Published online May 6, 2022. doi: 10.12998/wjcc.v10.i13.4153
Peer-review started: August 6, 2021
First decision: January 10, 2022
Revised: January 21, 2022
Accepted: March 17, 2022
Article in press: March 17, 2022
Published online: May 6, 2022
Processing time: 266 Days and 19.8 Hours
Primary hyperparathyroidism (PHPT) is the most common cause of pregnancy-related hypercalcemia. PHPT can cause maternal and fetal complications in pregnant women. General anesthesia for non-obstetric surgery in pregnant women is associated with maternal hazards and concerns regarding long-term neonatal neurocognitive effects. Surgical removal of the lesion in mid-pregnancy is currently the primary treatment option for pregnant patients with PHPT. However, the blood calcium concentration at which surgery should be considered remains under discussion due to the risk of miscarriage.
A 31-year-old nulliparous woman at 11 wk of gestation was admitted to our hospital for parathyroidectomy. The patient had a history of intrauterine fetal death with unknown etiology at 16 wk of gestation 1 year prior. Her blood test results showed that the serum calcium level was elevated to 12.9 mg/dL, and the parathyroid hormone level was elevated to 157 pg/mL. In a neck ultrasound, it revealed a 0.8 cm × 1.5 cm sized oval, hypoechoic mass in the upper posterior of the left thyroid gland, which was compatible with parathyroid adenoma. Superficial cervical plexus block (SCPB) for parathyroidectomy was performed. After surgery, the obstetrician checked the status of the fetus, and there were no abnormal signs. Since then her calcium level returned to normal values after one week of surgery and a healthy male neonate of 2910 g was delivered vaginally at 38 wk of gestation.
Our case suggests that SCPB can be an anesthetic option for parathyroidectomy during the first trimester of pregnancy.
Core Tip: Pregnant women undergoing general anesthesia for non-obstetric surgery have risks of maternal hazards. In addition, the surgery might affect the long-term development of the fetus in early pregnancy and cause premature birth in late pregnancy. Pregnant patient with severe primary hyperparathyroidism (PHPT), such as our case, should have their calcium concentration lowered before surgery. Surgical removal of the lesion in mid-pregnancy is the treatment-of-choice in PHPT. However, in case of persisting hypercalcemia despite of conservative treatment, superficial cervical plexus block can be an anesthetic option for parathyroidectomy during the first trimester of pregnancy.
- Citation: Chung JY, Lee YS, Pyeon SY, Han SA, Huh H. Bilateral superficial cervical plexus block for parathyroidectomy during pregnancy: A case report. World J Clin Cases 2022; 10(13): 4153-4160
- URL: https://www.wjgnet.com/2307-8960/full/v10/i13/4153.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i13.4153
The use of general anesthesia for non-obstetric surgery in pregnant women is associated with maternal hazards such as the potential for a difficult airway, aspiration, hypoxemia, and concern for long-term neonatal neurocognitive effects[1,2]. Cervical plexus blocks have been used in various head and neck surgeries to provide adequate anesthesia and analgesia for patients who cannot receive general anesthesia due to their comorbidities. While successful peripheral nerve blocks have been previously described in pregnant patients, the use of superficial cervical plexus block for parathyroidectomy has not been reported[3]. However, superficial cervical plexus block (SCPB) is a difficult technique because it is performed in a narrow space in the neck region that contains many sensitive structures, multiple fascial layers, and complicated innervation[4]. Therefore, it can result in many complications, such as phrenic nerve palsy, airway obstruction, Horner's syndrome, and local anesthetic systemic toxicity (LAST). Here, we report a successful bilateral SCPB using ultrasonography in a pregnant woman at 11 wk of gestation scheduled for parathyroidectomy.
A 31-year-old nulliparous woman was at 11 wk of gestation and admitted to our hospital for parathyroidectomy with gestational hypertension.
When she was at 5 wk of gestational age in this pregnancy, her blood pressure was high at 160/100 mmHg with mild tachycardia of 110/min. A detailed workup, including laboratory tests, was conducted to identify the secondary cause of hypertension.
The patient had a history of intrauterine fetal death with unknown etiology at 16 wk of gestation 1 year prior. The patient was diagnosed with gestational hypertension at that time, but she had not been evaluated for hypertension, and her blood pressure was not controlled by medication.
The patient had no abnormal specific personal or family history of other diseases.
Physical examination showed no positive signs.
Her blood test results showed that the serum calcium level was elevated to 12.9 mg/dL (reference range: 8.8-10.6), and the parathyroid hormone level was elevated to 157 pg/mL (reference range: 15.0-65.0). And the serum 25-hydroxy vitamin D(25(OH)D) level was decreased to 16.0 ng/mL (reference range: 25-80) and the serum Phosphorus level was decreased to 1.9 mg/dL (reference range: 2.5-4.5). Other laboratory test results, including complete blood cell count (CBC), electrolyte and glucose levels, and renal and hepatic function, were within normal limits.
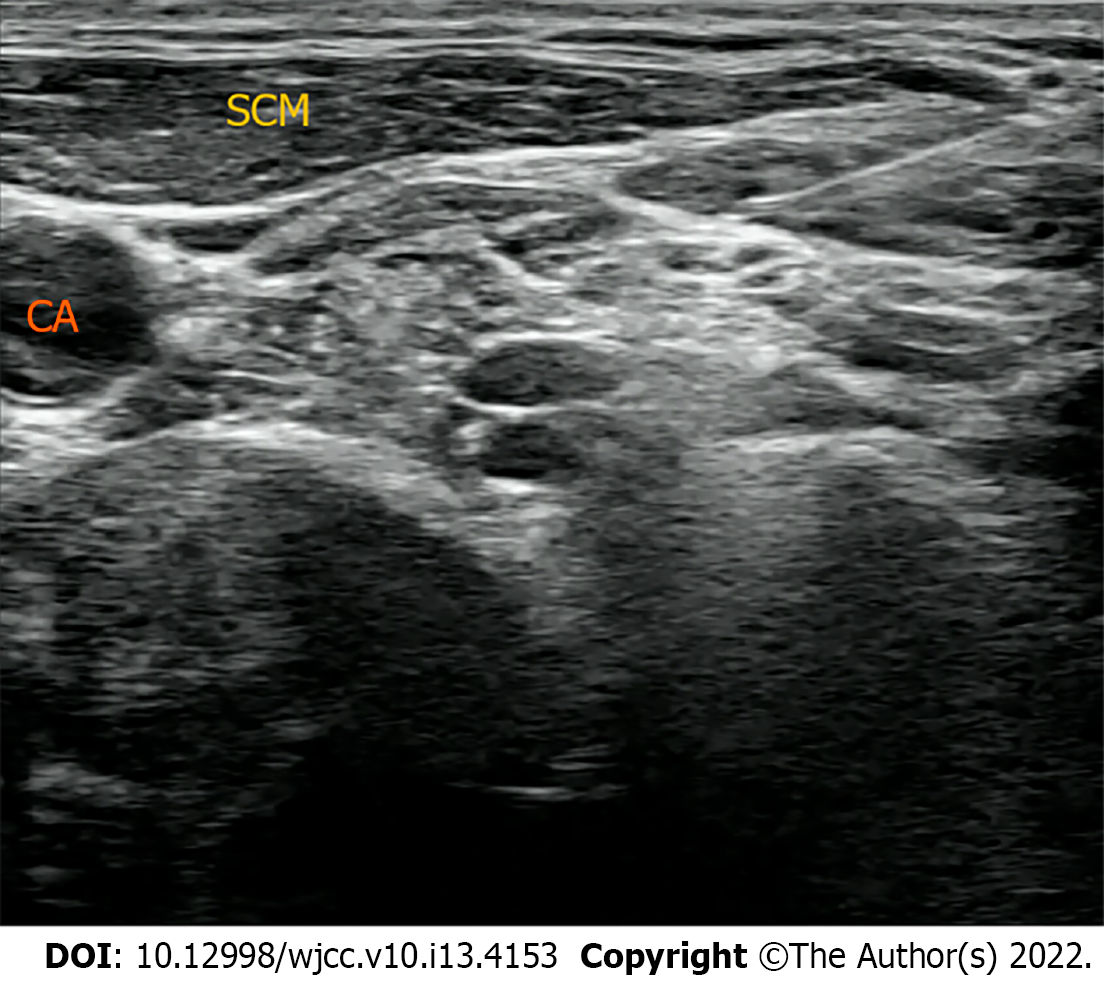
To identify the potential causes of hypercalcemia due to hyperparathyroidism, a neck ultrasound was conducted. It revealed a 0.8 cm × 1.5 cm sized oval, hypoechoic mass in the upper posterior of the left thyroid gland, which was compatible with parathyroid adenoma. Because the patient was pregnant, 99 mTc sestamibi scintigraphy for localization was contraindicated. Therefore, fine-needle aspiration (FNA) with parathyroid hormone (PTH) analysis was performed for confirmation of diagnosis. The PTH measured in the aspiration sample was 2802.0 pg/mL. Pathologic examination of FNA sample presented some giant cells and neutrophils and a few bland-looking follicular cells, which was insufficient for the confirmation of parathyroid adenoma. However, ultrasonographic findings and elevated PTH level of FNA sample highly supported the diagnosis of a parathyroid adenoma. Therefore, it was concluded clinically that her hypercalcemia was caused by parathyroid adenoma-induced primary hyperparathyroidism (PHPT).
The final diagnosis of the present case is the parathyroid adenoma on the upper posterior of the left thyroid gland.
As part of a multidisciplinary team specializing in maternal-fetal medicine, endocrinology surgeons and medical endocrinologists decided to surgically remove the parathyroid adenoma after the first-trimester when organogenesis of the embryo is completed because hyperparathyroidism and hypercalcemia are associated with poor perinatal outcomes such as miscarriage, stillbirth, preeclampsia, preterm birth, and postpartum hypercalcemic crisis[5,6]. The patient was educated on calcium restriction and hydration to control hypercalcemia until the scheduled operation because she did not want to receive any medical treatment due to fetal safety concerns. However, there were no changes in the serum calcium levels after several weeks of conservative treatment. Therefore, the multidisciplinary team planned the surgery at 11 wk of gestational age (the first-trimester). Anesthesiologists planned SCPB without general anesthesia, considering that the patient was in the first-trimester and due to concerns regarding the toxicity of the anesthetic to the fetus. Parathyroidectomy under SCPB was scheduled for March 12, 2020. The anesthesiologist visited the patient before surgery. In her preoperative laboratory test, there was no active lung lesion on chest radiography, and electrocardiography showed normal sinus rhythm. CBC, electrolyte, and glucose levels were within normal limits except calcium 14.02 mg/dL), PTH (139 pg/mL), and phosphorus (1.9 mg/dL). No other systematic abnormalities were observed. In the operating theater, the patient was monitored using three-lead electrocardiography, pulse oximetry and non-invasive blood pressure measurements. Her initial blood pressure was 164/118 mmHg, and heart rate was 85 beats per min (bpm). A peripheral vein was opened, and peripheral oxygen saturation was found to be 99% with room air. With the patient in a semi-sitting supine position, she turned her head contralateral to the procedural side. A 22-gauge needle was inserted once in a lateral-to-medial direction beneath the prevertebral fascia under ultrasound on both sides (Figure 1). After negative aspiration, 0.75% of ropivacaine 8 mL was injected for SCPB (16 mL in total). As soon as SCPB was performed, the patient complained about slight hoarseness, and her blood pressure and heart rate increased to 200/120 mmHg and 120–140 bpm, respectively. There was no difficulty in respiration, and saturation was 100% under nasal cannula oxygenation 2 L/min. The tubing of the invasive blood pressure monitor was placed via the left radial artery to monitor her blood pressure in real-time, and blood samples were collected for PTH level testing immediately.
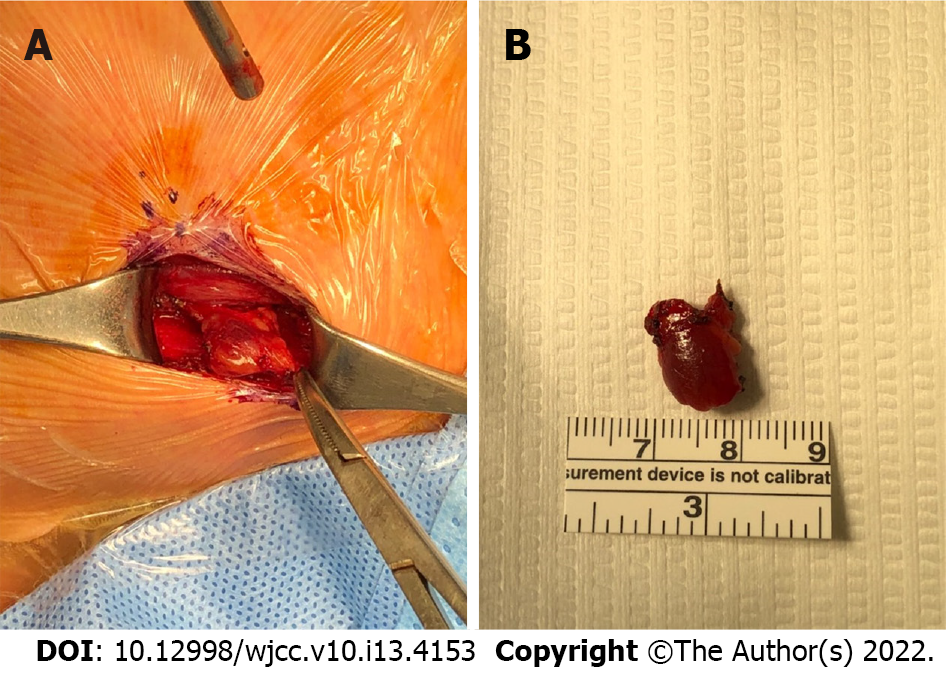
Anesthesiologists used nicardipine 1 mg twice, but her systolic blood pressure remained between 170 and 180 mmHg during surgery. After 15 min, satisfactory analgesic and anesthetic effects were achieved, and her vital signs were stabilized. Then, a parathyroidectomy was performed by the surgeon (Figure 2). The parathyroid adenoma confirmed by ultrasound and FNA was identified by ultrasound during the operation, and the adenoma was excised by making an incision directly above it. The PTH level, performed 10 min after parathyroidectomy, was 21.8 pg/mL and fell below 50% of the PTH level just before surgery (157 pg/mL) and was within the normal range. Because this finding supported that the remaining parathyroid glands secrete parathyroid hormone at a normal level, it was judged that all parathyroid adenomas in the patient's body were successfully resected. The patient was under anesthesia for 119 min, and the surgery was completed within 70 min. In total, 350 mL of Hartman solution was used, and blood loss was scanty. The patient did not complain about post-surgery pain, and her hoarseness improved in the post-anesthesia care unit. As soon as the patient arrived at the ward, the obstetrician checked the status of the fetus, and there were no abnormal signs. The laboratory test results at this time shows a calcium concentration of 12.7 mg/dL, phosphorus concentration of 1.6 mg/dL, and magnesium concentration of 1.5 mg/dL. The patient was released from the hospital on postoperative day 2.
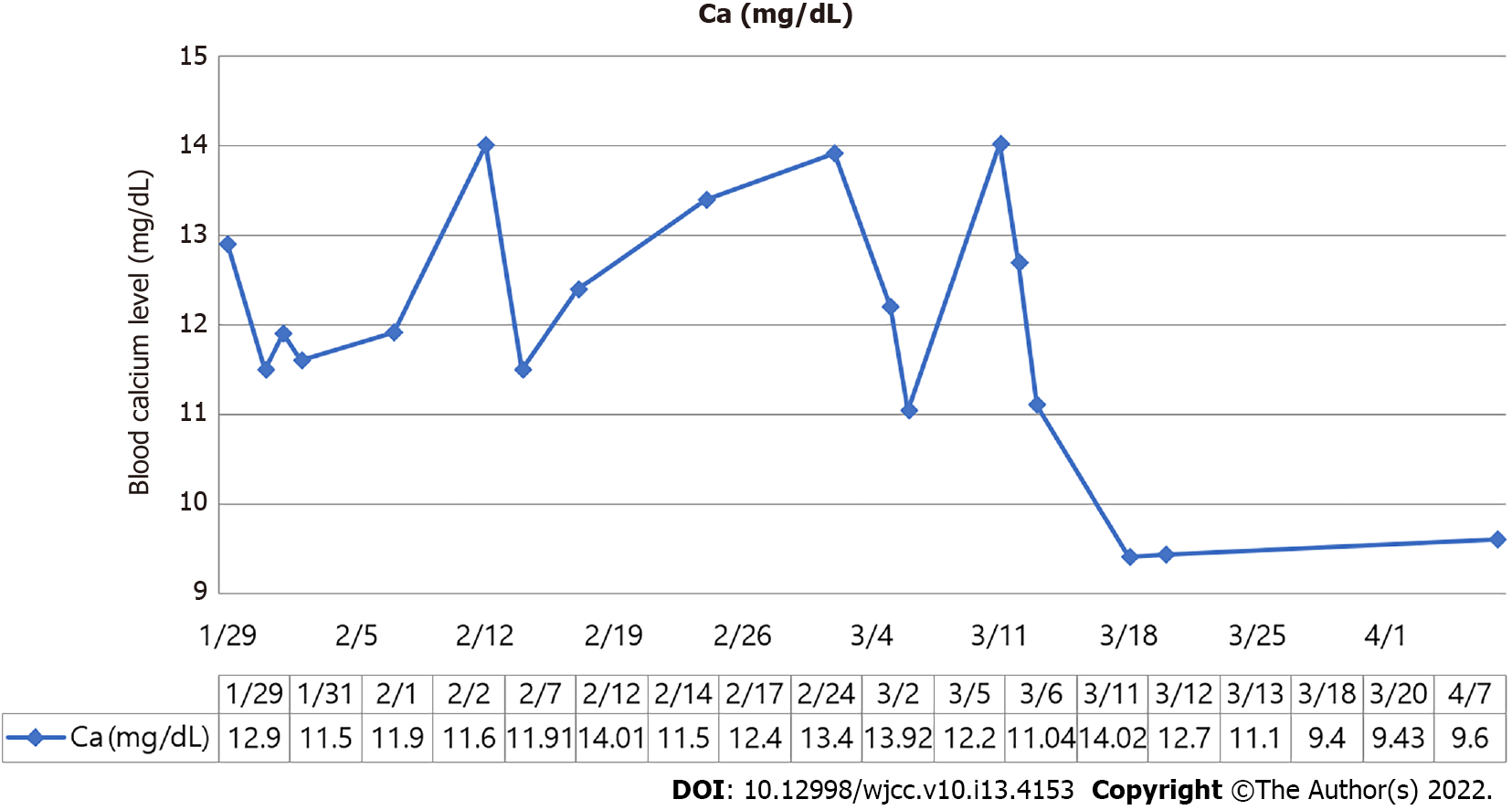
A follow-up examination on postoperative day 8 showed a calcium concentration of 9.4 mg/dL, PTH concentration of 43.6 pg/mL, potassium concentration of 3.7 mEq/L, sodium concentration of 134 mEq/dL, and chloride concentration of 102 mEq/dL, which returned to normal values. At this time, the patient was 12 wk and 2 d pregnant, and the fetus was healthy with a heart rate of 166 bpm. Figure 3 shows the fluctuations in the blood calcium concentration during the patient’s hospital stay. Since then, her blood pressure was stabilized to 120/80 mmHg during pregnancy, and a healthy male neonate of 2910 g was delivered vaginally at 38 wk of gestation with an Apgar score of 8/9 at 1/5 min. Her calcium level was 8.87 mg/dL on postpartum day 1, and her postpartum period was uneventful.
PHPT occurs in approximately 0.5% to 1.4% during pregnancy[7]. PHPT is the most common cause of pregnancy-related hypercalcemia. Hypercalcemic symptoms including nausea, fatigue, constipation, depression, renal impairment, and cardiac arrhythmia occurs in 20% of patients. Rates of relevant complications are as high as 67% and 80% among pregnant women and their infants, respectively[8]. Maternal complications include nephrolithiasis, pancreatitis, hyperemesis gravidarum, preeclampsia, and hypercalcemic crises. In addition, fetal complications include intrauterine growth restriction, preterm delivery, and a three to five times increased risk of miscarriage. There is also a direct relationship between serum calcium levels > 2.85 mmol/L (11.4 mg/dL)[6].
PHPT during pregnancy can be managed conservatively with oral or IV hydration with or without diuresis, a low calcium diet, and vitamin D supplementation[9]. Medical treatments, including calcitonin and cinacalcet, are not used in pregnancy due to limited safety data. In addition, the use of bisphosphonate therapy should be avoided due to the risk of adverse effects on fetal skeletal development[10]. There are several reasons why patients receive surgery and do not receive medical treatment. Due to radioactive exposure to the fetus, 99 mTC sestamibi scanning and computed tomography (CT) use is restricted. FNA biopsy was performed for this patient to diagnose hyper
Proper timing of surgery could reduce risks for pregnant patients and their fetuses, which is especially important for pregnant patients with PHPT. Generally, fetal organ development may be affected if surgery is performed during early pregnancy, and premature birth may result if surgery is performed late in pregnancy. Therefore, for pregnant patients with PHPT, the primary window for surgery is during mid-pregnancy (13-27 wk gestational age)[11]. Norman et al[12] reported a pregnancy loss rate of 48%, and the loss mainly occurred at 12.2 ± 4.5 wk, ranging from the late period of early pregnancy to the early period of mid-pregnancy. However, since her blood calcium concentration remained at 12.7 mg/dL despite 2 wk of conservative treatment, our patient underwent surgery at 11 wk of pregnancy, which was considered early pregnancy. And the surgery was successful with SCPB. Her postoperative blood calcium concentration returned to normal one week after surgery. Our case is remarkable because a delay in surgery to mid-pregnancy might have caused a miscarriage, and we used only SCPB instead of deep cervical plexus block and general anesthesia.
For PHPT-related surgery, options for anesthesia include general anesthesia, cervical plexus block, or a combination of the two. Lesion localization and diagnosis in patients with PHPT depend mostly on neck ultrasound, with a sensitivity of 69% and specificity of 94%, respectively, because CT and 99 mTc radioisotope application has radiation-associated risks. However, the performance is highly dependent on the experience of the operators. We found that the left upper gland had parathyroid adenoma by FNA, and the surgeon decided to perform minimal invasive parathyroidectomy rather than traditional exploration. Due to insufficient information regarding the effect of anesthetics on early pregnancy, anesthesiologists decided to administer SCPB instead of general anesthesia. Vincent Chan et al[13] suggested that a volume of 5-10 mL of local anesthesia is usually sufficient for ultra-guided cervical plexus block. Therefore, bilateral SCPB was performed with 8 mL of 0.75% ropivacaine for each side.
As soon as the block was placed, two events occurred. First, the patient reported vocal cord dysfunction with hoarseness. Although transitory, this side effect may have serious implications in patients with asymptomatic vocal cord paralysis. The patient underwent a vocal cord function test before surgery, although she had no history of vocal cord dysfunction or previous neck surgery, and the result was normal. SCPB can cause complications such as Horner's syndrome, partial brachial plexus block, and nerve blocks of phrenic and recurrent laryngeal types. These can occur as inadvertent complications of local anesthetic (LA) that spreads deeply following superficial injection[14]. Nash et al[15] reported that the deep cervical fascia's investing layer in the neck's anterior triangle was almost non-existent, which suggests that the fat and connective tissues that surround the structures of the neurovascular neck provide a means of direct communication between the prevertebral layer beneath the deep cervical fascia and the subcutaneous tissue. Another author suggested that it was unnecessary to use more than 5 mL of LA, and the use of larger volumes could promote a deeper spread of LA via the abovementioned anatomic pathways[16]. We used 8 mL of 0.75% ropivacaine on each side (total: 16 mL), which explains why, in our case, the patient experienced hoarseness. An awareness that complications similar to those associated with deep cervical plexus blocks may also be associated with SCPB is important.
Furthermore, the patient had an elevated heart rate. Her initial heart rate was 85/min, but her heart rate increased to 136/min with hypertension after block replacement. This may be due to the LAST of ropivacaine. LAST is a potentially life-threatening adverse event correlated with increasingly prevalent LA utilization, with an incidence currently estimated as 0.03%[17]. LAST is due to central nervous system (CNS) toxicity, including sensory and visual changes, muscular activation, seizure, and cardiovascular system (CVS) toxicity. The patient presented only CVS features, such as hypertension and tachycardia. Because pregnant women have reduced a1-acid glycoprotein concentrations in the plasma and increased cardiac output, accelerated perfusion of injection sites, rapid absorption of LA, and higher peaks of free LA concentrations can result[18]. Therefore, reduced doses of peripheral and central neuraxial LAs are recommended. Intravenous lipid emulsion therapy was administered for LAST, but we did not use it because the patient did not present with CNS symptoms, and she was 11 wks pregnant. Tachycardia was resolved in the post-anesthesia care unit, but hypertension remained because the patient had hypertension as an underlying disease.
PHPT during pregnancy is rare and complicated, and needs personalized management according to the gestational age, severity of hypercalcemia, and evaluation of the tradeoff between the risks and benefits. Pregnant patients with severe PHPT, such as our case, should have their calcium concentration lowered before surgery, firstly. And then the parathyroidectomy in mid-pregnancy is currently the primary treatment option for pregnant patients with PHPT. However, due to miscarriage risk, the range of blood calcium concentrations at which surgery is an option remains under discussion. Therefore our case suggests that SCPB can be an anesthetic option for parathyroidectomy during the first trimester of pregnancy.
The authors would like to thank the patient for her generous release of her clinical information for this paper.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Anesthesiology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: D'Orazi V, Italy; He XH, China; Lambrecht NW, United States; Mittal M, India S-Editor: Li X L-Editor: A P-Editor: Li X
| 1. | Reitman E, Flood P. Anaesthetic considerations for non-obstetric surgery during pregnancy. Br J Anaesth. 2011;107 Suppl 1:i72-i78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 164] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 2. | Flood P. Fetal anesthesia and brain development. Anesthesiology. 2011;114:479-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Vloka JD, Hadzic A, Drobnik L. Nerve blocks in the pregnant patient. In: Birnbach D, Gatt PS, Data S, editors. Textbook of obstetric anesthesia. Philadelphia, PA: Churchill Livingstone, 2000: 693–706. |
| 4. | Kim JS, Ko JS, Bang S, Kim H, Lee SY. Cervical plexus block. Korean J Anesthesiol. 2018;71:274-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 5. | Nash E, Ranka P, Tarigopula G, Rashid T. Primary hyperparathyroidism in pregnancy leading to hypercalcaemic crisis and uraemic encephalopathy. BMJ Case Rep. 2015;2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | McCarthy A, Howarth S, Khoo S, Hale J, Oddy S, Halsall D, Fish B, Mariathasan S, Andrews K, Oyibo SO, Samyraju M, Gajewska-Knapik K, Park SM, Wood D, Moran C, Casey RT. Management of primary hyperparathyroidism in pregnancy: a case series. Endocrinol Diabetes Metab Case Rep. 2019;2019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Adami S, Marcocci C, Gatti D. Epidemiology of primary hyperparathyroidism in Europe. J Bone Miner Res. 2002;17 Suppl 2:N18-N23. [PubMed] |
| 8. | Schnatz PF, Curry SL. Primary hyperparathyroidism in pregnancy: evidence-based management. Obstet Gynecol Surv. 2002;57:365-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 128] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Diaz-Soto G, Linglart A, Sénat MV, Kamenicky P, Chanson P. Primary hyperparathyroidism in pregnancy. Endocrine. 2013;44:591-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Djokanovic N, Klieger-Grossmann C, Koren G. Does treatment with bisphosphonates endanger the human pregnancy? J Obstet Gynaecol Can. 2008;30:1146-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Dochez V, Ducarme G. Primary hyperparathyroidism during pregnancy. Arch Gynecol Obstet. 2015;291:259-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Norman J, Politz D, Politz L. Hyperparathyroidism during pregnancy and the effect of rising calcium on pregnancy loss: a call for earlier intervention. Clin Endocrinol (Oxf). 2009;71:104-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 13. | Bendtsen TF, Abbas S, Chan V. Ultrasound-Guided Cervical Plexus Block. In: Hadzic A. eds. Hadzic's Textbook of Regional Anesthesia and Acute Pain Management, 2e. McGraw Hill; 2017. |
| 14. | Alzahrani T, Alnajjar M, Algarni AD, Al-Ahaideb A. Delayed Horner's syndrome following ultrasound- guided interscalene brachial plexus block. Saudi J Anaesth. 2014;8:121-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Nash L, Nicholson HD, Zhang M. Does the investing layer of the deep cervical fascia exist? Anesthesiology. 2005;103:962-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Flores S, Riguzzi C, Herring AA, Nagdev A. Horner's Syndrome after Superficial Cervical Plexus Block. West J Emerg Med. 2015;16:428-431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | El-Boghdadly K, Pawa A, Chin KJ. Local anesthetic systemic toxicity: current perspectives. Local Reg Anesth. 2018;11:35-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 270] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 18. | Rosenberg PH, Veering BT, Urmey WF. Maximum recommended doses of local anesthetics: a multifactorial concept. Reg Anesth Pain Med. 2004;29:564-75; discussion 524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 134] [Article Influence: 6.4] [Reference Citation Analysis (0)] |