Published online Jan 7, 2022. doi: 10.12998/wjcc.v10.i1.242
Peer-review started: December 27, 2020
First decision: July 8, 2021
Revised: July 12, 2021
Accepted: November 25, 2021
Article in press: November 25, 2021
Published online: January 7, 2022
Processing time: 367 Days and 9 Hours
Factor XI (FXI) deficiency, also known as hemophilia C, is a rare bleeding disorder of unpredictable severity that correlates poorly with FXI coagulation activity. This often poses great challenges in perioperative hemostatic management. Thromboelastography (TEG) is a method for testing blood coagulation using a viscoelastic hemostatic assay of whole blood to assess the overall coagulation status. Here, we present the successful application of intraoperative TEG monitoring in an FXI-deficient patient as an individualized blood transfusion strategy.
A 21-year-old male patient with FXI deficiency was scheduled to undergo reconstructive surgery for macrodactyly of the left foot under general anesthesia. To minimize his bleeding risk, he was scheduled to receive fresh frozen plasma (FFP) as an empirical prophylactic FXI replacement at a dose of 15-20 mL/kg body weight (900-1200 mL) before surgery. Subsequent FFP transfusion was to be adjusted according to surgical need. Instead, TEG assessment was used at the beginning and toward the end of his surgery. According to intraoperative TEG results, the normalization of coagulation function was achieved with an infusion of only 800 mL FFP, and blood loss was minimal. The patient showed an uneventful postoperative course and was discharged on postoperative day 8.
TEG can be readily applied in the intraoperative period to individualize transfusion needs in patients with rare inherited coagulopathy.
Core Tip: Factor XI (FXI) deficiency is a rare bleeding disorder of unpredictable severity that correlates poorly with FXI coagulation activity and that poses great challenges for perioperative hemostatic management. Thromboelastography (TEG) is a method for testing blood coagulation using a viscoelastic hemostatic assay of whole blood to assess overall coagulation status; it is readily available and provides real-time monitoring. This case report highlights the importance of using TEG in the intraoperative period to individualize transfusion needs for patients with rare inherited coagulopathy and to minimize transfusion-related risks.
- Citation: Guo WJ, Chen WY, Yu XR, Shen L, Huang YG. Intraoperative thromboelastography-guided transfusion in a patient with factor XI deficiency: A case report. World J Clin Cases 2022; 10(1): 242-248
- URL: https://www.wjgnet.com/2307-8960/full/v10/i1/242.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i1.242
Hemophilia C, or factor XI (FXI) deficiency, is a rare autosomal coagulation disorder[1]. Patients may be asymptomatic until they are hemodynamically challenged following trauma or surgery. In other cases, these coagulopathies are discovered as incidental laboratory findings along with other medical conditions. The unpredictability of bleeding patterns often poses perioperative challenges for clinicians[2]. Thromboelastography (TEG) is a method that is used to monitor and analyze the viscoelastic properties of blood clot formation and lysis. It has the advantages of working with the patient’s whole blood, providing real-time quantitative results on global hemostasis assessments[3]. Its adaptability for point-of-care (POC) testing makes this test particularly useful for intraoperative blood transfusion guidance. Here, we present a case in which the patient was diagnosed with FXI deficiency during a preoperative workup for macrodactyly reconstructive surgery. POC-TEG monitoring was successfully used to help assess the need for intraoperative transfusion.
A 21-year-old man was scheduled to undergo reconstructive surgery for macrodactyly of the left foot under general anesthesia.
The patient presented with significant enlargement of his left foot since birth, complicated by recurrent episodes of paronychia. He was scheduled to have reconstructive surgery at a local hospital. However, the surgery was deferred due to the unexpected perioperative discovery of abnormal coagulation studies.
The patient denied a previous history of easy bleeding or bruising.
There was significant swelling of the patient’s left foot without erythema, rash, or discoloration. The bilateral lower extremity pulses were equal. The patient had a normal gait. Motor and sensations were intact.
Preoperative laboratory workup showed an increased activated plasma thrombo
| APTT (normal) | APTT (normal-2 h) | APTT (patient) | APTT (patient-2 h) | APTT (1:1) | APTT (1:1-2 h) |
| 26.1 s | 27.4 s | 84.2 s | 83.1 s | 29.8 s | 31.3 s |
The diagnosis of FXI deficiency was confirmed by a hematologist.
Preoperative hematology consultation suggested empirically giving fresh frozen plasma (FFP) as prophylactic FXI replacement at a dose of 15-20 mL/kg body weight (patient weight 60 kg, prophylactic dose 900-1200 mL FFP) before surgery. Subsequent FFP transfusion would be adjusted per surgical need. Oral tranexamic acid was suggested for one week postoperatively.
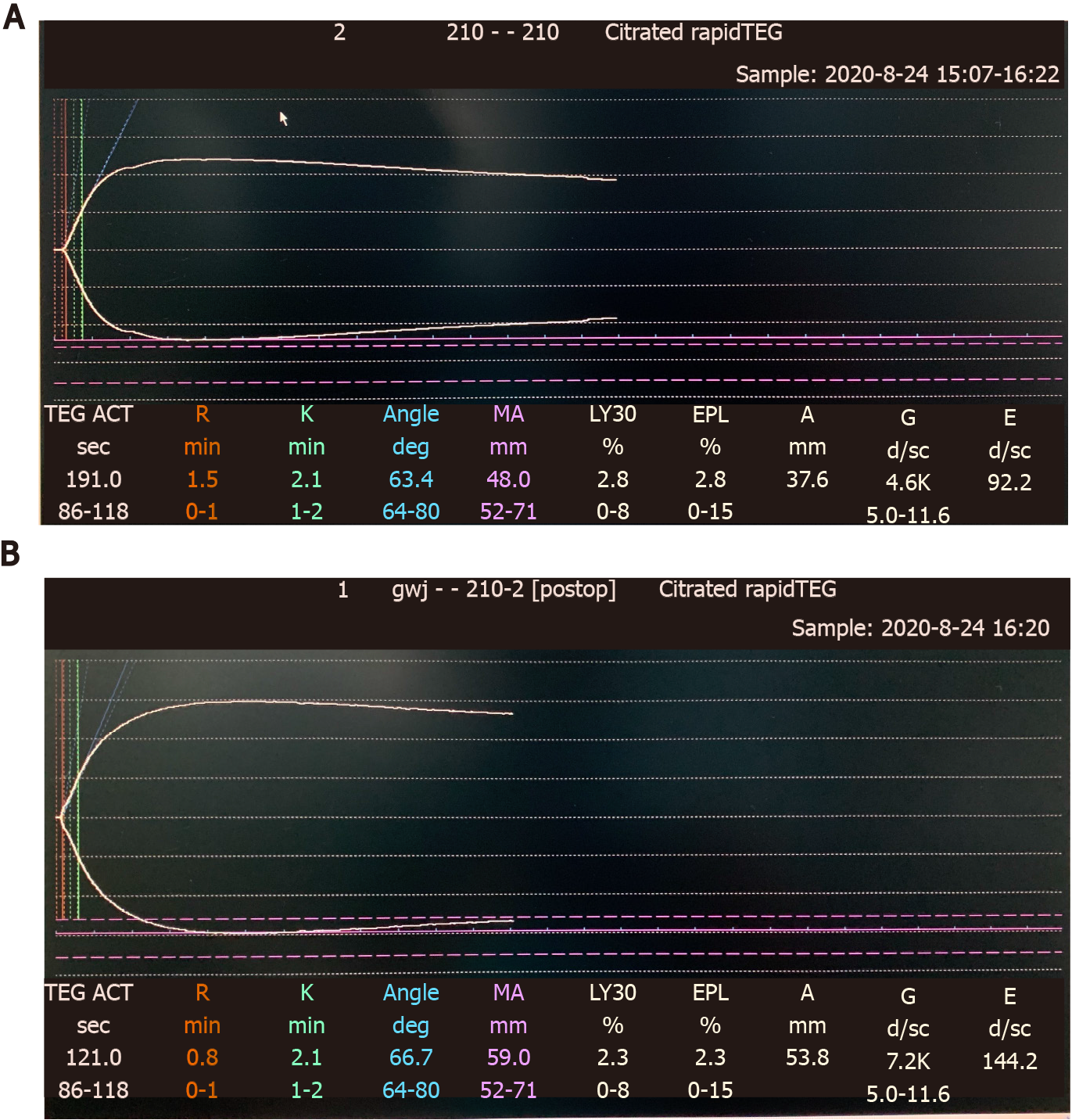
On the day of surgery, the patient received 400 mL FFP preoperatively. The first set of TEGs (Figure 1A) performed immediately after FFP transfusion showed moderately increased activated clotting time (ACT), R time, K time, max amplitude (MA), and alpha angle. The operation was performed under general anesthesia and lasted approximately 4 h. A tourniquet was applied above the knee to minimize blood loss. Continuous nasal temperature monitoring was used to ensure no intraoperative hypothermia was experienced. The patient received 2000 mL of Ringer’s lactate and 400 mL FFP intraoperatively. Urine output was 1400 mL, and blood loss was estimated to be approximately 300 mL. The second set of TEGs (Figure 1B) performed toward the end of surgery showed improvements in all parameters.
The patient had an uneventful postoperative course (Figure 2). Oral tranexamic acid 0.5 g three times per day was prescribed for one week. Surgical site drainage was 45 mL on postoperative day (POD) 1 and then decreased to a minimal level. The drain was removed on POD3. The patient received 400 mL FFP on POD 4 due to concerns of prolonged elevation of APTT levels (46.4 s, reference: 23.3-32.5 s), while the surgical dressing remained dry and clean. He was discharged on POD 8.
Hemophilia C caused by a deficiency of FXI is a rare autosomal inherited coagulopathy. FXI plays an important role not only in initiating clot formation but also in supporting clot consolidation. Conventional coagulation tests such as PT and APTT are less than satisfactory in the assessment of hemophilia C patients’ clinical profiles and bleeding risks. These tests are limited because they are endpoint assays that test only the speed of blood clot formation. However, they cannot reflect the process of further thrombin formation involved in clot consolidation and maintenance. Compared with hemophilia A and B, the clinical profile and bleeding management of hemophilia C is less clearly understood (Table 2). The relationship between bleeding phenotypes and baseline FXI level is poor, making perioperative bleeding risk hard to predict and manage[4].
| Hemophilia A | Hemophilia B | Hemophilia C | |
| Genetics | X-linked | X-linked | Autosomal |
| Pathophysiology | FVIII deficiency | FIX deficiency | FXI deficiency |
| Clinical manifestations | Bleeding of variable severity correlated with factor levels | Bleeding of variable severity correlated with factor levels | Variable |
| Routine management | Prophylactic factor replacement | Prophylactic factor replacement | None |
| Perioperative management | Factor replacement, Cryoprecipitate. The goal is to keep the levels of FVIII > 50% for major surgery | Factor replacement, Prothrombin complex concentrate. The goal is to keep the levels of factor IX > 50% for major surgery | Controversial. May include: FFP, antifibrinolytics, TPE, factor replacement. Optimal FXI level unclear |
TEG is a method of testing the efficiency of blood coagulation using a whole blood-based, viscoelastic hemostatic assay. It can provide a continuous assessment of the elastic properties of clot formation and lysis in both graphics and numbers. TEG measurements collected for analysis include reaction (R) time, coagulation (k) time, α angle, and maximum amplitude (MA), which are reflections of clotting factors, circulating inhibitory activity, fibrinogen and platelet levels and function, etc.[5] TEG’s short turnaround time makes it a promising measurement tool for the assessment of global hemostasis in trauma or perioperative settings. It is better than conventional coagulation tests in monitoring coagulation profiles and predicting transfusion requirements[5]. It reduces the total amount of blood products transfused compared with an empiric transfusion policy or a transfusion protocol guided by conventional coagulation tests[6]. Study results from trauma[7], liver transplant[8] and cardiac surgeries[9] have shown that the goal-directed allogeneic transfusion strategy is believed to provide better hemostatic competence. This was possibly due to the more timely administration of blood products such as plasma and platelets, which in turn resulted in less blood loss[3], reduced blood transfusion needs[10], lower costs, and fewer adverse events[11] in the TEG-guided transfusion group than in the conventional transfusion group. One study also suggested that TEG-guided transfusion could substantially affect patient outcomes, including length of hospital stay, odds of reoperation, and short-term mortality[9]. For inherited coagulopathies such as hemophilia A and B, a combination of standard coagulation laboratory tests and TEG tests results in a better understanding of hemostasis in an individual patient, giving insights into their long-term hemostatic management[12], as well as providing vital insights in more pressing situations such as traumas or surgeries. In later cases, studies from hemophilia A and B patients suggested that TEG could be successfully used in perioperative settings to evaluate the efficacy of various hemostatic agents, such as factor VIII concentrate, cryoprecipitate, and prothrombin complex concentrates[3]. TEG has the potential to assess the role FXI plays in global hemostasis. However, its application in perioperative transfusion management for hemophilia C patients has not been extensively studied.
Normally, FXI-deficient patients will require careful, individualized and multi
The patient we present here had no history of spontaneous bleeding and had no surgical history. This made the perioperative bleeding risk hard to predict and the prophylactic transfusion management strategy hard to plan. The consulting hematologists suggested a FFP loading dose of 15-20 mL/kg body weight to bring the FXI level within a satisfactory range (FXI: C, 30%–45%), which inevitably resulted in the need for a large volume of FFP. It is in this kind of situation that TEG monitoring is especially useful. TEG-guided prophylactic FFP replacement may allow for a more parsimonious use of replacement therapy in patients with severe FXI deficiency undergoing surgery. It can reduce the risks of volume overload, transfusion-related acute lung injury, transmission of infectious diseases, thrombosis, allergic reactions, and the development of inhibitors to FXI[13].Empirically, our patient was to receive a loading dose of 900-1200 mL FFP according to preoperative hematology consultation. In practice, however, based on the results from the intraoperative TEG monitoring, our patient received 800 mL FFP in total before and during the whole procedure with minimal blood loss and uneventful postoperative recovery. This experience is limited to a single case report. However, we believe that with improved TEG technology and accessibility, anesthesiologists and other medical practitioners will be able to provide transfusion therapy tailored to the need of each patient with FXI deficiency.
FXI deficiency is an underrecognized disorder with a wide range of clinical presentations and a poor correlation with coagulation studies. It poses great challenges for perioperative management. FXI concentrates, FFP, TPE and antifibrinolytic therapies are the mainstream treatments for FXI patients with surgical needs. POC-TEG could be readily applied in the perioperative period to individualize transfusion requirements on a case-by-case basis, providing guidance regarding the appropriate amount of blood products to be administered and thus minimizing transfusion needs and the associated risks. Further large-scale studies are needed to assess the potential for using TEG for perioperative transfusion guidance in the treatment of FXI patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Villalba R S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Jayakrishnan T, Shah D, Mewawalla P. Hemophilia C: A Case Report With Updates on Diagnosis and Management of a Rare Bleeding Disorder. J Hematol. 2019;8:144-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Gomez K, Bolton-Maggs P. Factor XI deficiency. Haemophilia. 2008;14:1183-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Fahrendorff M, Oliveri RS, Johansson PI. The use of viscoelastic haemostatic assays in goal-directing treatment with allogeneic blood products - A systematic review and meta-analysis. Scand J Trauma Resusc Emerg Med. 2017;25:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 4. | Santoro C, Di Mauro R, Baldacci E, De Angelis F, Abbruzzese R, Barone F, Bochicchio RA, Ferrara G, Guarini A, Foà R, Mazzucconi MG. Bleeding phenotype and correlation with factor XI (FXI) activity in congenital FXI deficiency: results of a retrospective study from a single centre. Haemophilia. 2015;21:496-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Peng HT, Nascimento B, Tien H, Callum J, Rizoli S, Rhind SG, Beckett A. A comparative study of viscoelastic hemostatic assays and conventional coagulation tests in trauma patients receiving fibrinogen concentrate. Clin Chim Acta. 2019;495:253-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Görlinger K, Dirkmann D, Hanke AA, Kamler M, Kottenberg E, Thielmann M, Jakob H, Peters J. First-line therapy with coagulation factor concentrates combined with point-of-care coagulation testing is associated with decreased allogeneic blood transfusion in cardiovascular surgery: a retrospective, single-center cohort study. Anesthesiology. 2011;115:1179-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 312] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 7. | Gonzalez E, Moore EE, Moore HB, Chapman MP, Chin TL, Ghasabyan A, Wohlauer MV, Barnett CC, Bensard DD, Biffl WL, Burlew CC, Johnson JL, Pieracci FM, Jurkovich GJ, Banerjee A, Silliman CC, Sauaia A. Goal-directed Hemostatic Resuscitation of Trauma-induced Coagulopathy: A Pragmatic Randomized Clinical Trial Comparing a Viscoelastic Assay to Conventional Coagulation Assays. Ann Surg. 2016;263:1051-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 447] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 8. | Graff JT, Cortez AR, Dhar VK, Wakefield C, Cuffy MC, Shah SA, Goodman MD. Perioperative thrombelastography serves as an important assessment tool of transfusion requirements during liver transplantation. Surg Open Sci. 2020;2:70-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Redfern RE, Fleming K, March RL, Bobulski N, Kuehne M, Chen JT, Moront M. Thrombelastography-Directed Transfusion in Cardiac Surgery: Impact on Postoperative Outcomes. Ann Thorac Surg. 2019;107:1313-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Schmidt AE, Israel AK, Refaai MA. The Utility of Thromboelastography to Guide Blood Product Transfusion. Am J Clin Pathol. 2019;152:407-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 11. | Sharp G, Young CJ. Point-of-care viscoelastic assay devices (rotational thromboelastometry and thromboelastography): a primer for surgeons. ANZ J Surg. 2019;89:291-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Nogami K. The utility of thromboelastography in inherited and acquired bleeding disorders. Br J Haematol. 2016;174:503-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 13. | Salomon O, Steinberg DM, Seligshon U. Variable bleeding manifestations characterize different types of surgery in patients with severe factor XI deficiency enabling parsimonious use of replacement therapy. Haemophilia. 2006;12:490-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 127] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 14. | Leff JD, Zumberg MS, Widyn JG, DeAnda A, Janelle GM. Hemophilia C in a patient undergoing cardiac surgery: perioperative considerations. Semin Cardiothorac Vasc Anesth. 2014;18:297-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Wheeler AP, Gailani D. Why factor XI deficiency is a clinical concern. Expert Rev Hematol. 2016;9:629-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 16. | Alsammak MS, Ashrani AA, Winters JL, Pruthi RK. Therapeutic plasma exchange for perioperative management of patients with congenital factor XI deficiency. J Clin Apher. 2017;32:429-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |