Copyright
©The Author(s) 2020.
World J Clin Cases. Mar 26, 2020; 8(6): 1065-1073
Published online Mar 26, 2020. doi: 10.12998/wjcc.v8.i6.1065
Published online Mar 26, 2020. doi: 10.12998/wjcc.v8.i6.1065
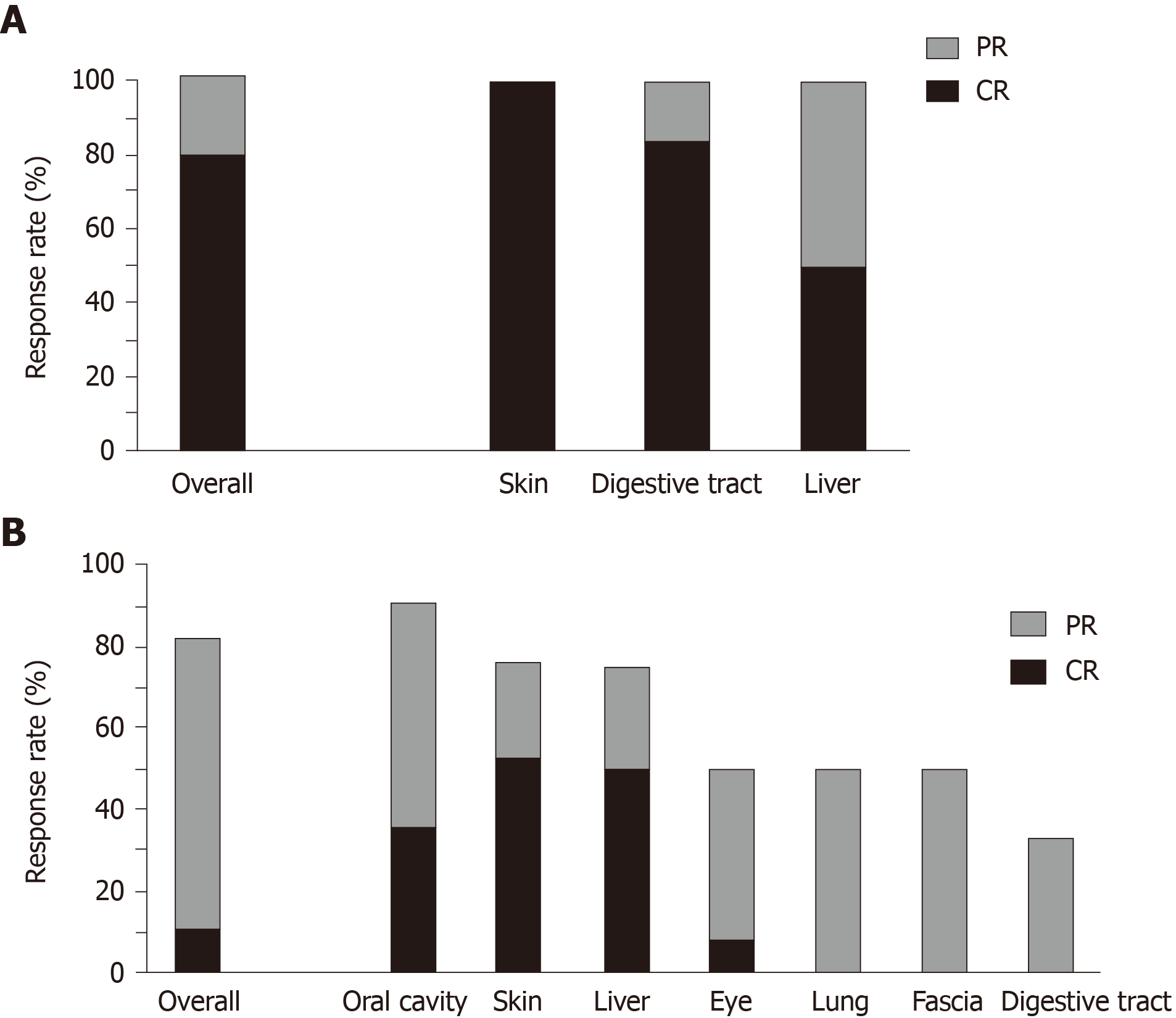
Figure 1 Response rates to ruxolitinib.
A: Overall response rates (n = 10) and response rates of the skin (n = 5), digestive tract (n = 6), and liver (n = 2) to ruxolitinib in patients with acute graft-vs-host disease; B: Overall response rates (n = 28) and response rates of the oral cavity (n = 11), skin (n = 21), liver (n = 4), eye (n = 12), lungs (n = 8), fascia (n = 10), and digestive tract (n = 6) to ruxolitinib in patients with chronic graft-vs-host disease. CR: Complete response; PR: Partial response.
- Citation: Dang SH, Liu Q, Xie R, Shen N, Zhou S, Shi W, Liu W, Zou P, You Y, Zhong ZD. Ruxolitinib add-on in corticosteroid-refractory graft-vs-host disease after allogeneic stem cell transplantation: Results from a retrospective study on 38 Chinese patients. World J Clin Cases 2020; 8(6): 1065-1073
- URL: https://www.wjgnet.com/2307-8960/full/v8/i6/1065.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i6.1065