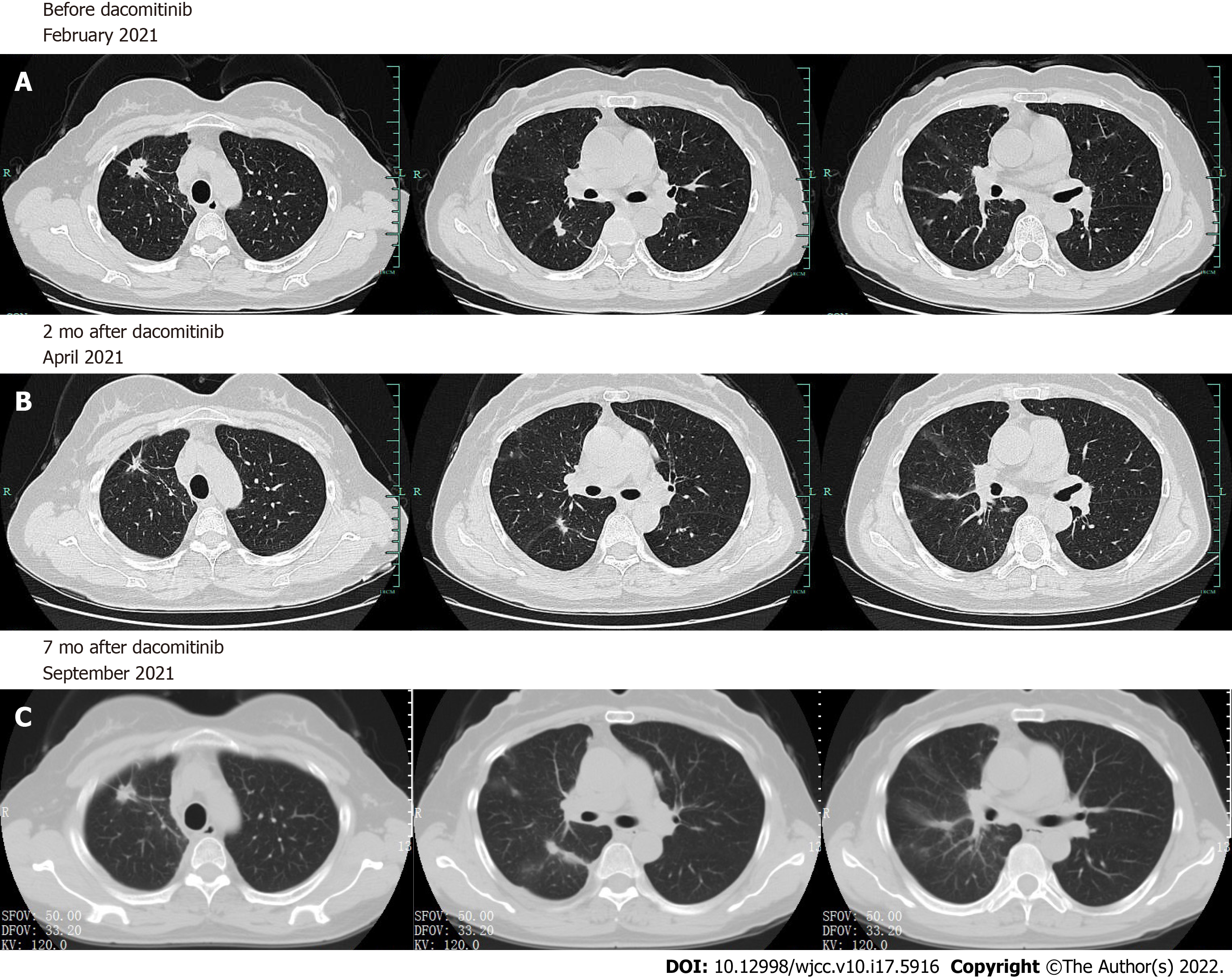
The patient then started chemotherapy with pemetrexed plus carboplatin and bevacizumab in September 2020. A CT scan after 2 cycles showed a reduction in the mass in the right upper lobe, but disease progression was observed in February 2021. The progression-free survival1 (PFS1) is 5 mo, and the best response was reduced stable disease based on Response Evaluation Criteria in Solid Tumors (RECIST) criteria. To seek more effective and potential treatment, CT-guided transthoracic lung biopsy was taken from the right upper lobe as her family demanded. A 12-gene NGS panel (Shanghai Yikon Genomics Inc. China) for lung cancer revealed the EGFR Del18 (delE709_T710insD) mutation. However, there are no recommended targeted drugs for this rare mutation. Dacomitinib 30 mg/d was administered as the second-line treatment, starting in February 2021.
A CT scan revealed that the primary lesion significantly decreased in size after 2 mo, and a partial response (PR) was achieved (Figure 2). There were no significant adverse effects of dacomitinib therapy. Nevertheless, recent CT showed that the mass of the right upper lobe grew larger, which met the RECIST criteria for progressive disease (PD) after 7.0 mo of dacomitinib treatment.
EGFR mutations are observed in up to 50% of Asian non-small-cell lung cancer (NSCLC) patients and approximately 10%-20% of non-Asian patients. EGFR-TKIs have become the standard first-line treatment for EGFR sensitizing mutations (del18 and L858R) NSCLC based on Phase III trials vs platinum-based doublet chemotherapy[4], which has revolutionized the management of EGFR-mutated NSCLC. Uncommon mutations or less frequent alterations involving exons 18 and 20 in EGFR account for 10-20% of all EGFR mutations in NSCLC. Individuals with uncommon EGFR mutations seem to be a heterogeneous group exhibiting differential sensitivity to EGFR inhibitors, but clinical evidence is scarce[5].
Studies on the delE709_T710insD mutation and its response to EGFR-TKIs, including gefitinib, erlotinib, and afatinib, have been reported sporadically in recent years (Table 1). Wu JY et al[6] reported that the prevalence of delE709_T710insD is 0.16% (5/3146) in EGFR mutations. Six gefitinib-treated patients harboring delE709_T710insD were nonresponders, with a median PFS of 2.65 mo[6-8]. Erlotinib was administered in previous case reports[8-12], which also seemed to be a frustrated treatment for delE709_T710insD. One had a PR, 5 had PD, and the response rate was only 25% (1/6). Afatinib was proven to be effective for such rare variants[13-18]. Among the 6 patients receiving afatinib, one achieved a complete response (CR), and 5 achieved a PR. More significantly, 1 patient with E709_T710delinsD mutations showed a survival benefit of afatinib after erlotinib treatment failed[19]. The overall response rate of afatinib for delE709_T710insD was 100% (7/7). According to the analysis by Rubiera-Pebe R et al[20], the median PFS comparison between first-generation TKIs and afatinib for patients with delE709_T710insD is 3.1 mo vs 7.0 mo, respectively. In vitro, a study by Kobayashi Y et al[19] investigated the sensitivities of exon 18 mutations to various EGFR-TKIs and suggested that second-generation EGFRi have broader inhibitory profiles than other TKIs for rare mutations.
| Ref. | Patient No. | Gender | Age (yr) | Smoking | Stage | Histologic type | TKI used/line | Response | PFS (m) | OS (m) |
| Wu et al[7], 2011 | 1 | F | 61 | No | IV | AD | Gefitinib/NA | SD | 5.1 | 22.7 |
| 2 | M | 65 | Yes | IV | AD | Gefitinib/NA | PD | 0.9 | 11.1 | |
| Ackerman et al[9], 2012 | 3 | F | 88 | No | IV | AD | Erlotinib/1st | PR | 6 | NA |
| Kobayashi et al[19], 2015 | 4 | M | 63 | NA | IV | AD | Erlotinib/3rd | SD | NA | NA |
| Afatinib/4th | PR | NA | ||||||||
| Wu et al[6], 2016 | 5 | F | 57 | No | IV | AD | Gefitinib/NA | PD | 0.6 | 24.1 |
| 6 | M | 79 | Yes | IV | AD | Gefitinib/NA | SD | 6.2 | 6.2 | |
| 7 | M | 68 | Yes | IV | AD | Gefitinib/NA | PD | 2.3 | 29.5 | |
| Klughammer et al[10], 2016 | 8 | F | 50 | No | III/IV | NSCLC | Erlotinib/2nd | PD | 1.3 | 1.7 |
| Ibrahim et al[13], 2017 | 9 | F | 52 | No | IV | AD | Afatinib/1st | PR | NA | NA |
| An et al[14], 2019 | 10 | M | 56 | No | IV | AD | Afatinib/2nd | PR | 11 | More than 21 |
| Iwamoto et al[15], 2019 | 11 | F | 56 | No | IV | AD | Afatinib/6th | PR | 7 | NA |
| D’Haene et al[16], 2019 | 12 | F | 57 | No | III | AD | Afatinib/2nd | PR | 12 | 36 |
| Martin et al[11], 2019 | 13 | M | 60 | No | IV | AD | Erlotinib/NA | PD | 1 | 3 |
| Isaksson et al[12], 2020 | 14 | NA | NA | NA | IV | NA | Erlotinib/1st | PD | 8 | NA |
| Sousa et al[8], 2020 | 15 | F | 66 | Yes | IV | AD | Gefitinib/1st | PD | 3 | 24 |
| 16 | F | 46 | Yes | II | AD | Erlotinib/2nd | PD | 4 | 26 | |
| 17 | F | 57 | No | IV | AD | Erlotinib/2nd | PD | 3 | 18 | |
| Wei et al[17], 2021 | 18 | F | 70 | No | II | NSCLC | Afatinib/1st | PR | 23 | On going |
| Jelli et al[18], 2021 | 19 | F | 57 | No | IV | AD | Afatinib/1st | CR | 17 (On going) | 17 (On going) |
| Xu et al, 2021 (this case) | 20 | F | 56 | No | IV | AD | Dacomitinib/2nd | PR | 7 | On going |
Like afatinib, dacomitinib is a second-generation pan-HER inhibitor that irreversibly binds to all three kinase-active members of the ErbB family (HER1/EGFR, HER2, and HER4), leading to more efficient EGFR inhibition. The efficacy of dacomitinib on patients acquiring Ex18 G719A as later-line therapy has been reported by Morita A et al[21]. In addition, dacomitinib in vitro has an IC50=29 nM for Ba/F3 cells expressing exon 18 delE709_T710insD[19], indicating the potential activity of this nonclassical mutation. The results of a phase 3 trial of dacomitinib (NCT01774721, ARCHER 1050) indicated that first-line dacomitinib significantly improved PFS and OS vs gefitinib, and the adverse events were manageable[22]. Based on these findings, dacomitinib seemed to be a promising candidate for EGFR-positive advanced NSCLC, including less common mutations. However, limited clinical data have shown the effect of dacomitinib on rare mutations.
In our study, we reported that a patient with EGFR delE709_T710insD achieved PR after the initiation of dacomitinib, with a PFS2 of 7 mo. To the best of our knowledge, this is the first report describing the clinical efficacy of dacomitinib for EGFR delE709_T710insD. The efficacy of dacomitinib on rare mutations needs to be evaluated in vivo or in vitro by further studies. In addition, appropriate genetic diagnosis methodologies will provide patients with more opportunities for targeted therapy. Our report may help to provide new treatment options for NSCLC patients with nonclassical variants.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Covantsev S, Russia; Suravajhala PN, India S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Han B, Tjulandin S, Hagiwara K, Normanno N, Wulandari L, Laktionov K, Hudoyo A, He Y, Zhang YP, Wang MZ, Liu CY, Ratcliffe M, McCormack R, Reck M. EGFR mutation prevalence in Asia-Pacific and Russian patients with advanced NSCLC of adenocarcinoma and non-adenocarcinoma histology: The IGNITE study. Lung Cancer. 2017;113:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 2. | Cheng C, Wang R, Li Y, Pan Y, Zhang Y, Li H, Zheng D, Zheng S, Shen X, Sun Y, Chen H. EGFR Exon 18 Mutations in East Asian Patients with Lung Adenocarcinomas: A Comprehensive Investigation of Prevalence, Clinicopathologic Characteristics and Prognosis. Sci Rep. 2015;5:13959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Atalogue of Somatic Mutations in Cancer, release version 94. [cited 20 January 2022]. Available from: https://cancer.sanger.ac.uk/cosmic. |
| 4. | Hsu WH, Yang JC, Mok TS, Loong HH. Overview of current systemic management of EGFR-mutant NSCLC. Ann Oncol. 2018;29:i3-i9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 262] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 5. | Russo A, Franchina T, Ricciardi G, Battaglia A, Picciotto M, Adamo V. Heterogeneous Responses to Epidermal Growth Factor Receptor (EGFR) Tyrosine Kinase Inhibitors (TKIs) in Patients with Uncommon EGFR Mutations: New Insights and Future Perspectives in this Complex Clinical Scenario. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 6. | Wu JY, Shih JY. Effectiveness of tyrosine kinase inhibitors on uncommon E709X epidermal growth factor receptor mutations in non-small-cell lung cancer. Onco Targets Ther. 2016;9:6137-6145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Wu JY, Yu CJ, Chang YC, Yang CH, Shih JY, Yang PC. Effectiveness of tyrosine kinase inhibitors on "uncommon" epidermal growth factor receptor mutations of unknown clinical significance in non-small cell lung cancer. Clin Cancer Res. 2011;17:3812-3821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 392] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 8. | Sousa AC, Silveira C, Janeiro A, Malveiro S, Oliveira AR, Felizardo M, Nogueira F, Teixeira E, Martins J, Carmo-Fonseca M. Detection of rare and novel EGFR mutations in NSCLC patients: Implications for treatment-decision. Lung Cancer. 2020;139:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Ackerman A, Goldstein MA, Kobayashi S, Costa DB. EGFR delE709_T710insD: a rare but potentially EGFR inhibitor responsive mutation in non-small-cell lung cancer. J Thorac Oncol. 2012;7:e19-e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Klughammer B, Brugger W, Cappuzzo F, Ciuleanu T, Mok T, Reck M, Tan EH, Delmar P, Klingelschmitt G, Yin AY, Spleiss O, Wu L, Shames DS. Examining Treatment Outcomes with Erlotinib in Patients with Advanced Non-Small Cell Lung Cancer Whose Tumors Harbor Uncommon EGFR Mutations. J Thorac Oncol. 2016;11:545-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 11. | Martin J, Lehmann A, Klauschen F, Hummel M, Lenze D, Grohé C, Tessmer A, Gottschalk J, Schmidt B, Pau HW, Witt C, Moegling S, Kromminga R, Jöhrens K. Clinical Impact of Rare and Compound Mutations of Epidermal Growth Factor Receptor in Patients With Non-Small-Cell Lung Cancer. Clin Lung Cancer. 2019;20:350-362.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Isaksson S, Hazem B, Jönsson M, Reuterswärd C, Karlsson A, Griph H, Engleson J, Oskarsdottir G, Öhman R, Holm K, Rosengren F, Annersten K, Jönsson G, Borg Å, Edsjö A, Levéen P, Brunnström H, Lindquist KE, Staaf J, Planck M. Clinical Utility of Targeted Sequencing in Lung Cancer: Experience From an Autonomous Swedish Health Care Center. JTO Clin Res Rep. 2020;1:100013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Ibrahim U, Saqib A, Atallah JP. EGFR exon 18 delE709_T710insD mutated stage IV lung adenocarcinoma with response to afatinib. Lung Cancer. 2017;108:45-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | An N, Wang H, Zhu H, Yan W, Jing W, Kong L, Zhang Y, Yu J. Great efficacy of afatinib on a patient with lung adenocarcinoma harboring uncommon EGFR delE709_T710insD mutations: a case report. Onco Targets Ther. 2019;12:7399-7404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Iwamoto Y, Ichihara E, Hara N, Nakasuka T, Ando C, Umeno T, Hirabae A, Maeda Y, Kiura K. Efficacy of afatinib treatment for lung adenocarcinoma harboring exon 18 delE709_T710insD mutation. Jpn J Clin Oncol. 2019;49:786-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | D'Haene N, Le Mercier M, Salmon I, Mekinda Z, Remmelink M, Berghmans T. SMAD4 Mutation in Small Cell Transformation of Epidermal Growth Factor Receptor Mutated Lung Adenocarcinoma. Oncologist. 2019;24:9-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Wei Y, Cui Y, Guo Y, Li L, Zeng L. A Lung Adenocarcinoma Patient With a Rare EGFR E709_T710delinsD Mutation Showed a Good Response to Afatinib Treatment: A Case Report and Literature Review. Front Oncol. 2021;11:700345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Jelli B, Taton O, D'Haene N, Remmelink M, Mekinda Z. Complete Response to Afatinib of an EGFR Exon 18 delE709_T710insD-Mutated Stage IV Lung Adenocarcinoma. Eur J Case Rep Intern Med. 2021;8:002749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Kobayashi Y, Togashi Y, Yatabe Y, Mizuuchi H, Jangchul P, Kondo C, Shimoji M, Sato K, Suda K, Tomizawa K, Takemoto T, Hida T, Nishio K, Mitsudomi T. EGFR Exon 18 Mutations in Lung Cancer: Molecular Predictors of Augmented Sensitivity to Afatinib or Neratinib as Compared with First- or Third-Generation TKIs. Clin Cancer Res. 2015;21:5305-5313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 159] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 20. | Rubiera-Pebe R, Hicks JK, Tanvetyanon T. Efficacy of tyrosine kinase inhibitors against lung cancer with EGFR exon 18 deletion: Case report and pooled analysis. Cancer Treat Res Commun. 2021;28:100407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Morita A, Hosokawa S, Yamada K, Umeno T, Kano H, Kayatani H, Shiojiri M, Sakugawa M, Bessho A. Dacomitinib as a retreatment for advanced non-small cell lung cancer patient with an uncommon EGFR mutation. Thorac Cancer. 2021;12:1248-1251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, Tsuji F, Linke R, Rosell R, Corral J, Migliorino MR, Pluzanski A, Sbar EI, Wang T, White JL, Nadanaciva S, Sandin R, Mok TS. Dacomitinib vs gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:1454-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 84] [Reference Citation Analysis (0)] |