Published online Sep 6, 2016. doi: 10.5527/wjn.v5.i5.437
Peer-review started: January 28, 2016
First decision: March 23, 2016
Revised: May 14, 2016
Accepted: June 27, 2016
Article in press: June 29, 2016
Published online: September 6, 2016
Processing time: 217 Days and 3 Hours
To evaluate the parathyroid ultrasonography and define parameters that can predict poor response to treatment in patients with secondary hyperparathyroidism due to renal failure.
This cohort study evaluated 85 patients with chronic kidney disease stage V with parathyroid hormone levels above 800 pg/mL. All patients underwent ultrasonography of the parathyroids and the following parameters were analyzed: Demographic characteristics (etiology of chronic kidney disease, gender, age, dialysis vintage, vascular access, use of vitamin D), laboratory (calcium, phosphorus, parathyroid hormone, alkaline phosphatase, bone alkaline phosphatase), and the occurrence of bone changes, cardiovascular events and death. The χ2 test were used to compare proportions or the Fisher exact test for small sample frequencies. Student t-test was used to detect differences between the two groups regarding continuous variables.
Fifty-three patients (66.4%) had parathyroid nodules with higher levels of parathyroid hormone, calcium and phosphorus. Sixteen patients underwent parathyroidectomy and had higher levels of phosphorus and calcium × phosphorus product (P = 0.03 and P = 0.006, respectively). They also had lower mortality (32% vs 68%, P = 0.01) and lower incidence of cardiovascular or cerebrovascular events (27% vs 73%, P = 0.02). Calcium × phosphorus product above 55 mg2/dL2 [RR 1.48 (1.06, 2.08), P = 0.03], presence of vascular calcification [1.33 (1.01, 1.76), P = 0.015] and previous occurrence of vascular events [RR 2.25 (1.27, 3.98), P < 0.001] were risk factors for mortality in this population. There was no association between the occurrence of nodules and mortality.
The identification of nodules at ultrasonography strengthens the indication for parathyroidectomy in patients with secondary hyperparathyroidism due to renal failure.
Core tip: We aimed to evaluate the parathyroid ultrasonography and defined parameters to predict poor response to treatment in 85 patients with chronic kidney disease stage V and parathyroid hormone (PTH) levels > 800 pg/mL. Fifty-three patients had nodules, higher PTH, calcium and phosphorus. Sixteen underwent parathyroidectomy and had significant higher levels of phosphorus and calcium phosphorus product; lower mortality and lower incidence of cardiovascular or cerebrovascular events. Calcium phosphorus product above 55, vascular calcification and previous vascular events were risk factors for mortality. There was no association between nodules and mortality. We concluded that nodules at ultrasonography strengthens the indication for parathyroidectomy in those patients.
- Citation: Ribeiro C, Penido MGMG, Guimarães MMM, Tavares MS, Souza BDN, Leite AF, de Deus LMC, Machado LJC. Parathyroid ultrasonography and bone metabolic profile of patients on dialysis with hyperparathyroidism. World J Nephrol 2016; 5(5): 437-447
- URL: https://www.wjgnet.com/2220-6124/full/v5/i5/437.htm
- DOI: https://dx.doi.org/10.5527/wjn.v5.i5.437
Chronic kidney disease-mineral and bone disorder (CKD-MBD) is a growing health care concern associated with secondary hyperparathyroidism (SHPT), mineral abnormalities and increased risk of cardiovascular disease[1]. Therefore, prevention and control of SHPT is one of the main objectives in the management of chronic kidney disease patients, particularly those on dialysis.
SHPT develops from the early stages of CKD due to disturbances in calcium (Ca), phosphorus (P) and vitamin D metabolism, characterized usually as high turnover bone disease[2]. Besides stimulating the synthesis and secretion of parathyroid hormone (PTH), hyperplasia of parathyroid cells, initially diffuse and then nodular[3], can also be observed. Patients with nodular parathyroid hyperplasia exhibit reduced number of Ca-sensing receptor (CaSR) and vitamin D receptor (VDR), and they are often resistant to vitamin D therapy[3,4]. In early stages of CKD, even without persistent hyperphosphatemia, elevated net body P load is associated with increased production of fibroblast-growth-factor 23 (FGF23) by bone osteoblasts and osteocytes. Serum FGF23 correlates to PTH in predialysis CKD patients and in patients with early CKD when levels of P and Ca are maintained within normal range[5-7]. However, in advanced stages of CKD, there is an inability of FGF23 to suppress PTH secretion.
A total transient elevation in P levels may be associated with reduction of serum Ca and thereby stimulate the secretion of PTH (tradeoff theory). Theories suggest that the hyperfiltration of the remaining nephrons could raise the P concentration in the proximal tubule cells, inhibiting the 1-alpha-hydroxylase. Experimental studies have shown a direct effect of P in increased PTH secretion[5] and have also demonstrated an independent association between phosphatemia and PTH in CKD patients[7,8].
Reduction of CaSR and VDR expression in parathyroid cells occurs with the progression of CKD[9,10]. This process is more prominent in patients with nodular parathyroid hyperplasia than those with diffuse hyperplasia[9,11-13]. However, it is unclear why diffuse parathyroid hyperplasia develops to nodular hyperplasia. In this kind of hyperplasia (nodular hyperplasia), the parathyroid gland contains nodules of rapidly proliferating parathyroid cells. Each nodule, formed by monoclonal proliferation of parathyroid cells, produces excessive amounts of PTH. Because of the low density of CaSR and VDR, these cells are refractory to medical treatment (activated vitamin D, calcimimetics, control of hyperphosphatemia). During the progression of parathyroid hyperplasia, the ability of PTH synthesis increases progressively while a quantitative reduction of VDR and CaSR occurs, resulting in autonomous function of this gland, which characterizes the tertiary hyperparathyroidism[9,13,14].
The Kidney Disease Outcomes Quality Initiative proposes strict targets for the control of PTH, Ca and P in CKD patients[15] considering studies showing that patients with serum PTH levels above 800 pg/mL have severe and refractory hyperparathyroidism[14]. Considering that parathyroid hyperplasia is resistant to the action of vitamin D, it is important to identify among CKD patients those with high PTH levels and low response to this treatment, since they may be candidates for parathyroidectomy (PTX) in case of hypercalcemia (Ca > 10 mg/dL) and/or hyperphosphatemia (P > 5.5 mg/dL) in association with clinical symptoms, such as bone pain, fractures or spontan tendon rupture, untreatable chronic pruritus, erythropoiesis stimulating agents (ESA) resistant anemia without other causes and calciphylaxis[16,17].
Ultrasonography (US) is an economically feasible and noninvasive imaging method able to identify nodular hyperplasia[18,19] and serves as a marker of prognosis and response to treatment of SHPT with vitamin D analogues[20,21]. Increased parathyroids size, despite several aspects of the US and possible ectopic locations, are generally characterized by a distinct echogenic capsule, independent of the thyroid capsule, surrounding a hypoechoic area due to progressive hypercellularity and the disappearance of adipose tissue[22,23]. Gland weighing more than 0.5 g (equivalent to 0.5 cm3) or larger than 1.0 cm or greater in diameter correspond to nodular hyperplasia in more than 90% of cases[24].
Severe SHPT is associated with higher mortality in patients with moderate and advanced CKD[25] as well as with disorders of bone metabolism (especially hyperphosphatemia and increased Ca × P product), and worse prognosis in CKD. Elevated PTH is responsible for serious long-term consequences, such as renal osteodystrophy, vascular and valvular calcification, changes in myocardial structure and function, immune dysfunction and anemia unresponsive to ESA[26,27], bone pain, abnormal bone histology and fractures among patients with SHPT[28,29].
The present study aimed to evaluate the usefulness of US of parathyroids in CKD patients on hemodialysis as a predictor of clinical outcome in severe hyperparathyroidism and correlate these sonographic findings and data related to CKD-MBD with clinical, epidemiological, laboratory and mortality data and response to treatment. As secondary endpoint, the study aimed to investigate the association between PTX and mortality of patients with CKD and BMD.
It was a cohort study of patients on hemodialysis regularly followed at the Nephrology Center of Santa Casa de Belo Horizonte, Brazil, from January 2005 to January 2009. Inclusion criteria were patients with CKD on hemodialysis, age equal to or older than 18 years and at least one measuring serum intact PTH above 800 pg/mL. Patients with severe neuropsychiatric disorders, anatomical and/or functional changes that would interfere with the US examination and patients acutely unstable, such as with uncontrolled infection, hemodynamic and/or metabolic instability, as well as those who refused to participate in the study were excluded.
During the clinical follow-up, patients were submitted to monthly clinical and laboratory examinations according to Resolution - No. 154 of June 15, 2004, the Brazilian National Health Surveillance Agency (ANVISA).
This study was submitted to and approved by the Research Ethics Committee of the Graduation Center of Santa Casa de Belo Horizonte, Minas Gerais, Brazil (011/2005), and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
CKD patients received calcitriol according to resolution SES # 267 of the State Health Authority of the State of Minas Gerais, Brazil (Table 1).
| PTH level | Vitamin D analogue dose (calcitriol) |
| PTH 200-499 pg/mL | 0.25 mcg qd once a day |
| PTH 500-999 pg/mL | 0.50 mcg qd or 1 mcg qd once a day/3 times a week |
| PTH > 1000 pg/mL | 2.0 mcg qd once a day/3 times a week |
In case of no response (reduction of 30%-50% of the initial PTH), patients received vitamin D analogues according to Table 2.
| PTH level | Vitamin D analogue dose (calcitriol) |
| PTH 200-299 pg/mL | 0.25 mcg qd once a day |
| PTH 300-399 pg/mL | 0.50 mcg qd or 0.75 mcg qd once a day, 3 times a week |
| PTH 400-999 pg/mL | 1.0 mcg qd once a day 3 times a week |
| PTH > 1000 pg/mL | 2.0 mcg oral or IV 3 times a week |
Vitamin D administration was withdrawn under the following conditions: Hypercalcemia (plasma Ca above 10.0 mg/dL), hyperphosphatemia (plasma P above 5.5 mg/dL), Ca × P product above 55 mg/dL or PTH less than 200 pg/mL.
The parathyroid US was performed by an examiner and a medical resident in training with a Siemens Versa Plus Sleep Line and 7.5 Mhz transducer. Both unaware of any patient data. The gland volume was considered increased when presented a dimension of 1.0 cm in any axis, or a volume higher than 0.5 cm[3,20,24].
Laboratory data were collected at Time 1, from January 2005 to January 2006, and at Time 2, from October 2008 to January 2009. The following parameters were evaluated: Total Ca, P, alkaline phosphatase (AP), bone specific AP (BAP) at Time 1 only, PTH and Ca × P product. Blood samples were collected immediately before the first dialysis session of the week. PTH was measured by chemoluminescence and bone AP by the method of thermal inactivation.
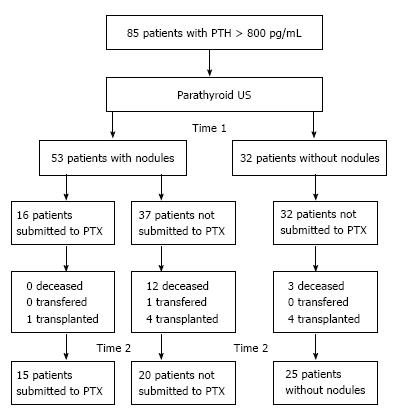
The clinical and demographic data were: Age, sex, time on hemodialysis, CKD etiology, type of access for hemodialysis and the presence of bone and/or vascular changes detected by imaging method. Figure 1 summarizes the patients selection and follow-up.
The two groups (with and without nodules) were subjected to a descriptive analysis, and nominal/categorical variables as well as frequency distribution were represented in tables. Continuous variables and measures of central tendency and variability were used.
Univariate analysis of categorical variables were performed with the χ2 test to compare proportions or the Fisher exact test when small sample frequencies were used. Student t-test was used to detect differences between the two groups regarding continuous variables. In all analyzes a significance level of 5% level was considered and SPSS v. 12.0 software was used for the analysis.
From January 2005 to January 2006 (Time 1), the 85 patients with PTH levels above 800 pg/mL underwent US parathyroid glands, 53 (62.4%) had at least one nodule and 32 (37.6%) showed no abnormality.
Mean age was 44.5 ± 13 years (range 18-76 years), 52% were female. Mean time on hemodialysis in patients without nodules (104.4 ± 54 mo) was similar to the group with parathyroid nodules (106 ± 65 mo; P = 0.91). There was no difference between groups regarding the type and dialysis access. Most patients were on hemodialysis with arteriovenous fistula as vascular access (89.4%, P = 0.47) (Table 3). Glomerular and vascular diseases were the most common causes of CKD in both groups.
Regarding the use of vitamin D-analogues, 6 patients (7%) did not follow the treatment, and four failed to achieve adequate levels of P and two did not achieve adequate levels of both Ca and P, which did not allow the treatment of SHPT. There was no difference between the groups regarding the use of calcitriol (P = 0.402). Patients with parathyroid nodules had higher PTH, Ca, P and Ca × P product. There was no significant difference between the levels of AP and BAP (Table 4).
| Surgery | PTH | Ca | P | Ca × P | AP | BAP |
| No nodule (n = 32) | 796.2 ± 229.7 | 8.6 ± 0.7 | 4.8 ± 1.2 | 41.5 ± 12.4 | 246.7 ± 128.7 | 100.8 ± 69.6 |
| With nodule (n = 53) | 1106.4 ± 462.5 | 9.0 ± 0.6 | 5.9 ± 1.0 | 53.1 ± 10.3 | 283 ± 254.4 | 106.4 ± 91.9 |
| P | < 0.001 | 0.006 | < 0.001 | < 0.001 | 0.387 | 0.783 |
There was no difference between groups when outcomes were analyzed, considering the 16 patients who underwent PTX in this period (Table 5).
| Nodule in US | P | RR (95%CI) | ||
| No (n = 32) | Yes (n = 53) | |||
| Deceased | ||||
| No | 29 (90.6%) | 41 (77.4%) | 0.1202 | 1.00 |
| Yes | 3 (9.4%) | 12 (22.6%) | 2.83 (0.73; 10.93) | |
| Transplant | ||||
| No | 28 (87.5%) | 48 (90.6%) | 0.7232 | 1.37 (0.54; 5.53) |
| Yes | 4 (12.5%) | 5 (9.4%) | 1.00 | |
| Cardiovascular and/or cerebrovascular event4 | ||||
| No | 25 (78.1%) | 43 (81.1%) | 0.7371 | 1.20 (0.41; 3.56) |
| Yes | 7 (21.9%) | 10 (18.9%) | 1.00 | |
| Vascular calcification5 | ||||
| No | 23 (71.9%) | 33 (62.3%) | 0.6331 | 1.00 |
| Yes | 7 (21.9%) | 13 (24.5%) | 1.29 (0.40; 4.28) | |
| No register | 2 (6.3%) | 7 (13.2%) | ||
| Bone disease6 | ||||
| No | 18 (56.3%) | 34 (64.2%) | 0.2021 | 1.89 (0.63; 5.56) |
| Yes | 12 (37.5%) | 12 (22.6%) | 1.00 | |
| No register | 2 (6.3%) | 7 (13.2%) | ||
| Surgery | ||||
| No | 32 (100%) | 37 (69.8%) | < 0.001 | 3 |
| Yes | 0 (0%) | 16 (30.2%) | ||
At Time 2 (October 2008 to January 2009), 60 patients remained in the study; 9 were transplanted, 15 deceased and one was transferred to another facility. In this period there was no change regarding PTH, Ca, P, Ca × P and AP between the groups with and without nodules (Table 6).
| Nodules | PTH | Ca | P | Ca × P | AP | |
| Without nodules (n = 25) | Mean | 833.2 | 8.70 | 4.80 | 41.4 | 249.6 |
| SD | 602.2 | 0.7 | 1.40 | 12.50 | 255.6 | |
| With nodules (n = 35) | Mean | 1031.2 | 8.70 | 5.10 | 44.60 | 396.2 |
| SD | 775.1 | 0.9 | 1.50 | 13.60 | 535.8 | |
| P1 | 0.271 | 0.974 | 0.341 | 0.350 | 0.210 |
In the comparison between Times 1 and 2, there was no difference in any variable in the group without nodule. In the group with nodules, there was a reduction in the levels of P (5.8 vs 5.1, P = 0.008 by paired t test) and Ca × P product (51.6 vs 44.6, P = 0.007 by paired t test).
At Time 1, there was a significant difference in AP and BAP variables, with higher values in the group submitted to PTX (Table 7). At Time 2, they got higher and lower values of P and Ca × P product, as well of AP, compared to non-operated group (Table 8).
| Surgery | PTH | Ca | P | Ca × P | AP | BAP |
| No (n = 37) | 1030.8 ± 391.8 | 9.0 ± 0.6 | 6.0 ± 1.0 | 53.7 ± 10.9 | 212.6 ± 121.5 | 82.7 ± 55.3 |
| Yes (n = 16) | 1281.1 ± 571.4 | 9.1 ± 0.6 | 5.7 ± 1.0 | 51.6 ± 9.0 | 445.9 ± 385.1 | 160.5 ± 132 |
| P1 | 0. 125 | 0. 605 | 0. 354 | 0. 497 | 0. 030 | 0. 050 |
| Surgery | PTH | Ca | P | Ca × P | AP | |
| No (n = 20) | Mean | 992.4 | 8.90 | 5.60 | 49.90 | 214.9 |
| SD | 639.5 | 0.80 | 1.20 | 11.70 | 251.7 | |
| Yes (n = 15) | Mean | 1082.9 | 8.40 | 4.50 | 37.60 | 638 |
| SD | 948.2 | 1.10 | 1.60 | 13.10 | 708.3 | |
| P1 | 0.752 | 0.126 | 0.030 | 0.006 | 0.041 | |
Comparing Time 1 with Time 2, in patients with nodular and non-operated, there were elevated levels of Ca (8.7 vs 8.9, P = 0.033 by Mann-Whitney test). Those submitted to PTX, a reduction of Ca, P and Ca × P product was observed (Table 9).
In relation to outcomes, a higher incidence of death and vascular events in patients with nodules not submitted to PTX was observed. Patients who died by the end of the study had higher mean values of Ca, P and Ca × P product at Time 1. Analysis of categorical variables, Ca × P product above 55 mg2/dL2 was associated with a 48% higher risk of death. Patients who experienced cardiovascular events and vascular calcification in imaging also had a higher risk of death. Those patients who died and had visible nodules in US, had higher mean values of Ca and Ca × P product at Time 1, but no demographic differences was observed in relation to those who did not die. In this group, patients who experienced cardiovascular and/or cerebrovascular events had almost 200% higher chance of death.
There was no difference in demographic and clinical characteristics between patients with and without nodule on US. At Time 1, patients with nodules had higher levels of Ca, P, Ca × P product and PTH. There was no difference in the occurrence of death, vascular events or bone disease. Patients with nodules submitted to surgery had longer time on dialysis. They also had significantly reduced levels of Ca, P and Ca × P product, without variation of PTH levels. Among patients with nodule, mortality and the occurrence of cardiovascular events was lower in those who underwent surgery. Those who died had higher levels of Ca, P and Ca × P product and highest occurrence of events and vascular calcification.
The present study shows that the identification of parathyroid nodules on US may be an indication criteria for PTX in patients with severe hyperparathyroidism associated with CKD, because this surgery is associated with lower morbidity and mortality in these patients. This finding is highly relevant in view of the simplicity and low cost of US and the great difficulty the treatment of SHPT in CKD. Despite the extensive knowledge about its pathophysiology, treatment of SHPT is complex, involving many factors to achieve therapeutic success. In this sense, achieving optimal levels of Ca, PTH and targets have been a challenge for everyone. The Dialysis Outcomes and Practice Patterns Study, which involves European countries and the United States, shows a large percentage of patients with controlled SHPT[16].
The presence of parathyroid nodules is associated with poorer bone metabolic profile in patients with severe hyperparathyroidism. In fact, corroborating the observation of other researchers[30-34], in the first moment of our study (Time 1) the levels of PTH, Ca, P and Ca × P product were higher in patients with parathyroid nodules. Furthermore, we did not find significant differences related to sex, age, time on renal replacement therapy (RRT), underlying disease or vascular access between groups with and without nodules. This in part could be attributed to the high cut-off level of PTH (> 800 pg/mL) in our study. These data would predict an increased difficulty in achieving the therapeutic goal in patients with severe hyperparathyroidism, despite the use of vitamin D-analogues. In fact, experiments have shown that the increase in parathyroid predict correlates with resistance to therapy with vitamin D-analogues (p.o. or parenteral)[3,20,35] .
However, the analysis of bone metabolic profile at Time 2 showed that our patients without nodules also demonstrated that resistance and did not show improvements in mean values of Ca, P and PTH. Nonpharmacological factors may have interfered as poor adherence, supply failures and errors in prescriptions and their interpretation, as well as the initial severity of bone metabolic disorder itself, suggested by the high cut-off level used as inclusion criteria. In fact, despite a better bone metabolic profile at Time 1, this initial severity is suggested by the same proportion of outcomes observed in patients with and without parathyroid nodules.
Nevertheless, the group with nodules showed a reduction of P and Ca × P product probably due to PTX. Patients undergoing PTX had a reduction of Ca, P and Ca × P product. Patients with nodules and non-submitted to surgery had increased Ca levels, while other parameters remanied stable. In any group a decrease of PTH was observed.
This observed differences concerning the osteometabolic profiles between the groups with and without parathyroid nodules cannot be attributed to a more severe osteometabolic disease observed in patients who died, as there was no difference in mortality between these groups. The declining profile among patients with nodules who were not operated, not observed among those without nodules, also not operated, suggests that this beneficial effect of PTX on bone metabolic profile in patients with severe hyperparathyroidism is more prominent and perhaps unique to patients with SHPT with nodules. In this sense, we must consider that PTX is the last therapeutical approach to severe SHPT, meaning there was clinical treatment failure. Patients undergoing PTX have high rates of early mortality, despite lower rates on long-term[36,37].
The PTX allowed an improvement of bone metabolic profile in our patients. It was not our objective to evaluate success of PTX rates, the surgical techniques used or pathology results. In general, studies show that different surgical techniques have different recurrence rates, persistence and varied complications. However, one study showed similar changes in postoperative patients undergoing total PTX with or without autograft, except for a greater need for Ca replacement[38]. Comparing patients with nodules, submitted or not to PTX, we observed that only dialysis vintage was different, higher in those submmited to PTX, with no difference concerning age, sex and type of vascular access. In other studies, different factors were associated to PTX: Younger age, female gender, non-diabetic etiology of CKD, longer dialysis vintage, parenteral use of vitamin D and peritoneal dialysis. Remarkable are younger age and the dialysis vintage[27,36,37,39]. In our study no predominance of a determined cause of CKD was observed, nor differences regarding the use of vitamin D analogues.
Our data showed that the group of patients with nodules was not associated with an increased risk of unfavourable outcome - death, or vascular events and bone changes or differences concerning the chance of transplantation. On the other hand, in the group with nodules not submitted to PTX, a higher mortality and further vascular events was observed. This could be attributed to a lower chance of surgery in patients with a critical state, who eventually died. However, patients with nodules and underwent surgery at Time 1 had poorer bone metabolic profile (higher levels of AP and BAP). The better metabolic control after surgery may have contributed to better survival and lower morbidity. In addition, other studies have shown that, in relation to clinical treatment, PTX is associated with higher early mortality (up to 6 mo), probably to a higher postoperative risk, but lower in the long-term, which could be explained by an improvement in the metabolic and cardiovascular profile with reduced vascular calcification in imaging methods[40,41]. There was also improvement in symptoms related to bone metabolic metabolism, among those submitted to surgery[42,43].
Patients who died in Time 1 had higher levels of Ca, P and Ca × P product. They did not differ, however, from those who survived regarding gender, age, dialysis vintage, type of vascular access, treatment with vitamin D-analogues, presence of parathyroid nodules and PTH levels. Within the group of patients with nodules, those who died presented at Time 1 higher values of Ca × P product, but did not differ from those who survived concerning the other parameters. A higher risk of death was observed in patients with cardiovascular events or vascular calcifications. In the population with nodules, the occurrence of death was higher among those with vascular events during the study. There was also a higher incidence of death and vascular events in patients with nodules and not submitted to PTX. These data suggest that bone metabolic profile can be a predictor of death in patients with severe HPT.
We cannot affirm that the presence of parathyroid nodule can also be a predictor of death in patients with severe hyperparathyroidism. However, as the operated group, with worse metabolic profile had improved this profile and had better outcomes, there is the possibility that the presence of parathyroid nodules can also be predictor of death.
In fact, studies have shown increased risk of death with P levels above 5 and 6.5 mg/dL, Ca × P product > 72 mg2/dL2 and PTH > 600 pg/mL[44,45]. The most common cause of death among patients on RRT is cardiovascular, with cardiac calcification in 60% of them. Joins P mortality, cardiovascular morbidity and mortality and progression of renal disease with or without CKD[46,47]. Elevated phosphate induces calcification of smooth muscle cells (SMC) in vitro. Pit-1, a sodium-dependent phosphate cotransporter is essential for SMC calcification and phenotypic modulation in response to elevated phosphate. P induces the expression of bone markers and extracelular matrix mineralization[46]. Calciphylaxis is the worst expression of the phenotypic modulation.
There is a strong association between high levels of P, Ca × P product and PTH and cardiovascular morbidity and mortality. The main mechanisms involved in this process are accelerated calcification and atherosclerotic instability, Ca accumulation in the tunica media, hypertension, left ventricular (LV) hypertrophy by direct trophic effects and secondary hypertension and coronary heart disease, anemia, and macro/microangiopatias[48,49]. PTH has been implicated in abnormalities in CKD-MBD: Immune dysfunction, refractory anemia, lower secretion of insulin[27]. Even in patients without CKD and SHPT, PTH has been associated with higher mortality[50,51] and P linked to higher mortality as well, and LV hypertrophy, cardiovascular events and coronary calcification[52,53].
Although it seems obvious the association of high levels of Ca, P and PTH in several possible combinations and morbidity and mortality, mainly related to cardiovascular events, it is unclear how the PTX can reduce these rates. In our study patients submitted to surgery and with a 49 mo follow-up had lower mortality rates and reduced levels of Ca and P. However, the effect on PTH was not significative and there was no significant correlation between PTH and mortality. It is plausible to question the correspondance between the success of a PTX as measured by PTH fall, and, therefore, the clinical improvement of a population in which the surgery was not effective. Other unevaluated factors had probably played a role.
The hormone fibroblast growth factor (FGF23) was first described in 2000 and it is mainly produced by osteocytes as a protein that reduces serum P through downregulation of the Na-P-cotransporter type 2 and leads to inhibition of the synthesis of 1-alpha-hydroxylase, with consequent reduction of calcitriol and increased serum PTH levels. The increase of FGF23 occurs in early stages of CKD, even before changes in serum levels of P and PTH and has been an independent factor of mortality in this population. In patients on hemodialysis, FGF23 levels can predict refractory SHPT[54-56]. Although not evaluated in our study, FGF23 could have explained some issues raised by the results.
Our study has limitations. It was retrospective and based on not standardized data and medical records which may be critical to the results. Moreover, another criticism is due to the fact that none of the patients have used other vitamin D analogs, such as paricalcitol and calcimimetics, whose results have demonstrated better clinical response than with traditional analogs of vitamin D. Two large studies have shown good responses to reduce PTH levels but failed to demonstrate reduction of morbidity and cardiovascular mortality: The EVOLVE and the PRIMO[54-56]. The first was done with cinacalcet and the second with paricalcitol. Although calcimimetics have reduced the need for surgical PTX, a Cochrane recent review showed no reduction in mortality in the population with CKD stage V with the use of cinacalcet[57].
In conclusion, patients with PTH > 800 pg/mL and parathyroid nodule presented worse bone metabolic profile than those without nodules. Hypercalcemia, hyperphosphatemia and elevated Ca × P product were associated with higher mortality in this population and PTX was associated with improvements in the control of Ca, P levels and Ca × P product, a lower occurrence of vascular events and longer survival in patients with severe SHPT.
Thus, the presence of nodules on US could be used as a criterion for PTX indication in this group of patients. Furthermore, it is possible that the presence of parathyroid nodule at US may be useful for the prediction of mortality or vascular events in patients with PTH levels higher than 800 pg/mL, although our study did not show this association.
We thank all patients for their great collaboration and their families for the valuable support.
Secondary hyperparathyroidism has a high prevalence in the chronic renal dialysis population as cause of cardiovascular morbidity and mortality. The presence of nodular hyperplasia in parathyroids in ultrasound could predict response to drug treatment and guides the surgical treatment.
To identify prognostic factors of response to pharmacological treatment with non invasive tests, in order to propose new therapies or indicate surgical treatment.
Ultrasonography could be an adjuvant exam in the follow-up of patients with severe hyperparathyroidism, in order to plan the treatment.
This study confirms the role of secondary hyperparathyroidism in cardiovascular mortality in chronic kidney disease patients in dialysis, associated with bone mineral disorders.
Secondary hyperparathyroidism is a hormonal disorder triggered by many metabolic abnormalities that are related to renal function impairment.
The authors support that “The identification of nodules at ultrasonography strengthens the indication for parathyroidectomy in patients with secondary hyperparathyroidism due to renal failure”.
Manuscript source: Unsolicited manuscript
Specialty type: Urology and nephrology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Nechifor G, Paraskevas KI S- Editor: Gong XM L- Editor: A E- Editor: Lu YJ
| 1. | Palmer SC, Hayen A, Macaskill P, Pellegrini F, Craig JC, Elder GJ, Strippoli GF. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA. 2011;305:1119-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 513] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 2. | Costa AF, dos Reis LM, Ribeiro MC, Moysés RM, Jorgetti V. Effects of calcitriol on parathyroid function and on bone remodelling in secondary hyperparathyroidism. Nephrol Dial Transplant. 2003;18:743-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Katoh N, Nakayama M, Shigematsu T, Yamamoto H, Sano K, Saito I, Nakano H, Kasai K, Kubo H, Sakai S. Presence of sonographically detectable parathyroid glands can predict resistance to oral pulsed-dose calcitriol treatment of secondary hyperparathyroidism. Am J Kidney Dis. 2000;35:465-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Tominaga Y, Tanaka Y, Sato K, Nagasaka T, Takagi H. Histopathology, pathophysiology, and indications for surgical treatment of renal hyperparathyroidism. Semin Surg Oncol. 1997;13:78-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Silver J, Naveh-Many T. FGF23 and the parathyroid glands. Pediatr Nephrol. 2010;25:2241-2245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Penido MG, Alon US. Phosphate homeostasis and its role in bone health. Pediatr Nephrol. 2012;27:2039-2048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 260] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 7. | Tsuchiya K, Nagano N, Nitta K. Klotho/FGF23 Axis in CKD. Contrib Nephrol. 2015;185:56-65. [PubMed] |
| 8. | Kates DM, Sherrard DJ, Andress DL. Evidence that serum phosphate is independently associated with serum PTH in patients with chronic renal failure. Am J Kidney Dis. 1997;30:809-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Drueke T, Martin D, Rodriguez M. Can calcimimetics inhibit parathyroid hyperplasia? Evidence from preclinical studies. Nephrol Dial Transplant. 2007;22:1828-1839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | de Francisco AL, Fresnedo GF, Rodrigo E, Piñera C, Amado JA, Arias M. Parathyroidectomy in dialysis patients. Kidney Int Suppl. 2002;161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Tokumoto M, Tsuruya K, Fukuda K, Kanai H, Kuroki S, Hirakata H. Reduced p21, p27 and vitamin D receptor in the nodular hyperplasia in patients with advanced secondary hyperparathyroidism. Kidney Int. 2002;62:1196-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Fukagawa M, Nakanishi S, Kazama JJ. Basic and clinical aspects of parathyroid hyperplasia in chronic kidney disease. Kidney Int Suppl. 2006;S3-S7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Gogusev J, Duchambon P, Hory B, Giovannini M, Goureau Y, Sarfati E, Drüeke TB. Depressed expression of calcium receptor in parathyroid gland tissue of patients with hyperparathyroidism. Kidney Int. 1997;51:328-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Cañadillas S, Canalejo A, Santamaría R, Rodríguez ME, Estepa JC, Martín-Malo A, Bravo J, Ramos B, Aguilera-Tejero E, Rodríguez M. Calcium-sensing receptor expression and parathyroid hormone secretion in hyperplastic parathyroid glands from humans. J Am Soc Nephrol. 2005;16:2190-2197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42:S1-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 904] [Cited by in RCA: 870] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 16. | Tominaga Y. Current status of parathyroidectomy for secondary hyperparathyroidism in Japan. NDT Plus. 2008;1:iii35-iii38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Schömig M, Ritz E. Management of disturbed calcium metabolism in uraemic patients: 2. Indications for parathyroidectomy. Nephrol Dial Transplant. 2000;15 Suppl 5:25-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Tomić-Brzac H, Pavlović D. [Ultrasonography methods in the diagnosis of renal osteodystrophy]. Acta Med Croatica. 2004;58:43-49. [PubMed] |
| 19. | Pavlovic D, Tomic Brzac H. Ultrasonographic evaluation of parathyroid hyperplasia in dialysis patients. ScientificWorldJournal. 2006;6:1599-1608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Fukagawa M, Kitaoka M, Yi H, Fukuda N, Matsumoto T, Ogata E, Kurokawa K. Serial evaluation of parathyroid size by ultrasonography is another useful marker for the long-term prognosis of calcitriol pulse therapy in chronic dialysis patients. Nephron. 1994;68:221-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 116] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Khati N, Adamson T, Johnson KS, Hill MC. Ultrasound of the thyroid and parathyroid glands. Ultrasound Q. 2003;19:162-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Randel SB, Gooding GA, Clark OH, Stein RM, Winkler B. Parathyroid variants: US evaluation. Radiology. 1987;165:191-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 30] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Vulpio C, Bossola M. Are parathyroid glands detectable by ultrasound also in normal PTH range haemodialysis patients? Nephrol Dial Transplant. 2008;23:4076-4077; author reply 4077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 24. | Fukagawa M, Tominaga Y, Kitaoka M, Kakuta T, Kurokawa K. Medical and surgical aspects of parathyroidectomy. Kidney Int Suppl. 1999;73:S65-S69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Kovesdy CP, Ahmadzadeh S, Anderson JE, Kalantar-Zadeh K. Secondary hyperparathyroidism is associated with higher mortality in men with moderate to severe chronic kidney disease. Kidney Int. 2008;73:1296-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 124] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 26. | Schwarz S, Trivedi BK, Kalantar-Zadeh K, Kovesdy CP. Association of disorders in mineral metabolism with progression of chronic kidney disease. Clin J Am Soc Nephrol. 2006;1:825-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 191] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 27. | Jofré R, López Gómez JM, Menárguez J, Polo JR, Guinsburg M, Villaverde T, Pérez Flores I, Carretero D, Rodríguez Benitez P, Pérez García R. Parathyroidectomy: whom and when? Kidney Int Suppl. 2003;S97-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Khosla S, Melton LJ, Wermers RA, Crowson CS, O’Fallon Wm, Riggs Bl. Primary hyperparathyroidism and the risk of fracture: a population-based study. J Bone Miner Res. 1999;14:1700-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 232] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 29. | Atsumi K, Kushida K, Yamazaki K, Shimizu S, Ohmura A, Inoue T. Risk factors for vertebral fractures in renal osteodystrophy. Am J Kidney Dis. 1999;33:287-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 220] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 30. | Lewin E, Olgaard K. Influence of parathyroid mass on the regulation of PTH secretion. Kidney Int Suppl. 2006;S16-S21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Tomić Brzac H, Pavlović D, Halbauer M, Pasini J. Parathyroid sonography in secondary hyperparathyroidism: correlation with clinical findings. Nephrol Dial Transplant. 1989;4:45-50. [PubMed] |
| 32. | Hamada C, Fukui M, Sakamoto T, Koizumi M, Ishiguro C, Osada S, Shou I, Hayashi K, Tomino Y. Evaluation of parathyroid hyperplasia by ultrasonographic examination in patients with end-stage renal failure before and at initiation of dialysis. Nephrology (Carlton). 2003;8:116-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Mendes V, Jorgetti V, Nemeth J, Lavergne A, Lecharpentier Y, Dubost C, Cournot-Witmer C, Bourdon R, Bourdeau A, Zingraff J. Secondary hyperparathyroidism in chronic haemodialysis patients: a clinico-pathological study. Proc Eur Dial Transplant Assoc. 1983;20:731-738. [PubMed] |
| 34. | Salem MM. Hyperparathyroidism in the hemodialysis population: a survey of 612 patients. Am J Kidney Dis. 1997;29:862-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Okuno S, Ishimura E, Kitatani K, Chou H, Nagasue K, Maekawa K, Izumotani T, Yamakawa T, Imanishi Y, Shoji T. Relationship between parathyroid gland size and responsiveness to maxacalcitol therapy in patients with secondary hyperparathyroidism. Nephrol Dial Transplant. 2003;18:2613-2621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Kestenbaum B, Seliger SL, Gillen DL, Wasse H, Young B, Sherrard DJ, Weiss NS, Stehman-Breen CO. Parathyroidectomy rates among United States dialysis patients: 1990-1999. Kidney Int. 2004;65:282-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 37. | Foley RN, Li S, Liu J, Gilbertson DT, Chen SC, Collins AJ. The fall and rise of parathyroidectomy in U.S. hemodialysis patients, 1992 to 2002. J Am Soc Nephrol. 2005;16:210-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 38. | Takagi H, Tominaga Y, Uchida K, Yamada N, Kawai M, Kano T, Morimoto T. Subtotal versus total parathyroidectomy with forearm autograft for secondary hyperparathyroidism in chronic renal failure. Ann Surg. 1984;200:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 44] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Malberti F, Marcelli D, Conte F, Limido A, Spotti D, Locatelli F. Parathyroidectomy in patients on renal replacement therapy: an epidemiologic study. J Am Soc Nephrol. 2001;12:1242-1248. [PubMed] |
| 40. | Costa-Hong V, Jorgetti V, Gowdak LH, Moyses RM, Krieger EM, De Lima JJ. Parathyroidectomy reduces cardiovascular events and mortality in renal hyperparathyroidism. Surgery. 2007;142:699-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 41. | Zaraca F, Mazzaferro S, Catarci M, Saputelli A, Alò P, Carboni M. Prospective evaluation of total parathyroidectomy and autotransplantation for the treatment of secondary hyperparathyroidism. Arch Surg. 1999;134:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Pasieka JL, Parsons LL. A prospective surgical outcome study assessing the impact of parathyroidectomy on symptoms in patients with secondary and tertiary hyperparathyroidism. Surgery. 2000;128:531-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Bleyer AJ, Burkart J, Piazza M, Russell G, Rohr M, Carr JJ. Changes in cardiovascular calcification after parathyroidectomy in patients with ESRD. Am J Kidney Dis. 2005;46:464-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 44. | Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208-2218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1878] [Cited by in RCA: 1883] [Article Influence: 89.7] [Reference Citation Analysis (0)] |
| 45. | Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31:607-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1705] [Cited by in RCA: 1646] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 46. | Kanbay M, Goldsmith D, Akcay A, Covic A. Phosphate - the silent stealthy cardiorenal culprit in all stages of chronic kidney disease: a systematic review. Blood Purif. 2009;27:220-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 47. | Llach F. Hyperphosphatemia in end-stage renal disease patients: pathophysiological consequences. Kidney Int Suppl. 1999;73:S31-S37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 48. | Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK. Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol. 2001;12:2131-2138. [PubMed] |
| 49. | Noordzij M, Korevaar JC, Bos WJ, Boeschoten EW, Dekker FW, Bossuyt PM, Krediet RT. Mineral metabolism and cardiovascular morbidity and mortality risk: peritoneal dialysis patients compared with haemodialysis patients. Nephrol Dial Transplant. 2006;21:2513-2520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 50. | Carlstedt F, Lind L, Wide L, Lindahl B, Hänni A, Rastad J, Ljunghall S. Serum levels of parathyroid hormone are related to the mortality and severity of illness in patients in the emergency department. Eur J Clin Invest. 1997;27:977-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 51. | Hagström E, Hellman P, Larsson TE, Ingelsson E, Berglund L, Sundström J, Melhus H, Held C, Lind L, Michaëlsson K. Plasma parathyroid hormone and the risk of cardiovascular mortality in the community. Circulation. 2009;119:2765-2771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 309] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 52. | Foley RN. Phosphate levels and cardiovascular disease in the general population. Clin J Am Soc Nephrol. 2009;4:1136-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 53. | Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA. Serum phosphorus levels associate with coronary atherosclerosis in young adults. J Am Soc Nephrol. 2009;20:397-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 279] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 54. | Pritchett Y, Jemiai Y, Chang Y, Bhan I, Agarwal R, Zoccali C, Wanner C, Lloyd-Jones D, Cannata-Andía JB, Thompson T. The use of group sequential, information-based sample size re-estimation in the design of the PRIMO study of chronic kidney disease. Clin Trials. 2011;8:165-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 55. | Chertow GM, Correa-Rotter R, Block GA, Drueke TB, Floege J, Goodman WG, Herzog CA, Kubo Y, London GM, Mahaffey KW. Baseline characteristics of subjects enrolled in the Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE) trial. Nephrol Dial Transplant. 2012;27:2872-2879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 56. | Moe SM, Thadhani R. What have we learned about chronic kidney disease-mineral bone disorder from the EVOLVE and PRIMO trials? Curr Opin Nephrol Hypertens. 2013;22:651-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 57. | Ballinger AE, Palmer SC, Nistor I, Craig JC, Strippoli GF. Calcimimetics for secondary hyperparathyroidism in chronic kidney disease patients. Cochrane Database Syst Rev. 2014;12:CD006254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |