Copyright
©The Author(s) 2023.
World J Clin Cases. Feb 6, 2023; 11(4): 797-808
Published online Feb 6, 2023. doi: 10.12998/wjcc.v11.i4.797
Published online Feb 6, 2023. doi: 10.12998/wjcc.v11.i4.797
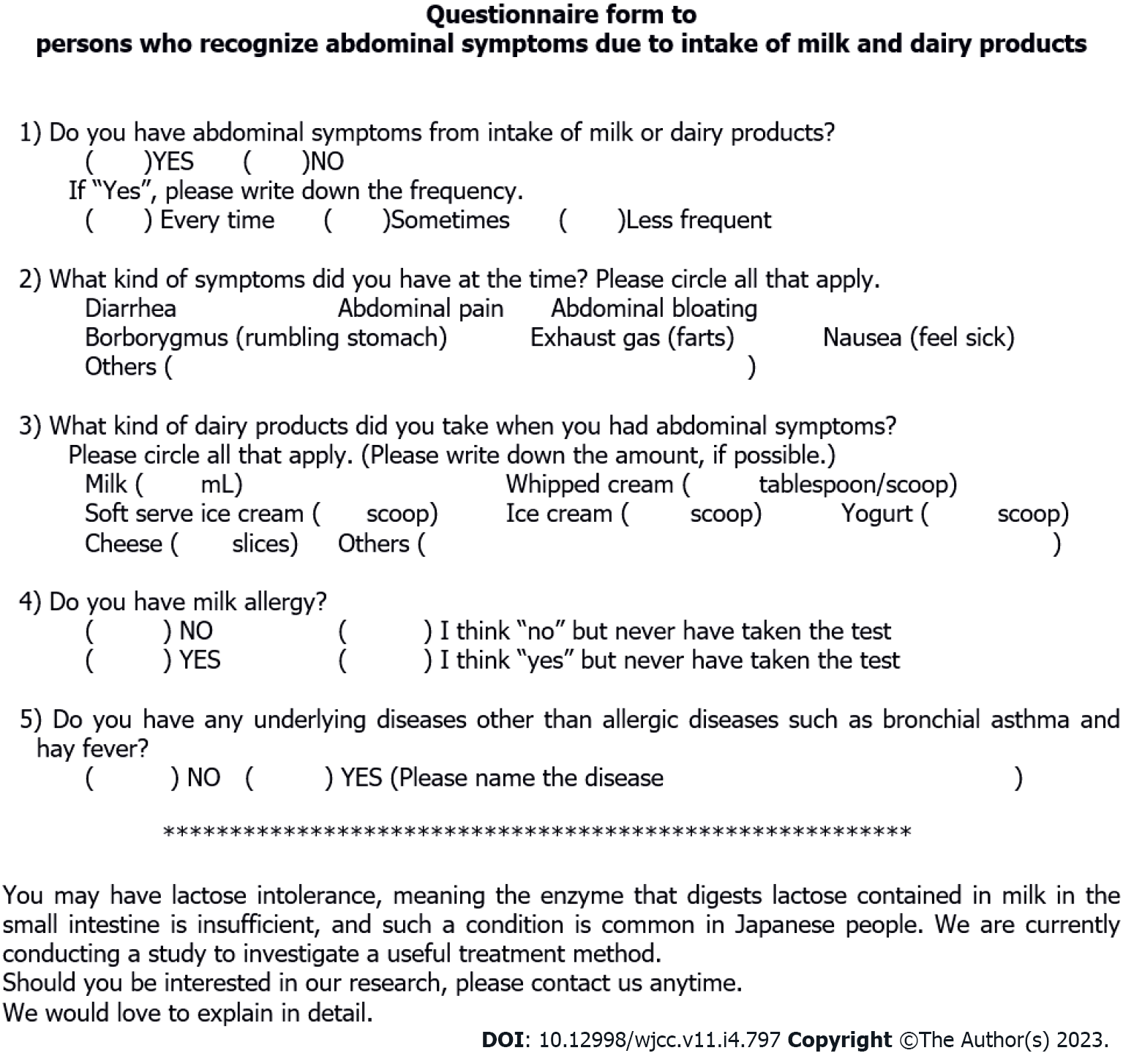
Figure 1 Questionnaire form to persons who recognize abdominal symptoms due to intake of milk and dairy products.
The questionnaire asks for the frequency and severity of LI symptoms, milk allergy, or other underlying diseases. There is also a brief introduction of our study to the subjects.
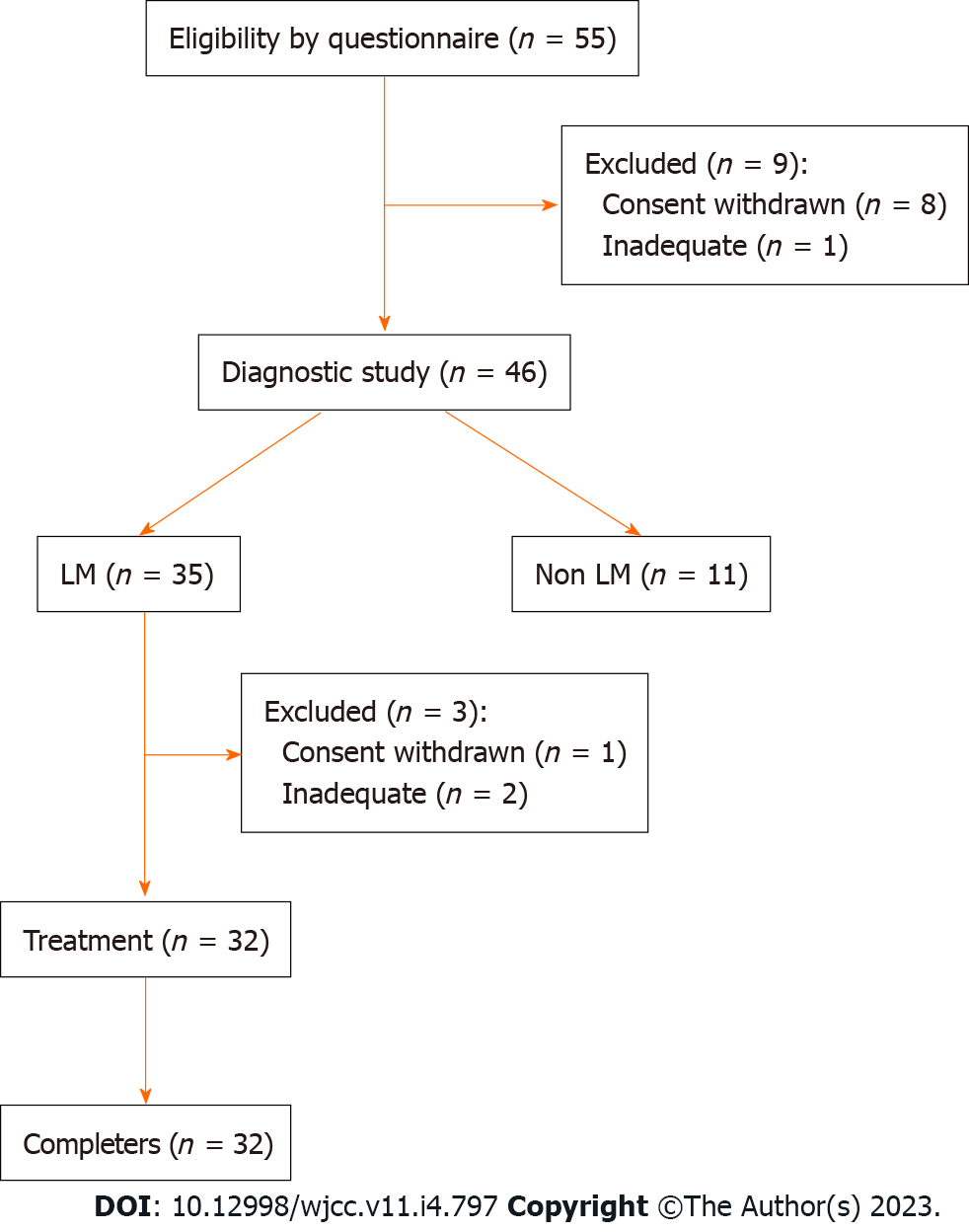
Figure 2 Flowchart of participant recruitment and study processes.
A questionnaire survey was conducted on 55 participants, and 20 g lactose hydrogen breath test was performed on 46 subjects who were assumed to have lactose intolerance symptoms. Thirty-five subjects were diagnosed with lactose malabsorption, of which 32 underwent and completed the treatment study without dropping out. LM: Lactose malabsorption.
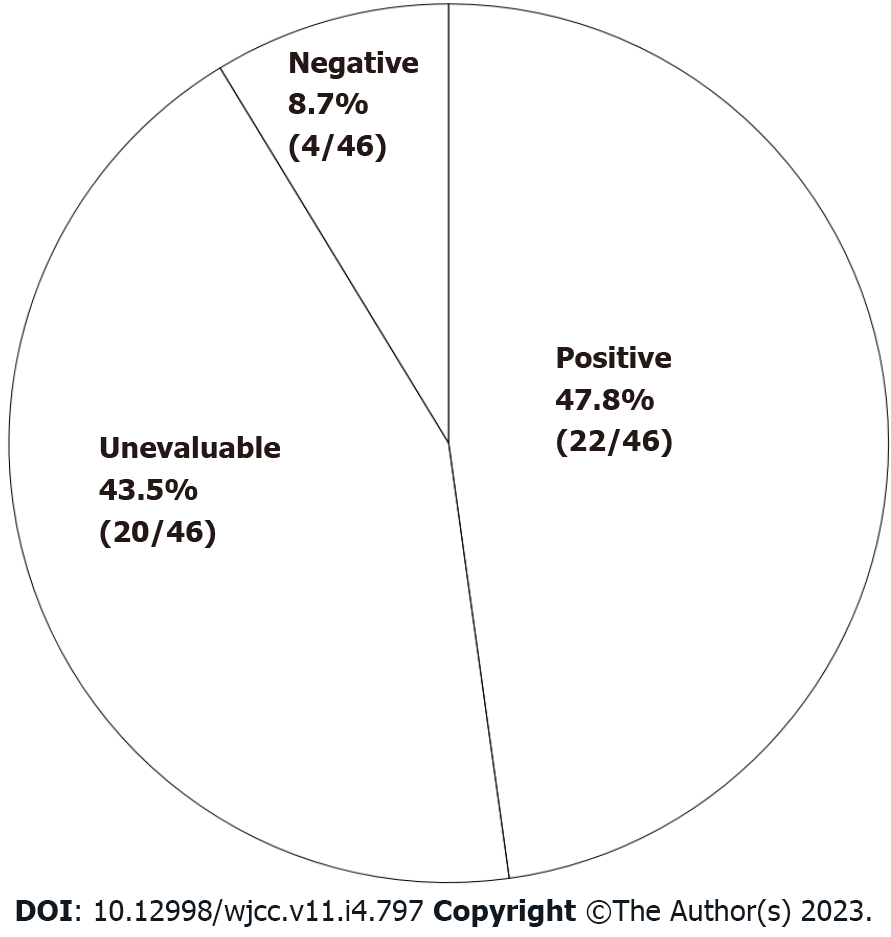
Figure 3 Outcomes of 200 mL single-blind comparative study.
Positive: More obvious symptoms induced by general milk than by lactose-reduced milk. Unevaluable: Symptoms induced by lactose-reduced milk, or unclear difference between the two materials. Negative: No symptoms induced by either material.
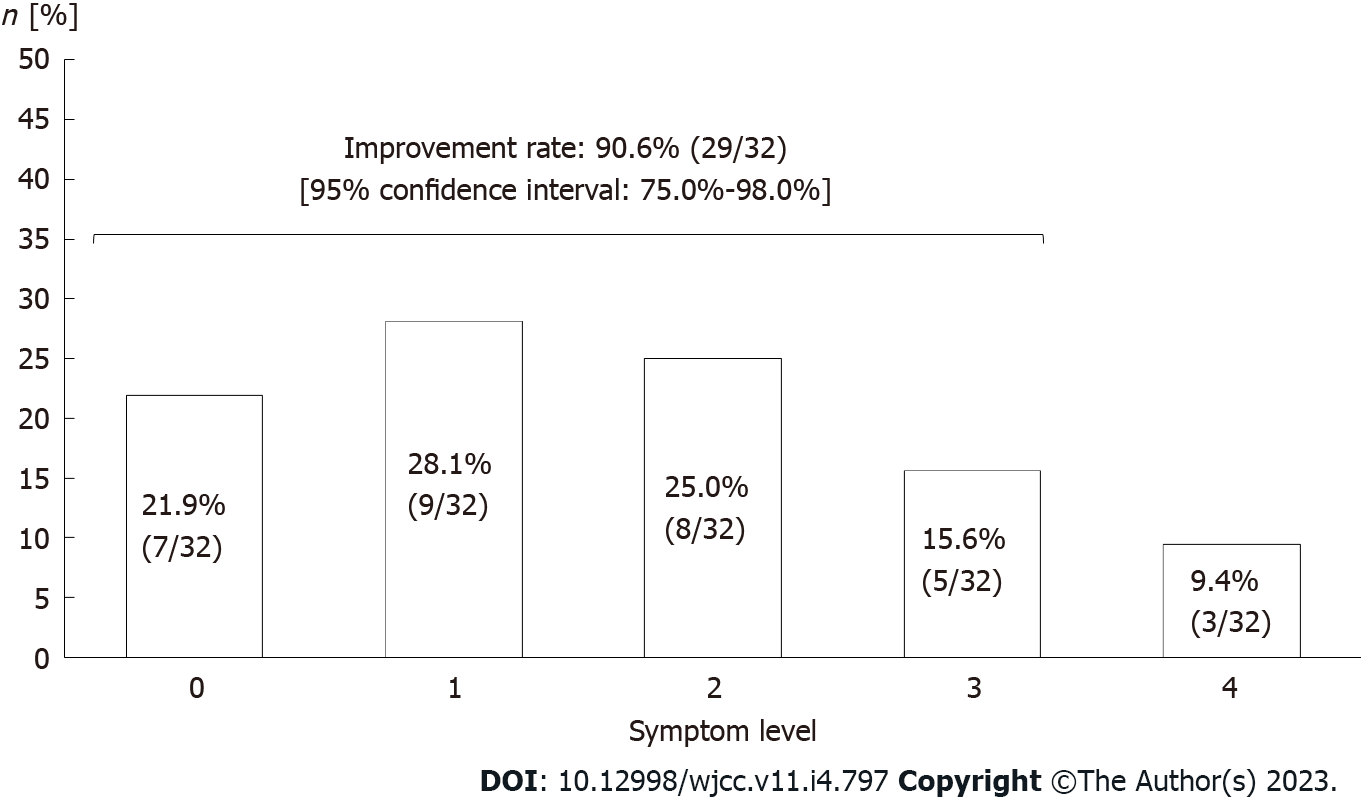
Figure 4 Evaluation of symptom improvement after the incremental milk treatment of lactose malabsorption subjects.
Grades of symptom level: 0 = no symptoms; 1 = trivial symptoms; 2 = mild symptoms but improved; 3 = moderate symptoms but improved; 4 = no improvement. Symptom improvement was defined in grades from 0-3.
Figure 5 Comparison of diagnostic values of lactose malabsorption by 20 g lactose hydrogen breath test before and after the incremental milk treatment in subjects with improved symptoms.
Decrease (improved): More than 15 ppm decrease; No change: Within 15 ppm difference; Increase: More than 15 ppm increase. LHBT: Lactose hydrogen breath test.
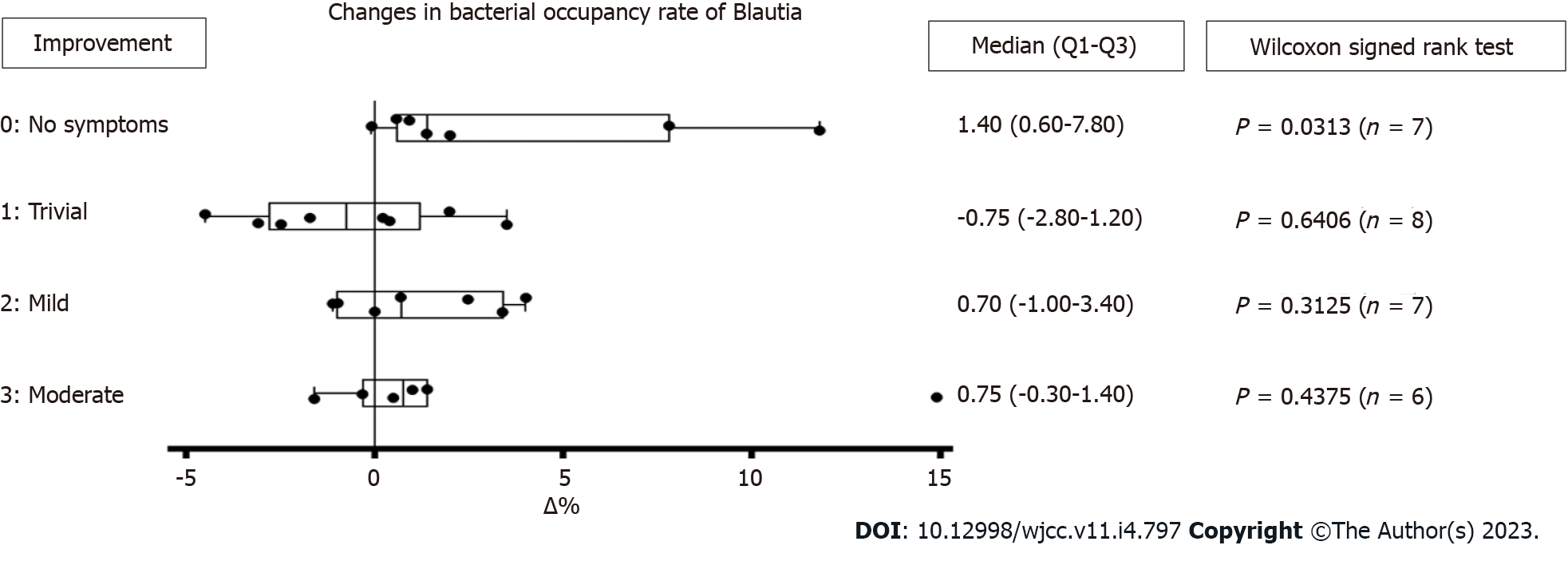
Figure 6 Analysis of Blautia in fecal microbiota before and after the incremental milk treatment.
Change in bacterial occupancy rate of Blautia based on the degree of symptom improvement was observed in 28 subjects. Blautia was not detected in one out of 29 subjects. Degree of improvement: 0 = no symptoms; 1 = trivial symptoms; 2 = mild symptoms but improved; 3 = moderate symptoms but improved.
- Citation: Hasegawa M, Okada K, Nagata S, Sugihara S. Efficacy of incremental loads of cow’s milk as a treatment for lactose malabsorption in Japan. World J Clin Cases 2023; 11(4): 797-808
- URL: https://www.wjgnet.com/2307-8960/full/v11/i4/797.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i4.797