Published online Jun 6, 2022. doi: 10.12998/wjcc.v10.i16.5502
Peer-review started: December 27, 2021
First decision: February 8, 2022
Revised: February 18, 2022
Accepted: April 3, 2022
Article in press: April 3, 2022
Published online: June 6, 2022
Processing time: 157 Days and 8.2 Hours
Gastric neuroendocrine carcinoma (GNEC) is a rare histological subtype of gastric cancer, which is categorized into small cell and large cell neuroendocrine car
Herein, we first present a 57-year-old patient diagnosed with L/SCNEC of the stomach. A 57-year-old Chinese male presented with epigastric discomfort. Outpatient gastroscopic biopsy was performed, and pathological examination revealed that the cardia was invaded by adenocarcinoma. The patient underwent laparoscopic-assisted radical proximal subtotal gastrectomy and was diagnosed with L/SCNEC. He refused adjuvant treatment and was followed up every 3 mo. Eight months after the operation, the patient showed no evidence of local re
We advocate conducting further genomic studies to explore the origin of gastric large cell and small cell neuroendocrine carcinoma and using different chemo
Core Tip: To the best of our knowledge, there have been no previous reports on mixed large and small cell neuroendocrine carcinoma of the stomach. This case might contribute to improving our understanding of gastric neuroendocrine carcinoma. More basic and clinical researches are warranted to clarify the heterogeneity of gastric neuroendocrine carcinoma.
- Citation: Li ZF, Lu HZ, Chen YT, Bai XF, Wang TB, Fei H, Zhao DB. Mixed large and small cell neuroendocrine carcinoma of the stomach: A case report and review of literature. World J Clin Cases 2022; 10(16): 5502-5509
- URL: https://www.wjgnet.com/2307-8960/full/v10/i16/5502.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i16.5502
It has been reported that the incidence rate of gastric neuroendocrine carcinoma (GNEC) is relatively low and accounts for 0.1% to 0.6% of all gastric cancers[1]. However, the incidence rate has been increasing in the past 20 years[2]. Due to its high degree of malignancy and poor prognosis, GNEC is receiving increasing attention. In 2019, the World Health Organization (WHO) listed poorly differentiated GNEC separately from the type 4 gastric neuroendocrine tumor and further subdivided it into two subtypes: Gastric large cell neuroendocrine carcinoma and gastric small cell neuroendocrine carcinoma[3]. Herein, we first report a 57-year-old male diagnosed with mixed large and small cell neuroendocrine carcinoma (L/SCNEC) of the stomach.
A 57-year-old man was referred to our hospital for the treatment of gastric cancer.
Two months prior, he visited a clinic complaining of upper abdominal discomfort. Pathologic examination of the biopsy under esophagogastroduodenoscopy revealed cardiac adenocarcinoma in another hospital.
He had diabetes for 30 years, for which he was taking metformin daily.
There was no relevant personal or family history.
Physical assessment revealed no abnormalities.
Laboratory examinations, including the tumor marker levels, revealed no abnormalities.
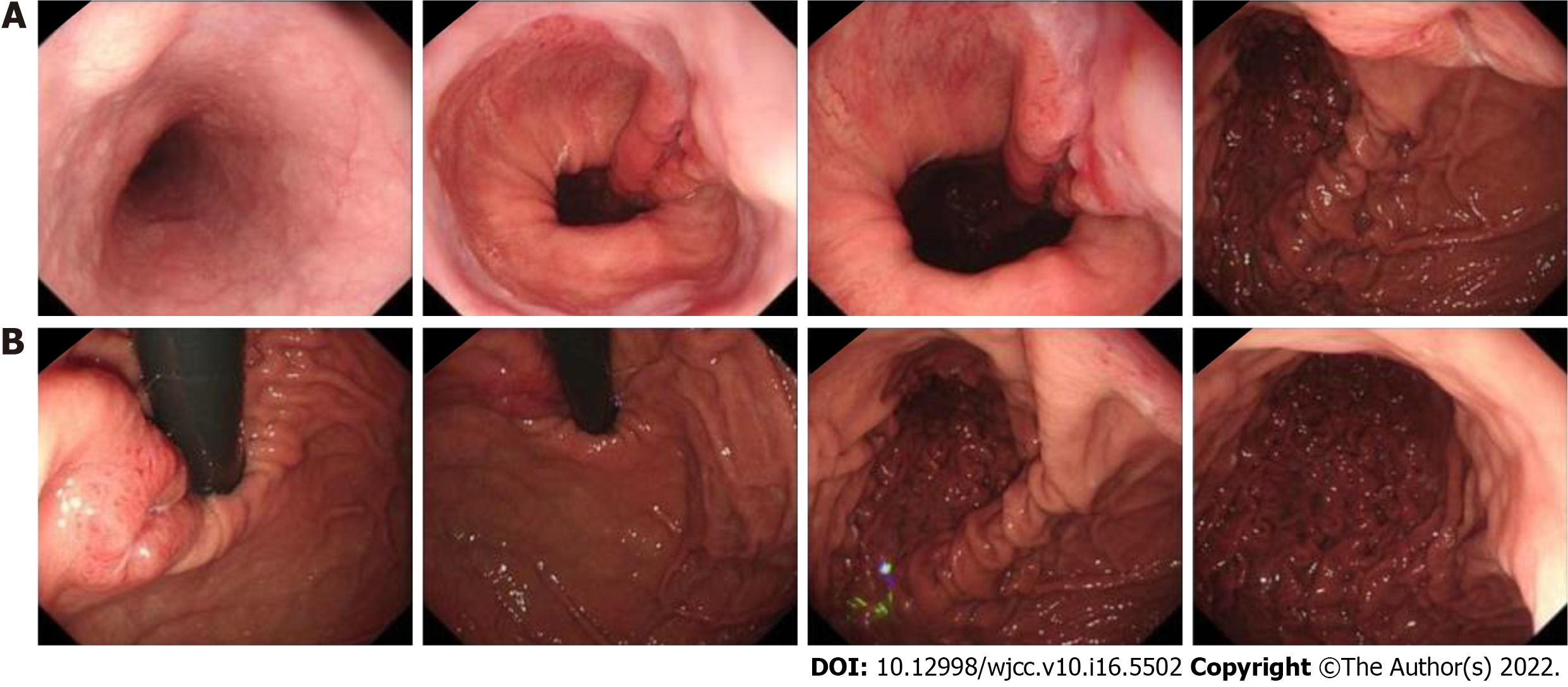
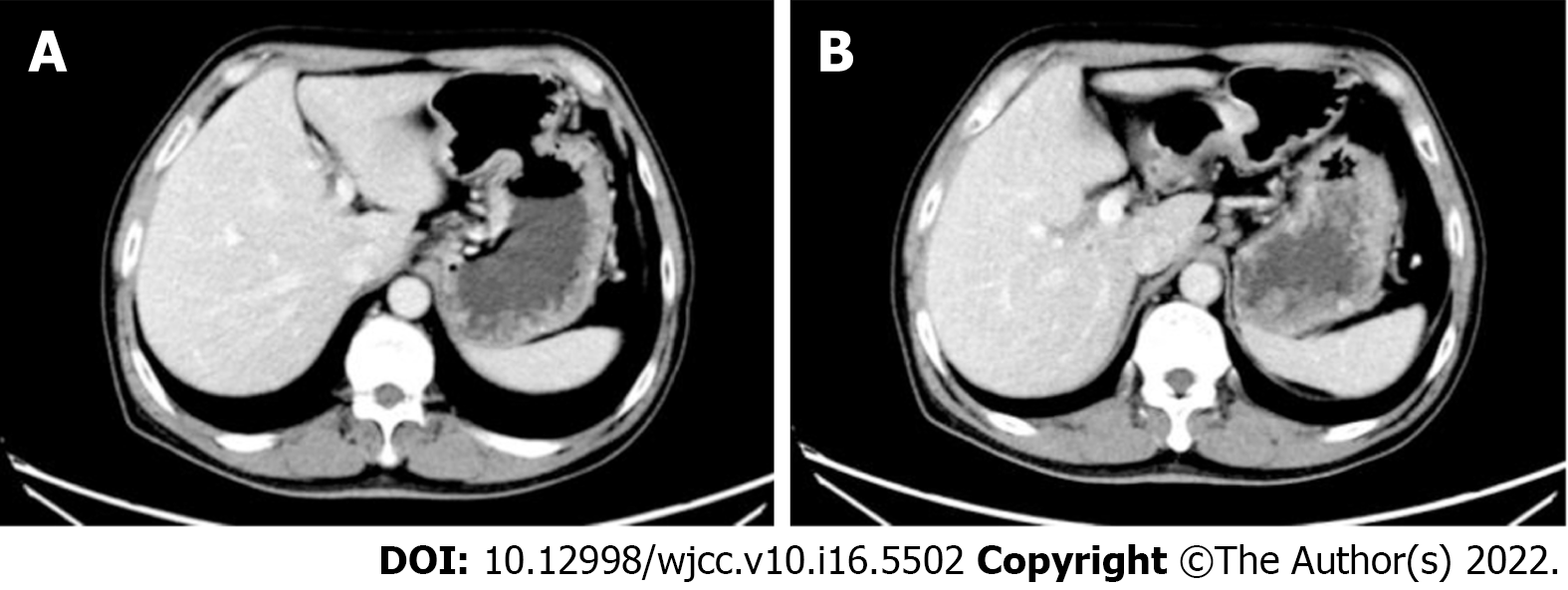
Esophagogastroduodenoscopy showed that an ulcerative tumor was approximately 1-3 cm away from the esophagogastric junction with a deep ulcer bottom and covered with dirt and white moss on the surface (Figure 1). Contrast-enhanced computed tomography scans revealed uneven thickening of the lesser curvature of the cardia and corpus, in accordance with gastric cancer, and coalesced lymph nodes in the cardiac area, approximately 0.8 cm in diameter (Figure 2).
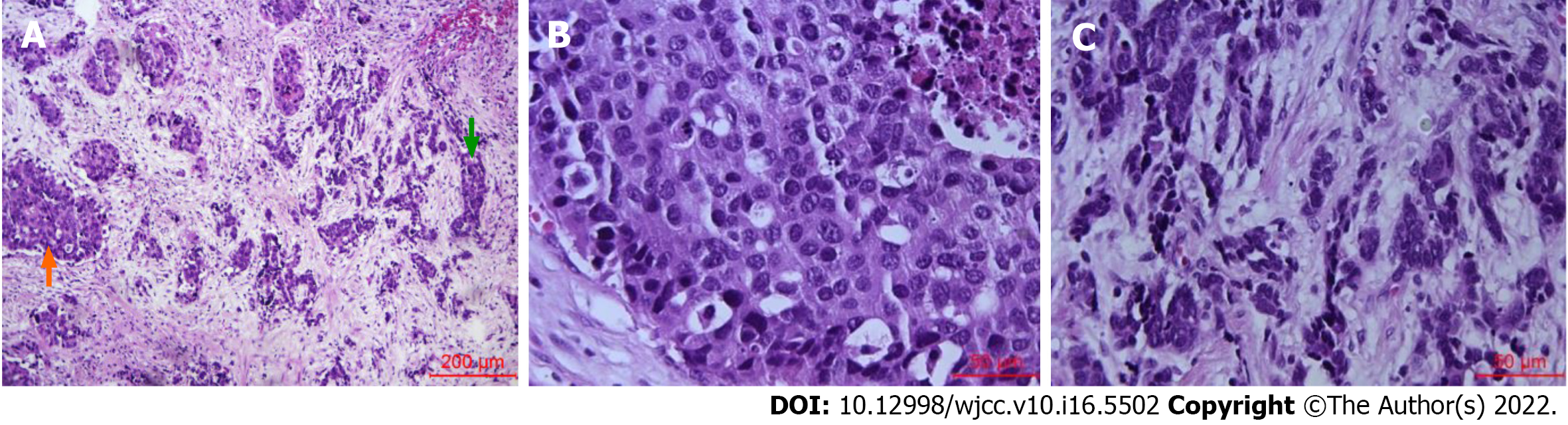
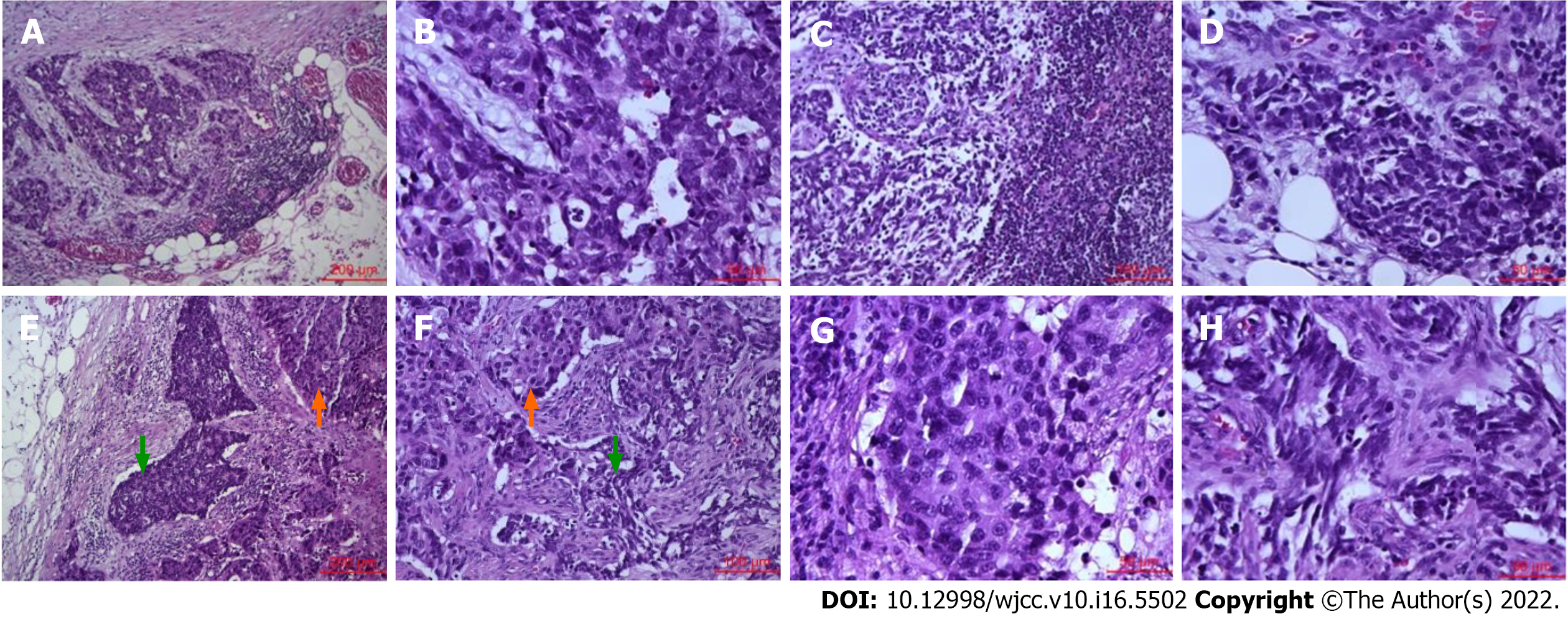
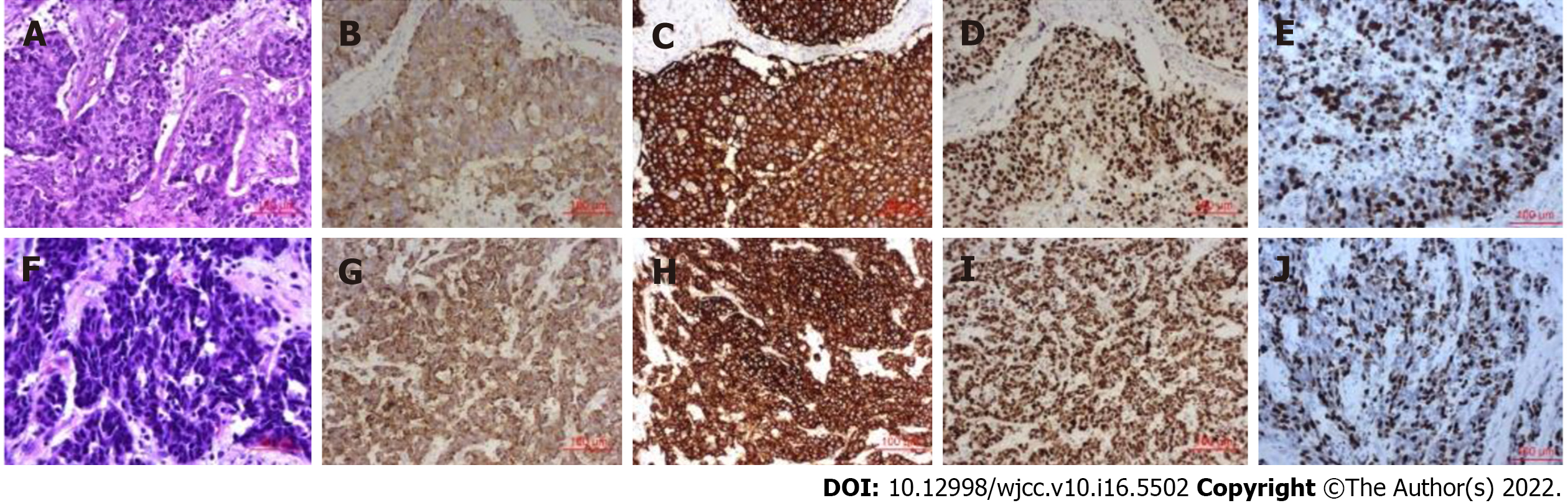
A gross examination of the surgically resected specimen showed that a protuberant tumor with a size of 3 cm × 1 cm × 0.6 cm could be seen at the esophagogastric junction. Microscopically, mixed large (70%) and small (30%) carcinoma cells invaded the propria muscularis layer, with a negative margin (Figure 3). Vascular tumor thrombus and nerve invasion could be seen. Some lymph nodes were found to have metastatic carcinoma (5/21). One of them was large cell carcinoma components. One of them was mixed large and small cell carcinoma components. Three lymph nodes were small cell carcinoma components (Figure 4). Immunohistochemistry (Figure 5) showed AE1/AE3 (2+), Syn (3+), CD56 (3+), CgA (2+), Ki-67 (60-70%), p53 (80%), AFP (-), c-Met (-), EGFR (-), GPC3 (-), HER2 (0), MLH1 (+), MSH2 (+), MSH6 (+), PMS2 (+), Sall4 (2+), and S-100 (-). In situ hybridization showed EBER (-). The pTNM classification was T2N2M0 (stage IIB).
The patient was diagnosed with gastric cancer (L/SCNEC) pT2N2M0 (stage IIB), accompanied by diabetes.
We performed a laparoscopic-assisted subtotal gastrectomy with D2 lymphadenectomy. The patient refused adjuvant treatment.
The patient remained recurrence- and metastasis-free 8 mo after surgery.
GNEC is a malignant tumor with poor biological behavior. The incidence rate of GNEC has been increasing in recent years[2]. In 2019, the WHO listed poorly differentiated GNEC separately from the type 4 gastric neuroendocrine tumor and further subdivided it into two types: large cell neuroendocrine carcinoma and small cell neuroendocrine carcinoma. Mixed adenoneuroendocrine carcinoma (MANEC) has also been expanded to mixed neuroendocrine non-neuroendocrine neoplasms (MiNEN), and it is stipulated that both neuroendocrine and non-neuroendocrine components should exceed 30%[3]. However, the cutoff point of 30% has been controversial for a long time[4]. Jiang et al[5] believed that more than 20% of neuroendocrine components could affect the prognosis in gastric adenocarcinoma, and Park et al[6] advocated that the cutoff value should be set at 10%. Even though the neuroendocrine component accounts for a relatively low proportion in the primary focus, it can become the main component in the metastatic lymph nodes, suggesting that the GNEC component has higher malignant behavior, and the vessels and lymphatic vessels could be invaded in the early stage[7]. This case of GNEC did not receive neoadjuvant therapy and was mixed with large cell and small cell neuroendocrine components, both of which were more than 30%. A few reports of L/SCNEC have been seen in the lung, uterus and other organs in the past[8,9]. However, to the best of our knowledge, this is the first time it has been reported in the digestive system.
The origin of GNEC and MiNEN has not been determined. One view is that during the proliferation of normal enterochromaffin-like cells, superimposed gene mutations result in gastric neuroendocrine tumor formation, which further progresses to GNEC, diffuse gastric adenocarcinoma and finally signet ring cell carcinoma[10-12]. Another view is that gastric neuroendocrine cells predominantly arise from neuroendocrine precursor cell clones occurring in preceding adenocarcinoma components, which transform into neuroendocrine cells during rapid clonal expansion. The adenocarcinoma component may become necrotic or desquamate, while the neuroendocrine component rapidly develops. Thus, MANEC seems to be a transitional stage in the transformation from gastric adenocarcinoma to GNEC. Gene sequencing[13-15] and mucin phenotype expression[16] of GNEC, gastric adenocarcinoma and two components in MANEC have tested and supported the hypothesis. GNEC is usually diagnosed at an advanced stage, which also supports this viewpoint. There is a potential consensus that adenocarcinoma cells and neuroendocrine cancer cells can originate from the same kind of precursor cells. However, Makuuchi et al[17] found that there were significant differences in gene expression between GNEC and gastric adenocarcinoma by whole exon sequencing. The vast majority of mutated genes in GNEC (517/557, 92.8%) were not mutated in gastric adenocarcinoma. Lewin[18] histologically divided MANEN into a combination type (two components adjacent but not mixed), collision type (two components cross mixed with each other) and double secretion type (tumor cells secreting mucus and expressing neuroendocrine markers at the same time). The tumorigenesis of different types may be distinct, which may explain the differences in the above research results.
For GNEC patients without distant metastasis, surgical resection of the lesion is still the first choice. At present, platinum-based chemotherapy is often used as the first-line treatment for patients with advanced GNEC who have lost the opportunity for radical operation. FOLFIRI (fluorouracil, leucovorin, and irinotecan) or FOLFOX (leucovorin, fluorouracil, and oxaliplatin) can be used as the second-line treatment. The effectiveness of molecular targeted therapy[19], immunotherapy[20] and peptide receptor radionuclide therapy in patients with GNEC needs to be further tested[21]. Okita et al[22] found that after receiving EP (cisplatin plus irinotecan) chemotherapy, the response rate of 12 GNEC patients with distant metastasis or postoperative recurrence was 75%. The median progression-free survival time was 212 d, and the median survival time was 679 d. Thus, the EP regimen showed good therapeutic effects. Ma et al[23] found that neoadjuvant therapy can improve the prognosis of patients with GNEC (the 5-year survival rates of the neoadjuvant therapy group and direct surgery group were 57.4% vs 28.5%, respectively). However, there was no effect between the two subgroups of neoadjuvant chemotherapy using regimens based on platinum agents or not. In addition, there has been much discussion about whether adjuvant chemotherapy after radical resection can improve the prognosis of patients with GNEC. In 2020, a multicenter study in China found that after propensity score matching, neither chemotherapy based on platinum agents nor chemotherapy based on 5-fluorouracil agents can improve the prognosis of these patients[24]. The heterogeneity of GNEC may be the reason for the difference in treatment response.
The prognosis of GNEC is worse than that of gastric adenocarcinoma[4], and the prognosis of MANEC is worse than that of gastric adenocarcinoma but better than that of GNEC[25]. A multicenter retrospective study included 503 patients with GNEC, 401 patients with MANEC and 2875 patients with gastric adenocarcinoma. After propensity score matching, the 5-year disease-free survival rates of GNEC and gastric adenocarcinoma were 47.6% vs 57.6%, respectively (P < 0.001); the 5-year disease-free survival rates of MANEC and gastric adenocarcinoma were 51.1% and 57.8%, respectively (P = 0.02)[26]. The high proportion of neuroendocrine components in MANEC often indicates poor prognosis[27,28]. This may be related to the fact that the components of GNEC are more prone to distant metastasis and lack of responsive chemotherapy.
In our case, although small cell neuroendocrine carcinoma components accounted for a lower ratio in the primary focus, there were more lymph node metastases. Compared with large cell neuroendocrine carcinoma, small cell neuroendocrine carcinoma may have worse biological behavior, at least in this case. However, there are few studies comparing the incidence rate, biological behavior, treatment modalities and prognosis of large cell GNEC and small cell GNEC. Xie et al[29] found that in 132 cases of GNEC, small cell carcinoma accounted for 23.7%, and the 3-year survival rate was 63.3%, while large cells accounted for 77.3%, and the 3-year survival rate was 41.6%. A retrospective clinical study also suggested that the prognosis of large cell GNEC was worse in Korea[30]. Whether the prognosis of L/SCNEC is different needs to be further explored in the future. In lung cancer with a higher incidence rate, next-generation sequencing studies have shown that large cell neuroendocrine carcinoma can be further subdivided into two mutually exclusive groups based on their mutational patterns: the small cell carcinoma-like type, characterized by TP53+RB1 co-mutation/loss and other small cell carcinoma-type alterations, including MYCL amplification; and the non-small cell carcinoma-like type, characterized by the lack of co-altered TP53+RB1 and nearly universal occurrence of non-small cell carcinoma-type mutations (STK11, KRAS, and KEAP1)[31]. The prognosis of lung large cell neuroendocrine carcinoma may be further improved by selecting the corresponding chemotherapy regimen according to different molecular subtypes[32].
At present, many scientists believe that some precursor cells in well-differentiated adenocarcinoma can differentiate into neuroendocrine cancer cells[33]. The tumor as a whole gradually becomes MANEC. Then, as adenocarcinoma cells undergo necrosis, they gradually progress to pure GNEC. In view of the two molecular subtypes of lung large cell neuroendocrine carcinoma, we believe that gastric large cell neuroendocrine carcinoma may also have two subtypes: "small cell carcinoma-like" and "adenocarcinoma-like". However, there are few gene sequencing studies in GNEC. The above hypothesis needs to be further verified by histology and genomics.
This report is the first case report on L/SCNEC of the stomach. There is no corresponding classification in the WHO 2019 classification of digestive system neuroendocrine neoplasms. Clinically, most of patients with GNEC did not receive different chemotherapy schemes according to large cells or small cells, which may cause confusion in clinical treatment. We report the first case of L/SCNEC of the stomach and advocate using different chemotherapy regimens according to large or small cell neuroendocrine carcinoma of the stomach for clinical research to clarify the heterogeneity of GNEC and improve the prognosis of patients with GNEC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fagundes RB, Brazil; Masaki S, Japan; Masaki S, Japan S-Editor: Gao CC L-Editor: A P-Editor: Zhang YL
| 1. | Iwamoto M, Gotoda T, Noda Y, Esaki M, Moriyama M, Yoshida N, Takayama T, Kobayashi H, Masuda S. Gastric Neuroendocrine Carcinoma with Rapid Progression. Intern Med. 2020;59:1271-1276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3:1335-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1510] [Cited by in RCA: 2480] [Article Influence: 310.0] [Reference Citation Analysis (4)] |
| 3. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2428] [Article Influence: 485.6] [Reference Citation Analysis (3)] |
| 4. | Chen J, Wang A, Ji K, Bu Z, Ji J. Comparison of overall survival of gastric neoplasms containing neuroendocrine carcinoma components with gastric adenocarcinoma: a propensity score matching study. BMC Cancer. 2020;20:777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Jiang SX, Mikami T, Umezawa A, Saegusa M, Kameya T, Okayasu I. Gastric large cell neuroendocrine carcinomas: a distinct clinicopathologic entity. Am J Surg Pathol. 2006;30:945-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Park JY, Ryu MH, Park YS, Park HJ, Ryoo BY, Kim MG, Yook JH, Kim BS, Kang YK. Prognostic significance of neuroendocrine components in gastric carcinomas. Eur J Cancer. 2014;50:2802-2809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Xie JW, Lu J, Wang JB, Lin JX, Chen QY, Cao LL, Lin M, Tu RH, Huang ZN, Lin JL, Zheng CH, Li P, Huang CM. Prognostic factors for survival after curative resection of gastric mixed adenoneuroendocrine carcinoma: a series of 80 patients. BMC Cancer. 2018;18:1021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Lei Y, Feng H, Qiang H, Shang Z, Chang Q, Qian J, Zhang Y, Zhong R, Fan X, Chu T. Clinical characteristics and prognostic factors of surgically resected combined small cell lung cancer: a retrospective study. Lung Cancer. 2020;146:244-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Hu R, Jiang J, Song G, Zhu C, Chen L, Wang C, Wang X. Mixed large and small cell neuroendocrine carcinoma of the endometrium with serous carcinoma: A case report and literature review. Medicine (Baltimore). 2019;98:e16433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Waldum H, Mjønes PG. Correct Identification of Cell of Origin May Explain Many Aspects of Cancer: The Role of Neuroendocrine Cells as Exemplified from the Stomach. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Bakkelund K, Fossmark R, Nordrum I, Waldum H. Signet ring cells in gastric carcinomas are derived from neuroendocrine cells. J Histochem Cytochem. 2006;54:615-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Ronellenfitsch U, Ströbel P, Schwarzbach MH, Staiger WI, Gragert D, Kähler G. A composite adenoendocrine carcinoma of the stomach arising from a neuroendocrine tumor. J Gastrointest Surg. 2007;11:1573-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Nishikura K, Watanabe H, Iwafuchi M, Fujiwara T, Kojima K, Ajioka Y. Carcinogenesis of gastric endocrine cell carcinoma: analysis of histopathology and p53 gene alteration. Gastric Cancer. 2003;6:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Ishida S, Akita M, Fujikura K, Komatsu M, Sawada R, Matsumoto H, Saegusa J, Itoh T, Kakeji Y, Zen Y. Neuroendocrine carcinoma and mixed neuroendocrine‒non-neuroendocrine neoplasm of the stomach: a clinicopathological and exome sequencing study. Hum Pathol. 2021;110:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Scardoni M, Vittoria E, Volante M, Rusev B, Bersani S, Mafficini A, Gottardi M, Giandomenico V, Malleo G, Butturini G, Cingarlini S, Fassan M, Scarpa A. Mixed adenoneuroendocrine carcinomas of the gastrointestinal tract: targeted next-generation sequencing suggests a monoclonal origin of the two components. Neuroendocrinology. 2014;100:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 16. | Domori K, Nishikura K, Ajioka Y, Aoyagi Y. Mucin phenotype expression of gastric neuroendocrine neoplasms: analysis of histopathology and carcinogenesis. Gastric Cancer. 2014;17:263-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Makuuchi R, Terashima M, Kusuhara M, Nakajima T, Serizawa M, Hatakeyama K, Ohshima K, Urakami K, Yamaguchi K. Comprehensive analysis of gene mutation and expression profiles in neuroendocrine carcinomas of the stomach. Biomed Res. 2017;38:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Lewin K. Carcinoid tumors and the mixed (composite) glandular-endocrine cell carcinomas. Am J Surg Pathol. 1987;11 Suppl 1:71-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 169] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Mishima S, Kawazoe A, Matsumoto H, Kuboki Y, Bando H, Kojima T, Doi T, Ohtsu A, Yoshino T, Nonte EM, Chintharlapalli S, Nasir A, Kuwata T, Shitara K. Efficacy and safety of ramucirumab-containing chemotherapy in patients with pretreated metastatic gastric neuroendocrine carcinoma. ESMO Open. 2018;3:e000443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Yang MW, Fu XL, Jiang YS, Chen XJ, Tao LY, Yang JY, Huo YM, Liu W, Zhang JF, Liu PF, Liu Q, Hua R, Zhang ZG, Sun YW, Liu DJ. Clinical significance of programmed death 1/programmed death ligand 1 pathway in gastric neuroendocrine carcinomas. World J Gastroenterol. 2019;25:1684-1696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Thomas KEH, Voros BA, Boudreaux JP, Thiagarajan R, Woltering EA, Ramirez RA. Current Treatment Options in Gastroenteropancreatic Neuroendocrine Carcinoma. Oncologist. 2019;24:1076-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Okita NT, Kato K, Takahari D, Hirashima Y, Nakajima TE, Matsubara J, Hamaguchi T, Yamada Y, Shimada Y, Taniguchi H, Shirao K. Neuroendocrine tumors of the stomach: chemotherapy with cisplatin plus irinotecan is effective for gastric poorly-differentiated neuroendocrine carcinoma. Gastric Cancer. 2011;14:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Ma F, Wang B, Xue L, Kang W, Li Y, Li W, Liu H, Ma S, Tian Y. Neoadjuvant chemotherapy improves the survival of patients with neuroendocrine carcinoma and mixed adenoneuroendocrine carcinoma of the stomach. J Cancer Res Clin Oncol. 2020;146:2135-2142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 24. | Lin JP, Zhao YJ, He QL, Hao HK, Tian YT, Zou BB, Jiang LX, Lin W, Zhou YB, Li Z, Xu YC, Zhao G, Xue FQ, Li SL, Fu WH, Li YX, Zhou XJ, Li Y, Zhu ZG, Chen JP, Xu ZK, Cai LH, Li E, Li HL, Xie JW, Huang CM, Li P, Lin JX, Zheng CH. Adjuvant chemotherapy for patients with gastric neuroendocrine carcinomas or mixed adenoneuroendocrine carcinomas. Br J Surg. 2020;107:1163-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | La Rosa S, Marando A, Sessa F, Capella C. Mixed Adenoneuroendocrine Carcinomas (MANECs) of the Gastrointestinal Tract: An Update. Cancers (Basel). 2012;4:11-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 190] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 26. | Lin J, Zhao Y, Zhou Y, Tian Y, He Q, Lin J, Hao H, Zou B, Jiang L, Zhao G, Lin W, Xu Y, Li Z, Xue F, Li S, Fu W, Li Y, Xu Z, Chen J, Zhou X, Zhu Z, Cai L, Li E, Li H, Zheng C, Li P, Huang C, Xie J. Comparison of Survival and Patterns of Recurrence in Gastric Neuroendocrine Carcinoma, Mixed Adenoneuroendocrine Carcinoma, and Adenocarcinoma. JAMA Netw Open. 2021;4:e2114180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 27. | Chen MH, Kuo YJ, Yeh YC, Lin YC, Tzeng CH, Liu CY, Chang PM, Chen MH, Jeng YM, Chao Y. High neuroendocrine component is a factor for poor prognosis in gastrointestinal high-grade malignant mixed adenoneuroendocrine neoplasms. J Chin Med Assoc. 2015;78:454-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Nie L, Li M, He X, Feng A, Wu H, Fan X. Gastric mixed adenoneuroendocrine carcinoma: correlation of histologic characteristics with prognosis. Ann Diagn Pathol. 2016;25:48-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Xie JW, Sun YQ, Feng CY, Zheng CH, Li P, Wang JB, Lin JX, Lu J, Chen QY, Cao LL, Lin M, Tu RH, Yang YH, Huang CM. Evaluation of clinicopathological factors related to the prognosis of gastric neuroendocrine carcinoma. Eur J Surg Oncol. 2016;42:1464-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Choi NY, Kim BS, Oh ST, Yook JH. Comparative Outcomes in Patients With Small- and Large-Cell Neuroendocrine Carcinoma (NEC) and Mixed Neuroendocrine-Non-Neuroendocrine Neoplasm (MiNEN) of the Stomach. Am Surg. 2021;87:631-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Rekhtman N, Pietanza MC, Hellmann MD, Naidoo J, Arora A, Won H, Halpenny DF, Wang H, Tian SK, Litvak AM, Paik PK, Drilon AE, Socci N, Poirier JT, Shen R, Berger MF, Moreira AL, Travis WD, Rudin CM, Ladanyi M. Next-Generation Sequencing of Pulmonary Large Cell Neuroendocrine Carcinoma Reveals Small Cell Carcinoma-like and Non-Small Cell Carcinoma-like Subsets. Clin Cancer Res. 2016;22:3618-3629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 342] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 32. | Derks JL, Leblay N, Thunnissen E, van Suylen RJ, den Bakker M, Groen HJM, Smit EF, Damhuis R, van den Broek EC, Charbrier A, Foll M, McKay JD, Fernandez-Cuesta L, Speel EM, Dingemans AC; PALGA-Group. Molecular Subtypes of Pulmonary Large-cell Neuroendocrine Carcinoma Predict Chemotherapy Treatment Outcome. Clin Cancer Res. 2018;24:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 146] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 33. | Quintanal-Villalonga Á, Chan JM, Yu HA, Pe'er D, Sawyers CL, Sen T, Rudin CM. Lineage plasticity in cancer: a shared pathway of therapeutic resistance. Nat Rev Clin Oncol. 2020;17:360-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 356] [Article Influence: 71.2] [Reference Citation Analysis (0)] |