Published online Jan 7, 2022. doi: 10.12998/wjcc.v10.i1.371
Peer-review started: August 12, 2021
First decision: November 11, 2021
Revised: November 19, 2021
Accepted: November 30, 2021
Article in press: November 30, 2021
Published online: January 7, 2022
Processing time: 139 Days and 17.4 Hours
Air embolism is a very rare, yet serious and potentially fatal complication of digestive endoscopic treatment. Air embolism is the result of air directly entering the arteries or veins. However, to recognize neurological dysfunction under sedation can be difficult. Therefore, it is extremely important to identify high-risk groups and take preventive measures.
Herein, we report a 74-year-old female patient with esophageal varices who suffered from consciousness disturbance after the third endoscopic ligation of esophageal varices under sedation. Combined with the patient’s imaging examination results and medical history, we highly suspected that the patient had developed paradoxical cerebral air embolism during endoscopic ligation. We learned that the patient died at a later follow-up. In order to be able to identify and prevent the occurrence of air embolism early, we summarize and analyze the risk factors, pathogenesis, clinical manifestations, prevention and treatment options of gastrointestinal endoscopy complicated by cerebral air embolism.
Electroencephalographic monitoring helps to recognize the occurrence of air embolism in time and increase the patient's chance of survival.
Core Tip: We present a rare case of suspected paradoxical cerebral air embolism during endoscopic ligation of esophageal varices. Anesthetic management and identification are challenging in patients with air embolism during digestive endoscopic treatment. We summarize and analyze the risk factors, pathogenesis, clinical manifestations, prevention and treatment options of gastrointestinal endoscopy complicated by cerebral air embolism, and propose the application of electroencephalogram monitoring during sedation or anesthesia to detect and identify abnormal brain function in time.
- Citation: Zhang CMJ, Wang X. Suspected cerebrovascular air embolism during endoscopic esophageal varices ligation under sedation with fatal outcome: A case report. World J Clin Cases 2022; 10(1): 371-380
- URL: https://www.wjgnet.com/2307-8960/full/v10/i1/371.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i1.371
Air embolism is a very rare yet serious complication of digestive endoscopic treatment that is potentially fatal with death occurring in 15.4% of cases. It includes venous air embolism, paradoxical air embolism, and systemic air embolism, which can manifest as an unstable heart and pulmonary circulation, and important organ ischemia and nerve damage[1]. If air embolism occurs during sedation or anesthesia, its clinical symptoms overlap with the effects of sedation, and the two are difficult to distinguish. Therefore, it is important to improve clinicians’ ability to evaluate and recognize air embolisms. In this case, a patient with esophageal varices developed a paradoxical cerebral air embolism during endoscopic ligation. This article summarizes and analyzes the risk factors, pathogenesis, clinical manifestations, and prevention and treatment options of gastrointestinal endoscopy complicated by cerebral air embolism, as well as the use of electroencephalogram (EEG) monitoring during sedation or anesthesia to promptly detect and identify abnormal brain function. Written authorization for the case report was obtained from the patient’s family.
A 74-year-old female patient (height, 160 cm; weight, 77.5 kg) was admitted to the hospital for ligation of esophageal varices that developed because of a 2-year history of decompensated primary biliary cirrhosis (Child-Pugh grade B) and had started to bleed.
The patient’s previous medical history included grade 2 hypertension with a maximum blood pressure of 170/90 mmHg. She took one 2.5-mg levamlodipine besylate tablet per day; nevertheless, blood pressure control was poor. The patient underwent painless esophageal venous ligation/injection treatment with gastroscopy 2 years and again 4 mo before the reported admission.
The patient had no history of past illness.
The patient had a disease-free personal and family history.
When the patient entered the operating room, the vital signs were stable, a baseline blood pressure of 158/75 mmHg, a baseline pulse rate of 75 beats per min, and a baseline respiratory rate of 20 breaths per min, the baseline oxygen saturation was maintained at about 98% (Figure 1).
Preoperative laboratory test results were as follows: C-reactive protein, 6.56 mg/L; procalcitonin, 0.12 ng/mL; interleukin 6 (IL-6), 29.7 pg/mL; and D-dimer, 1.06 mg/L; other laboratory test results were within the normal range.
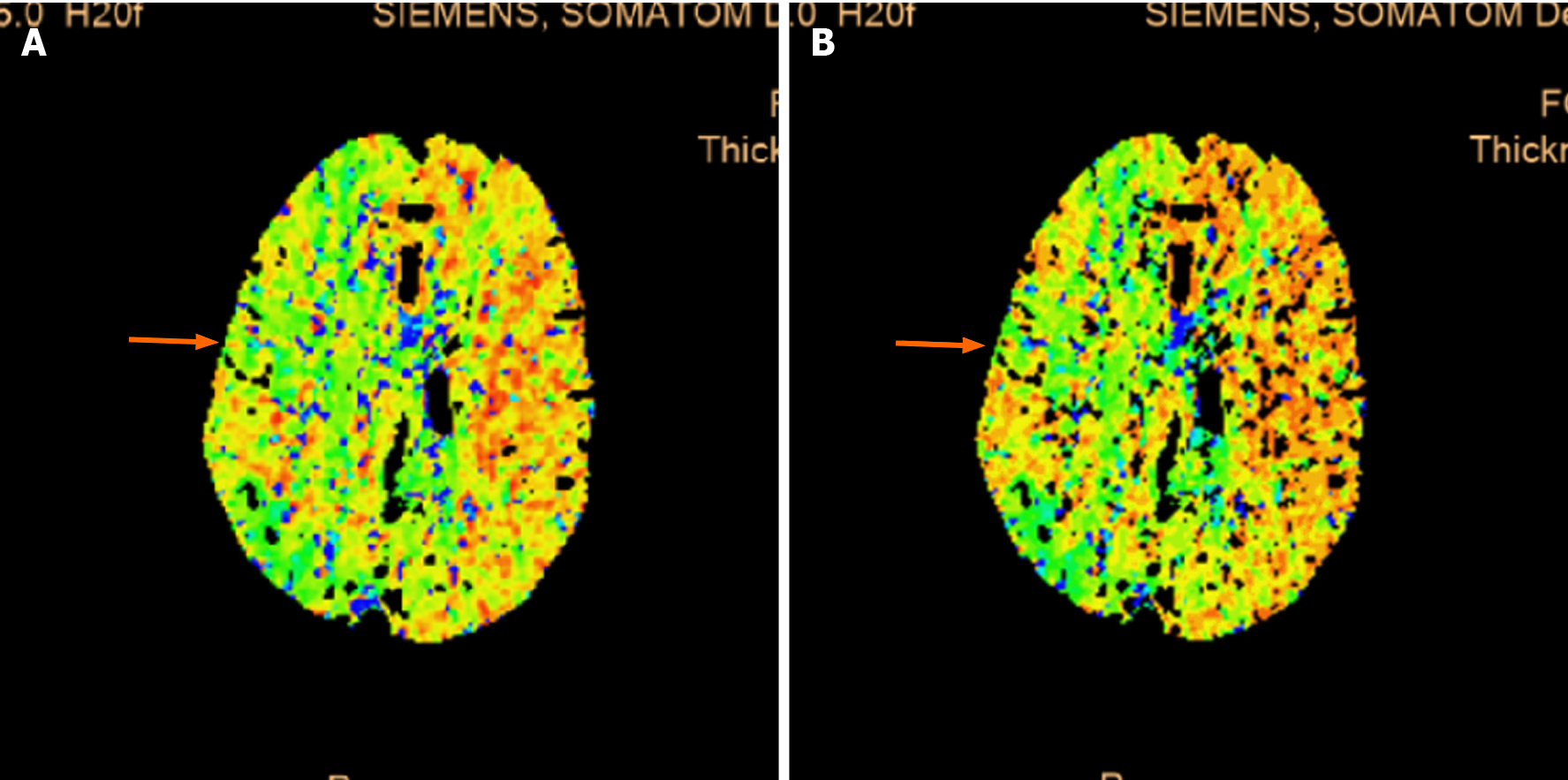
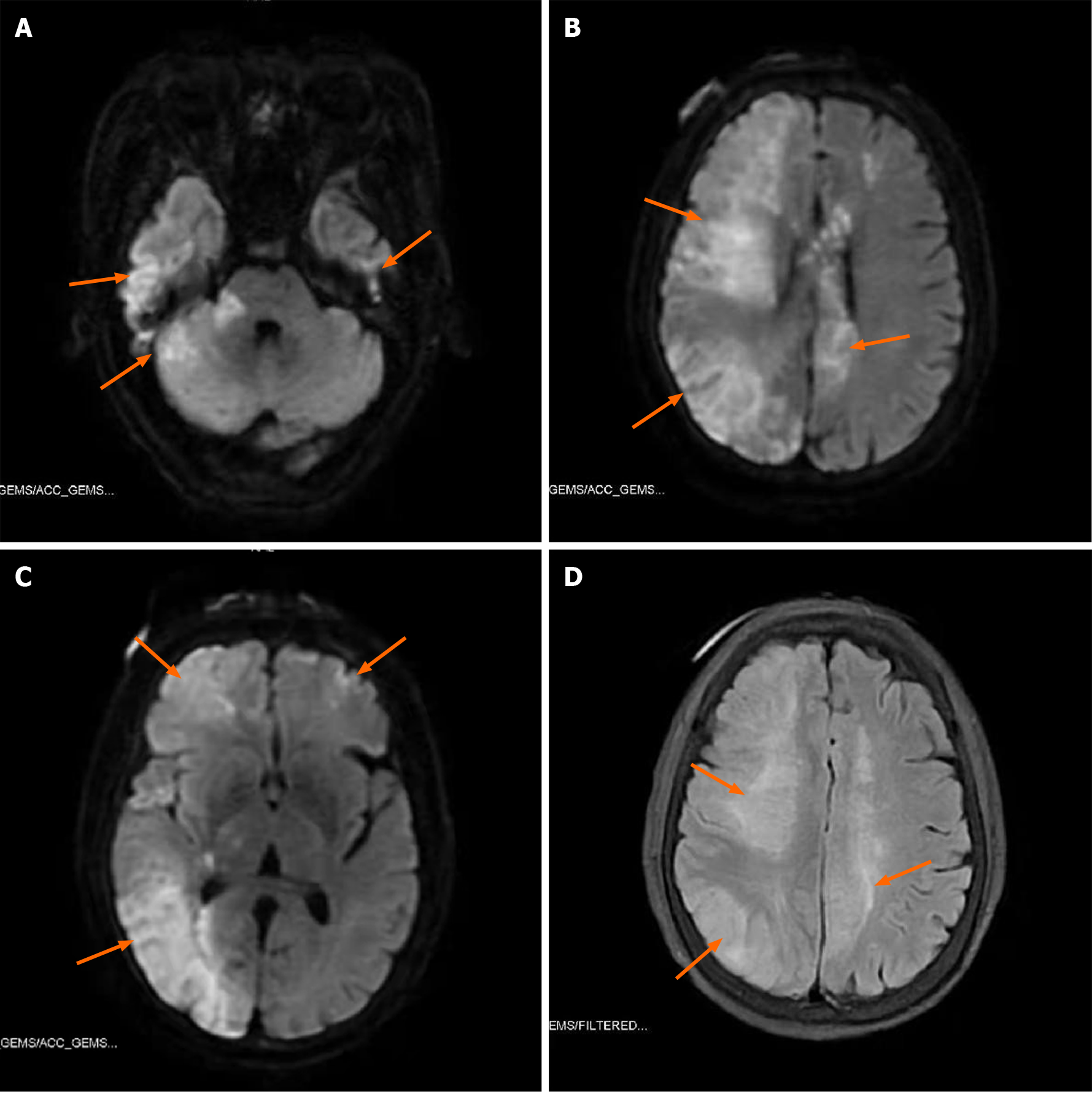
Ultrasonic Cardiogram revealed enlargement of the whole heart (left ventricle, 58 cm2; left atrium, 37 cm2; right ventricle, 20 cm × 41 cm; right atrium, 47 cm2), slightly widened ascending aorta and pulmonary artery, slightly thickened basal segment of the ventricular septum, and mild tricuspid regurgitation. The measured left ventricular systolic function was within the normal range; however, diastolic function was reduced (left ventricular ejection fraction, 64%; pressure gradient of tricuspid regurgitation, 32 mmHg; stroke volume, 108 mL). Computed tomography (CT) plain scans of the chest and abdomen revealed an enlarged heart, calcification of the aorta and left coronary artery wall, liver cirrhosis, splenomegaly, portal hypertension with open collateral circulation, a small amount of ascites, mild peritonitis, and calcification of the wall of the abdominal aorta and its branches. The electrocardiograph (ECG) was normal. CT perfusion (Figure 2) showed that the right hemisphere mean transit time and time to peak were prolonged, and the cerebral blood flow was slightly decreased in some areas. Head magnetic resonance imaging (Figure 3) revealed long T1 and T2 signals and hyperintense FLAIR phase signals in bilateral frontal and parietal lobes, right occipital lobe, and right cerebellar hemisphere. Diffusion-weighted imaging revealed limited diffusion in the same areas.
The final diagnosis of the present case was cerebral infarction, decompensated primary biliary cirrhosis (Child-Pugh grade B), grade 2 hypertension.
Before the surgery, the patient was lying on her left side, peripheral venous access was obtained, and oxygen was supplied through a nasal catheter (oxygen flow, 5 L/min). Monitoring included non-invasive arterial blood pressure, finger pulse oxygen saturation, and three-lead ECG, which were all within normal limits. End-tidal carbon dioxide (EtCO2) monitoring was not used because the patient was not breathing through a mask, endotracheal tube, or laryngeal mask airway, and an EtCO2 sampling nasal cannula was not available. Sedation was induced with intravenous injection of midazolam 1 mg, sufentanil 4 µg, and propofol 40 mg (cannot wake up state, MOAA/S ≤ 1), and maintained with propofol 3 mg/kg/h administered using a continuous intravenous pump[2]. During gastroscopic esophageal varices ligation, the gastrointestinal tract was filled with air. The patient's vital signs were stable throughout the procedure. Bleeding occurred during the ligation; however, it was quickly stopped by the ligation. The operation time was approximately 15 min (Figure 1). After the operation, the patient was returned to the supine position and sent to the anesthesia recovery room to regain consciousness.
The hemodynamic indicators of the patient were normal after the operation, but the patient could not be awakened 15 min after the operation, and there was no response to the stimulation. Initially, we considered that the sedative or anesthetic drugs had not been completely metabolized by the liver of the patient, and we then continued to observe and wait for the patient's consciousness to recover. After 30 min, the patient's consciousness had not recovered. At the same time, she exhibited binocular gaze to the upper left, poor recovery of language expression and orientation, and profuse sweating all over the body. On physical examination, bilateral pupils were circular and approximately 2 mm in diameter, which were sensitive to light reflection. The patient could complete the command actions such as opening eyes, nodding, and sticking tongue out. The patient's vital signs remained stable at this time. We immediately performed an emergency arterial blood gas analysis (oxygen flow 5 L/min) (Figure 1). At this time, we considered that the patient may have hepatic encephalopathy caused by decompensated cirrhosis, and immediately drew blood to recheck liver and kidney function and blood ammonia levels (subsequent results were within normal limits) and continued to observe the patient.
After 30 min, the patient's consciousness still showed no signs of improvement. She was still unreactive and exhibited poor responses to stimuli. She could open her eyes voluntarily without complying with instructions. Physical examination found that the left limb can move autonomously and the Babinski sign was negative. The muscle strength of the right limb was higher than that of the left side, and the pain stimulus response was weaker than that of the left side. The Babinski sign of the right limb was also negative. We performed another arterial blood gas analysis (oxygen flow 5 L/min) (Figure 1), which did not have significantly different results from those of the previous test.
At this point, we considered that the patient may have an intracranial disease, and immediately sent the patient to the emergency department for a head CT scan. The CT revealed no obvious bleeding or infarction. The patient's state of consciousness then improved. After consulting the doctor in charge and the patient’s family members, the patient was returned to the Gastroenterology High Dependency Unit for continued monitoring and observation. Two hours later, the patient developed shortness of breath. The patient was intubated within a few minutes and sent to the intensive care unit for further treatment.
A few days later, the patient’s condition did not improve, and her family chose for her to be discharged voluntarily due to financial reasons. Follow up later revealed that the patient died at home a few days after being discharged from the hospital.
Although cerebral air embolism is rare after endoscopic esophageal varix ligation, it can occur following any digestive endoscopic surgery, including esophagogastroduodenoscopy, colonoscopy, endoscopy ultrasound, endoscopic retrograde cholangiopancreatography, and sigmoidoscopy. Air embolism is the result of air directly entering the arteries or veins, and its pathophysiological causes are twofold. On the one hand, there must be an increased pressure gradient (expanding the lumen of the digestive tract during any endoscopic operation) to promote air into the circulation channel. On the other hand, destruction of the mucosal-vascular barrier (direct vascular system defect or organ wall defect) must also be present[3-6]. When treating esophageal varices with ligation under endoscopy, the endoscopist controls the intermittent pressurized air flow to expand the intestines. If the mucosal-vascular barrier is damaged, it can accelerate the arrival of cerebrovascular air embolisms.
Pathways of endoscopic gas entering the human body include the following: (1) Venous system: via the portal vein or portal vein collaterals, via the duodenal vein, via the hepatic vein or inferior vena cava, via the superior vena cava (directly into the brain), via a fistula or shunt between the biliary tract and venous system, via the transvertebral venous plexus, and via transesophageal varicose veins; and (2) Arterial system: following percutaneous arterial intervention, venous air embolism can also pass right to left through shunts in the heart, right to left shunts in the lungs, enter the left atrium through the pulmonary vein, and eventually develop into a systemic air embolism[7,8].
The reported risk factors for gas embolism include: (1) Previous intervention or surgery on the biliary system; (2) Transjugular intrahepatic portosystemic shunt (TIPS); (3) Blunt or penetrating liver injury; (4) Inflammation of the digestive system (cholangitis, pyloritis, liver abscess, inflammatory bowel disease, necrotizing enteritis, mesenteric ischemia); (5) Postoperative gastrointestinal fistula; (6) Gastrointestinal tumors; (7) Biliary atresia; and (8) Special interventional techniques (choledochoscopy, biliary sphincterotomy, metal stent placement, liver biopsy, high-pressure gas insufflation operations, operations where the surgical site is higher than the level of the heart, and the use of N2O).
The patient’s previous echocardiography did not find a cardiac shunt or intra-atrial thrombosis; the patient also had no atrial fibrillation, diabetes, hyperlipidemia, or other diseases, and subsequent CTA results suggested the absence of carotid atherosclerotic plaque and intracranial artery stenosis. The patient did not have intraoperative hypotension; therefore, the patient did not have any high-risk factors for cerebral infarction. But the patient had a history of multiple endoscopic esophageal varix ligations or gluing treatments. There was a high risk of air embolisms in the past; traffic arteries were formed due to past disease. Air may have flowed back into the left ventricle along the injured intestinal artery, and then into the systemic circulation to form an air embolism.
The diagnosis of air embolism is usually difficult and complicated because air entering the systemic circulation may be rapidly distributed to various organs; therefore, it is typically necessary to exclude other life-threatening processes. The patient's blood ammonia level was normal, which ruled out the possibility of hepatic encephalopathy, and there was no cerebral ischemia or hypoxia caused by hypotension, hypoperfusion and hypoxia, and brain dysfunction caused by hypoglycemia during the operation. Because early recognition of air embolism increases the patients’ chances of survival, anesthesiologists should be highly trained to recognize this complication as early as possible and distinguish it from any sedation related to central nervous system ischemia, bleeding, or cardiopulmonary problems. Nervous system symptoms are common in patients with systemic air embolism and are often difficult to distinguish from ischemic or hemorrhagic cerebrovascular disease. At this time, priority should be given to echocardiography to confirm whether air embolism has occurred, rather than head CT to assess cerebrovascular events. If the underlying problem is air embolism, CT scans may delay diagnosis and correct symptomatic treatment, leading to undesirable consequences. The causes of air embolism are relatively hidden. Transesophageal ultrasound, intraoperative precardiac Doppler ultrasonography (PDU), transcranial Doppler ultrasonography, and related EEG monitoring can observe a higher incidence of air embolism. Intraoperative PDU would have been appropriate in this particular case considering the patient had an enlarged heart with tricuspid regurgitation. But some air embolisms are found only in postmortem autopsy[9]. It is very important for anesthesiologists to identify whether patients with a poor response after sedation or general anesthesia have air embolism and to prevent air embolism during surgery. This can not only provide accurate information for families to help them choose the best treatment plan, but also improve the survival rate of patients. However, it is difficult to recognize neurological dysfunction under general sedation or anesthesia, and the signs of nervous system embolism may be masked and not detected in time. It usually manifests as a lack of regaining consciousness after anesthesia or disturbance of consciousness[6]. In this difficult situation, electrophysiological records, namely EEG and evoked potentials, can help promptly identify the occurrence of air embolism.
At first glance, EEG—as a functional method for studying changes in brain electrical activity—is a unique and ideal method for evaluating brain function in severely disabled patients; at the same time, it is the only electrophysiology tool available in most hospitals. It can be easily recorded at the patient's bedside, allowing a valuable estimate of changing clinical status, and some centers even propose continuous EEG monitoring[10]. EEG is also a key method of determining some reversible causes of coma, such as cerebral air embolism[11].
Reasons for intraoperative EEG monitoring include: (1) Preventing intraoperative awareness; (2) Maintaining as light anesthesia as possible; (3) Avoiding deep anesthesia; and (4) Detecting cerebral ischemia and epilepsy.
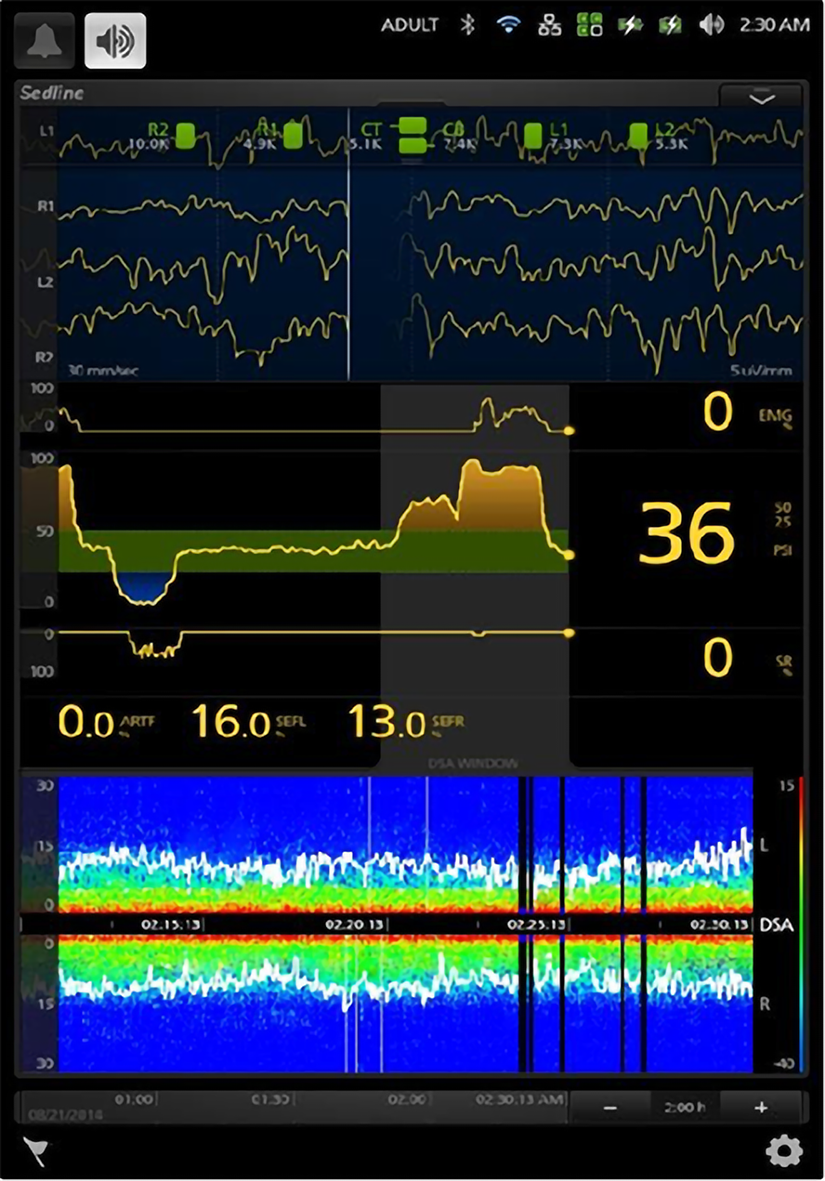
We can therefore use the brain function monitoring instrument, SedLine (Figure 4), to evaluate brain function during the operation. SedLine is an EEG monitoring platform specially designed for the operating room. It focuses on the assessment of the depth of sedation. It displays the following monitoring information through the Root platform: (1) Four-channel real-time EEG monitoring for both sides of the brain; (2) Patient state index; (3) Density spectrum array; (4) Spectral edge frequency; (5) Additional information: EMG, SR, ARTF; and (6) Electrode status.
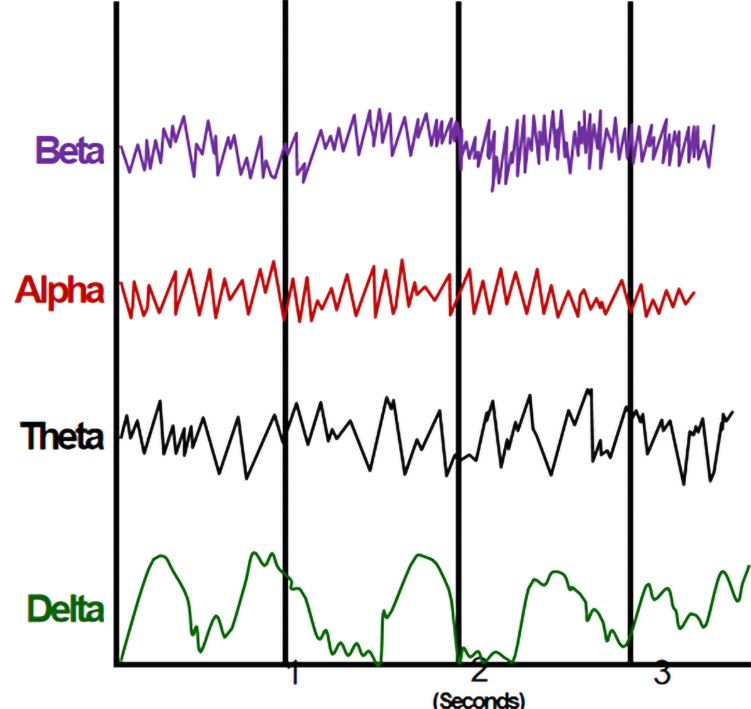
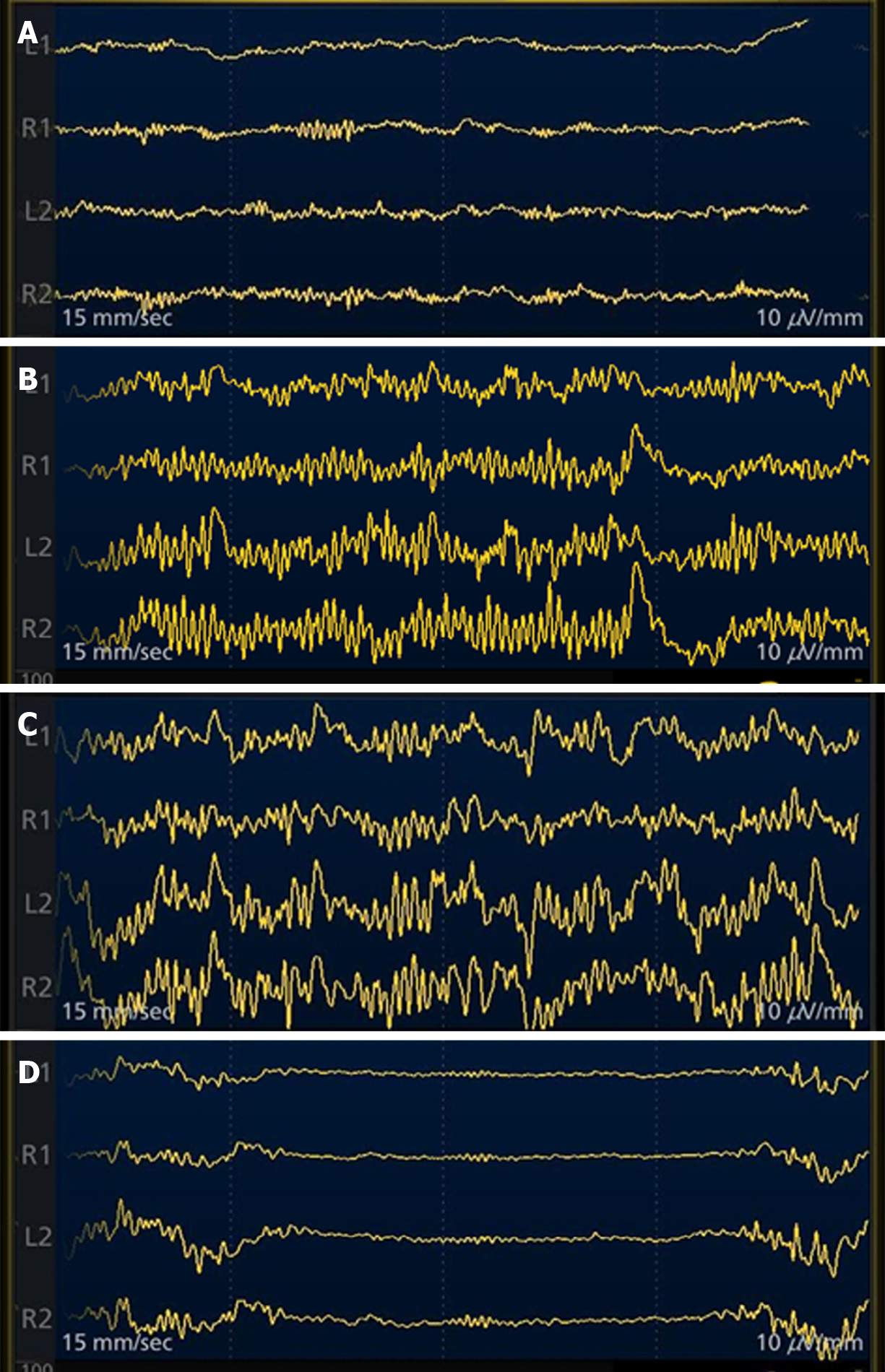
We can judge the patient's state of consciousness based on the frequency and amplitude of the EEG waveform (Figure 5). If the fast wave with fast frequency and small amplitude is dominant, the patient is awake; if the slow wave with slow frequency and large amplitude is dominant, the patient is unconscious. We can observe the intraoperative EEG waveform to determine whether the patient is sedated or anesthetized, or has neurological disease (Figure 6). Once the burst suppressed state appears on the intraoperative EEG, the endoscopic operation can be stopped immediately to avoid or reduce adverse consequences, such as air embolism.
In addition to the simple and easy-to-operate non-invasive brain function monitors such as SedLine, there is standard neurosurgery intraoperative electrophysiological monitoring (involving SEP and MEP), but this method requires the electrode tip to be placed in the skin muscle, which is traumatic, cumbersome to operate, and requires a neurologist. Although cortical and subcortical ischemia can be monitored more accurately and identified earlier than when using imaging, it is still not suitable for endoscopic and endoscopic surgery with a short operation time.
In fact, the evolution of EEG signals in the first few days after brain injury is always the most critical stage[12,13]. Therefore, we can continue to monitor the patient's EEG changes after the occurrence of cerebral air embolism.
The most critical step in patient management is to maintain a high degree of suspicion of air embolism. Once air embolism is found during the operation, emergency measures should be taken. First, the operation and gastrointestinal inflation should be stopped immediately. Second, high-flow pure oxygen at a concentration of 100% should be inhaled to reduce bubble expansion. Third, a large amount of normal saline should be infused for expansion. Fourth, the patients should be positioned so their head is low and their feet are high (Trumper format); lying on their left side, to minimize the migration of air to the brain, keep the air in the right atrium, and prevent the gas in the right heart from entering the systemic circulation. Fifth, if necessary, advanced cardiac life support should be initiated. Sixth, if conditions permit, emergency bedside echocardiography or transesophageal echocardiography should be performed. If echocardiography confirms that there is air in the right heart (diagnostic gold standard), a central venous catheter should be inserted and the air aspirated. Seventh, it is recommended that all patients with air embolism be given hyperbaric oxygen therapy as soon as possible to minimize heart, lung, and brain damage and preserve nerve function as much as possible[3].
Air embolism is iatrogenic and preventable. A lethal volume of entrained air is approximately 200 mL to 300 mL or 3-5 mL/kg, with smaller volumes required for embolisms in veins closer to the heart[14]. Once air embolism occurs, the condition is often dangerous, and the prognosis is poor. Therefore, it is extremely important to identify high-risk groups and take preventive measures. First, for the patients themselves, optimizing their own volume state and maintaining a normal volume can prevent air embolism in the endoscope[15]. Second, when conditions permit, it is recommended to routinely use CO2 instead of air in endoscopic surgery (the rate of dissolution and absorption of CO2 is higher than that of air), which can greatly reduce the severity of gas embolism in patients[14]. Nowadays, carbon dioxide has replaced air as the “gold standard” for expansion in gastrointestinal endoscopic surgery. CO2 significantly increases the safety margin for accidental gas migration into the systemic circulation; nevertheless, there are still reports of CO2 embolism[16-18]. The amount of inhaled air and the speed at which air is injected into the body play a decisive role in the prognosis of patients[19]. Shorter acting narcotics, such as fentanyl or remifentanil infusion, are better suited for procedures in high-risk patients who already experienced cerebral infarction pre-operatively than sufentanil. Nalbuphine can be considered a reasonable alternative to sufentanil in high-risk patients undergoing colonoscopy. Doses in the range of 0.1 mg/kg - 0.2 mg/kg are recommended. The decreased risks of respiratory depression and apnea make nalbuphine suitable for patients with respiratory problems[20].
The early recognition of air embolism increases patients’ chances of survival; for patients at high risk, it is recommended to perform echocardiography before surgery to exclude all patients with right-to-left shunts in whom endoscopy is planned[21]. Precordial Doppler probe and EEG monitors can be used during surgery; precordial Doppler monitoring can quickly detect air in the heart and pulmonary blood vessels before clinical symptoms appear, and EEG monitoring equipment can promptly detect cerebral ischemia and epilepsy in time[22].
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Anesthesiology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: DeSousa K, Mastrantonakis K S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Bessereau J, Genotelle N, Chabbaut C, Huon A, Tabah A, Aboab J, Chevret S, Annane D. Long-term outcome of iatrogenic gas embolism. Intensive Care Med. 2010;36:1180-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 2. | Xu ZY, Wang X, Si YY, Wu JC, Zuo YX, Xue FS, Liu J. Intravenous remifentanil and propofol for gastroscopy. J Clin Anesth. 2008;20:352-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Donepudi S, Chavalitdhamrong D, Pu L, Draganov PV. Air embolism complicating gastrointestinal endoscopy: A systematic review. World J Gastrointest Endosc. 2013;5:359-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 4. | Maqsood MH, Mirza N, Hanif MA, Hanif H, Saleem M, Maqsood MA, Fatima I, Tahir MM. Clinical Presentation, Diagnosis, and Management of Air Embolism During Endoscopic Retrograde Cholangiopancreatography. Gastroenterology Res. 2019;12:283-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Lanke G, Adler DG. Gas embolism during endoscopic retrograde cholangiopancreatography: diagnosis and management. Ann Gastroenterol. 2019;32:156-167. [PubMed] |
| 6. | Finsterer J, Stöllberger C, Bastovansky A. Cardiac and cerebral air embolism from endoscopic retrograde cholangio-pancreatography. Eur J Gastroenterol Hepatol. 2010;22:1157-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Lowdon JD, Tidmore TL Jr. Fatal air embolism after gastrointestinal endoscopy. Anesthesiology. 1988;69:622-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 27] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Desmond PV, MacMahon RA. Fatal air embolism following endoscopy of a hepatic portoenterostomy. Endoscopy. 1990;22:236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Afreen LK, Bryant AS, Nakayama T, Ness TJ, Jones KA, Morgan CJ, Wilcox CM, Phillips MC. Incidence of Venous Air Embolism During Endoscopic Retrograde Cholangiopancreatography. Anesth Analg. 2018;127:420-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Azabou E, Fischer C, Guerit JM, Annane D, Mauguiere F, Lofaso F, Sharshar T. Neurophysiological assessment of brain dysfunction in critically ill patients: an update. Neurol Sci. 2017;38:715-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Drenthen J, van Hulst RA, Blok JH, van Heel MD, Haitsma JJ, Lachmann B, Visser GH. Quantitative EEG monitoring during cerebral air embolism and hyperbaric oxygen treatment in a pig model. J Clin Neurophysiol. 2003;20:264-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Berkhoff M, Donati F, Bassetti C. Postanoxic alpha (theta) coma: a reappraisal of its prognostic significance. Clin Neurophysiol. 2000;111:297-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 61] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Fossi S, Amantini A, Grippo A, Cossu C, Boni N, Pinto F. Anoxic-ischemic alpha coma: prognostic significance of the incomplete variant. Neurol Sci. 2004;24:397-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Prielipp RC, Brull SJ. Vascular Air Embolism and Endoscopy: Every Bubble Matters. Anesth Analg. 2018;127:333-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Ghannam M, Beran A, Ghazaleh D, Ferderer T, Berry B, Banna MA, Mohl L, Streib C, Thacker T, Matos I. Cerebral Air Embolism after Esophagogastroduodenoscopy: Insight on Pathophysiology, Epidemiology, Prevention and Treatment. J Stroke Cerebrovasc Dis. 2019;28:104403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Wang WL, Wu ZH, Sun Q, Wei JF, Chen XF, Zhou DK, Zhou L, Xie HY, Zheng SS. Meta-analysis: the use of carbon dioxide insufflation vs. room air insufflation for gastrointestinal endoscopy. Aliment Pharmacol Ther. 2012;35:1145-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Dellon ES, Hawk JS, Grimm IS, Shaheen NJ. The use of carbon dioxide for insufflation during GI endoscopy: a systematic review. Gastrointest Endosc. 2009;69:843-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 18. | Seifert H, Biermer M, Schmitt W, Jürgensen C, Will U, Gerlach R, Kreitmair C, Meining A, Wehrmann T, Rösch T. Transluminal endoscopic necrosectomy after acute pancreatitis: a multicentre study with long-term follow-up (the GEPARD Study). Gut. 2009;58:1260-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 305] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 19. | Katzgraber F, Glenewinkel F, Fischler S, Rittner C. Mechanism of fatal air embolism after gastrointestinal endoscopy. Int J Legal Med. 1998;111:154-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Deng C, Wang X, Zhu Q, Kang Y, Yang J, Wang H. Comparison of nalbuphine and sufentanil for colonoscopy: A randomized controlled trial. PLoS One. 2017;12:e0188901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Wills-Sanin B, Cárdenas YR, Polanco L, Rivero O, Suarez S, Buitrago AF. Air embolism after endoscopic retrograde cholangiopancreatography in a patient with budd Chiari syndrome. Case Rep Crit Care. 2014;2014:205081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Siddiqui J, Jaffe PE, Aziz K, Forouhar F, Sheppard R, Covault J, Bonkovsky HL. Fatal air and bile embolism after percutaneous liver biopsy and ERCP. Gastrointest Endosc. 2005;61:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |