Published online May 16, 2022. doi: 10.12998/wjcc.v10.i14.4380
Peer-review started: August 29, 2021
First decision: December 17, 2021
Revised: December 30, 2021
Accepted: March 25, 2022
Article in press: March 25, 2022
Published online: May 16, 2022
Processing time: 256 Days and 21.2 Hours
The neutrophil-lymphocyte ratio (NLR) is often used to predict a poor prognosis in patients with tumors. This study investigated the preoperative peripheral blood NLR in predicting postoperative survival (POS) in patients with multiple myeloma bone disease (MMBD).
To evaluate whether NLR can be used to predict the prognosis of MMBD patients after surgery.
The clinical data of 82 MMBD patients who underwent surgical treatments in Beijing Chao-yang Hospital were collected. The NLR was obtained from the absolute number of neutrophils and lymphocytes, calculated by the number of neutrophils and divided by the number of lymphocytes. The peripheral blood lymphocyte percentage was used as the major marker to analyze the change in characteristics of the immune statuses of multiple myeloma patients.
The NLR cut-off values of NLR ≥ 3 patients and NLR ≥ 4 patients were significantly correlated with POS. The 3- and 5-year cumulative survival rates of the high NLR group (NLR ≥ 3 patients) were 19.1% and 0.0%, respectively, which were lower than those of the low NLR group (NLR < 3 patients) (67.2% and 48.3%) (P = 0.000). In the high NLR group, POS (14.86 ± 14.28) was significantly shorter than that in the low NLR group (32.68 ± 21.76). Univariate analysis showed that the lymphocyte percentage 1 wk after the operation (19.33 ± 9.08) was significantly lower than that before the operation (25.72 ± 11.02). Survival analysis showed that postoperative chemotherapy, preoperative performance status and preoperative peripheral blood NLR ≥ 3 were independent risk factors for POS.
The preoperative peripheral blood NLR can predict POS in MMBD patients. MMBD patients with a high preoperative NLR (NLR ≥ 3) showed poor prognosis.
Core Tip: The clinical data of 82 multiple myeloma bone disease (MMBD) patients who underwent operations in our hospital were collected. By observing the change characteristics of neutrophil-lymphocyte ratio (NLR) at different stages after the operation, to make a preliminary analysis of the effect of the operation on the immune status of multiple myeloma patients and study the prognostic value of peripheral blood NLR in predicting the treatment of MMBD. Data showed that NLR was significant for the prediction of the postoperative survival (POS) of MMBD patients treated with the operation, and MMBD patients with high preoperative NLR (NLR ≥ 3) had a poorer prognosis and shorter POS.
- Citation: Xu ZY, Yao XC, Shi XJ, Du XR. Significance of preoperative peripheral blood neutrophil-lymphocyte ratio in predicting postoperative survival in patients with multiple myeloma bone disease. World J Clin Cases 2022; 10(14): 4380-4394
- URL: https://www.wjgnet.com/2307-8960/full/v10/i14/4380.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i14.4380
Multiple myeloma (MM) is a malignant proliferative disease of plasma cells with the second highest incidence among blood tumor diseases. Due to the growth of the aging population the incidence of MM has increased and there are many studies on prognosis, especially on prognostic factors and relevant stratification data. Such studies are based on the characteristics of patients, tumor load and cytogenetics[1], and mainly target the selection of chemotherapy regimens and other medical treatments. However, to date, only a few studies have reported MM surgical treatment prognosis stratification[2-4].
As a marker of systematic inflammation, the neutrophil-lymphocyte ratio (NLR) has been used to diagnose infectious diseases. The NLR has also been used as an immune status evaluation indicator to assess the prognosis of solid tumors, such as hepatocellular carcinoma, gastric cancer, renal cell carcinoma, colorectal cancer, ovarian cancer and prostate cancer. These studies suggested that a high NLR may predict a poor prognosis[5-7]. Although some retrospective studies have initially explored the influence of NLR on the prognosis of MM in recent years, these studies have been inadequate due to a small number of cases included in the study and shorter follow-up times[8,9]. These studies did not report on whether there was any change in the immune status of MM patients before and after the operation. Can NLR be used for the prognostic evaluation of surgically treated MM bone disease (MMBD)? Thus, the effect of the NLR on the prognosis of MM patients remains to be studied further.
This study reviewed the general conditions, indicators, surgical information and follow-up of MMBD patients from 2007 to 2017 to analyze the value of the preoperative peripheral blood NLR in predicting the prognosis of patients and its impact on postoperative survival (POS). According to the changes in the absolute neutrophil count, absolute lymphocyte count and lymphocyte percentage at different time points before and after the surgical treatments, the effect of the surgical treatments on the immune status of patients was discussed.
The institutional review board of our hospital approved this retrospective study. A total of 82 MMBD patients from the Beijing Chao-Yang Hospital were enrolled between 2007 and 2017. The sites and number of their lesions were determined according to computed tomography and magnetic resonance imaging findings. General information of patients, including age, sex, Durie/Salmon (DS) stage, international staging system (ISS) stage, isotype, lesion site, preoperative/postoperative treatment, chemotherapy regimens, radiotherapy, operation scheme and stem cell transplantation therapy were collected.
The general physical condition of patients before surgical treatments including nutrition status, body mass index (BMI), performance status and MM activity were collected. The classification criteria of nutrition status are as follows: (1) “Well”, the mucous membrane is ruddy, the skin is shiny and elastic, the subcutaneous fat is plump and elastic, the skinfold thickness is normal or enlarged, the muscle is strong, the nails and hair are moist, the intercostal space and supraclavicular fossa are moderate and the scapular and abdominal muscles are plump. Body weight and body mass index are in the normal range or slightly above normal; (2) “Poorly”, the skin and mucous membrane is dry, the elasticity is reduced, the subcutaneous fat is thin, the skinfold thickness is lower than normal, the muscle is loose and weak, the nails are rough, the hair is sparse and dull, the intercostal space and supraclavicular fossa are sunken and the shoulder, ribs and iliac bones are prominent. Body weight and body mass index were significantly lower than normal; and (3) “Fairly”, between the above two classification criteria.
The evaluation of the patient’s performance status is based on the Zubrod-ECOG-WHO score (ZPS score, 5-point method), as follows: Grade 0 normal activity; Grade 1 symptoms but nearly ambulatory; Grade 2 some bed time but needs to be in bed less than 50% of the normal daytime; Grade 3 needs to be in bed more than 50% of the normal daytime; Grade 4 unable to get out of bed; Grade 5 death.
According to routine blood examination, the NLR was obtained using the absolute number of neutrophils and lymphocytes, calculated by the number of neutrophils divided by the number of lymphocytes. According to their NLR values, patients were grouped into a high NLR group (NLR ≥ 3 patients) and a low NLR group (NLR < 3 patients). Moreover, their cumulative survival rates were calculated according to their survival 1 year, 3 years and 5 years after the operation.
Patient clinical data were analyzed by the Chi-square test and independent sample t-test. The risk factors that may affect the POS in patients were analyzed by Cox model analysis. The survival time was assessed by the Kaplan-Meier method and examined by the log-rank test. The chi-square test was used to analyze the 1-year, 3-year and 5-year cumulative survival rates of patients with different preoperative peripheral blood NLRs to calculate the cut-off value and select the NLR cut-off value with the most significant difference for grouping. Peripheral blood lymphocyte percentage was used as the evaluation indicator to preliminarily assess the immune status of 82 MMBD patients before and after the operation and to perform a univariate analysis on the lymphocyte percentages of the 82 postoperative patients at different stages and preliminarily assess the effect of operations on the immune status of MMBD patients. One-way ANOVA and the Mann-Whitney U test for further comparisons between specific group pairs were used. All data were analyzed by SPSS 19.0 statistical software (IBM SPSS Statistics 19.0). The measurement data are expressed as the mean ± SD, and P < 0.05 was considered as a significant difference.
Among the 82 MMBD patients with an average age of 60.33 ± 9.62 years, 44 patients were male (53.7%) and 38 patients were female (46.3%). The spine was involved in 56 patients (68.3%), long bone of the limb in 19 (23.2%) patients and the soft tissue in 7 (8.5%) patients. The average time from diagnosis to treatment of 82 patients was 19.48 mo (19.48 ± 26.69). Nine patients (11%) underwent complete excision, 72 (87.8%) underwent partial excision/intra-lesion curettage and 1 patient (1.2%) underwent tissue biopsy. Twenty patients (24.4%) received autologous stem cell transplantation while sixty-two patients (75.6%) did not. The average follow-up period of 82 patients was 27.03 ± 21.31 mo (range, 0.25-84 mo) (Table 1).
| Characteristic | Value n (%) or n [SD] |
| Age (yr) | 60.33 [9.620] |
| Gender | |
| Male | 44 (53.7) |
| Female | 38 (46.3) |
| ISS stage | |
| ISS-I | 15 (18.3) |
| ISS-II | 37 (45.1) |
| ISS-III | 30 (36.6) |
| Number of lesions | |
| 1 | 53 (64.6) |
| 2 | 18 (22.0) |
| ≥ 3 | 11 (13.4) |
| Site of lesion | |
| Spine | 56 (68.3) |
| Long bone of limb | 19 (23.2) |
| Soft tissue | 7 (8.5) |
| Isotype | |
| IgA | 23 (28) |
| IgD | 3 (3.7) |
| IgG | 45 (54.9) |
| Light chain κ/λ | 11 (13.4) |
| Preoperative chemotherapy | |
| Yes | 48 (58.5) |
| No | 34 (41.5) |
| Postoperative chemotherapy | |
| Yes | 71 (86.6) |
| No | 11 (13.4) |
| Preoperative radiotherapy | |
| Yes | 6 (7.3) |
| No | 76 (92.7) |
| Time from a diagnosis to surgery (mo) | 19.48 [26.69] |
| Operation scheme | |
| Complete excision1 | 9 (11) |
| Partial excision/intramedullary curettage2 | 72 (87.8) |
| Tissue biopsy3 | 1 (1.2) |
| Autologous stem cell transplantation | |
| Yes | 20 (24.4) |
| No | 62 (75.6) |
| POS (mo) | 27.03 [21.31] |
| Total survival (mo) | 46.63 [31.04] |
The results showed that the preoperative BMI of 82 patients was 23.14 ± 3.36. The preoperative nutrition status classification showed “Well” in 68 patients, “Fairly” in 6 patients and “Poorly” in 8 patients. The results of the preoperative performance status of 82 patients showed that 16 patients (19.5%) were grade 0-2, and 66 patients (80.5%) were grade 3-4. The data on MM activity at the time of surgery of 82 patients showed that there were 2 cases (2.4%) of partial remission, 8 cases (9.8%) of stable disease and 72 cases (87.8%) of progressive disease (Table 2).
| Characteristic | Value n (%) or n [SD] |
| Preoperative nutrition | |
| Well | 68 (82.9) |
| Fairly | 6 (7.3) |
| Poorly | 8 (9.8) |
| Preoperative BMI (kg/m2) | 23.14 [3.36] |
| Preoperative performance status | |
| Grade 0-2 | 16 (19.5) |
| Grade 3-4 | 66 (80.5) |
| Preoperative MM activity | |
| Partial remission | 2 (2.4) |
| Stable disease | 8 (9.8) |
| Progression disease | 72 (87.8) |
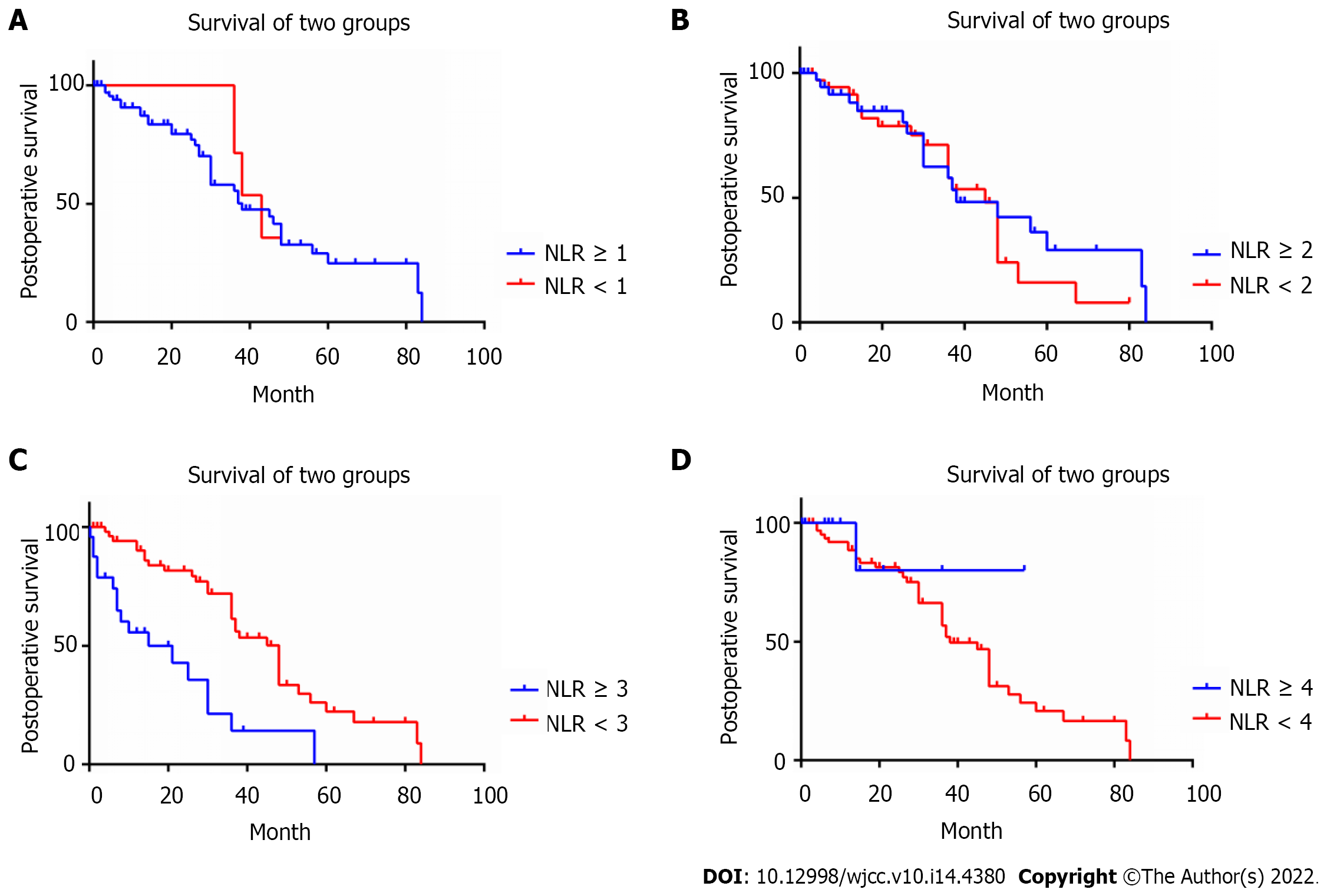
After statistically analyzing the correlation between the preoperative peripheral blood NLR and POS, it was observed that the NLR cut-off values of the preoperative peripheral blood NLR ≥ 3 and NLR ≥ 4 were significantly correlated with the postoperative cumulative survival rate (Table 3). In particular, when the NLR cut-off value was set to 3, the difference was most significant. The POS of NLR ≥ 3 patients (n = 26) (14.86 ± 14.28 mo) was significantly lower than that of NLR < 3 patients (n = 56) (32.68 ± 21.76 mo) (P = 0.000) (Table 4, Figure 1). Among the 82 patients, 48 patients have received chemotherapy before surgical treatments. There were 11 (22.9%) patients in the immunomodulator-based treatment group, 20 (41.7%) patients in the proteasome inhibitor-based treatment group and 17 (35.4%) patients in the immunomodulator and proteasome inhibitor treatment groups. Seventy-one patients have received chemotherapy after surgical treatments (Table 4).
| Cutoff value of NLR | 1-yr CSRa | 3-yr CSRa | 5-yr CSRa | Chi-square | P value |
| NLR ≥ 4 | 41.7% vs 82.5% | 10.4% vs 60.9% | 0.0% vs 41.7% | 15.969 | 0.000a |
| NLR ≥ 3 (n = 26) | 52.0% vs 87.3% | 19.1% vs 67.2% | 0.0% vs 48.3% | 22.192 | 0.000a |
| NLR ≥ 2 | 65.8% vs 88.9% | 46.5% vs 61.1% | 31.0% vs 34.0% | 2.015 | 0.156 |
| NLR ≥ 1 | 75.3% vs 85.7% | 52.6% vs - | 30.6% vs - | 0.459 | 0.498 |
| Characteristic | NLR < 3 (n = 56) | NLR ≥ 3 (n = 26) | P value |
| Age (yr) | 60.93 ± 9.88 | 59.04 ± 9.10 | 0.411 |
| Gender, n (%) | 0.981 | ||
| Male | 30 (53.6) | 14 (53.8) | |
| Female | 26 (46.4) | 12 (46.2) | |
| ISS stage, n (%) | 0.693 | ||
| ISS-I | 10 (17.9) | 5 (19.2) | |
| ISS-II | 27 (48.2) | 10 (38.5) | |
| ISS-III | 19 (33.9) | 11 (42.3) | |
| Number of lesions, n (%) | 0.033a | ||
| 1 | 41 (73.2) | 12 (46.2) | |
| 2 | 8 (14.3) | 10 (38.5) | |
| ≥ 3 | 7 (12.5) | 4 (15.4) | |
| Site of lesion, n (%) | 0.982 | ||
| Spine | 38 (67.9) | 18 (69.2) | |
| Long bone of limb | 13 (23.2) | 6 (23.1) | |
| Soft tissue | 5 (8.9) | 2 (7.7) | |
| Time from a diagnosis to surgery (mo) | 21.18 ± 29.75 | 15.81 ± 18.44 | 0.321 |
| Preoperative nutrition, n (%) | 0.071 | ||
| Well | 50 (89.3) | 18 (69.2) | |
| Fairly | 3 (5.4) | 3 (11.5) | |
| Poorly | 3 (5.4) | 5 (19.2) | |
| Preoperative BMI (kg/m2) | 23.80 ± 3.29 | 21.74 ± 3.13 | 0.009a |
| Preoperative performance status, n (%) | 0.066 | ||
| Grade 0-2 | 14 (25.0) | 2 (7.7) | |
| Grade 3-4 | 42 (75.0) | 24 (92.3) | |
| MM activity at the time of surgery, n (%) | 0.590 | ||
| Partial response | 2 (3.6) | 0 (0.0) | |
| Stable disease | 5 (8.9) | 3 (11.5) | |
| Progressive disease | 49 (87.5) | 23 (88.5) | |
| Preoperative chemotherapy, n (%) | 0.707 | ||
| Yes | 32 (57.1) | 16 (61.5) | |
| No | 24 (42.9) | 10 (38.5) | |
| Preoperative chemotherapy regimens (n = 48), n (%) | 0.216 | ||
| Immunomodulator-based | 6 (37.5) | 5 (15.6) | |
| Proteasome inhibitor-based | 6 (37.5) | 14 (43.8) | |
| Both immunomodulator and proteasome inhibitor | 4 (25.0) | 13 (40.6) | |
| Postoperative chemotherapy, n (%) | 0.080 | ||
| Yes | 51 (91.1) | 20 (76.9) | |
| No | 5 (8.9) | 6 (23.1) | |
| Postoperative chemotherapy regimens (n = 71), n (%) | 0.159 | ||
| Immunomodulator-based | 3 (6.0) | 4 (19.0) | |
| Proteasome inhibitor-based | 17 (34.0) | 4 (19.0) | |
| Both immunomodulator and proteasome inhibitor | 30 (60.0) | 13 (61.9) | |
| Autologous stem cell transplantation, n (%) | 0.458 | ||
| Yes | 15 (26.8) | 5 (19.2) | |
| No | 41 (73.2) | 21 (80.8) | |
| POS (mo) | 32.68 ± 21.76 | 14.86 ± 14.28 | 0.001 |
Univariate analysis showed that the preoperative BMI in the high NLR group was significantly lower than that in the low NLR group (21.74 ± 3.13 vs 23.79 ± 3.29, P = 0.009). There was no significant difference in preoperative chemotherapy regimens (P = 0.216), the time from diagnosis of MM to surgery (P = 0.321), the performance status (P = 0.066), MM activity at the time of surgery (P = 0.590) and postoperative chemotherapy regimens (P = 0.159) between the high NLR group and the low NLR group (Table 4).
Univariate analysis showed that the number of lesions affected NLR to some extent (P = 0.033), while sex, ISS stage, lesion sites, chemotherapy and autologous stem cell transplantation did not affect the NLR (Table 4). Differences in preoperative Hb and aspartate aminotransferase (AST) were found between the high NLR group and the low NLR group (P = 0.047, P = 0.007), while no difference was found for PLT and pre/postoperative C-reactive protein (CRP) (Table 5).
| Preoperative laboratory examination | NLR < 3 (n = 56) | NLR ≥ 3 (n = 26) | P value |
| Hb (g/L) | 112.66 ± 24.88 | 100.58 ± 25.91 | 0.047a |
| PLT (109/L)a | 208.38 ± 77.39 | 196.89 ± 103.64 | 0.577 |
| ALb (g/L) | 32.88 ± 6.02 | 31.16 ± 6.29 | 0.237 |
| AST (U/L) | 22.89 ± 8.39 | 33.96 ± 18.79 | 0.007a |
| ALT (U/L) | 19.89 ± 10.28 | 28.46 ± 24.17 | 0.093 |
| Γ-GT (U/L) | 34.11 ± 27.52 | 47.58 ± 38.19 | 0.073 |
| Blood β2-MG (mg/L) | 5.43 ± 9.08 | 4.10 ± 2.85 | 0.475 |
| Cr (umol/L) | 84.54 ± 73.73 | 89.38 ± 85.84 | 0.794 |
| Preoperative CRP (mg/L) | 2.60 ± 3.84 | 3.67 ± 3.93 | 0.411 |
| Post-operative CRP (mg/L) | 1.98 ± 2.79 | 1.83 ± 2.06 | 0.877 |
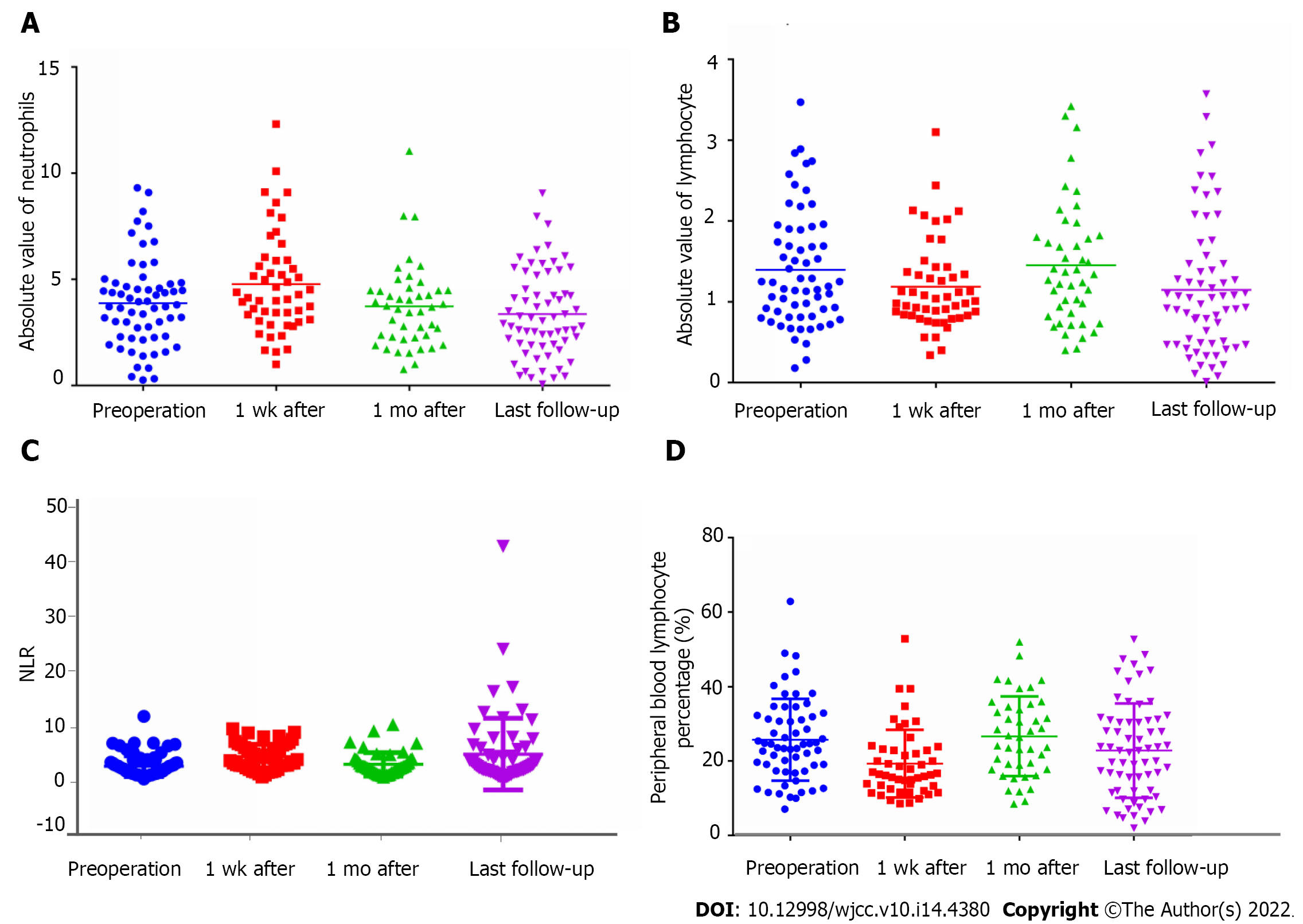
The absolute neutrophil count 1 wk after the operation significantly increased (4.78 ± 2.37 × 109/L). One month after the operation (3.72 ± 1.96 × 109/L), the levels returned to those before the operation (Table 6, Figure 2A and B). The peripheral blood NLR after the operation significantly increased (2.78 ± 1.97 vs 4.56 ± 2.33, P = 0.002) and then declined 1 mo after the operation (3.14 ± 2.19). The levels increased again at the last follow-up visit (5.03 ± 6.73) (Table 6, Figure 2C).
| Characteristic | Before operation | 1 wk after operation | 1 mo after operation | Last follow-up visit | F | P value |
| Absolute blood lymphocyte counts | 1.34 ± 0.75 | 1.19 ± 0.55 | 1.45 ± 0.76 | 1.15 ± 0.821,2 | 2.382 | 0.070 |
| Absolute neutrophil counts | 3.87 ± 2.07 | 4.79 ± 2.373-5 | 3.72 ± 1.96 | 3.36 ± 2.04 | 4.376 | 0.005 |
| NLR | 2.78 ± 1.976,7 | 4.56 ± 2.33 | 3.14 ± 2.198 | 5.03 ± 6.73 | 4.998 | 0.002 |
| Lymphocyte percentages (%) | 25.09 ± 9.96 | 18.19 ± 7.193,4 | 26.66 ± 10.70 | 22.83 ± 12.68 | 5.770 | 0.001 |
When preliminarily analyzing the immune status of the 82 MMBD patients before and after the operation with the peripheral lymphocyte percentage as the evaluation indicator, the results showed that the peripheral lymphocyte percentage of MMBD patients before operation (25.09% ± 9.96%) was at a normal (low) level; 1 wk after operation, it (18.19% ± 7.19%) significantly declined; 1 mo after operation, it (26.66% ± 10.70%) returned to that before operation; at the last follow-up visit, it (22.83% ± 12.68%) returned to a low level again (Table 6, Figure 2D).
Kaplan-Meier analysis showed that the number of lesions, site of lesion, postoperative chemotherapy, preoperative performance status and preoperative peripheral blood NLR ≥ 3 were risk factors affecting POS (Table 7). Multivariate survival analysis showed that postoperative chemotherapy (P = 0.000), preoperative performance status (P = 0.002), and preoperative peripheral blood NLR ≥ 3 (P = 0.000) were independent risk factors affecting POS (Table 8).
| Characteristic | Chi-square | P value |
| Gender | 1.703 | 0.192 |
| ISS stage | 1.966 | 0.374 |
| Number of lesions | 7.055 | 0.029a |
| Site of lesion | 8.160 | 0.017a |
| Isotype | 1.526 | 0.676 |
| Preoperative chemotherapy | 0.387 | 0.534 |
| Postoperative chemotherapy | 35.837 | 0.000a |
| Preoperative radiotherapy | 0.908 | 0.341 |
| Time from a diagnosis to surgery | 1.841 | 0.606 |
| Operation scheme | 3.583 | 0.167 |
| Stem cell transplantation | 2.836 | 0.092 |
| Preoperative peripheral blood NLR ≥ 3 | 22.192 | 0.000a |
| Preoperative nutrition | 0.782 | 0.676 |
| Preoperative performance status | 20.775 | 0.000a |
| Preoperative MM activity | 1.634 | 0.652 |
There is clinical value in using peripheral blood NLR in predicting the prognosis of MMBD patients treated with an operation. Multiple myeloma is a malignant proliferative disease of plasma cells, where its occurrence and development are strongly dependent on the microenvironment. The interaction between MM cells and the microenvironment has a crucial role in the pathogenesis of MM[10-12]. The MM microenvironment is mainly composed of inflammatory cells (including macrophages, dendritic cells, mast cells and myeloid-derived suppressor cells). Inflammatory cells are the main source of MM-infiltrating bone marrow cytokines and also mediate immune suppression of MM[13-16]. Inflammation is one of the characteristics of malignant tumors and tumor-related inflammation has an important role in promoting tumorigenesis by inducing tumor cell growth, angiogenesis and genomic instability[17]. As a marker of systematic inflammation, NLR has also been used as an immune status indicator for the prognosis of solid tumors[18].
Our study showed that compared with MMBD patients in the low NLR group, those in the high NLR group had a different number of lesions, preoperative hemoglobin levels, preoperative glutamate transaminase and preoperative absolute lymphocyte count. Multivariate survival analysis showed that postoperative chemotherapy and preoperative peripheral blood NLR were independent risk factors affecting the POS of MMBD patients.
Some studies have also reported on NLR prognosis prediction in patients with hematologic tumors[19-23]. Zhou et al[24] found that an elevated NLR is associated with decreased overall survival in MM patients receiving bortezomib induction therapy. Wongrakpanich et al[25] reported that an elevated NLR and a decreased platelet-lymphocyte ratio are independent prognostic factors for progression-free survival in MM patients. Shi et al[26] indicated that an elevated NLR, a decreased monocyte-lymphocyte ratio and a decreased platelet-lymphocyte ratio predict adverse clinical outcomes in MM patients and may serve as cost-effective and readily available prognostic biomarkers. Lee et al[27] analyzed 179 MM patients treated with the VMP regimen and found that a high NLR was an independent poor prognostic factor. Solmaz et al[28] reviewed 150 MM patients who underwent autologous stem cell transplantation (ASCT) and found that a high NLR on the 100th d post transplantation was associated with inferior overall survival (OS) and Progression-free survival (PFS). Romano et al[29] showed that the median PFS was significantly shorter for MM patients with NLR ≥ 2 when considering the clinical outcomes of ASCT. In summary, MM patients have shortened OS with a high NLR.
To date, there are no reports on the prognostic value of NLR in MM patients who undergo an operation. We think that elevated NLR may be used as a prognostic biomarker for the prediction of survival and help predict clinical outcome in patients with MMBD. Before it can be used in clinical practice, the time to detect NLR and the cut-off value should be further standardized.
Due to the great range of tumor tissue removal during surgery, the tumor load significantly decreased, and the stimulating effect of tumor cells on the immune and inflammatory reaction was reduced resulting in great changes in the NLR before and after the operation. A previous study found that the NLR of a non-small cell lung cancer patient at 1 mo after operation increased by over 0.27, and the DFS and OS of the same patient were significantly shorter than those whose NLR declined or increased by less than 0.27 thus suggesting that NLR changes before and after the operation could be used for the early evaluation of the effectiveness of tumor therapy[30].
In this study, we found that MM patients who had a high NLR before the operation, had a significantly increased NLR 1 wk after the operation. In the 2nd wk after the operation, the absolute neutrophil count significantly increased while the absolute lymphocyte count declined; in the 2nd mo, the NLR declined while the absolute neutrophil count and absolute lymphocyte count returned to levels before the operation. At the last follow-up visit, the NLR returned to a high level while the absolute neutrophil count significantly increased and the absolute lymphocyte count significantly declined. This change indicates that a high preoperative NLR, which may be the result of the imbalance of inflammatory reactions between antitumor and protumor effects, may be used to predict poor prognosis in MM patients[26]. An elevated NLR indicates that the neutrophils in an MM patient's blood increased while the lymphocytes decreased, resulting in an imbalance between the antitumor effect of lymphocytes and the protumor effect of neutrophils, thus affecting the prognosis of tumor patients.
Cellular immunity and humoral immunity play a key role in the occurrence and progression of tumors. The function and status of cellular immunity largely reflects the progression of tumors and the prognosis of patients. At present, antitumor cell immunotherapy based on the correlation between cellular immunologic function and status and tumors is commonly applied in clinical practice.
In this study, we found normal levels of neutrophils in MM patients before surgery. These levels significantly increased 1 wk after the operation and then returned to normal levels 1 mo after the operation; at the last follow-up visit, the levels were lower than before the operation. We believe that 1 wk after the operation, patients were in the postoperative inflammatory reaction stage, so their neutrophils were high. However, with time, the immune status recovered, which led to a decrease in neutrophils. This may be a normal body reaction; however, whether such a reaction affects tumors needs to be further explored. At the last follow-up visit, the tumor status of patients was improved, and the level of their neutrophils declined over that before operation due to the application of treatment measures such as operations and chemotherapy. Peripheral blood NLR before and 1 mo after the operation may reflect the immune status of patients from one side and can be used as one of the indicators for predicting operative prognoses.
Correct judgment and evaluation of the cellular immunologic function of patients with malignant tumors have an important role in evaluating disease development and guiding clinical treatment. Multiple myeloma is characterized by immunologic dysfunction. MM immune injury has been considered as another tool to predict prognosis[30]. Long-term survival in MM patients may result from unique immunologic characteristics[31]. Lymphocytes are an important part of the immune system. They contain subsets of different functions, which can be roughly divided into T cells, B cells and NK cells. Domestic and foreign studies have found various defects in the immune system of MM patients, including abnormal quantities and functional defects in B cells, T cells, NK cells and DC cells, as well as abnormal regulatory Tregs[32,33]. Bernal et al[34] found that the number of NK cells in the peripheral blood of MM patients was increased, while their activity declined. Furthermore, Jurisic et al[35] proved that the decline in NK cell activity in patients was related to disease progression and the degree of plasma cell infiltration.
T cells have a major role in the antitumor immune reaction of tumor patients and regulate the immune reaction and maintain immune stability. T cell subset expression anomalies have an important role in the pathogenesis of MM. Koike et al[36] and Oken et al[37] have found a decrease in CD4(+) T/CD8(+) T cells in MM patients, which disrupts the immune balance of MM patients and weakens their existing antitumor functions. Tregs are a subset of T cells that control autoimmunity and have a negative immunoregulatory function, which maintains the immune system stability of the body together with Th1 and Th2 cells. It is related to the immune tolerance and tumor immune escape mechanisms[38,39]. Recent studies have found a lower proportion of Th1 and Th2 cells in MM patients than in healthy controls, suggesting that both humoral and cellular immunity of MM patients are impaired to varying degrees[40]. Therefore, lymphocyte subsets and Th cells are of great significance in the prognostic evaluation of MM patients. Lymphocytes have a key role in cell-mediated antitumor immune reactions and their amount reflects the degree of reaction of the host immune system.
Whether operations affect the immune status of MM patients has not yet been clarified. Our data showed that the lymphocyte percentage of MM patients was within normal (low) levels before the operation. These levels declined 1 mo after surgery and returned to normal levels later on (at the last follow-up visit), which suggests that operations affect the immune status of patients with MM. After the operation, the immune balance of MM patients is disrupted, which in turn affects the immune status. However, with time, immune status is recovered. Therefore, for MM patients who undergo surgery, adjuvant therapy to enhance their immunity may be given when necessary to allow patients to build up their immune status, which may be helpful for the overall treatment of MM patients. The correlation between such results and the timing of chemotherapy after surgery needs further study.
Neutrophils have a multivariate role in tumor diseases. However, their role in cancer has not been fully understood[41]. Neutrophils reflect the inflammatory state of tumor patients and have different roles in different stages of tumor diseases[42]. Neutrophils release reactive nitrogen species, reactive oxygen species and proteases that promote the occurrence of tumors[43]. Nitric oxide synthase or arginase 1 released by neutrophils can inhibit the antitumor reaction of CD8(+) T lymphocytes[44] by weakening the immune system, promoting tumor proliferation, and invasion and stimulating TGF-β[45]. Moreover, some researchers believe that an increase in neutrophil count is one of the adverse prognostic factors of head and neck cancer progression[46].
Nonetheless, this study had some limitations. Due to the length of diagnosis and treatment and numerous follow-up visits, the lymphocyte subset information at different time points was not available for some patients. Second, due to different treatment regimens of patients during the treatment period, the detection of peripheral blood-related cells alone cannot fully reflect the body’s immune status.
The lesion site, number of lesions, postoperative chemotherapy, preoperative peripheral blood NLR and preoperative performance status are the risk factors affecting POS in MMBD patients. Postoperative chemotherapy, preoperative peripheral blood NLR and preoperative performance status are independent risk factors.
Performance status, as a score to evaluate the physical activity status of patients, can reveal their general health status and tolerance to treatment based on their physical strength. For patients with poor general health before surgery, their tolerance to surgery is worse, recovery time is slower, postoperative complications are more likely to occur and the survival time is often shorter. However, as a subjective scoring system, some results do not fully respond to the true condition of patients. Comprehensive evaluation of other indicators such as BMI, muscle fat ratio and bone density is required to determine this.
Chemotherapy is another independent risk factor affecting the POS of patients with MMBD. Chemotherapy is the most important treatment method for multiple myeloma and helps to control tumors and extend the survival period. However, there is often a large disparity in treatment tolerance that affects the statistical analysis. If some patients are insensitive to chemotherapy or change chemotherapy regimens frequently, this may affect the accuracy of the statistical results. To a certain extent, NLR is an indicator of systemic inflammatory reactions which can reflect the balance between inflammation and immunity in tumor patients. We can obtain relevant values directly from the peripheral blood. However, NLR easily interferes with other factors. For patients with other infectious diseases or those who are using certain drugs, the values from the peripheral blood are not completely accurate. If the numerical difference is found in the measurement, it should be rerecorded in time.
MMBD patients with a high preoperative NLR had a poorer prognosis and shorter POS. The immune status of MMBD patients was at a normal low level and then significantly declined after surgical treatments. The NLR can predict the prognosis of MM patients undergoing surgical treatments.
This study investigated the preoperative peripheral blood neutrophil-lymphocyte ratio (NLR) in predicting postoperative survival (POS) in patients with multiple myeloma bone disease (MMBD).
As a marker of systematic inflammation, NLR has been used to diagnose infectious diseases. Although some retrospective studies have initially explored the influence of NLR on the prognosis of multiple myeloma (MM) in recent years, these studies didn't report on whether there is any change in the immune status of MM patients before and after the operation.
To analyze the effect of preoperative peripheral blood NLR on the prognosis of MMBD patients and evaluate the immune status of MMBD patients in different time periods.
The clinical data of 82 MMBD patients who underwent surgical treatments in Beijing Chaoyang hospital were collected.
Data showed that the NLR cut-off values of the NLR ≥ 3 group and NLR ≥ 4 group were significantly correlated with POS. The POS of NLR ≥ 3 patients (14.86 ± 14.28) was significantly shorter than that of NLR < 3 patients (32.68 ± 21.76). The lymphocyte percentage 1 wk after operation (19.329 ± 9.083) was significantly lower than that before operation (25.723 ± 11.016). Survival analysis showed that postoperative chemotherapy and preoperative peripheral blood NLR were independent risk factors for POS.
MMBD patients with high preoperative NLR had a poorer prognosis and shorter POS. NLR can predict the prognosis of MM patients undergoing surgical treatments.
Preoperative peripheral blood NLR can predict POS in MMBD patients. The immune status of MMBD patients was at a normal low level and then it significantly declined after surgical treatments.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Arigami T, Japan; Fonteh Fru PN, South Africa S-Editor: Chen YL L-Editor: Filipodia P-Editor: Chen YL
| 1. | Rajkumar SV. Multiple myeloma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91:719-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 328] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 2. | Miller JA, Balagamwala EH, Chao ST, Emch T, Suh JH, Djemil T, Angelov L. Spine stereotactic radiosurgery for the treatment of multiple myeloma. J Neurosurg Spine. 2017;26:282-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Surgeon's Committee of the Chinese Myeloma Working Group of the International Myeloma Foundation. Consensus on Surgical Management of Myeloma Bone Disease. Orthop Surg. 2016;8:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Yao X, Xu Z, Du X. PKP/PVP combine chemotherapy in the treatment of multiple myeloma patients with vertebral pathological fractures: minimum 3-year follow-up of 108 cases. J Orthop Surg Res. 2019;14:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, Templeton AJ, Früh M. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017;111:176-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 659] [Article Influence: 82.4] [Reference Citation Analysis (0)] |
| 6. | Sun S, Wang X, Chen J. Using Pre-Treatment Neutrophil-to-Lymphocyte Ratio to Predict the Prognosis of Young Patients with Hepatocellular Carcinoma Implemented Minimally Invasive Treatment. J Adolesc Young Adult Oncol. 2020;9:85-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Luvira V, Kamsa-Ard S, Pugkhem A, Luvira V, Srisuk T, Titapun A, Silsirivanit A, Wongkham S, Khuntikeo N, Pairojkul C, Bhudhisawasdi V. Predictive utility of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in intraductal papillary neoplasm of the bile duct. Clin Exp Hepatol. 2019;5:250-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Zeng Q, Liu Z, Li Q, Liu T. Prognostic value of neutrophil to lymphocyte ratio and clinicopathological characteristics for multiple myeloma: A meta-analysis. Medicine (Baltimore). 2018;97:e12678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Liu S, Shi J, Guo H, Xu F, Wei M, Sun K, Chen Y. Prognostic Significance Of The Inflammatory Index-Based Scoring System In Patients Preliminarily Diagnosed With Multiple Myeloma In The Bortezomib-Based Chemotherapy Era. Cancer Manag Res. 2019;11:9409-9420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Kumar S. Multiple myeloma - current issues and controversies. Cancer Treat Rev. 2010;36 Suppl 2:S3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Spicka I. Advances in multiple myeloma therapy during two past decades. Comput Struct Biotechnol J. 2014;10:38-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1742] [Cited by in RCA: 1892] [Article Influence: 135.1] [Reference Citation Analysis (0)] |
| 13. | Sun Y, Pan J, Mao S, Jin J. IL-17/miR-192/IL-17Rs regulatory feedback loop facilitates multiple myeloma progression. PLoS One. 2014;9:e114647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Chauhan D, Singh AV, Brahmandam M, Carrasco R, Bandi M, Hideshima T, Bianchi G, Podar K, Tai YT, Mitsiades C, Raje N, Jaye DL, Kumar SK, Richardson P, Munshi N, Anderson KC. Functional interaction of plasmacytoid dendritic cells with multiple myeloma cells: a therapeutic target. Cancer Cell. 2009;16:309-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 228] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 15. | Tai YT, Anderson KC. B cell maturation antigen (BCMA)-based immunotherapy for multiple myeloma. Expert Opin Biol Ther. 2019;19:1143-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 16. | Görgün GT, Whitehill G, Anderson JL, Hideshima T, Maguire C, Laubach J, Raje N, Munshi NC, Richardson PG, Anderson KC. Tumor-promoting immune-suppressive myeloid-derived suppressor cells in the multiple myeloma microenvironment in humans. Blood. 2013;121:2975-2987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 331] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 17. | Musolino C, Allegra A, Innao V, Allegra AG, Pioggia G, Gangemi S. Inflammatory and Anti-Inflammatory Equilibrium, Proliferative and Antiproliferative Balance: The Role of Cytokines in Multiple Myeloma. Mediators Inflamm. 2017;2017:1852517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 18. | Cummings M, Merone L, Keeble C, Burland L, Grzelinski M, Sutton K, Begum N, Thacoor A, Green B, Sarveswaran J, Hutson R, Orsi NM. Preoperative neutrophil: lymphocyte and platelet: lymphocyte ratios predict endometrial cancer survival. Br J Cancer. 2015;113:311-320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 19. | Luo G, Guo M, Liu Z, Xiao Z, Jin K, Long J, Liu L, Liu C, Xu J, Ni Q, Yu X. Blood neutrophil-lymphocyte ratio predicts survival in patients with advanced pancreatic cancer treated with chemotherapy. Ann Surg Oncol. 2015;22:670-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 20. | Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88:218-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 784] [Cited by in RCA: 1091] [Article Influence: 90.9] [Reference Citation Analysis (0)] |
| 21. | Feng JF, Liu JS, Huang Y. Lymphopenia predicts poor prognosis in patients with esophageal squamous cell carcinoma. Medicine (Baltimore). 2014;93:e257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 22. | Kokcu A, Kurtoglu E, Celik H, Tosun M, Malatyalıoglu E, Ozdemir AZ. May the platelet to lymphocyte ratio be a prognostic factor for epithelial ovarian cancer? Asian Pac J Cancer Prev. 2014;15:9781-9784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Gungorduk K, Ertas IE, Ozdemir A, Akkaya E, Telli E, Taskin S, Gokcu M, Guzel AB, Oge T, Akman L, Toptas T, Solmaz U, Dogan A, Terek MC, Sanci M, Ozsaran A, Simsek T, Vardar MA, Yalcin OT, Ozalp S, Yildirim Y, Ortac F. Prognostic Significance of Retroperitoneal Lymphadenectomy, Preoperative Neutrophil Lymphocyte Ratio and Platelet Lymphocyte Ratio in Primary Fallopian Tube Carcinoma: A Multicenter Study. Cancer Res Treat. 2015;47:480-488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Zhou X, Wang J, Xia J, Cheng F, Mao J, Zhu J, Guo H. Evaluation of neutrophil-to-lymphocyte ratio in newly diagnosed patients receiving borte- zomib-based therapy for multiple myeloma. Cancer Biomark. 2018;22:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Wongrakpanich S, George G, Chaiwatcharayut W, Biso S, Candelario N, Mittal V, Pomerantz S, Varadi G. The Prognostic Significance of Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios in Patients With Multiple Myeloma. J Clin Lab Anal. 2016;30:1208-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Shi L, Qin X, Wang H, Xia Y, Li Y, Chen X, Shang L, Tai YT, Feng X, Acharya P, Acharya C, Xu Y, Deng S, Hao M, Zou D, Zhao Y, Ru K, Qiu L, An G. Elevated neutrophil-to-lymphocyte ratio and monocyte-to-lymphocyte ratio and decreased platelet-to-lymphocyte ratio are associated with poor prognosis in multiple myeloma. Oncotarget. 2017;8:18792-18801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 27. | Uz B. The Prognostic Value of the Derived Neutrophil-to-Lymphocyte Ratio in Transplantation-Ineligible Patients with Multiple Myeloma. Acta Haematol. 2018;140:157-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Solmaz Medeni S, Acar C, Olgun A, Acar A, Seyhanlı A, Taskıran E, Sevindik OG, Alacacıoglu I, Piskin O, Ozcan MA, Demirkan F, Undar B, Ozsan GH. Can Neutrophil-to-Lymphocyte Ratio, Monocyte-to-Lymphocyte Ratio, and Platelet-to-Lymphocyte Ratio at Day +100 be used as a prognostic marker in Multiple Myeloma patients with autologous transplantation? Clin Transplant. 2018;32:e13359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Romano A, Parrinello NL, Consoli ML, Marchionni L, Forte S, Conticello C, Pompa A, Corso A, Milone G, Di Raimondo F, Borrello I. Neutrophil to lymphocyte ratio (NLR) improves the risk assessment of ISS staging in newly diagnosed MM patients treated upfront with novel agents. Ann Hematol. 2015;94:1875-1883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | Pessoa de Magalhães RJ, Vidriales MB, Paiva B, Fernandez-Gimenez C, García-Sanz R, Mateos MV, Gutierrez NC, Lecrevisse Q, Blanco JF, Hernández J, de las Heras N, Martinez-Lopez J, Roig M, Costa ES, Ocio EM, Perez-Andres M, Maiolino A, Nucci M, De La Rubia J, Lahuerta JJ, San-Miguel JF, Orfao A; Spanish Myeloma Group (GEM); Grupo Castellano-Leones de Gammapatias Monoclonales, cooperative study groups. Analysis of the immune system of multiple myeloma patients achieving long-term disease control by multidimensional flow cytometry. Haematologica. 2013;98:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 31. | Bryant C, Suen H, Brown R, Yang S, Favaloro J, Aklilu E, Gibson J, Ho PJ, Iland H, Fromm P, Woodland N, Nassif N, Hart D, Joshua DE. Long-term survival in multiple myeloma is associated with a distinct immunological profile, which includes proliferative cytotoxic T-cell clones and a favourable Treg/Th17 balance. Blood Cancer J. 2013;3:e148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 32. | Feyler S, von Lilienfeld-Toal M, Jarmin S, Marles L, Rawstron A, Ashcroft AJ, Owen RG, Selby PJ, Cook G. CD4(+)CD25(+)FoxP3(+) regulatory T cells are increased whilst CD3(+)CD4(-)CD8(-)alphabetaTCR(+) Double Negative T cells are decreased in the peripheral blood of patients with multiple myeloma which correlates with disease burden. Br J Haematol. 2009;144:686-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 33. | Pratt G, Goodyear O, Moss P. Immunodeficiency and immunotherapy in multiple myeloma. Br J Haematol. 2007;138:563-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 264] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 34. | Bernal M, Garrido P, Jiménez P, Carretero R, Almagro M, López P, Navarro P, Garrido F, Ruiz-Cabello F. Changes in activatory and inhibitory natural killer (NK) receptors may induce progression to multiple myeloma: implications for tumor evasion of T and NK cells. Hum Immunol. 2009;70:854-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Jurisic V, Srdic T, Konjevic G, Markovic O, Colovic M. Clinical stage-depending decrease of NK cell activity in multiple myeloma patients. Med Oncol. 2007;24:312-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 36. | Koike M, Sekigawa I, Okada M, Matsumoto M, Iida N, Hashimoto H, Oshimi K. Relationship between CD4(+)/CD8(+) T cell ratio and T cell activation in multiple myeloma: reference to IL-16. Leuk Res. 2002;26:705-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Oken MM, Kay NE. T-cell subpopulations in multiple myeloma: correlation with clinical disease status. Br J Haematol. 1981;49:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Tu JF, Ding YH, Ying XH, Wu FZ, Zhou XM, Zhang DK, Zou H, Ji JS. Regulatory T cells, especially ICOS+ FOXP3+ regulatory T cells, are increased in the hepatocellular carcinoma microenvironment and predict reduced survival. Sci Rep. 2016;6:35056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 39. | Fan L, Strasser-Weippl K, Li JJ, St Louis J, Finkelstein DM, Yu KD, Chen WQ, Shao ZM, Goss PE. Breast cancer in China. Lancet Oncol. 2014;15:e279-e289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 835] [Cited by in RCA: 1098] [Article Influence: 99.8] [Reference Citation Analysis (0)] |
| 40. | Beyer M, Kochanek M, Giese T, Endl E, Weihrauch MR, Knolle PA, Classen S, Schultze JL. In vivo peripheral expansion of naive CD4+CD25high FoxP3+ regulatory T cells in patients with multiple myeloma. Blood. 2006;107:3940-3949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 247] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 41. | Grecian R, Whyte MKB, Walmsley SR. The role of neutrophils in cancer. Br Med Bull. 2018;128:5-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 42. | Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16:431-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 997] [Cited by in RCA: 1288] [Article Influence: 143.1] [Reference Citation Analysis (0)] |
| 43. | Jamieson T, Clarke M, Steele CW, Samuel MS, Neumann J, Jung A, Huels D, Olson MF, Das S, Nibbs RJ, Sansom OJ. Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. J Clin Invest. 2012;122:3127-3144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 309] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 44. | Bodogai M, Moritoh K, Lee-Chang C, Hollander CM, Sherman-Baust CA, Wersto RP, Araki Y, Miyoshi I, Yang L, Trinchieri G, Biragyn A. Immunosuppressive and Prometastatic Functions of Myeloid-Derived Suppressive Cells Rely upon Education from Tumor-Associated B Cells. Cancer Res. 2015;75:3456-3465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 45. | Spiegel A, Brooks MW, Houshyar S, Reinhardt F, Ardolino M, Fessler E, Chen MB, Krall JA, DeCock J, Zervantonakis IK, Iannello A, Iwamoto Y, Cortez-Retamozo V, Kamm RD, Pittet MJ, Raulet DH, Weinberg RA. Neutrophils Suppress Intraluminal NK Cell-Mediated Tumor Cell Clearance and Enhance Extravasation of Disseminated Carcinoma Cells. Cancer Discov. 2016;6:630-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 375] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 46. | Valero C, Pardo L, López M, García J, Camacho M, Quer M, León X. Pretreatment count of peripheral neutrophils, monocytes, and lymphocytes as independent prognostic factor in patients with head and neck cancer. Head Neck. 2017;39:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |