Published online Jun 6, 2022. doi: 10.12998/wjcc.v10.i16.5230
Peer-review started: September 14, 2021
First decision: October 25, 2021
Revised: January 7, 2022
Accepted: April 2, 2022
Article in press: April 2, 2022
Published online: June 6, 2022
Processing time: 261 Days and 1.7 Hours
The prognosis of cerebrovascular diseases treated with mechanical ventilation during perioperative has not been clearly reported.
To analyze mortality and functional disability and to determine predictors of unfavorable outcome in the patients with cerebrovascular diseases treated with mechanical ventilation.
A retrospective follow-up study of 111 cerebrovascular disease patients who underwent mechanical ventilation during the perioperative period in the First Hospital of Jilin University from June 2016 to June 2019 was performed. Main measurements were mortality and functional outcome in-hospital and after 3-month follow-up. According to the modified rankin scale (mRS), the functional outcome was divided into three groups: Good recovery (mRS ≤ 3), severe disability (mRS = 4 or 5) and death (mRS = 6). Univariate analysis was used to compare the differences between three functional outcomes. Multivariate logistic regression analysis was used to for risk factors of mortality and severe disability.
The average age of 111 patients was 56.46 ± 12.53 years, 59 (53.15%) were males. The mortality of in-hospital and 3-month follow-up were 36.9% and 45.0%, respectively. Of 71 discharged patients, 46.47% were seriously disabled and 12.67% died after three months follow-up. Univariate analysis showed that preoperative glasgow coma scale, operation start time and ventilation reasons had statistically significant differences in different functional outcomes. Multiple logistic regression analysis showed that the cause of ventilation was related to the death and poor prognosis of patients with cerebrovascular diseases. Compared with brainstem compression, the risk of death or severe disability of pulmonary disease, status epilepticus, impaired respiratory center function, and shock were 0.096 (95%CI: 0.028-0.328), 0.026 (95%CI: 0.004-0.163), 0.095 (95%CI: 0.013-0.709), 0.095 (95%CI: 0.020-0.444), respectively.
The survival rate and prognostic outcomes of patients with cerebrovascular diseases treated with mechanical ventilation during the perioperative period were poor. The reason for mechanical ventilation was a statistically significant predictor for mortality and severe disability.
Core Tip: We were aimed to analyze mortality and functional disability and to determine predictors of unfavorable outcome in the patients with cerebrovascular diseases treated with mechanical ventilation. Our study indicated that survival and functional outcome in the patients of cerebrovascular diseases who treated with mechanical ventilation during the perioperative period were poor. The reason for mechanical ventilation (pulmonary disease, status epilepticus, shock, impaired respiratory center function and brainstem compression) could be a predictor for mortality and severe disability in these patients.
- Citation: Zhang JZ, Chen H, Wang X, Xu K. Risk factors of mortality and severe disability in the patients with cerebrovascular diseases treated with perioperative mechanical ventilation. World J Clin Cases 2022; 10(16): 5230-5240
- URL: https://www.wjgnet.com/2307-8960/full/v10/i16/5230.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i16.5230
Cerebrovascular diseases remain major cause of disability in the world. Studies showed that approximately 60% of the stroke patients die during the acute phase of therapy and the majority of the survivors suffered from severe disability[1-3], especially, the patients require intensive care therapy and mechanical ventilation. Patients with intracerebral hemorrhage, arteriovenous malformations, subarachnoid hemorrhage require mechanical ventilation because of pneumonia, pulmonary edema, brainstem ischemia or compression, and status epilepticus[4-6]. Mechanical ventilation often has been shown to be cost-ineffective at extending life with good recovery in the patients with stroke or other cerebrovascular diseases[7,8]. However, the dilemma is that although mechanical ventilation could sustain life in patients with acute respiratory failure[9], the survival rate and functional outcome for the patients of cerebrovascular diseases who treated with mechanical ventilation had a poor prognosis. Therefore, identification of risk factors affecting long-term survival and functional outcome in these patients could be useful to improve management after mechanical ventilation, to help family making decision on continuation or withdrawal of care, and to guide orientation after discharge.
Previous studies indicated that mortality of the patients with cerebrovascular disease was 18%-19% and majority of the survivors remained severely disabled[10,11]. Several factors, including age (> 65 years), unconsciousness at admission [glasgow coma scale (GCS) score], intubation, and disappearance of brainstem reflexes, have been identified as independent predictors for the long-term survival and functional outcome in the patients with cerebrovascular diseases who required mechanical ventilation [1,2,12,13]. However, the prognosis of cerebrovascular diseases treated with mechanical ventilation during perioperative has not been clearly reported.
The aim of the current study was to evaluate mortality and severe disability of patients with cerebrovascular diseases who required mechanical ventilation during acute phase of treatment at an intensive care unit (ICU) (in-hospital), and to explore the predictors of death and functional disability of these patients.
This cross-sectional study focuses on patients with cerebrovascular disease who underwent ventilator-assisted respiration during perioperative period. 113 patients from the neurovascular surgery ICU of the First hospital of Jilin University were collected from June 2016 to June 2019. The inclusion criteria included: (1) Patients had neurovascular surgery perioperative ventilator assisted breathing; (2) patients were on mechanical ventilation at least for 48 h; and (3) age ≥ 18 years old. The exclusion criteria are the patient’s family members gave up treatment or the patient died of disease. Two patients under the age of 18 were excluded. Finally total of 111 patients were included. The patient diagnoses cerebral hemorrhage, cerebral arteriovenous malformation, subarachnoid hemorrhage, cerebral vascular stenosis or occlusion, cerebral infarction, etc. through head computerized tomography (CT), CT angiography and cerebrovascular angiography. This study was adhered to the strengthening the reporting of observational studies in epidemiology statement for reporting[14].
The study protocol was approved by the Ethics Committee of the First Hospital of Jilin University. Informed consent was waived due to the retrospective nature of this study.
Basic demographic information and following clinical information were collected and analyzed: Age, gender, smoking, hypertension, diabetes, diagnosis of cerebrovascular diseases[15], tracheotomy, subarachnoid hemorrhage (SAH) gradings[16]. Preoperative GCS score, surgical operation methods, reason for mechanical ventilation, time from admission to start using ventilator, ventilation initiation, operation hours, mechanical ventilation time, and reason for ventilation.
The primary outcome of this study was death or functional outcome in patients with cerebrovascular disease 3 mo after discharge. The secondary outcome was death or functional outcome at discharge. Patient’s outcome was scaled with modified rankin scale (mRS) score[17], mRS ≤ 3 was defined as good recovery; mRS = 4 or 5 as severe disability; mRS = 6 as dead. Functional outcome assessment was carried out by a physician through telephone call.
Discrete variables were expressed by frequency (%), χ2 test and fisher exact test were used to compare outcomes of the patients with various features. The Kolmogorov-Smirnov test was used to test the normality of the continuous variables. Continuous variables that fit the normal distribution were expressed by either mean ± SD otherwise as median and interquartile range (IQR). Wilcoxon rank sum test or Kruskal-Wallis rank sum test was used to compare outcomes of the patients with various features. We used ordinal Logistic Regression to analyze the association of mortality and functional outcome in patients with cerebrovascular disease and related factors. R software (R version 4.1.2) was used to perform all analysis and P value < 0.05 was considered as significant.
Average age of the 111 patients, who received surgical operation and peri-operative mechanical ventilation at a neurovascular surgical department during the 3-month study period, was 56.46 ± 12.53 years old, the median preoperative GCS was 9 (8, 15), and operation hour was 3.30 h (IQR: 2.14-4.70). Of them, 53.15% were male and 30.63% were smokers. Majority of them had comorbidities with 72.07% of hypertension and 16.22% diabetes (Table 1). Of the 111 patients, 96 (86.48%) were diagnosed as hemorrhagic cerebrovascular disease, 4 (3.6%) were brain tumor, and 10 (9.01%) were malformed or narrowed intracerebral blood vessels. The following operation methods were applied in this study: Aneurysm clipping in 29 (26.13%) cases; aneurysm embolization in 23 (20.27%) patients; craniotomy for hematoma removal in 30 (27.03%) patients; external ventricular drainage in 12 (10.81%) patients; cranial drilling and hemorrhage drainage in 8 (7.21%) patients, and other surgeries in the rest of 9 (8.11%) cases. Of the 111 patient, 16 (55.2%) were non-SAH, 5 (17.2%) were SAH grading I, 3 (10.3%) were SAH grading II and 5 (17.2%) were SAH grading III (Table 1).
| Characteristic | Number |
| Age (mean ± SD) | 55.46 ± 12.53 |
| Preoperative GCS score, median (IQR) | 9 (8, 15) |
| Male gender, n (%) | 59 (53.15) |
| Smoking, n (%) | |
| Yes | 34 (30.63) |
| Hypertension, n (%) | |
| Yes | 80 (72.07) |
| Diabetes, n (%) | |
| Yes | 18 (16.22) |
| Diagnosis of neurovascular diseases, n (%) | |
| Ischemic cerebrovascular disease | 1 (0.90) |
| Hemorrhagic cerebrovascular disease | 96 (86.48) |
| Brain tumor | 4 (3.60) |
| Malformed or narrowed intracerebral blood vessels | 10 (9.01) |
| SAH grading, n (%) | |
| Non-SAH | 62 (55.86) |
| I | 12 (10.81) |
| II | 13 (11.71) |
| III | 18 (16.22) |
| IV | 6 (5.41) |
| Surgical operation methods, n (%) | |
| Aneurysm clipping | 29 (26.13) |
| Aneurysm embolization | 23 (20.72) |
| Craniotomy for hematoma removal | 30 (27.03) |
| External ventricular drainage | 12 (10.81) |
| Cranial drilling and drainage | 8 (7.21) |
| Others | 9 (8.11) |
| Mechanical ventilation time, median (IQR) | 113 (69, 187) |
| Tracheotomy, n (%) | |
| Yes | 56 (50.45) |
| Time from admission to start using ventilator (h), median (IQR) | 40 (4, 86) |
| Ventilation initiation, n (%) | |
| Post-operation | 94 (84.68) |
| Pre-operation | 17 (15.32) |
| Operation hours (h), median (IQR) | 3.3 (2.1, 4.7) |
| Reason for ventilation, n (%) | |
| Pulmonary disease | 55 (49.55) |
| Status epilepticus | 9 (8.11) |
| Impaired respiratory center function | 6 (5.41) |
| Shock | 14 (12.61) |
| Brainstem compression | 27 (24.32) |
Median mechanical ventilation hour was 113 h with 69-187 h of IQR. Over half of the patients (50.45%) had tracheotomy for the mechanical ventilation. Majority (94, 84.68%) patients were given mechanical ventilation before the surgery and rest 17 (15.32%) were on ventilation after the surgery. There were variety kinds of reasons for the patients to have mechanical ventilation. Of them, 55 (49.55%) cases were due to pulmonary disease; 9 (8.11%) were status epilepticus; 6 (5.41%) were due to impaired function of respiratory center function; 14 (9.73%) were shock; 27 (24.32%) were because of brainstem compression with cerebral hemorrhage and brain herniation (Table 1).
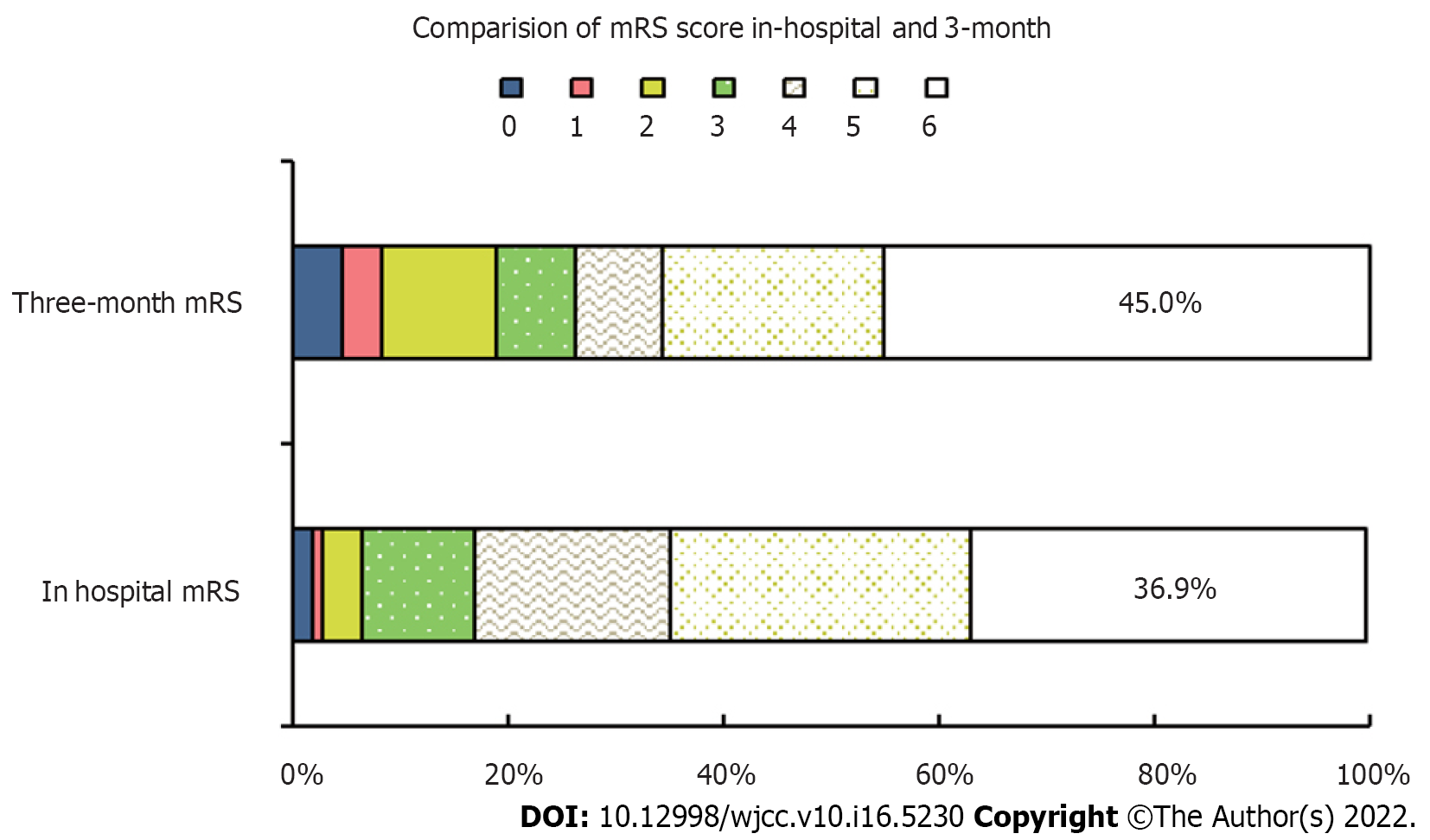
Figure 1 showed the comparison of mRS score with cerebrovascular disease at discharge and 3 mo after discharge. Of 71 survivors, 46.47% were seriously disabled and 12.67% died after three months of follow-up. Compared with the period of hospitalization, 11 patients with poor prognosis turned to good, and 9 deaths were added. In general, compared with hospitalization, the proportion of patients with good prognosis (MRS ≤ 3) after 3 mo of discharge has increased. However, the number of deaths continues to increase, and the total mortality rate reaches 45.0% after three months.
After 3 mo of follow-up, 29 (26.1%) of the 111 patients had good recovery, there were 32 (28.8%) patients with poor prognosis and 50 deaths (45.0%). The results of univariate analysis showed that there was also no significant difference in age, gender, smoking, hypertension, diabetes, diagnosis of neurovascular diseases, tracheotomy, SAH gradings, surgical operation methods, ventilation initiation, operation hours, and mechanical ventilation time (P > 0.05). There were significant differences in preoperative GCS score (P = 0.002), time from admission to start using ventilator (P = 0.038) and reason for ventilation (P < 0.001) among different prognostic groups (Table 2).
| Characteristic | Good recovery (n = 29) | Severe disability (n = 32) | Death (n = 50) | Statistical value | P value |
| Age, median (IQR) | 55 (46, 63) | 61 (50.5, 65) | 59 (51.2, 66) | 2.984 | 0.225 |
| Male gender, n (%) | 17 (58.6) | 19 (59.4) | 23 (46.0) | 1.873 | 0.409 |
| Smoking, n (%) | |||||
| Yes | 10 (34.5) | 12 (37.5) | 12 (24.0) | 1.948 | 0.407 |
| Hypertension, n (%) | 4.524 | 0.104 | |||
| Yes | 19 (65.5) | 20 (62.5) | 41 (82.0) | ||
| Diabetes, n (%) | 1.271 | 0.544 | |||
| Yes | 3 (10.4) | 5 (15.6) | 10 (20.0) | ||
| Tracheotomy, n (%) | 4.298 | 0.115 | |||
| Yes | 12 (41.4) | 21 (65.6) | 23 (46.0) | ||
| Diagnosis of neurovascular diseases, n (%) | 0.690 | 0.406 | |||
| Ischemic cerebrovascular disease | 1 (3.4) | 0 (0.0) | 0 (0.0) | ||
| Hemorrhagic cerebrovascular disease | 22 (75.9) | 29 (90.6) | 45 (90.0) | ||
| Brain tumor | 2 (6.9) | 1 (3.1) | 1 (2.0) | ||
| Malformed or narrowed intracerebral blood vessels | 4 (13.8) | 2 (6.2) | 4 (8.0) | ||
| SAH, n (%) | 5.609 | 0.234 | |||
| Non-SAH | 16 (55.2) | 19 (59.4) | 27 (54.0) | ||
| I | 5 (17.2) | 3 (9.4) | 4 (8.0) | ||
| II | 3 (10.3) | 2 (6.3) | 8 (16.0) | ||
| III | 5 (17.2) | 6 (18.8) | 7 (14.0) | ||
| IV | 0 (0) | 2 (6.3) | 4 (8.0) | ||
| Preoperative GCS score, median (IQR) | 11 (9, 15) | 9 (9, 10) | 9 (8, 10) | 12.575 | 0.002 |
| Surgical operation methods, n (%) | 15.956 | 0.082 | |||
| Aneurysm clipping | 8 (27.6) | 13 (40.6) | 8 (16.0) | ||
| Aneurysm embolization | 9 (31.0) | 2 (6.3) | 12 (24.0) | ||
| Craniotomy for hematoma removal | 5 (17.2) | 9 (28.1) | 16 (32.0) | ||
| External ventricular drainage | 1 (3.4) | 3 (9.4) | 8 (16.0) | ||
| Cranial drilling and drainage | 3 (10.3) | 2 (6.2) | 4 (8.0) | ||
| Others | 3 (10.3) | 3 (9.4) | 2 (4.0) | ||
| Time from admission to start using ventilator (h), median (IQR) | 50 (9, 80) | 71.0 (26.2, 102.8) | 27.0 (1.0, 74.5) | 6.537 | 0.038 |
| Operation hour (h), median (IQR) | 3.5 (2.0, 5.3) | 3.5 (2.8, 4.7) | 3.1 (2.0, 4.4) | 1.694 | 0.249 |
| Ventilation initiation, n (%) | 1.235 | 0.559 | |||
| Postoperation | 24 (82.8) | 29 (90.6) | 41 (82.0) | ||
| Preoperation | 5 (17.2) | 3 (9.4) | 9 (18.0) | ||
| Mechanical ventilation time (h), median (IQR) | 111.5 (64.5, 223.0) | 127.0 (75.0, 182.5) | 102.5 (69.5, 181.0) | 0.98 | 0.613 |
| Reason for ventilation, n (%) | 11.033 | < 0.001 | |||
| Pulmonary disease | 13 (44.8) | 24 (75.0) | 18 (36.0) | ||
| Status epilepticus | 6 (20.7) | 2 (6.3) | 1 (2.0) | ||
| Impaired respiratory center function | 3 (10.3) | 1 (3.1) | 2 (4.0) | ||
| Shock | 7 (24.1) | 1 (3.1) | 6 (12.0) | ||
| Brainstem compression | 0 (0.0) | 4 (12.5) | 23 (46.0) |
Multiple logistic regression analysis showed that preoperative GCS score and time from admission to start using ventilator were not related to the death and prognosis of patients with cerebrovascular diseases (P > 0.05), and the reason of ventilation was related to the death and poor prognosis of patients with cerebrovascular diseases. Compared with brainstem compression, The risk of death or severe disability of pulmonary diseases was 0.096 times (P < 0.001, 95%CI: 0.028-0.328); The risk of death or severe disability of status epilepticus was 0.026 times (P < 0.001, 95%CI: 0.004-0.163), The risk of death or severe disability of impaired respiratory center function was 0.095 times (P = 0.022, 95%CI: 0.013-0.709), The risk of death or severe disability of shock was 0.095 times (P = 0.003, 95%CI: 0.020-0.444) (Table 3).
| Value | Estimate | Standard error | Wald | P value | OR | 95%CI | |
| [Outcome = 1] | -4.692 | 1.05 | 20.13 | 0.000 | 0.009 | 0.001 | 0.071 |
| [Outcome = 2] | -3.128 | 1.00 | 9.80 | 0.002 | 0.044 | 0.006 | 0.311 |
| Preoperative GCS score | -0.149 | 0.09 | 2.89 | 0.089 | 0.861 | 0.725 | 1.023 |
| Time from admission to start using ventilator | 0.000 | 0.00 | 0.03 | 0.865 | 1.000 | 0.995 | 1.006 |
| Pulmonary disease | -2.345 | 0.63 | 13.95 | < 0.001 | 0.096 | 0.028 | 0.328 |
| Status epilepticus | -3.668 | 0.94 | 15.08 | < 0.001 | 0.026 | 0.004 | 0.163 |
| Impaired respiratory center function | -2.352 | 1.02 | 5.27 | 0.022 | 0.095 | 0.013 | 0.709 |
| Shock | -2.359 | 0.79 | 8.94 | 0.003 | 0.095 | 0.020 | 0.444 |
| Brainstem compression | - | - | - | - | - | - | - |
In the current study, we found that mechanical ventilation was required for the patients who underwent surgical operation with various kinds of cerebrovascular diseases. Outcome of mechanical ventilation in these patients, however, revealed that mortality and occurrence of severe disability were high. Prognosis of the patients treated with mechanical ventilation in this study was associated with the comorbidities that required mechanical ventilation. Compared with brainstem compression, the survival and functional outcome of pulmonary disease, status epilepticus, status epilepticus, impaired respiratory center function, and shock are relatively well.
Patients with cerebrovascular diseases requiring ventilator support treatment have poor prognosis with high mortality and severe disability even though they were treated aggressively in the ICU[18]. Cerebrovascular diseases such as intracerebral hemorrhage, subarachnoid hemorrhage, and cerebral arteriovenous malformations often cause severe intracerebral hemorrhage, which blocks normal circulation of cerebrospinal fluid and results in brain herniation and even death. These patients, therefore, often need to be mechanically ventilated before and/or after surgery. Comorbidity rate of surgery in the patients with cerebrovascular diseases were 14% and some of them require mechanical ventilation[19]. In this regard, Mayer et al[12]reported that 5% of ischemic stroke patients, 26% of intracerebral hemorrhage patients, and 47% of subarachnoid hemorrhage patients required mechanical ventilation, with two third of mortality rate and majority of neuro-dysfunction. In the current study, we found that in-hospital mortality of the patients with cerebrovascular diseases was 36.9% and 45.0% during the 3-month follow-up period, and that 46.8% of them had 4-5 mRS score at discharge from the hospital and 29.7% of them had 4-5 mRS score 3 mo after discharge.
Steiner et al[2]reported that GCS (< 10) at admission was one of the seven independent factors that influenced 2-month fatality for the stroke patients who received mechanical ventilation. Although our univariate analysis showed that preoperative GSC had statistically significant differences in different prognostic outcomes, considering the confounding factors among multiple variables, further multiple regression analysis did not find a correlation between GSC and poor prognosis. Similarly, Fugate[19] also believe that preoperative GCS score could not predict the patient’s outcome because the intervention of surgical operation could affect the outcome, which could be good recovery, severe disability or even death. It has been reported that mechanical ventilation treatment in the comatose patients resulting from inoperative acute intracerebral hemorrhage, especially patients had brainstem compression due to brain herniation, could only prolong unresponsive life[20]. Brainstem compression causes changes in respiratory rhythm and even respiratory arrest, it is the second reason (24.3%) in this study for the patients required mechanical ventilation and majority of them (85.2%) died. Brainstem compression occurred in the patients with acute hydrocephalus following aneurysm subarachnoid hemorrhage. Although external ventricular drainage might release the pressure, difficulty in weaning from the mechanical ventilator was a problem. The retrospective cohort study of Chang et al[21] concluded that brainstem compression is the predictor of mortality within 6-months in patients with spontaneous cerebellar hemorrhage, which is consistent with our findings.
Prognosis of the patients with perioperative mechanical ventilation largely depends on the comorbidities[22]. Pneumonia or other pulmonary complications are often the cause of mechanical ventilation in the cerebrovascular patients following surgeries. It has been reported that mortality of the stroke patients with pneumonia was three times higher than that of the patients without pneumonia[23-25]. The main cause of stroke associated pneumonia is aspiration caused by swallowing dysfunction[26], pneumonia was closely associated with GCS score at admission, that is, patients with lower GCS score were often unconsciousness, vomiting and aspiration may occur when the disease occurs. In severe cases, mechanical ventilation and tracheotomy may be required[27,28]. In the current study, 49.5% of the patients were mechanically ventilated due to pulmonary disease. Of them, mortality was 32.7% and severe disability was 43.6%.
It has also been reported that status epilepticus was an independent risk factor for fatality of the patients with spontaneous cerebral hemorrhage[29], especially, in the patients with refractory and nonconvulsive epilepsy. These patients often require intubation and mechanical ventilation because these patients might stop breathing and heart-beating. In our study, 9 patients had status epilepticus with unexpected unconsciousness, stop breathing, but normal brain CT examination. Of them, 3 patients had status epilepticus before the surgery and the rest 6 patients had it after the surgery. One of the six patients had it 334 h after the surgery. The treatment of status epilepticus is to ensure the airway, maintain circulation, and give benzodiazepines, such as diazepam, lorazepam, midazolam, etc.[30].
We also identified that shock could be a prediction of the outcome for the patients with mechanical ventilation. In the early stage of shock, due to the excitement of the patient's respiratory center and the increase of ventilation, it can cause hypocapnia and respiratory alkalosis. Generally, it can be used as an early indicator of shock before the decrease of blood pressure and the increase of lactate. However, in the late stage of shock, acute respiratory failure often occurs, which is characterized by progressive hypoxemia and dyspnea, which is called shock lung. Those with hypoxemia in the early and late stage of shock need ventilator assisted respiration[31]. In this regard, total 11 patients were ventilated after being diagnosed as shocked. Of them, 4 patients who were diagnosed in early stage of shock and given treatment such as raising blood pressure and improving circulation. The patients recovered well. While 5 patients who were diagnosed at a late stage of shock, died, suggesting identification of shock at its early stage is crucial for the patient’s outcome. Myint et al[32] reported that shock index at presentation to the emergency department predicts patient-related clinical outcomes in ischemic and hemorrhagic stroke.
There are some limitations in the current study. First, the study design was observational and follow-up period was short. Second, relatively small sample size might limit significant effects for some predictive factors that potentially influence outcome. Third, quality of life in those patients, who had severe disability, was not evaluated. Fourth, the retrospective collection of the data may have introduced bias, especially, patients ventilated for less than 48 h were excluded from this study, and thus, this result may not easily be extrapolated to a general elderly population. Finally, the data was from only one department of single center. In the future, we will include more samples, fully consider various confounding factors, and conduct a prospective cohort study to verify the causal relationship between various ventilation causes and the poor prognosis of patients with cerebrovascular diseases undergoing perioperative mechanical ventilation.
Taken together, mortality and severe disability rate were high in the cerebrovascular patients who had perioperative mechanical ventilation. The outcome of these patients was not associated with time-length of mechanical ventilation, primary diagnosis of cerebrovascular diseases, surgical operation method, and whether ventilated before or after the surgery. However, comorbidities that require mechanical ventilation significantly affected mortality and functional outcome of the patients in this study. Compared with brainstem compression, the survival and functional outcome of pulmonary disease, status epilepticus, status epilepticus, impaired respiratory center function, and shock are relatively well.
The dilemma is that although mechanical ventilation could sustain life in patients with acute respiratory failure, the survival rate and functional outcome for the patients of cerebrovascular diseases who treated with mechanical ventilation had a poor prognosis. Therefore, identification of risk factors affecting long-term survival and functional outcome in these patients could be useful to improve management after mechanical ventilation, to help family making decision on continuation or withdrawal of care, and to guide orientation after discharge.
The survival rate and prognostic outcomes of patients with cerebrovascular diseases treated with mechanical ventilation during the perioperative period were poor. The reason for mechanical ventilation was a statistically significant predictor for mortality and severe disability.
The average age of 111 patients was 56.46 ± 12.53 years, 59 (53.15%) were males. The mortality of in-hospital and 3-month follow-up were 36.9% and 45.0%, respectively. Of 71 discharged patients, 46.47% were seriously disabled and 12.67% died after three months follow-up. Univariate analysis showed that preoperative glasgow coma scale, operation start time and ventilation reasons had statistically significant differences in different functional outcomes. Multiple logistic regression analysis showed that the cause of ventilation was related to the death and poor prognosis of patients with cerebro
A retrospective follow-up study of 111 cerebrovascular disease patients who underwent mechanical ventilation during the perioperative period in the First Hospital of Jilin University from June 2016 to June 2019 was performed. Main measurements were mortality and functional outcome in-hospital and after 3-month follow-up. The functional outcome was divided into three groups based the modified rankin scale. Univariate analysis was used to compare the differences between three functional outcomes. Multivariate logistic regression analysis was used to for risk factors of mortality and severe disability.
To analyze mortality and functional disability and to determine predictors of unfavorable outcome in the patients with cerebrovascular diseases treated with mechanical ventilation.
To analyze mortality and functional disability and to determine predictors of unfavorable outcome in the patients with cerebrovascular diseases treated with mechanical ventilation.
The prognosis of cerebrovascular diseases treated with mechanical ventilation during perioperative has not been clearly reported.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Clinical neurology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cabezuelo AS, Spain; Karim HMR, India S-Editor: Guo XR L-Editor: A P-Editor: Guo XR
| 1. | Gujjar AR, Deibert E, Manno EM, Duff S, Diringer MN. Mechanical ventilation for ischemic stroke and intracerebral hemorrhage: indications, timing, and outcome. Neurology. 1998;51:447-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 159] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 2. | Steiner T, Mendoza G, De Georgia M, Schellinger P, Holle R, Hacke W. Prognosis of stroke patients requiring mechanical ventilation in a neurological critical care unit. Stroke. 1997;28:711-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 98] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Wijdicks EF, Scott JP. Causes and outcome of mechanical ventilation in patients with hemispheric ischemic stroke. Mayo Clin Proc. 1997;72:210-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Kolak J, van Saene HK, de la Cal MA, Silvestre L, Peric M. Control of bacterial pneumonia during mechanical ventilation. Croat Med J. 2005;46:183-196. [PubMed] |
| 5. | Hawkes MA, English SW, Mandrekar JN, Rabinstein AA, Hocker S. Causes of Death in Status Epilepticus. Crit Care Med. 2019;47:1226-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 6. | Clark SB, Soos MP. Noncardiogenic Pulmonary Edema. 2021 Nov 3. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. [PubMed] |
| 7. | Wachter RM. Intensive care for patients with AIDS: clinical and ethical issues. Schweiz Med Wochenschr. 1995;125:1119-1122. [PubMed] |
| 8. | Bouvet P, Murgier M, Pons B, Darmon M. Long-term Outcomes of Critically Ill Patients With Stroke Requiring Mechanical Ventilation. Am J Crit Care. 2019;28:477-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Brochard L, Slutsky A, Pesenti A. Mechanical Ventilation to Minimize Progression of Lung Injury in Acute Respiratory Failure. Am J Respir Crit Care Med. 2017;195:438-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 774] [Article Influence: 96.8] [Reference Citation Analysis (0)] |
| 10. | Broessner G, Helbok R, Lackner P, Mitterberger M, Beer R, Engelhardt K, Brenneis C, Pfausler B, Schmutzhard E. Survival and long-term functional outcome in 1,155 consecutive neurocritical care patients. Crit Care Med. 2007;35:2025-2030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | el-Ad B, Bornstein NM, Fuchs P, Korczyn AD. Mechanical ventilation in stroke patients--is it worthwhile? Neurology. 1996;47:657-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Mayer SA, Copeland D, Bernardini GL, Boden-Albala B, Lennihan L, Kossoff S, Sacco RL. Cost and outcome of mechanical ventilation for life-threatening stroke. Stroke. 2000;31:2346-2353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Burtin P, Bollaert PE, Feldmann L, Nace L, Lelarge P, Bauer P, Larcan A. Prognosis of stroke patients undergoing mechanical ventilation. Intensive Care Med. 1994;20:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3667] [Cited by in RCA: 6888] [Article Influence: 626.2] [Reference Citation Analysis (0)] |
| 15. | Wu J, Yang Y, Rao ML. Chinese Classification of Cerebrovascular Diseases 2015. Chinese Journal of Neurology. 2017;50:168-171. [DOI] [Full Text] |
| 16. | Fernandez A, Schmidt JM, Claassen J, Pavlicova M, Huddleston D, Kreiter KT, Ostapkovich ND, Kowalski RG, Parra A, Connolly ES, Mayer SA. Fever after subarachnoid hemorrhage: risk factors and impact on outcome. Neurology. 2007;68:1013-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 17. | Bamford JM, Sandercock PA, Warlow CP, Slattery J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1989;20:828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3704] [Cited by in RCA: 4416] [Article Influence: 119.4] [Reference Citation Analysis (0)] |
| 18. | Lahiri S, Mayer SA, Fink ME, Lord AS, Rosengart A, Mangat HS, Segal AZ, Claassen J, Kamel H. Mechanical Ventilation for Acute Stroke: A Multi-state Population-Based Study. Neurocrit Care. 2015;23:28-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Fugate JE. Complications of Neurosurgery. Continuum (Minneap Minn). 2015;21:1425-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Fukuhara T, Aoi M, Namba Y. Mechanical ventilation for comatose patients with inoperative acute intracerebral hemorrhage: possible futility of treatment. PLoS One. 2014;9:e103531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Chang CY, Lin CY, Chen LC, Sun CH, Li TY, Tsai TH, Chang ST, Wu YT. The Predictor of Mortality within Six-Months in Patients with Spontaneous Cerebellar Hemorrhage: A Retrospective Study. PLoS One. 2015;10:e0132975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Doshi VS, Say JH, Young SH, Doraisamy P. Complications in stroke patients: a study carried out at the Rehabilitation Medicine Service, Changi General Hospital. Singapore Med J. 2003;44:643-652. [PubMed] |
| 23. | Wartenberg KE, Schmidt JM, Claassen J, Temes RE, Frontera JA, Ostapkovich N, Parra A, Connolly ES, Mayer SA. Impact of medical complications on outcome after subarachnoid hemorrhage. Crit Care Med. 2006;34:617-23; quiz 624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 363] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 24. | Guo R, Yang J, Yu Z, Chen R, You C, Li H, Ma L. Risk Factors and Outcomes of Pneumonia After Primary Intraventricular Hemorrhage. World Neurosurg. 2019;127:e979-e985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | de Montmollin E, Ruckly S, Schwebel C, Philippart F, Adrie C, Mariotte E, Marcotte G, Cohen Y, Sztrymf B, da Silva D, Bruneel F, Gainnier M, Garrouste-Orgeas M, Sonneville R, Timsit JF; OUTCOMEREA Study Group. Pneumonia in acute ischemic stroke patients requiring invasive ventilation: Impact on short and long-term outcomes. J Infect. 2019;79:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Kuo YW, Huang YC, Lee M, Lee TH, Lee JD. Risk stratification model for post-stroke pneumonia in patients with acute ischemic stroke. Eur J Cardiovasc Nurs. 2020;19:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Namale G, Kamacooko O, Makhoba A, Mugabi T, Ndagire M, Ssanyu P, Ddamulira JBM, Yperzeele L, Cras P, Ddumba E, Seeley J, Newton R. Predictors of 30-day and 90-day mortality among hemorrhagic and ischemic stroke patients in urban Uganda: a prospective hospital-based cohort study. BMC Cardiovasc Disord. 2020;20:442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Alsumrain M, Melillo N, Debari VA, Kirmani J, Moussavi M, Doraiswamy V, Katapally R, Korya D, Adelman M, Miller R. Predictors and outcomes of pneumonia in patients with spontaneous intracerebral hemorrhage. J Intensive Care Med. 2013;28:118-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Mehta A, Zusman BE, Shutter LA, Choxi R, Yassin A, Antony A, Thirumala PD. The Prevalence and Impact of Status Epilepticus Secondary to Intracerebral Hemorrhage: Results from the US Nationwide Inpatient Sample. Neurocrit Care. 2018;28:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Seinfeld S, Goodkin HP, Shinnar S. Status Epilepticus. Cold Spring Harb Perspect Med. 2016;6:a022830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 31. | Maccagnan Pinheiro Besen BA, Tomazini BM, Pontes Azevedo LC. Mechanical ventilation in septic shock. Curr Opin Anaesthesiol. 2021;34:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Myint PK, Sheng S, Xian Y, Matsouaka RA, Reeves MJ, Saver JL, Bhatt DL, Fonarow GC, Schwamm LH, Smith EE. Shock Index Predicts Patient-Related Clinical Outcomes in Stroke. J Am Heart Assoc. 2018;7:e007581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |