Published online Jun 6, 2022. doi: 10.12998/wjcc.v10.i16.5456
Peer-review started: October 14, 2021
First decision: January 18, 2022
Revised: February 1, 2022
Accepted: April 3, 2022
Article in press: April 3, 2022
Published online: June 6, 2022
Processing time: 230 Days and 20.5 Hours
Computed tomography (CT)-guided percutaneous lung biopsy is a common protocol in the context of diagnostic thoracic oncology, but entails a risk of complications including systematic air embolism (SAE). While SAE is often well tolerated, it can be difficult to treat and may result in rapid mortality in some cases.
We report a rare case of left atrial SAE in a 71-year-old woman who underwent a CT-guided lung biopsy of a pulmonary nodule in the posterior basal segment of the right lower lobe. Shortly following needle extraction, she experienced a mild cough, hemoptysis, rapid-onset unconsciousness, and cardiopulmonary arrest. Cardiopulmonary resuscitation was immediately performed, but the patient died 40 min after the procedure. A closer review of collected CT scans revealed the presence of a large volume of air within the left atrium.
Although SAE is generally well tolerated and asymptomatic, interventional radiologists must be aware of the risk of fatal outcomes and establish appropriate emergency management protocols. In this report, the characteristics, mechanisms, and treatment recommendations associated with SAE are discussed in an effort to improve the survival of affected patients.
Core Tip: Systemic air embolism (SAE) is a rare but potentially fatal complication of certain procedures. Although some risk factors and emergency treatments for SAE have been proposed, a proportion of patients nonetheless suffer from catastrophic SAE even if procedures are performed by experienced operators, ultimately experiencing poor outcomes. Lesion localization above the level of the left atrium is a risk factor for SAE following percutaneous lung biopsy. Positive pressure ventilation may exacerbate SAE-related episodes in patients suffering from catastrophic air embolism, particularly in those who required cardiopulmonary resuscitation.
- Citation: Li YW, Chen C, Xu Y, Weng QP, Qian SX. Fatal left atrial air embolism as a complication of percutaneous transthoracic lung biopsy: A case report. World J Clin Cases 2022; 10(16): 5456-5462
- URL: https://www.wjgnet.com/2307-8960/full/v10/i16/5456.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i16.5456
Computed tomography (CT)-guided lung biopsy is a widely accepted, minimally invasive procedure with a high diagnostic yield for a variety of peripheral lung nodules. The most frequently reported complications of this procedure include pneumothorax, pulmonary hemorrhage, and hemoptysis, which are generally managed conservatively[1]. Systemic air embolism (SAE) is a very rare complication of percutaneous CT-guided lung biopsy, with reported air embolism incidence rates of 0.02%-0.07% to 0.21%-4.8% when including undiagnosed asymptomatic patients following systematic thoracic CT scans[2]. One recent study explored the rate of air embolism in 2026 patients that had undergone percutaneous CT-guided lung biopsy, all of whom underwent general post-biopsy lung and brain CT scans. This analysis revealed an incidence rate of 0.9%, while just three cases (0.14%) presented with obvious clinical symptoms[3]. Owing to these extremely low incidence rates, only case reports and small retrospective studies on this topic have been published to date.
The presentations and outcomes of SAE are linked to the degrees of end-organ involvement and associated anoxic damage, which are, in turn, determined by the volume of the air embolism and the site where it ultimately becomes lodged. The most vulnerable systems include the cerebral, spinal cord, and coronary circulatory systems[4]. When affected by an air embolism within the coronary arteries, patients may experience arrhythmia and acute coronary syndrome, while cerebral involvement can cause seizures, hemiparesis, pupillary dilation, or altered mental status. While SAE is generally well tolerated and asymptomatic, symptoms can manifest within minutes, resulting in hemodynamic instability, shock, and, in some cases, unconsciousness. When SAE is suspected, brain and chest CT scans should thus be performed to confirm the presence of an air embolism after terminating the associated procedure.
Here, we describe the case of a rare, catastrophic SAE that occurred immediately after percutaneous CT-guided lung biopsy in a 71-year-old woman. The clinical presentation and mechanistic basis for this complication are discussed, and practical tips for the management of SAE are reviewed.
A 71-year-old woman visited the Hangzhou First People’s Hospital for the evaluation of a lung lesion.
The patient was admitted for an incidental finding of an 11-mm partially-solid ground-glass nodule in the right lower lobe of the lung during a screening CT scan conducted 1 mo previously. The patient had no complaints other than a productive cough and a mild degree of dyspnea over the previous week.
The patient had a 6-year history of hypertension and had been taking amlodipine regularly.
The patient was free of any known congenital disease.
Vital signs were within normal limits at time of admission, with a heart rate of 90 bpm, blood pressure of 151/61 mmHg, respiratory rate of 20 breaths per minute, and temperature of 36.8℃. Her physical examination was normal.
The following examinations were performed: Electrocardiogram, blood cell count, C-reactive protein, and coagulation function tests, including activated partial thromboplastin and prothrombin time tests. All results were found to be within the normal ranges.
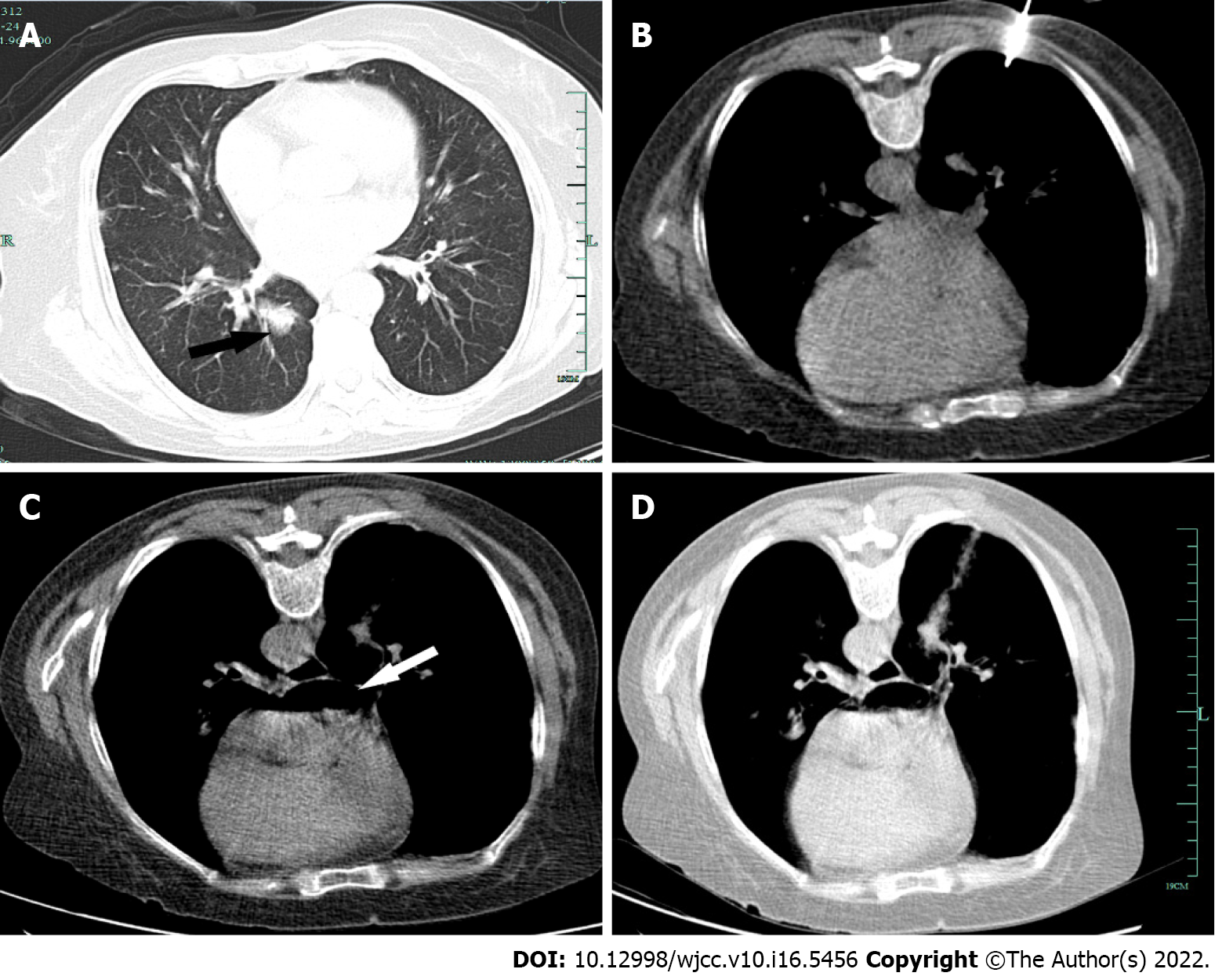
A CT scan identified a region of ground-glass opacity in the right lower lobe of the lung and scattered dense consolidation (Figure 1A). Echocardiography revealed moderate aortic regurgitation and mild mitral regurgitation.
The patient was thus diagnosed with pulmonary nodules and pulmonary infection.
Prior to the procedure, CT scanning was conducted to determine an appropriate needle trajectory, and the patient was instructed to cease full inspiration when directed. The nodule of interest was located on the posterior basal segment of the right lower lobe of the lung, and the prone position thus allowed for the best access route to this nodule. Under CT guidance, a co-axial 15-gauge needle was used to access the nodule with an Argon disposable core biopsy instrument (Argon Medical Devices, United States), and three biopsy samples were collected (Figure 1B). The introducer needle was then removed. The patient remained cooperative throughout the procedure without any coughing or deep breathing. The extraction procedure was performed two times. Shortly after needle extraction, she developed a mild cough and hemoptysis followed by breathlessness, further hemoptysis, near-immediate uncon
The patient did not respond to resuscitation, and was declared dead 40 min after the procedure. A closer postprocedural review of collected CT scans revealed a massive volume of air in the left atrium (Figure 1C) and a faint connection between blood vessels and airways (Figure 1D). Her relatives declined a postmortem study.
SAE is a potentially life-threatening event that requires prompt diagnosis and effective emergency treatment in all cases. Early animal experiments revealed that lethal air doses for rabbits and dogs were 0.5–0.75 mL/kg and 7.5–15.0 mL/kg, respectively[5]. However, given the clear variability among species, these data cannot be readily translated to humans. In 2001, Thomas JK first documented a precise lethal air volume in humans (200 mL) rather than retrospectively estimating this lethal volume[6]. Another study found that, in cases where specific vessels or organs were involved such as the cerebral circulation of the pulmonary vein, small volumes of air (2 mL and 0.5-1.0 mL, respectively) were sufficient to result in cardiac arrest[7,8]. This suggests that a smaller volume of gas is necessary to cause mortality when the air embolism is proximal to the right heart. It is thus likely that even a small volume of air within the coronary and cerebrovascular system can cause severe symptoms and complications owing to the vulnerability of these systems to hypoxia.
The factors that can give rise to SAE during transthoracic needle core biopsy are not fully understood, with three potential mechanisms having been described to date. First, a bronchovenous fistula between the intra-alveolar or intrabronchial air space and the adjacent pulmonary vein can be created when the needle passes through the lung parenchyma. Second, when the opening of the needle is not immediately sealed following stylet withdrawal, this can result in direct communication between the atmosphere and the pulmonary vein, particularly in any context that induces a negative pressure gradient and when there is a communication channel between the vasculature and the airway, as may occur upon coughing during a lung biopsy (Valsalva), in the context of positive end-expiratory pressure (PEEP) ventilation, or in those with obstructive pulmonary disease[9,10]. Third, air may be sucked into the pulmonary arterial system and may reach the pulmonary venous circulation by traversing the pulmonary microvasculature. Evidence from case reports suggests that certain factors can increase the risk of SAE, including the use of a larger biopsy needle, smaller lesions, procedures performed for a cystic or cavitary lesion, and patients with vasculitis, inflammation, or coagulopathy, although the relevance of these risk factors remains controversial[11-13].
As demonstrated in this report, the patient suspended full inspiration during the biopsy procedure, and the hollow portion of the needle remained occluded at all times through this procedure. As such, the formation of a transient bronchovenous fistula between a pulmonary vein and a small bronchus or alveolus may be the most likely explanation for these results, with cough, prone positioning, and lesion location all being relevant risk factors. In this patient, a mild cough and hemoptysis developed that, in turn, stimulated forceful coughing, resulting in the introduction of air into the damaged pulmonary vein[14]. Furthermore, the target lesion was located in the posterior basal segment of the right lower lobe, and the patient was thus placed in the prone position, resulting in a puncture site at the level of the heart such that the pulmonary venous pressure at this location was lower than left atrial pressure[14]. This position is more likely to allow for air entry into the left atrium through a bronchovenous fistula[15]. If the patient had instead been placed in an ipsilateral dependent position, which is a supine position with partial left side elevation, it is possible that the risk of this outcome could have been reduced[16].
This patient experienced rapid-onset unconsciousness and cardiac arrest towards the end of this biopsy procedure. Given her clinical signs and rapid deterioration, together with postprocedural chest CT images, we posit that a rapid and considerable volume of air was introduced into the pulmonary vein and the left atria, leading to obstructed cardiac inflow and outflow. As more air became trapped, this led to a critical reduction in cardiac output that ultimately resulted in systemic cardiovascular collapse, potentially complicating Stokes-Adams attacks. Unfortunately, the possibility of cranial air embolism could not be confirmed because the severity of the condition did not allow further examination and an autopsy was refused.
Hyperbaric oxygen treatment and the Trendelenburg position are currently recognized as a first-line treatments for air embolisms located within the coronary and cerebral vasculature. However, in clinical contexts, some patients are unable to undergo such interventions owing to rapid and severe cardiovascular collapse, as in the present case. Most patients exhibit adverse outcomes even if cardiopulmonary resuscitation and the inhalation of 100% oxygen are immediately provided when catastrophic air embolism is suspected[17]. One possible explanation for this fact is that PEEP and continuous positive airway pressure at resuscitation are considered to be independent risk factors with the potential to worsen SAE-related episodes. After a positive gradient between alveolar pressure and pulmonary venous pressure has been established, positive pressure ventilation increases pulmonary pressure through the application of high inflation pressures, thereby exacerbating the risk of air becoming trapped in addition to potentially facilitating air entry. Available data regarding the appropriate treatment of hemodynamically unstable patients suffering from SAE following lung biopsy are very limited. When basic therapy is unavailable, some reports suggest that the aspiration of air directly from the circulation via intracardiac catheter aspiration can be a safe and effective treatment for this condition, and studies using animal models have confirmed the feasibility of air embolism aspiration[5,18,19]. Furthermore, extracorporeal membrane oxygenation (ECMO) as a rescue therapy can be implemented to provide cardiopulmonary support and adequate gas exchange or perfusion pending etiologic SAE treatment. In a case report published by Seong et al[19], immediate resuscitation and ECMO were used to treat a 61-year-old man who suffered from paradoxical SAE during the removal of a central venous catheter. However, ECMO entails team management issues and requires a multidisciplinary approach. Moreover, the evidence regarding the value of ECMO use is limited to small case series, and its role in this context is not well established[20,21]. Even so, there may be a treatment benefit with ECMO when patients exhibit persistent cardiovascular collapse and are unresponsive to CPR.
In summary, percutaneous CT-guided lung biopsy is a widely accepted approach to the targeted analysis of lung lesions, permitting a range of downstream pathological and mutational analyses. While awareness of SAE has grown in recent years, diagnosing it remains challenging, and mortality rates remain high. Even when this procedure is performed by trained radiologists and patients are cooperative, SAE can inevitably occur in rare cases. There is thus an urgent need for high-quality prospective studies of SAE-related risk factors in order to guide appropriate patient risk stratification when selecting management strategies. Some SAE-related risk factors are inevitable. For example, when lesions are located in the dorsal and basal segments of the lower lobe of the lung, a prone position is always considered to be the preferred choice. Biopsies for pathological pulmonary abnormalities are more likely to be accompanied by prolonged exposure of the vessel lumen to the airway. To increase the safety of this procedure, clinicians should thus be aware of the courses of the airway and the adjacent vasculature in high-risk patients. Positive pressure ventilation also has the potential to exacerbate SAE progression. Further research is necessary to determine whether a lack of PEEP or temporary PEEP reductions are beneficial in the context of SAE-related cardiac arrest incidence.
Limitations of this report include the fact that no autopsy was performed, and as such, the cause of death was determined based upon clinical speculation. Furthermore, owing to the rarity of catastrophic SAE, our experience with optimal treatment is very limited and this patient ultimately experienced a poor outcome.
Herein, we have described a rare case of left atrial SAE following CT-guided percutaneous lung biopsy that resulted in sudden death. As this case demonstrates, SAE can be a life-threatening condition, making it essential that high-risk patients be identified. There may be an interval of just minutes between symptom onset and circulatory collapse, and as such, if this complication is suspected, prompt and effective emergency intervention is essential. It is critical that appropriate emergency equipment and medications be made available in first-aid kits. If possible, immediate trans-catheter removal of the SAE or ECMO support has the potential to reduce mortality rates. However, owing to the rarity of symptomatic SAE, there will be few future opportunities to pool studies for future prospective randomized trials. More clinical data and high-quality systematic reviews are expected to provide further insight into the most appropriate treatment of patients affected by SAE in the coming years.
We thank all the reviewers for their assistance and support.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Emergency medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hazafa A, Pakistan; Tumminello G, Italy; Vermeersch P, Belgium S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Heerink WJ, de Bock GH, de Jonge GJ, Groen HJ, Vliegenthart R, Oudkerk M. Complication rates of CT-guided transthoracic lung biopsy: meta-analysis. Eur Radiol. 2017;27:138-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 490] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 2. | Monnin-Bares V, Chassagnon G, Vernhet-Kovacsik H, Zarqane H, Vanoverschelde J, Picot MC, Bommart S. Systemic air embolism depicted on systematic whole thoracic CT acquisition after percutaneous lung biopsy: Incidence and risk factors. Eur J Radiol. 2019;117:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Liu SH, Fu Q, Yu HL, Yang Q, Hu YB, Zhang ZX, Zhang BP, Zhang CY. A retrospective analysis of the risk factors associated with systemic air embolism following percutaneous lung biopsy. Exp Ther Med. 2020;19:347-352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Hare SS, Gupta A, Goncalves AT, Souza CA, Matzinger F, Seely JM. Systemic arterial air embolism after percutaneous lung biopsy. Clin Radiol. 2011;66:589-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Mirski MA, Lele AV, Fitzsimmons L, Toung TJ. Diagnosis and treatment of vascular air embolism. Anesthesiology. 2007;106:164-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 376] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 6. | Toung TJ, Rossberg MI, Hutchins GM. Volume of air in a lethal venous air embolism. Anesthesiology. 2001;94:360-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 116] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Ohashi S, Endoh H, Honda T, Komura N, Satoh K. Cerebral air embolism complicating percutaneous thin-needle biopsy of the lung: complete neurological recovery after hyperbaric oxygen therapy. J Anesth. 2001;15:233-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Ho AM, Ling E. Systemic air embolism after lung trauma. Anesthesiology. 1999;90:564-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 90] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Kazimirko DN, Beam WB, Saleh K, Patel AM. Beware of positive pressure: coronary artery air embolism following percutaneous lung biopsy. Radiol Case Rep. 2016;11:344-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Glodny B, Schönherr E, Freund MC, Haslauer M, Petersen J, Loizides A, Grams AE, Augustin F, Wiedermann FJ, Rehwald R. Measures to Prevent Air Embolism in Transthoracic Biopsy of the Lung. AJR Am J Roentgenol. 2017;208:W184-W191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Mokhlesi B, Ansaarie I, Bader M, Tareen M, Boatman J. Coronary artery air embolism complicating a CT-guided transthoracic needle biopsy of the lung. Chest. 2002;121:993-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Arnold BW, Zwiebel WJ. Percutaneous transthoracic needle biopsy complicated by air embolism. AJR Am J Roentgenol. 2002;178:1400-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Rehwald R, Loizides A, Wiedermann FJ, Grams AE, Djurdjevic T, Glodny B. Systemic air embolism causing acute stroke and myocardial infarction after percutaneous transthoracic lung biopsy - a case report. J Cardiothorac Surg. 2016;11:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Hung WH, Chang CC, Ho SY, Liao CY, Wang BY. Systemic air embolism causing acute stroke and myocardial infarction after percutaneous transthoracic lung biopsy-a case report. J Cardiothorac Surg. 2015;10:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Rott G, Boecker F. Influenceable and Avoidable Risk Factors for Systemic Air Embolism due to Percutaneous CT-Guided Lung Biopsy: Patient Positioning and Coaxial Biopsy Technique-Case Report, Systematic Literature Review, and a Technical Note. Radiol Res Pract. 2014;2014:349062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Shetty PG, Fatterpekar GM, Manohar S, Sujit V, Varsha J, Zarir U. Fatal cerebral air embolism as a complication of transbronchoscopic lung biopsy: a case report. Australas Radiol. 2001;45:215-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Her AY, Kim YH, Moon DH, Kim JH, Jeong JH, Park SH, Jeong JS. Successful treatment of intracardiac air embolism using intracardiac catheter aspiration. J Geriatr Cardiol. 2017;14:151-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Colley PS, Artru AA. Bunegin-Albin catheter improves air retrieval and resuscitation from lethal venous air embolism in upright dogs. Anesth Analg. 1989;68:298-301. [PubMed] |
| 19. | Seong GM, Lee J, Kim M, Choi JC, Kim SW. Massive air embolism while removing a central venous catheter. Int J Crit Illn Inj Sci. 2018;8:176-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 20. | Shin S, Nam B, Soh S, Koo BN. Percutaneous cardiopulmonary support to treat suspected venous air embolism with cardiac arrest during open eye surgery: a case report. Korean J Anesthesiol. 2014;67:350-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Kuo CT, Yang Y, Tseng SH, Hsu MY, Wu NY, Wu YL, Tsai TP. Treat Venous Air Embolism Induced Acute Hypoxemic Respiratory Failure during Retinal Surgery by ECMO (V-V Mode). Acta Cardiol Sin. 2021;37:208-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |