Published online May 16, 2022. doi: 10.12998/wjcc.v10.i14.4446
Peer-review started: March 8, 2022
First decision: March 24, 2022
Revised: March 27, 2022
Accepted: April 20, 2022
Article in press: April 20, 2022
Published online: May 16, 2022
Processing time: 66 Days and 0.9 Hours
Colorectal cancer (CRC) is a common malignant tumor of the gastrointestinal tract. Lipid metabolism, as an important part of material and energy circulation, is well known to play a crucial role in CRC.
To explore the relationship between serum lipids and CRC development and identify aberrantly expressed cholesterol metabolism genes in CRC.
We retrospectively collected 843 patients who had confirmed CRC and received surgical resection from 2013 to 2015 at the Cancer Hospital of the Chinese Academy of Medical Sciences as our research subjects. The levels of serum total cholesterol (TC), triglycerides, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), LDL-C/HDL-C and clinical features were collected and statistically analyzed by SPSS. Then, we used the data from Oncomine to screen the differentially expressed genes (DEGs) of the cholesterol metabolism pathway in CRC and used Gene Expression Profiling Interactive Analysis to confirm the candidate DEGs. PrognoScan was used to analyze the prognostic value of the DEGs, and Search Tool for the Retrieval of Interacting Genes was used to construct the protein–protein interaction network of DEGs.
The serum HDL-C level in CRC patients was significantly correlated with tumor size, and patients whose tumor size was more than 5 cm had a lower serum HDL-C level (1.18 ± 0.41 mmol/L vs 1.25 ± 0.35 mmol/L, P < 0.01) than their counterparts. In addition, TC/HDL (4.19 ± 1.33 vs 3.93 ± 1.26, P < 0.01) and LDL-C/HDL-C (2.83 ± 1.10 vs 2.61 ± 0.96, P < 0.01) were higher in patients with larger tumors. The levels of HDL-C (P < 0.05), TC/HDL-C (P < 0.01) and LDL-C/HDL-C (P < 0.05) varied in different stages of CRC patients, and the differences were significant. We screened 14 differentially expressed genes (DEGs) of the cholesterol metabolism pathway in CRC and confirmed that lipoprotein receptor-related protein 8 (LRP8), PCSK9, low-density lipoprotein receptor (LDLR), MBTPS2 and FDXR are upregulated, while ABCA1 and OSBPL1A are downregulated in cancer tissue. Higher expression of LDLR (HR = 3.12, 95%CI: 1.77-5.49, P < 0.001), ABCA1 (HR = 1.66, 95%CI: 1.11-2.48, P = 0.012) and OSBPL1A (HR = 1.38, 95%CI: 1.01-1.89, P = 0.041) all yielded significantly poorer DFS outcomes. Higher expression of FDXR (HR = 0.7, 95%CI: 0.47-1.05, P = 0.002) was correlated with longer DFS. LDLR, ABCA1, OSBPL1A and FDXR were involved in many important cellular function pathways.
Serum HDL-C levels are associated with tumor size and stage in CRC patients. LRP8, PCSK9, LDLR, MBTPS2 and FDXR are upregulated, while ABCA1 and OSBPL1A are downregulated in CRC. Among them, LDLR, ABCA1, OSBPL1A and FDXR were valuable prognostic factors of DFS and were involved in important cellular function pathways.
Core Tip: Cholesterol metabolism is closely related to colorectal cancer (CRC). In this study, we explored the association between serum high-density lipoprotein cholesterol and tumor characteristics in patients with CRC, identified differentially expressed genes in the cholesterol metabolism pathway and analyzed the prognostic value of these differentially expressed genes in CRC. These findings provide clues for further research on cholesterol metabolism and CRC.
- Citation: Tao JH, Wang XT, Yuan W, Chen JN, Wang ZJ, Ma YB, Zhao FQ, Zhang LY, Ma J, Liu Q. Reduced serum high-density lipoprotein cholesterol levels and aberrantly expressed cholesterol metabolism genes in colorectal cancer. World J Clin Cases 2022; 10(14): 4446-4459
- URL: https://www.wjgnet.com/2307-8960/full/v10/i14/4446.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i14.4446
Colorectal cancer (CRC) is a common malignant tumor of the gastrointestinal tract. It ranks third in the incidence of male malignant tumors in the world and second in female malignant tumors[1]. The occurrence and development of CRC involve multiple dysregulated genes and complicated physiological processes. Lipid metabolism, as an important part of material and energy circulation, is well known to play a crucial role in CRC.
Numerous studies have found that lipid abnormalities are closely related to CRC. There have been a number of reports in the literature regarding the relationship between abnormal serum lipid levels, including total cholesterol (TC), triglycerides (TGs), low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C), and CRC[2,3], but there is no consistent conclusion at present. Most of these studies focus on the different lipid levels between healthy people and patients with CRC, but it is difficult to clarify the specific causal relationship between lipid alterations and cancer. Additionally, research on the underlying mechanism is relatively scarce.
Many studies have shown that some key enzymes and transporters in metabolic pathways play very important roles in various cancers[4]. Dysregulation of these genes causes altered metabolic phenotypes that influence the nutrient, energy and signal transduction balance in cells. However, only a few metabolic genes are presently known to be directly implicated in CRC, especially in the cholesterol metabolism pathway, and there is still much more to learn about the causal role of metabolic genes in cancer.
In this study, we conducted a retrospective analysis of the relationship between serum TC, TG, HDL-C, LDL-C and clinical characteristics in patients with CRC and explored potential CRC-associated cholesterol metabolic genes using a series of bioinformatics databases and tools. We aimed to provide more evidence on the alterations in cholesterol metabolism and identify potential cancer-associated metabolic genes in CRC.
The current research was approved by the Institutional Review Board. Informed consent was obtained from all participants for the use of the clinical information in this research.
This retrospective study enrolled a total of 843 patients with CRC who underwent surgical resection between January 2013 and December 2015 at the Cancer Hospital of the Chinese Academy of Medical Sciences (Beijing, China). The average patient age at surgery was 59 years, 498 were male, 345 were female, 348 cases were colon cancer, and 495 cases were rectal cancer. Tumor staging conformed to the eighth edition of the American Joint Committee on Cancer/Union International Control Center (AJCC/UICC) TNM staging manual (2017). A total of 151, 296, 340 and 56 cases were found to be stages I, II, III and IV, respectively. Patients with diabetes or hyperlipidemia were excluded. The serum lipid information (TC, TG, HDL-C, and LDL-C), body mass index (BMI (weight/height2 and kg/m2)), tumor size (< 5 cm/≥ 5 cm), and smoking and drinking history (yes/no) of these patients were collected.
The levels of TC, TG, HDL-C, LDL-C, TC/LDL-C, TC/HDL-C and LDL-C/HDL-C are presented as the mean ± SD and were compared using the independent sample t test between different groups of tumor size (< 5 cm/≥ 5 cm). An analysis of variance (ANOVA) was used to compare the differences among stages. A covariance analysis was used to compare multiple variables. Differences between groups with P < 0.05 were regarded as statistically significant.
The Oncomine database (https://www.oncomine.org), an online database consisting of previously published and open-access microarray data[5], was used to identify the transcription level of cholesterol metabolism pathway genes in CRC. The analysis type selected was “Cancer vs Normal Analysis”, the cancer type as “Colorectal cancer”, the data type was “mRNA”, and the GO concept was "Cholesterol metabolism" in the analysis of differentially expressed genes (DEGs) in the cholesterol metabolic pathway in CRC compared with normal tissues.
The expression of DEGs in CRC was analyzed using Gene Expression Profiling Interactive Analysis (GEPIA). GEPIA is an interactive web server for estimating the mRNA expression data in The Cancer Genome Atlas (TCGA) and Genotype-Tissue Expression (GTEx) dataset projects[6].
PrognoScan (http://www.prognoscan.org/) is a comprehensive online platform for assessing potential tumor biomarkers and therapeutic targets[7]. To analyze the prognostic values of specific DEGs in CRC, the PrognoScan platform was used to display disease-free survival (DFS). The HRs and log-rank P values are presented on the webpage.
We built a protein–protein interaction (PPI) network for low-density lipoprotein receptor (LDLR), FDXR, ABCA1 and OSBPL1A using Search Tool for the Retrieval of Interacting Genes (STRING, http://string-db.org/), an online resource search tool for the retrieval of interacting genes, which include physical and functional associations[8].
Several well-known factors are associated with serum lipid levels, such as sex, age, BMI and history of smoking and drinking. We considered all of these factors and found that serum HDL-C level, TC/HDL-C and LDL-C/HDL-C were significantly correlated with tumor size and stage in patients with CRC (Table 1).
| Project | Gender | Age (60 year) | BMI (24 kg/m2) | Smoking | Drinking | Stage | Tumor size (5 cm) |
| TC | 0.001 | 0.373 | 0.502 | 0.575 | 0.606 | 0.448 | 0.360 |
| TG | 0.188 | 0.912 | < 0.001 | 0.917 | 0.378 | 0.768 | 0.852 |
| HDL-C | < 0.001 | < 0.001 | < 0.001 | 0.008 | 0.496 | 0.042 | 0.016 |
| LDL-C | 0.027 | 0.498 | 0.078 | 0.630 | 0.879 | 0.250 | 0.929 |
| TC/LDL-C | 0.544 | 0.575 | 0.118 | 0.755 | 0.750 | 0.797 | 0.259 |
| TC/HDL-C | 0.031 | 0.001 | < 0.001 | 0.020 | 0.099 | 0.008 | 0.019 |
| LDL-C/HDL-C | 0.067 | 0.014 | < 0.001 | 0.075 | 0.254 | 0.018 | 0.008 |
We defined tumor size as a tumor single diameter more or no more than 5 cm according to some previous studies in CRC. The results showed that the serum HDL-C (1.18 ± 0.41 mmol/L vs 1.25 ± 0.35 mmol/L, P < 0.01) levels were lower in patients with a larger tumor, while TC/HDL-C (4.19 ± 1.33 vs 3.93 ± 1.26, P < 0.01) and LDL-C/HDL-C (2.83 ± 1.10 vs 2.61 ± 0.96, P < 0.01) were higher, as shown in Table 2.
| Project (size) | < 5 cm (n = 560) | ≥ 5 cm (n = 283) | P value |
| TC | 4.62 ± 0.95 | 4.56 ± 0.91 | 0.354 |
| TG | 1.38 ± 0.8 | 1.36 ± 0.95 | 0.782 |
| HDL-C | 1.25 ± 0.35 | 1.18 ± 0.41 | 0.009 |
| LDL-C | 3.06 ± 0.86 | 3.06 ± 0.82 | 0.965 |
| TC/LDL-C | 1.56 ± 0.26 | 1.53 ± 0.24 | 0.242 |
| TC/HDL-C | 3.93 ± 1.26 | 4.19 ± 1.33 | 0.009 |
| LDL-C/HDL-C | 2.61 ± 0.96 | 2.83 ± 1.1 | 0.004 |
The analysis of variance regarding serum lipid levels of patients with CRC and stages in Table 3 revealed significant correlations between the levels of HDL-C, TC/HDL-C and LDL-C/HDL-C and tumor stages. The levels of HDL-C in patients with stage I (1.3 ± 0.35 mmol/L), II (1.22 ± 0.38 mmol/L) and III (1.2 ± 0.37 mmol/L) disease were gradually reduced, and the serum HDL-C level in patients with stage IV disease (1.24 ± 0.37 mmol/L) was slightly higher than that in patients with stage II and III disease. The differences were significant (P < 0.05). TC/HDL-C and LDL-C/HDL-C gradually increased in stage I to stage III in patients with CRC and decreased in stage IV patients, with significant differences (P < 0.05).
| Project (stage) | I (n = 151) | II (n = 296) | III (n = 340) | IV (n = 56) | P value |
| TC | 4.61 ± 0.86 | 4.55 ± 0.88 | 4.65 ± 1.01 | 4.30 ± 0.94 | 0.395 |
| TG | 1.44 ± 0.95 | 1.31 ± 0.79 | 1.4 ± 0.87 | 1.09 ± 0.85 | 0.228 |
| HDL-C | 1.3 ± 0.35 | 1.22 ± 0.38 | 1.2 ± 0.37 | 1.24 ± 0.37 | 0.045 |
| LDL-C | 3.01 ± 0.78 | 3.04 ± 0.8 | 3.11 ± 0.91 | 2.81 ± 0.85 | 0.427 |
| TC/LDL-C | 1.57 ± 0.18 | 1.54 ± 0.22 | 1.55 ± 0.3 | 1.58 ± 0.25 | 0.689 |
| TC/HDL-C | 3.78 ± 1.17 | 3.96 ± 1.15 | 4.19 ± 1.44 | 3.65 ± 1.29 | 0.006 |
| LDL-C/HDL-C | 2.5 ± 0.95 | 2.66 ± 0.97 | 2.8 ± 1.06 | 2.4 ± 1.01 | 0.014 |
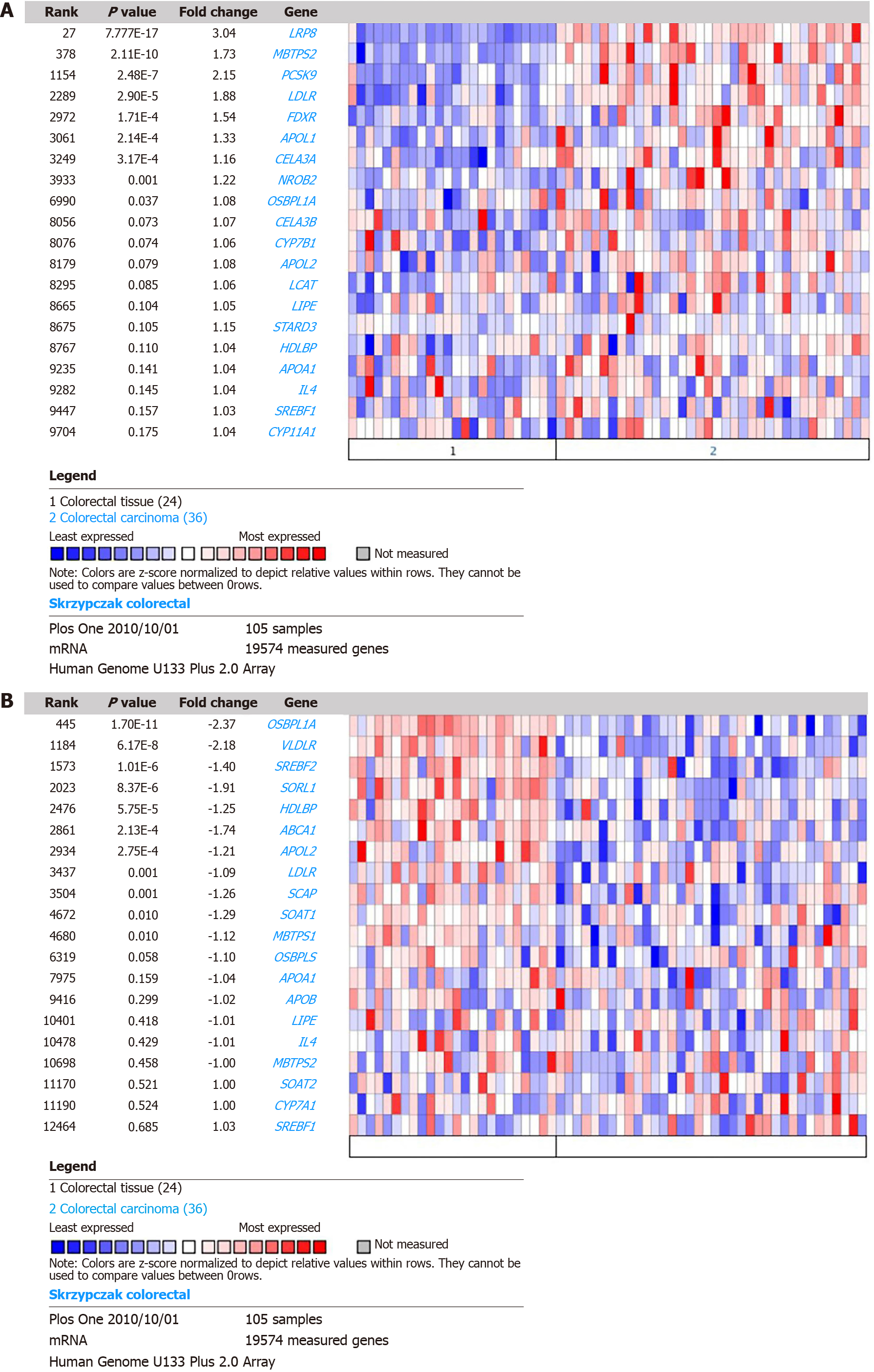
According to the case analysis results, we deduce that changes in HDL-C levels in patients with CRC may be associated with abnormal expression of cholesterol metabolism pathway genes in CRC tissues. An online analysis was performed using Oncomine. By comparing the DEGs of the cholesterol metabolism pathway in Skrzypczak CRC, we found that the mRNA levels of lipoprotein receptor-related protein 8 (LRP8) (P = 7.77E-17, fold change = 3.04), MBTPS2 (P = 2.11E-10, fold change = 1.73), PCSK9 (P = 2.48E-7, fold change = 2.15), LDLR (P = 2.90E-5, fold change = 1.88), FDXR (P = 1.71E-4, fold change = 1.54), APOL1 (P = 2.14E-4, fold change = 1.33), and CELA3A (P = 3.17E-4, fold change = 1.16) were upregulated in CRC tissue, as shown in Figure 1A. The mRNA levels of OSBPL1A (P = 1.7E-11, fold chance = -2.37), VLDLR (P = 6.17E-8, fold change = -2.18), SREBF2 (P = 1.01E-6, fold change = -1.4), SORL1 (P = 8.37E-6, fold change = -1.91), HDLBP (P = 5.75E-5, fold change = -1.25), ABCA1 (P = 2.13E-4, fold change = -1.74) and APOL2 (P = 2.75E-4, fold change = -1.21) were downregulated in CRC tissue (Figure 1B).
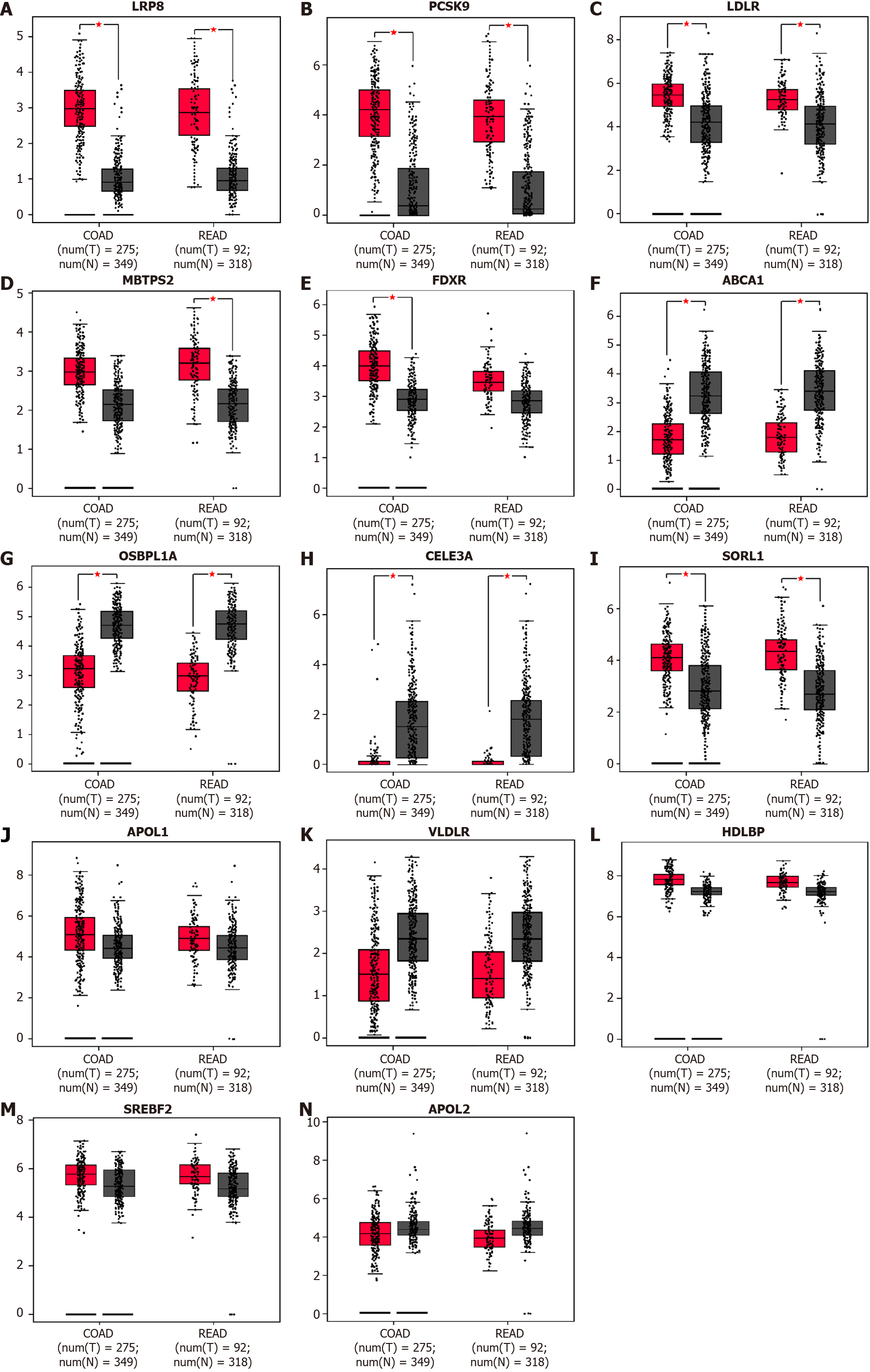
DEGs in CRC were reanalyzed using GEPIA based on TCGA and GTEx datasets for transcriptomic analysis. The results demonstrated that LRP8, PCSK9, and LDLR were upregulated in CRC tissue compared with normal tissue (Figure 2A-C), while MBTPS2 and FDXR showed significantly higher expression only in rectal cancer and colon cancer, respectively (Figure 2D and E). ABCA1 and OSBPL1A were downregulated in CRC tissue (Figure 2F and G), consistent with the results from Oncomine. CELA3A was downregulated (Figure 2H), and SORL1 was upregulated in cancer tissue (Figure 2I), which is the reverse of the above results. The expression levels of APOL1, VLDLR, HDLBP, SREBF2 and APOL2 were comparable between cancer and normal tissues according to the GEPIA analysis results (Figure 2J-N). Therefore, we identified LRP8, PCSK9, LDLR, MBTPS2, FDXR, ABCA1 and OSBPL1A as dysregulated in CRC.
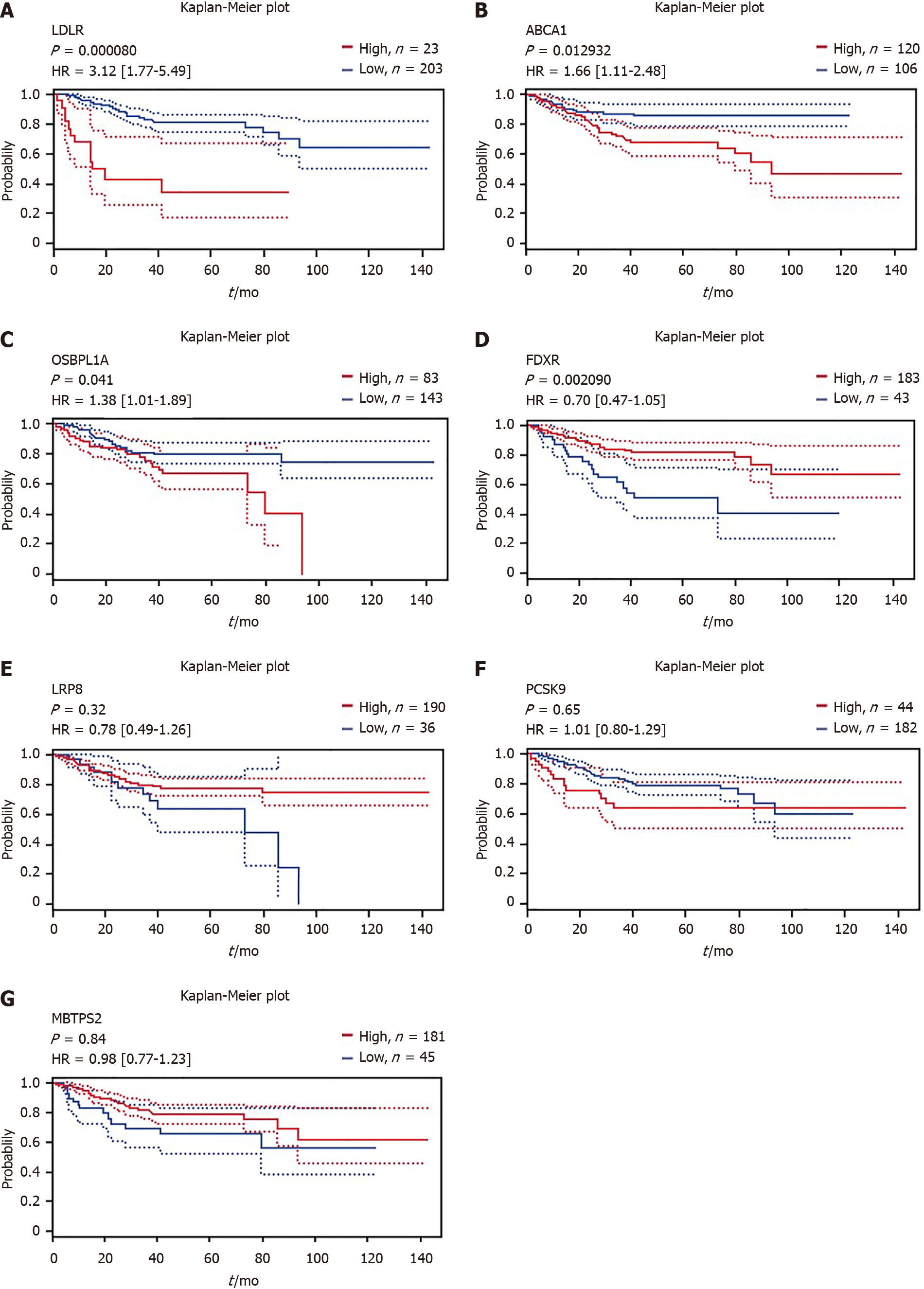
Using the online Kaplan–Meier survival analysis tool PrognoScan, we discovered that high mRNA expression of LDLR [hazard ratio (HR) = 3.12, 95% confidence interval (CI): 1.77-5.49, P < 0.001], ABCA1 (HR = 1.66, 95%CI: 1.11-2.48, P = 0.012) and OSBPL1A (HR = 1.38, 95%CI: 1.01-1.89, P = 0.041) was an unfavorable prognostic factor for disease-free survival (DFS) in CRC patients (Figure 3A-C), while high mRNA expression of FDXR (HR = 0.7, 95%CI: 0.47-1.05, P = 0.002) was a favorable prognostic factor for DFS (Figure 3D). Unfortunately, LRP8, PCSK9 and MBTPS2 expression could not be used to predict DFS outcome according to the results of this analysis, as shown in Figure 3E-G.
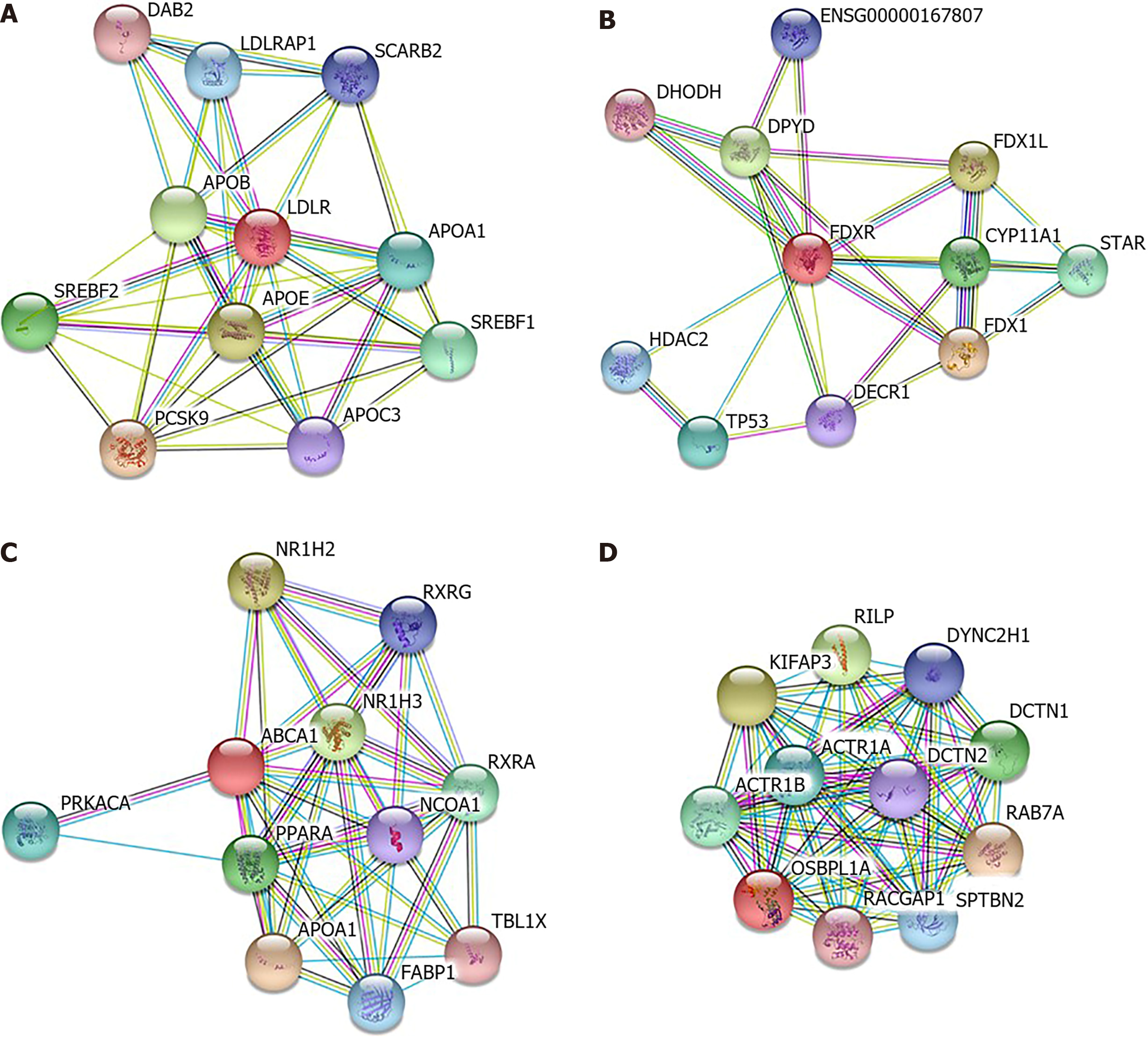
LDLR, FDXR, ABCA1 and OSBPL1A were analyzed by STRING to construct protein–protein interaction networks (Figure 4) and predict other possible roles that they may play in addition to cholesterol metabolism. The results implied that FDXR was also involved in xenobiotic metabolic processes, cellular responses to xenobiotic stimuli and oxidation–reduction processes. ABCA1 was shown to be involved in the steroid hormone-mediated signaling pathway, and OSBPL1A was involved in antigen processing and presentation of exogenous peptide antigens via MHC class II, microtubule-based movement and vesicle-mediated transport.
Cholesterol is the most abundant steroid compound in the human body and is essential for membrane biogenesis, signal transduction, cell proliferation and differentiation[9]. When cholesterol is deficient in humans, the normal cell physiological process is disrupted, cellular rigidity is increased, and the cells are easily fractured. Cholesterol is provided by the diet but can also be synthesized by the liver in humans and distributed throughout the body via low-density lipoprotein (LDL) and high-density lipoprotein (HDL) transporters[10], such as LDL-cholesterol (LDL-C) and HDL-cholesterol (HDL-C). Cancer has been associated with cholesterol, as cholesterol can directly influence cell physiological function and is also the obligatory precursor of steroid hormones, which are involved in tumor promotion and tumor death[10]. Hypercholesterolemia was shown to promote mammary tumor growth and invasiveness in several mouse transgenic models[11], suggesting that cholesterol or its metabolites promote CRC.
A large number of epidemiological statistics and basic research suggest that the uptake of cholesterol and serum cholesterol levels are closely related to the occurrence, development and prognosis of CRC, but there is no consistent conclusion thus far. The main focus of these studies was the association of blood concentrations of total cholesterol (TC) in relation to CRC risk. Findings from three prospective studies on TC concentrations have been inconsistent, showing either a positive association with CRC, colon and rectal cancer risk[12], no association with the risk of colon cancer but a positive association with the risk of rectal cancer in men only[13], or no association at all[14]. Regarding triglyceride (TG) concentrations, three cohort studies found no significant associations with the risk of CRC, colon or rectal cancer. Data on their relationship with the clinical features in CRC patients are scarce. In this study, we found that both TC and TG levels were not associated with the cancer type (colon/rectal, data not shown), tumor size or tumor stage in CRC patients.
HDL-C, as an indispensable form of cholesterol, plays a very important role in some diseases, including arteriosclerosis[15], and has been a subject of intense research in cancer. However, studies of the link between HDL-C levels and CRC have also led to contrasting results. Yang et al[16] found that serum HDL-C level reduction was associated with an increased risk of cancer, including CRC. Jafri et al[17] conducted a large meta-analysis of randomized lipid-altering trials and observed a significant inverse association between baseline HDL-C levels and the risk of developing cancer. In our study, we found that HDL-C is decreased in CRC patients with larger tumors and advanced stages. Although these findings do not seem to have reached a definitive conclusion on whether low serum HDL-C levels should be considered a marker of the presence of cancer or a possible causative factor, several possible mechanisms exist by which serum concentrations of HDL-C may be directly or indirectly involved in colorectal carcinogenesis. Decreased concentrations of HDL-C have been related to increased circulating concentrations of proinflammatory cytokines such as interleukin 6 (IL-6) and tumor necrosis factor-a receptors, which can stimulate cell growth and cellular proliferation and inhibit apoptosis[18]. Increased HDL-C levels are associated with increased concentrations of anti-inflammatory cytokines such as IL-10[19], which inhibit the production of these proinflammatory cytokines[20]. These observations suggest that HDL may modulate colon carcinogenesis through inflammatory pathways. Another proposed pathway is through modulation of oxidative stress because HDL displays antioxidative activities and is believed to confer protection against oxidation of LDL-C[21,22]. A low concentration of HDL-C leads to more oxidized LDL-C[23], which has been described as a cause of increased intracellular oxidative stress, a process that is involved in the pathogenesis of cancer[24]. However, as oncogenic processes enable cancer cells to synthesize their own cholesterol, which can be further metabolized and take part in whole-body circulation, and tumor development may also disrupt intestinal homeostasis, the change in serum HDL-C levels can be a result of CRC from this perspective.
Investigations at the laboratory level have revealed a more complex map of the influence of cholesterol metabolism on the promotion or suppression of CRC that could account for these conflicting studies. Metabolic reprogramming is a common hallmark of tumors[25]. In addition to the Warburg effect, tumor cells also undergo lipid remodeling, which is mostly characterized by aberrant de novo lipogenesis and cholesterogenesis due to oncogenic-driven lipogenic enzyme overexpression[26]. In this study, analysis of abnormally expressed cholesterol metabolism-related genes in CRC showed that the upregulated genes are mainly involved in the synthesis and uptake of cholesterol, while the downregulated genes are mainly concentrated in the channels of cholesterol transport. Related to the active characteristics, the abnormal expression of these genes is conducive to maintaining intracellular high cholesterol levels to ensure the growth of material and energy needs.
Low-density LRP8 and LDLR are cell surface receptors and function in signal transduction and endocytosis of specific ligands. LRP8 participates in the development and metastasis of several cancers, such as gastric cancer[27] and melanoma[28]. Some studies have reported the relationship between LDLR and CRC at the in vitro and animal levels[29,30]. However, research on the underlying mechanism is still scarce. MBTPS2 encodes a membrane-bound zinc metalloprotease, an endoplasmic reticulum membrane protein, that exhibits a dual function[31]. On the one hand, MBTPS2 activates sterol regulatory element-binding proteins (SREBPs), which are key transcription factors that subsequently promote the expression of cholesterol-related genes[32]. On the other hand, as MBTPS2 can respond to stress in the endoplasmic reticulum and cause cells to cope with stressful conditions, the dysregulation of MBTPS2 can lead to severe disease in humans[33,34]. PCSK9 is a secreted serine protease that is involved in the posttranscriptional regulation of LDR, which can promote intracellular degradation in acidic subcellular compartments[35]. Regarding its LDLR-decreasing function, a study in cancer research showed its inverse correlation with LDLR expression[36]. However, in our study, we found that PCSK9 and LDLR are both upregulated in CRC, which is very intriguing. The role of FDXR in CRC has been explored in some studies[37,38] before; in our analysis, we also confirmed an increase in FDXR expression, while the high expression level is a favorable prognostic factor for DFS in CRC. ATP-binding cassette transporter (ABCA1) is a transmembrane protein responsible for reverse cholesterol transport and synthesis of HDL-C[39,40]. Both the overexpression and the decrease in ABCA1 are associated with tumorigenesis[41,42]. OSBPL1A was reported to be involved in ABCA1-mediated pathways of cholesterol efflux and could also impact the biogenesis of HDL-C in the liver and intestine[43]; thus, the relationship between the downregulated expression of these two genes and the decrease in serum HDL-C in CRC is worthy of attention.
Overall, in this study, we found that serum HDL-C levels are different in CRC patients with different stages and tumor sizes. LRP8, PCSK9, LDLR, MBTPS2 and FDXR are upregulated, while ABCA1 and OSBPL1A are downregulated in CRC. Among them, LDLR, ABCA1, OSBPL1A and FDXR were valuable prognostic factors of DFS. Our findings provide hypothetical and biological characteristic insight into the role of cholesterol metabolism in CRC, and further molecular-level studies are needed to elucidate potential mechanisms.
Colorectal cancer (CRC) is a common malignant tumor of the gastrointestinal tract. It ranks third in the incidence of male malignant tumors in the world and second in female malignant tumors. The occurrence and development of CRC involve multiple dysregulated genes and complicated physiological processes. Lipid metabolism, as an important part of material and energy circulation, is well known to play a crucial role in CRC. Numerous studies have found that lipid abnormalities are closely related to CRC. Most of these studies focus on the different lipid levels between healthy people and patients with CRC, but it is difficult to clarify the specific causal relationship between lipid alterations and cancer. Additionally, research on the underlying mechanism is relatively scarce.
In previous studies, we found that CRC is closely related to serum cholesterol levels, but the specific key genes that affect the occurrence, development and prognosis of CRC are unknown. The aim of this study was to explore more evidence for altered cholesterol metabolism and to identify potential cancer-related metabolic genes in CRC.
The objective is to explore the relationship between serum lipids and CRC development and identify aberrantly expressed cholesterol metabolism genes in CRC.
We reviewed 843 CRC patients and collected serum total cholesterol (TC), triglycerides (TGs), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), LDL-C/HDL- Statistical analysis of C levels and clinical characteristics was performed by SPSS. Meanwhile, we screened the differentially expressed genes (DEGs) of cholesterol metabolism pathways in CRC using the database data of Oncomine, and confirmed candidate DEGs using GEPIA. PrognoScan was used to analyze the prognostic value of DEGs, and Search Tool for the Retrieval of Interacting Genes was used to construct the protein–protein interaction network of DEGs to finally understand the relationship between CRC and cholesterol metabolism.
Serum HDL-C levels in CRC patients were significantly correlated with tumor size, and serum HDL-C levels were lower in patients with tumors larger than 5 cm, on the contrary, TC/HDL [4.19 ± 1.33 vs HDL-C (2.83 ± 1.10 vs 2.61 ± 0.96, P < 0.01)] was higher. There were significant differences in the levels of HDL-C (P < 0.05), TC/HDL-C (P < 0.01) and LDL-C/HDL-C (P < 0.05) in CRC patients of different stages, and the differences were statistically significant. The authors screened 14 differentially expressed genes (DEGs) with the most significant cholesterol metabolic pathways in CRC and confirmed that lipoprotein receptor-related protein 8 (LRP8), PCSK9, low-density lipoprotein receptor (LDLR), MBTPS2 and FDXR were up-regulated in cancer tissues, while ABCA1 and OSBPL1A were down-regulated. LDLR (HR = 3.12, 95%CI: 1.77-5.49, P < 0.001), ABCA1 (HR = 1.66, 95%CI: 1.11-2.48, P = 0.012) and OSBPL1A (HR = 1.38, 95% CI: 1.01-1.89, P = 0.041)) in cancer tissue high expression of all produced significantly poorer DFS results. Higher expression of FDXR (HR = 0.7, 95%CI: 0.47-1.05, P = 0.002) was associated with longer DFS. LDLR, ABCA1, OSBPL1A, and FDXR are also involved in many other important cellular functional pathways.
Serum HDL-C levels are closely related to tumor size and stage in CRC patients. In CRC, LRP8, PCSK9, LDLR, MBTPS2, and FDXR genes were up-regulated, while ABCA1 and OSBPL1A genes were down-regulated. Meanwhile, LDLR, ABCA1, OSBPL1A and FDXR genes are valuable prognostic factors for DFS and participate in other important functional pathways of cells.
Only a few metabolic genes are presently known to be directly implicated in CRC, especially in the cholesterol metabolism pathway, and there is still much more to learn about the causal role of metabolic genes in CRC. By studying the mechanism of key genes in the cholesterol metabolism pathway in CRC, more treatment options for CRC can be provided.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bordonaro M, United States; Cheng KC, Taiwan S-Editor: Ma YJ L-Editor: A P-Editor: Li X
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21370] [Article Influence: 2137.0] [Reference Citation Analysis (3)] |
| 2. | Barrington WE, Schenk JM, Etzioni R, Arnold KB, Neuhouser ML, Thompson IM Jr, Lucia MS, Kristal AR. Difference in Association of Obesity With Prostate Cancer Risk Between US African American and Non-Hispanic White Men in the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA Oncol. 2015;1:342-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 3. | Liu B, Wen P, Gu X, Weng R, Liu S. Elevated serum triglyceride predicts recurrence of colorectal polyps in patients with advanced adenomas. Lipids Health Dis. 2020;19:211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Pastushenko I, Mauri F, Song Y, de Cock F, Meeusen B, Swedlund B, Impens F, Van Haver D, Opitz M, Thery M, Bareche Y, Lapouge G, Vermeersch M, Van Eycke YR, Balsat C, Decaestecker C, Sokolow Y, Hassid S, Perez-Bustillo A, Agreda-Moreno B, Rios-Buceta L, Jaen P, Redondo P, Sieira-Gil R, Millan-Cayetano JF, Sanmatrtin O, D'Haene N, Moers V, Rozzi M, Blondeau J, Lemaire S, Scozzaro S, Janssens V, De Troya M, Dubois C, Pérez-Morga D, Salmon I, Sotiriou C, Helmbacher F, Blanpain C. Fat1 deletion promotes hybrid EMT state, tumour stemness and metastasis. Nature. 2021;589:448-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 290] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 5. | Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, Barrette T, Pandey A, Chinnaiyan AM. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2463] [Cited by in RCA: 2831] [Article Influence: 134.8] [Reference Citation Analysis (0)] |
| 6. | Tang Z, Li C, Kang B, Gao G, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98-W102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5550] [Cited by in RCA: 7088] [Article Influence: 886.0] [Reference Citation Analysis (0)] |
| 7. | Zheng H, Zhang G, Zhang L, Wang Q, Li H, Han Y, Xie L, Yan Z, Li Y, An Y, Dong H, Zhu W, Guo X. Comprehensive Review of Web Servers and Bioinformatics Tools for Cancer Prognosis Analysis. Front Oncol. 2020;10:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 8. | Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, von Mering C. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447-D452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6477] [Cited by in RCA: 7627] [Article Influence: 693.4] [Reference Citation Analysis (0)] |
| 9. | Vona R, Iessi E, Matarrese P. Role of Cholesterol and Lipid Rafts in Cancer Signaling: A Promising Therapeutic Opportunity? Front Cell Dev Biol. 2021;9:622908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 10. | Silvente-Poirot S, Poirot M. Cancer. Cholesterol and cancer, in the balance. Science. 2014;343:1445-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 176] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 11. | Tie G, Yan J, Khair L, Messina JA, Deng A, Kang J, Fazzio T, Messina LM. Hypercholesterolemia Increases Colorectal Cancer Incidence by Reducing Production of NKT and γδ T Cells from Hematopoietic Stem Cells. Cancer Res. 2017;77:2351-2362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Loke YL, Chew MT, Ngeow YF, Lim WWD, Peh SC. Colon Carcinogenesis: The Interplay Between Diet and Gut Microbiota. Front Cell Infect Microbiol. 2020;10:603086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 13. | Mayengbam SS, Singh A, Pillai AD, Bhat MK. Influence of cholesterol on cancer progression and therapy. Transl Oncol. 2021;14:101043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 14. | Fang HJ, Shan SB, Zhou YH, Zhong LY. Diabetes mellitus and the risk of gastrointestinal cancer in women compared with men: a meta-analysis of cohort studies. BMC Cancer. 2018;18:422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Stadler JT, Wadsack C, Marsche G. Fetal High-Density Lipoproteins: Current Knowledge on Particle Metabolism, Composition and Function in Health and Disease. Biomedicines. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Yang C, Tian G, Mi J, Wei X, Li X, Wang W, Wang B. Causal relevance of circulating high-density lipoprotein cholesterol with cancer: a Mendelian randomization meta-analysis. Sci Rep. 2015;5:9495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Gu JN, Yao S, Cao YH, Deng SH, Mao FW, Jiang HY, He YT, Li XY, Ke SQ, Li HL, Li H, Liu XH, Liu HL, Wang JL, Wu K, Liu L, Cai KL. Novel parameter based on lipid indicators ratio improves prognostic value of plasma lipid levels in resectable colorectal cancer patients. World J Gastrointest Surg. 2021;13:689-701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Song M, Mehta RS, Wu K, Fuchs CS, Ogino S, Giovannucci EL, Chan AT. Plasma Inflammatory Markers and Risk of Advanced Colorectal Adenoma in Women. Cancer Prev Res (Phila). 2016;9:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Dong Y, Zhou J, Zhu Y, Luo L, He T, Hu H, Liu H, Zhang Y, Luo D, Xu S, Xu L, Liu J, Zhang J, Teng Z. Abdominal obesity and colorectal cancer risk: systematic review and meta-analysis of prospective studies. Biosci Rep. 2017;37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 142] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 20. | Chen H, Zheng X, Zong X, Li Z, Li N, Hur J, Fritz CD, Chapman W Jr, Nickel KB, Tipping A, Colditz GA, Giovannucci EL, Olsen MA, Fields RC, Cao Y. Metabolic syndrome, metabolic comorbid conditions and risk of early-onset colorectal cancer. Gut. 2021;70:1147-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 142] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 21. | Zeljkovic A, Vekic J, Mihajlovic M, Gojkovic T, Vladimirov S, Zeljkovic D, Spasojevic-Kalimanovska V, Trifunovic B. Revealing the Role of High-Density Lipoprotein in Colorectal Cancer. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 22. | Valencia C SY, Isaza M CA, Henao B J, Beltrán A L, Loango N, Landázuri P. Arylesterase activity of paraoxonase 1 (PON1) on HDL3 and HDL2: Relationship with Q192R, C-108T, and L55M polymorphisms. Biochem Biophys Rep. 2021;26:100971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Tabata S, Yamamoto M, Goto H, Hirayama A, Ohishi M, Kuramoto T, Mitsuhashi A, Ikeda R, Haraguchi M, Kawahara K, Shinsato Y, Minami K, Saijo A, Toyoda Y, Hanibuchi M, Nishioka Y, Sone S, Esumi H, Tomita M, Soga T, Furukawa T, Akiyama SI. Thymidine catabolism promotes NADPH oxidase-derived reactive oxygen species (ROS) signalling in KB and yumoto cells. Sci Rep. 2018;8:6760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Bleve A, Durante B, Sica A, Consonni FM. Lipid Metabolism and Cancer Immunotherapy: Immunosuppressive Myeloid Cells at the Crossroad. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 25. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47147] [Article Influence: 3367.6] [Reference Citation Analysis (5)] |
| 26. | Cotte AK, Aires V, Fredon M, Limagne E, Derangère V, Thibaudin M, Humblin E, Scagliarini A, de Barros JP, Hillon P, Ghiringhelli F, Delmas D. Lysophosphatidylcholine acyltransferase 2-mediated lipid droplet production supports colorectal cancer chemoresistance. Nat Commun. 2018;9:322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 248] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 27. | Wolfe K, Kamata R, Coutinho K, Inoue T, Sasaki AT. Metabolic Compartmentalization at the Leading Edge of Metastatic Cancer Cells. Front Oncol. 2020;10:554272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Pencheva N, Tran H, Buss C, Huh D, Drobnjak M, Busam K, Tavazoie SF. Convergent multi-miRNA targeting of ApoE drives LRP1/LRP8-dependent melanoma metastasis and angiogenesis. Cell. 2012;151:1068-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 328] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 29. | Wang C, Li P, Xuan J, Zhu C, Liu J, Shan L, Du Q, Ren Y, Ye J. Cholesterol Enhances Colorectal Cancer Progression via ROS Elevation and MAPK Signaling Pathway Activation. Cell Physiol Biochem. 2017;42:729-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 159] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 30. | Chaudhary J, Bower J, Corbin IR. Lipoprotein Drug Delivery Vehicles for Cancer: Rationale and Reason. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Nemer G, Safi R, Kreidieh F, Usta J, Bergqvist C, Ballout F, Btadini W, Hamzeh N, Abbas O, Kibbi AG, Shimomura Y, Kurban M. Understanding the phenotypic similarities between IFAP and Olmsted syndrome from a molecular perspective: the interaction of MBTPS2 and TRPV3. Arch Dermatol Res. 2017;309:637-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Caengprasath N, Theerapanon T, Porntaveetus T, Shotelersuk V. MBTPS2, a membrane bound protease, underlying several distinct skin and bone disorders. J Transl Med. 2021;19:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Saral S, Vural A, Wollenberg A, Ruzicka T. A practical approach to ichthyoses with systemic manifestations. Clin Genet. 2017;91:799-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Wang H, Humbatova A, Liu Y, Qin W, Lee M, Cesarato N, Kortüm F, Kumar S, Romano MT, Dai S, Mo R, Sivalingam S, Motameny S, Wu Y, Wang X, Niu X, Geng S, Bornholdt D, Kroisel PM, Tadini G, Walter SD, Hauck F, Girisha KM, Calza AM, Bottani A, Altmüller J, Buness A, Yang S, Sun X, Ma L, Kutsche K, Grzeschik KH, Betz RC, Lin Z. Mutations in SREBF1, Encoding Sterol Regulatory Element Binding Transcription Factor 1, Cause Autosomal-Dominant IFAP Syndrome. Am J Hum Genet. 2020;107:34-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 35. | Eslami SM, Nikfar S, Ghasemi M, Abdollahi M. Does Evolocumab, as a PCSK9 Inhibitor, Ameliorate the Lipid Profile in Familial Hypercholesterolemia Patients? J Pharm Pharm Sci. 2017;20:81-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | He M, Hou J, Wang L, Zheng M, Fang T, Wang X, Xia J. Actinidia chinensis Planch root extract inhibits cholesterol metabolism in hepatocellular carcinoma through upregulation of PCSK9. Oncotarget. 2017;8:42136-42148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 37. | Haerian MS, Haerian BS, Molanaei S, Kosari F, Sabeti S, Bidari-Zerehpoosh F, Abdolali E. MTRR rs1801394 and its interaction with MTHFR rs1801133 in colorectal cancer: a case-control study and meta-analysis. Pharmacogenomics. 2017;18:1075-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Druck T, Cheung DG, Park D, Trapasso F, Pichiorri F, Gaspari M, Palumbo T, Aqeilan RI, Gaudio E, Okumura H, Iuliano R, Raso C, Green K, Huebner K, Croce CM. Fhit-Fdxr interaction in the mitochondria: modulation of reactive oxygen species generation and apoptosis in cancer cells. Cell Death Dis. 2019;10:147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 39. | Huang K, Jo H, Echesabal-Chen J, Stamatikos A. Combined LXR and RXR Agonist Therapy Increases ABCA1 Protein Expression and Enhances ApoAI-Mediated Cholesterol Efflux in Cultured Endothelial Cells. Metabolites. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Song G, Lin Q, Zhao H, Liu M, Ye F, Sun Y, Yu Y, Guo S, Jiao P, Wu Y, Ding G, Xiao Q, Qin S. Hydrogen Activates ATP-Binding Cassette Transporter A1-Dependent Efflux Ex Vivo and Improves High-Density Lipoprotein Function in Patients With Hypercholesterolemia: A Double-Blinded, Randomized, and Placebo-Controlled Trial. J Clin Endocrinol Metab. 2015;100:2724-2733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 41. | Bi DP, Yin CH, Zhang XY, Yang NN, Xu JY. MiR-183 functions as an oncogene by targeting ABCA1 in colon cancer. Oncol Rep. 2016;35:2873-2879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 42. | Chou JL, Huang RL, Shay J, Chen LY, Lin SJ, Yan PS, Chao WT, Lai YH, Lai YL, Chao TK, Lee CI, Tai CK, Wu SF, Nephew KP, Huang TH, Lai HC, Chan MW. Hypermethylation of the TGF-β target, ABCA1 is associated with poor prognosis in ovarian cancer patients. Clin Epigenetics. 2015;7:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 43. | Ilnytska O, Lai K, Gorshkov K, Schultz ML, Tran BN, Jeziorek M, Kunkel TJ, Azaria RD, McLoughlin HS, Waghalter M, Xu Y, Schlame M, Altan-Bonnet N, Zheng W, Lieberman AP, Dobrowolski R, Storch J. Enrichment of NPC1-deficient cells with the lipid LBPA stimulates autophagy, improves lysosomal function, and reduces cholesterol storage. J Biol Chem. 2021;297:100813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |