Published online Jul 6, 2015. doi: 10.5527/wjn.v4.i3.423
Peer-review started: November 18, 2014
First decision: February 7, 2015
Revised: March 8, 2015
Accepted: April 28, 2015
Article in press: April 30, 2015
Published online: July 6, 2015
Processing time: 234 Days and 21.1 Hours
AIM: To quantify changes in urinary excretion of aquaporin2 water channels (u-AQP2), the sodium-potassium-chloride co-transporter (u-NKCC2) and the epithelial sodium channels (u-ENaC) during treatment with bendroflumethiazide (BFTZ), amiloride and placebo.
METHODS: In a randomized, double-blinded, placebo-controlled, 3-way crossover study we examined 23 healthy subjects on a standardized diet and fluid intake. The subjects were treated with amiloride 5 mg, BFTZ 1.25 mg or placebo twice a day for 4.5 d before each examination day. On the examination day, glomerular filtration rate was measured by the constant infusion clearance technique with 51Cr-EDTA as reference substance. To estimate the changes in water transport via AQP2 and sodium transport via NKCC2 and ENaC, u-NKCC2, the gamma fraction of ENaC (u-ENaCγ), and u-AQP2 were measured at baseline and after infusion with 3% hypertonic saline. U-NKCC2, u-ENaCγ, u-AQP2 and plasma concentrations of vasopressin (p-AVP), renin (PRC), angiotensin II (p-ANG II) and aldosterone (p-Aldo) were measured, by radioimmunoassay. Central blood pressure was estimated by applanation tonometry and body fluid volumes were estimated by bio-impedance spectroscopy. General linear model with repeated measures or related samples Friedman’s two-way analysis was used to compare differences. Post hoc Bonferroni correction was used for multiple comparisons of post infusion periods to baseline within each treatment group.
RESULTS: At baseline there were no differences in u-NKCC2, u-ENaCγ and u-AQP2. PRC, p-Ang II and p-Aldo were increased during active treatments (P < 0.001). After hypertonic saline, u-NKCC2 increased during amiloride (6% ± 34%; P = 0.081) and increased significantly during placebo (17% ± 24%; P = 0.010). U-AQP2 increased significantly during amiloride (31% ± 22%; P < 0.001) and placebo (34% ± 27%; P < 0.001), while u-NKCC2 and u-AQP2 did not change significantly during BFTZ (-7% ± 28%; P = 0.257 and 5% ± 16%; P = 0.261). U- ENaCγ increased in all three groups (P < 0.050). PRC, AngII and p-Aldo decreased to the same extent, while AVP increased, but to a smaller degree during BFTZ (P = 0.048). cDBP decreased significantly during BFTZ (P < 0.001), but not during amiloride or placebo. There were no significant differences in body fluid volumes.
CONCLUSION: After hypertonic saline, u-NKCC2 and u-AQP2 increased during amiloride, but not during BFTZ. Lower p-AVP during BFTZ potentially caused less stimulation of NKCC2 and AQP2 and subsequent lower reabsorption of water and sodium.
Core tip: Measurements of urinary sodium-potassium chloride co-transporter (NKCC2), epithelial sodium channel (ENaC) and aquaporin2 (AQP2) can be used as biomarkers of water- and sodium transport in the nephron. However, it has never been studied to what extent the function of NKCC2, ENaC and AQP2 is simultaneously affected in response to diuretics. The present study showed that infusion of 3% saline increased u-NKCC2 and u-AQP2 during amiloride and placebo, while u-NKCC2 and u-AQP2 remained unchanged during bendroflumethiazide. Therefor, in contrast to amiloride, bendroflumethiazide caused the absence of a compensatory reabsorption of sodium via NKCC2 and water via AQP2.
- Citation: Jensen JM, Mose FH, Kulik AEO, Bech JN, Fenton RA, Pedersen EB. Changes in urinary excretion of water and sodium transporters during amiloride and bendroflumethiazide treatment. World J Nephrol 2015; 4(3): 423-437
- URL: https://www.wjgnet.com/2220-6124/full/v4/i3/423.htm
- DOI: https://dx.doi.org/10.5527/wjn.v4.i3.423
During normal conditions, approximately 60% of filtered sodium is absorbed in the proximal tubules and 30% of sodium is absorbed in the kidneys via the sodium-potassium chloride co-transporter (NKCC2) in the thick ascending limb of Henle’s loop (TAL). The distal convoluted tubules are responsible for 5%-10% of sodium reabsorption via the sodium chloride co-transporter (NCC)[1]. Thiazides inhibit NCC in distal tubules and decrease sodium reabsorption[2]. In the collecting duct the epithelial sodium channel (ENaC) is responsible for the reabsorption of 3%-5% of filtered sodium[1]. Amiloride is a potassium sparing selective inhibitor of ENaC channels[3]. Water is predominantly reabsorbed in the proximal tubules and thin descending limb of Henle’s loop[1]. In the collecting ducts water absorption depends on passive transport via AQP2 water channels and is regulated by vasopressin (AVP)[4]. AQP2 can be excreted into urine[5,6], and may be used as a biomarker of collecting duct water transport[7-9]. Similarly, urinary excretion of beta ENaC correlates with changes in urinary sodium excretion[10]. Recently, our group documented changes in transport of water via AQP2 and sodium via ENaC in healthy subjects after infusion of isotonic glucose or hypertonic and isotonic saline, by measurements of urinary excretion of AQP2 (u-AQP2) and gamma ENaC (u-ENaCγ)[11] and abnormal urinary excretion of NKCC2 (u-NKCC2) and u-AQP2 in patients with chronic kidney disease[12].
In the present randomised, placebo-controlled study present study in healthy young subjects, we hypothesize that excretion of NKCC2 will not be effected but compensatory increases in distal transporter activity will occur during thiazide treatment but not during amiloride. This is compared to placebo both at baseline and in response to a saline load. Therefore, the aim was to quantify changes in urinary excretion of NKCC2, u-ENaCγ and u-AQP2 as estimates of tubular water and sodium handling at baseline conditions and after 3% saline infusion, during treatment with bendroflumethiazide (BFTZ), amiloride and placebo. In addition, changes in renal tubular function, vasoactive hormones, body fluid volumes and blood pressure were measured. The novelty of this study is due to; measurements of u-NKCC2 and the interplay with ENaC, AQP2 and the regulating mechanisms involved in water and sodium homeostasis, while simultaneously antagonizing NCC with BFTZ and ENaC with amiloride. Quantification of sodium- and water channel proteins in urine during different conditions may provide important information of the mechanisms involved in water and sodium balance in the nephron.
The trial was conducted as a randomized, double-blinded, placebo-controlled, 3-way crossover study. Subjects were randomized to tablet BFTZ, amiloride or placebo for 4.5 d. Each treatment period was followed by an examination day, separated by at least 3 wk.
Eligible participants were healthy non-smoking men and women aged between 18-45 years. Exclusion criteria were clinical signs or history of heart, lung, kidney, endocrine or malignant disease; abnormal findings in ECG, urine dipstick or biochemistry [blood cell count, plasma concentrations of glucose, bilirubin, alanine aminotransferase, alkaline phosphatase, sodium, potassium, creatinine, albumin, cholesterol and haemoglobin; arterial hypertension (24 h ambulatory BP > 130/80 mmHg); medical treatment (except oral contraceptives); alcohol and substance abuse, i.e., more than 21 alcoholic drinks per week for males and 14 drinks for females]; current smoking; pregnancy, breast feeding; donation of blood within one month prior to the study and obesity (BMI > 32 kg/m2). Withdrawal criteria were, development of conditions given in exclusion criteria during the study, withdrawal of informed consent and poor compliance.
Participants were recruited through advertisement at public institutions in Holstebro, Denmark.
The study took place at Department of Medical Research, University Clinic of Nephrology and Hypertension, Regional Hospital Holstebro, Denmark, from 1st of August 2012 until 13th of September 2013.
This study was reviewed and approved by the Regional Committees on Health Research Ethics, Skottenborg 26, Viborg, Denmark (j.no 1-10-72-178-12) and was carried out in accordance with the Helsinki Declaration. All study participants provided informed written consent prior to study enrolment.
The main effect variable was u-NKCC2. Secondary effect variables were: u-AQP2, u- ENaCγ, glomerular filtration rate (GFR), free water clearance (CH2O), urine output (UO), urinary excretion of sodium (u-Na) and potassium (u-K), fractional excretion of sodium (FENa) and potassium (FEK), plasma sodium (p-Na) and potassium (p-K), plasma osmolality (p-osm) and plasma albumin (p-alb), plasma concentration of renin (PRC), angiotensin II (p-AngII), aldosterone (p-Aldo), vasopressin (p-AVP), extracellular fluid volume (ECV), intracellular fluid volume (ICV), total body water (TBW), brachial systolic- (bSBP) and diastolic blood pressure (bDBP), pulse wave velocity (PWV) and central systolic- (cSBP) and diastolic blood pressure (cDBP).
Using a significance level of 5% and a power of 80% it was calculated that the number of subjects should be 16, when the minimal relevant difference in u-NKCC2 was 0.3 ng/min and SD was 0.3 ng/min. In this study, incomplete voiding during study days was expected in some subjects; therefore, 20 subjects were included as a minimum.
Subjects were randomized to treatment using block randomization conducted at http://www.randomization.com. Aarhus Hospital Pharmacy, Denmark, generated the randomization sequence into five blocks of six from 01-30 and labeled the bottles. Five days prior to each examination day, participants received a numbered bottle containing BFTZ, amiloride or placebo tablets. BFTZ, amiloride and placebo were capsulated in grey DB Caps® size B (Capsugel) with click effect to obtain blinding. The randomization code was kept at Aarhus Hospital Pharmacy during the trial. Individual randomization codes were kept in sealed envelopes at Department of Medical Research if necessary for the investigator to know the given treatment. Investigators, participants and other study personnel were blinded to treatment assignment for the duration of the study.
Experimental procedure prior to the study day: Four days prior to each study day, subjects consumed a standardized diet regarding calories, sodium and fluid. The diet consisted of 11000 (kJ/d) with an energy distribution of 55% carbohydrates, 30% fat and 15% protein in accordance to general dietary guidelines. The sodium content was approximately 120-150 mmol pr. day. The subjects were asked to drink 2500 mL/d. No alcohol or soft drink consumption was allowed while on the standardized diet. A maximum of two cups (6 oz.) of coffee or tea was allowed daily. Subjects were instructed to keep their usual physical activity during the experiments but to abstain from hard training the day prior to the examination. A 24-h urine collection from 7:00 AM to 7:00 AM on the examination day was used to assess water and sodium balance. A 24-h ambulatory BP measurement was performed to evaluate the effect of the intervention on blood pressure (Table 1).
| Before the study day | On the study day | |||||||||||||||
| Day-4 | Day-3 | Day-2 | Day-1 | 6:00-8:00 | 8:00-08:30 | 8:30-09:00 | 9:00-9:30 | 9:30-10:00 | 10:00-10:30 | 10:30-11:00 | 11:00-11:30 | 11:30-12:00 | 12:00-12:30 | 12:30-13:00 | 13:00-13:30 | |
| Periods | Baseline | Infusion | Post Infusion | |||||||||||||
| Time | 0 | 30 | 60 | 90 | 120 | 150 | 180 | 210 | 240 | |||||||
| Diet | x | x | x | x | ||||||||||||
| Study drug | xx | xx | xx | xx | x | |||||||||||
| 24-h BP | x------ | -----x | ||||||||||||||
| 24-h urine | x------ | -----x | ||||||||||||||
| IV access | x | |||||||||||||||
| Weight | x | x | x | x | ||||||||||||
| Water load | x--------------------------------------------------------------------------- 175 mL every 30 min ----------------------------------------------------------------------------------------------- x | |||||||||||||||
| Urine sample | (x) | x | x | x | x | x | x | x | x | |||||||
| Blood samples | x | x | x | x | x | x | x | x | ||||||||
| Blood pressure | x | x | x | x | x | x | x | x | x | |||||||
| 51Cr-EDTA | x ------------------------------------------------------------------------------------------------------------------------------------------------------------------ x | |||||||||||||||
| IV. fluid | x-----------------x | |||||||||||||||
| App.Ton | x | x | ||||||||||||||
| BIS | x | x | x | x | ||||||||||||
During the four-day diet and the morning of the examination day, participants were randomized to capsules containing either 1.25 mg BFTZ, 5 mg amiloride or matching placebo twice daily at 7 AM and 6 PM.
Table 1 shows the time points of study interventions. Following an overnight fast, subjects arrived at our research facility at 8:00 AM. Two indwelling catheters for blood sampling and administration of 51Cr-EDTA and fluid were placed in both cubital veins. Every 30 min, starting at arrival, participants received an oral water load of 175 mL. Urine was collected in standing or sitting position. Otherwise, subjects were kept in a supine position in a quiet temperature-controlled room (22 °C-25 °C). At 9:00 AM a priming dose of 51Cr-EDTA was administered, followed by sustained infusion. Three 30-min baseline clearance periods were obtained from 9:30 AM to 11:00 AM. The baseline periods were followed by an infusion period from 11:00 AM to 12:00 PM during which a sustained infusion of 3% hypertonic saline was administered. The post infusion period consisted of three 30-min periods from 12:00 PM to 1:30 PM. Blood and urine samples were collected every 30 min from 8:30 AM to 1:30 PM.
Blood samples were drawn and analyzed for 51Cr-EDTA, p-sodium, p-potassium, p-albumin and p-osmolality. Analysis of PRC, p-Ang II, p-Aldo and p-AVP were conducted from blood samples drawn at 11:00 AM, 12:00 PM and 1:30 PM.
Urine samples were analyzed for u-51Cr-EDTA, u-sodium, u-potassium, u-creatinine and u-osmolality. Analysis of u-AQP2, u-NKCC2 and u-ENaCγ was conducted from the 24-h urine collection and clearance period 10:30-11:00 AM (basal); 11:00-12:00 AM (fluid infusion), 12:00-12:30 PM (30 min after cessation of fluid infusion) and 1:00-1:30 PM (90 min after cessation of fluid infusion). For data analysis, the 30-min periods from 9:30 AM to 1:30 PM were subdivided into: baseline (0-90 min), infusion period (90-150 min) and three post infusion period 150-180 min, 180-210 min and 210-240 min).
Measurements of PWV, augmentation index (Aix) and cBP were performed at 11:00 AM (before infusion) and 12:00 AM (after infusion). Body composition was measured at 8:30 AM, 11:00 AM, 12:00 PM and 1:30 PM (end of examination day).
Renal function: Glomerular filtration rate was measured by the constant infusion clearance technique with 51Cr-EDTA as reference substance. More than 15% variation in GFR between the three baseline periods led to the exclusion of clearance related analysis.
Fractional excretion of sodium and potassium was calculated as: [Sodium/potassium clearance (CNa/K)/GFR x 100%]. Free water clearance was calculated as: [Urine output (UO) - osmolar clearance (COSM)]. COSM was calculated as: [Urine osmolality/plasma osmolality x UO].
Blood samples: were centrifuged for 10 min at 2200 x g at 4 °C. Plasma hormone samples were kept frozen at -20 °C (AngII) and -80 °C (PRC, Aldo, and AVP) until assayed. Renin in plasma was determined using an immunoradiometric assay (CIS Bio International, Gif-Sur-Yvette Cedex, France). Minimal detection level was 1 pg/mL. The coefficients of variation were 14.5% (interassay) and 4.5% (intra assay). Aldosterone in plasma was determined by radioimmunoassay (Demeditec Diagnostics Systems Laboratories Inc., Webster, TX, United States). Minimal detection level was 22 pmol/L. The coefficients of variation were 8.2% (inter-assay) and 3.9% (intra-assay). Arginine vasopressin and Angiotensin II were extracted from plasma with C18 Sep-Pak (Water associates, Milford, MA, United States) and subsequently measured using radioimmunoassay as previously described[13]. The antibody against angiotensin II was obtained from the Department of Clinical Physiology, Glostrup Hospital, Glostrup, Denmark. Minimal detection level was 2 pmol/L. The coefficients of variation were 12% (inter-assay) and 8% (intra-assay). The antibody against AVP was a gift from Professor Jacques Dürr (Miami, FL, United States). Minimal detection level was 0.2 pmol/L. The coefficients of variation were 13% (inter-assay) and 9% (intra-assay).
Generation of NKCC2 specific antibody: A novel rabbit polyclonal antiserum against human NKCC2 (Slc12a2) was generated against the following peptide: CNITKTTPKKDGSIN by Genscript® (New Jersey, United States). The N-terminal cysteine was added for conjugation to carrier protein and for attaching the peptide to the affinity purification column. The immune serum from two rabbits (#593 and #594) was affinity purified using immunizing peptides, resulting in NKCC2-specific antibodies. NKCC2 antibody characterization has previously been described[12].
Urine sample immunoassays: Urines were stored frozen at -20 °C until assayed.
U-NKCC2 was measured in urine by a newly developed radioimmunoassay[12]. Antibodies were raised in rabbits against human NKCC2 (Slc12a2) against the peptide CNITKTTPKKDGSIN. The N-terminal cysteine was added for conjugation to carrier protein and affinity purification. Minimal detection level was 0.5 ng/tube. The coefficients of variation were 14% (inter-assay) and 6.8% (intra-assay).
U-AQP2 was measured by radioimmunoassay as previously described[9,14]. Antibodies were raised in rabbits to a synthetic peptide corresponding to the 15 COOH-terminal amino acids in human AQP2 to which was added an NH2-terminal cysteine for conjugation and affinity purification. Minimal detection level was 34 pg/tube per tube. The coefficients of variation were 11.7% (inter-assay) and 5.9% (intra-assay).
U-ENaCγ was measured by radioimmunoassay as previously described[15,16]. Antibodies were raised against a synthetic ENaCγ peptide in rabbits and affinity purified[17]. Minimal detection level was 48 pg/tube. The coefficients of variation were 14% (inter-assay) and 6.7% (intra-assay).
Blood pressure measurement: Twenty-four hours BP was measured using Kivex TM-2430 (Kivex, Hoersholm, Denmark). Measurements were taken every 15 min during daytime and every 30 min overnight. Brachial blood pressure was recorded using a semiautomatic oscillometric device (Omron 705IT, Omron Matsusaka, Japan).
Plasma and urine: Concentrations of sodium, potassium, creatinine and albumin were measured using routine methods at the Department of Clinical Biochemistry, Holstebro Hospital.
Plasma and urine osmolality was measured by freezing point depression (Advanced Model 3900 multisampling osmometer).
Bioimpedance spectroscopy: Was performed at 50 frequencies, from 5 to 1000 kHz using the Fresenius Body Composition Monitor and the Fluid Management Tool, version 3.
Applanation tonometry: Recordings of PWA and carotid-femoral PWV were obtained by applanation tonometry (SphygmoCor® CPV system®, AtCor Medical, Sydney, Australia) as double-recordings by a trained observer. Only duplicate recording meeting the quality requirements were included in the final analysis. An operator index of 80 or more was required to accept recordings of a peripheral pulse-wave form[18].
Bendroflumethiazide [Tablet Salures 2.5 mg (1/2 tablet)] were obtained from Pfizer AB, Sollentuna, Sweden. Amiloride (Tablet Amilorid Mylan 5 mg) were obtained from Mylan AB, Stockholm, Sweden via Tjellesen Max Jenne A/S, Medilink A/S, Roedovre, Denmark.
Statistical analyses were performed by the authors using IBM SPSS statistics version 20.0.0 (IBM Corp.; Armonk, NY, United States).
As clearance data from the three baseline periods were very similar, single baseline values were obtained by taking the average of the measurements from the three baseline periods. Parametric data are presented as means ± SD and nonparametric data as medians with 25th and 75th percentiles. General linear model (GLM) with repeated measures was performed, with time as within-subject factor and intervention as between subject factor, to test for differences within and between groups. One-way ANOVA was used for comparison of means between groups when differences were found. For non-parametric data, related samples Friedman’s two-way analysis was used. Post hoc Bonferroni correction was used for multiple comparisons of post infusion periods to baseline within each treatment group. Statistical significance was defined as P < 0.050 in all analyses.
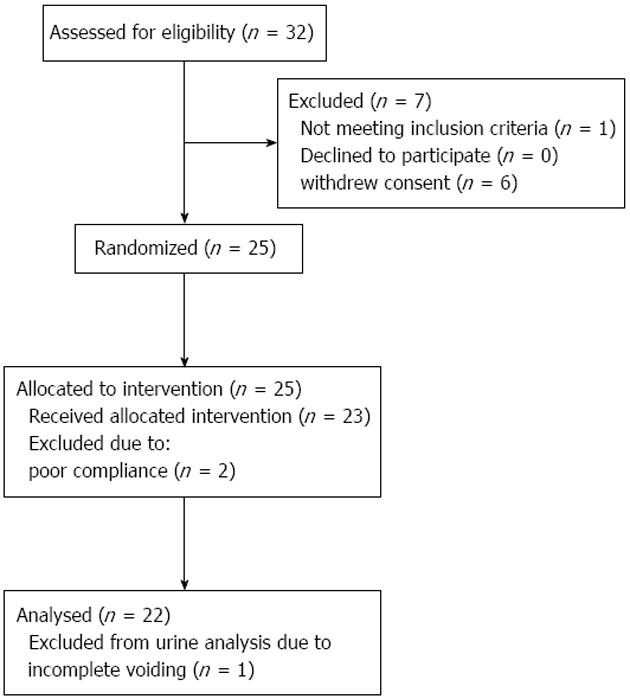
Thirty-two healthy women and men were assessed for eligibility. Nine were excluded due to: withdrawal of informed consent (6), non-compliance (2) or 24-h BP above 130/80 mmHg (1). Thus, 23 were initially allocated to and completed the study. One was not able to void satisfactorily during baseline clearance experiments and was excluded from urine analysis (Figure 1).
The 23 subjects (8 males; 15 females) who completed the trial had a mean age of 26 ± 4 years, BMI 24 ± 3 kg/m2, 24-h BP 117/69 ± 7/4 mmHg. Screening blood values were b-haemoglobin 8.4 ± 0.7 mmol/L, p-sodium 141 ± 1 mmol/L, p-potassium 3.7 ± 0.4 mmol/L, p-creatinine 79 ± 14 μmol/L, eGFR 93 ± 16 mL/min, p-albumin 43 ± 3 g/L, p-glucose 5±1 mmol/L, p-alanine transaminase 25 ± 19 U/L and p-cholesterol 4.4 ± 0.8 mmol/L. Baseline values did not differ between males and females, apart from p-crea (males: 91 ± 15 μmol/L vs females: 73 ± 7 μmol/L, P < 0.012), b-haemoglobin (males: 8.9 ± 0.6 mmol/L vs females: 8.2 ± 0.5 mmol/L, P < 0.016) and p-albumin (males: 45 ± 2 g/L vs females: 42 ± 3 g/L, P < 0.003).
UO, u-osm, CH2O, Creatinine-Clearance, u-Na, u-AQP2 and ENaCγ in 24-h urine were not significantly different between treatments. During BFTZ treatment u-NKCC2 and u-K were significant higher than both amiloride and placebo treatment (Table 2). Twenty-four hour ambulatory bSBP did not differ between treatments, however there was a small but significant lower bDBP during amiloride treatment (Table 2).
| Examination day | P (ANOVA) | |||
| Thiazide | Amilorid | Placebo | ||
| Urine output (mL/24 h) | 2527 ± 728 | 2418 ± 469 | 2316 ± 700 | 0.481 |
| u-osm (mosm/24 h) | 865 ± 158 | 835 ± 176 | 761 ± 187 | 0.087 |
| CH2O (mL/min) | -0.40 ± 0.33 | -0.37 ± 0.47 | -0.24 ± 0.53 | 0.534 |
| Cr.Cl (mL/min per m2) | 113 ± 25 | 118 ± 32 | 110 ± 26 | 0.549 |
| u-NKCC (ng/mmol) | 0.35 ± 0.07 | 0.30 ± 0.05 | 0.32 ± 0.06 | 0.025 |
| u-AQP2 (ng/mmol) | 113.2 ± 39.2 | 103.3 ± 25.5 | 98.5 ± 17.4 | 0.244 |
| u-ENaCγ (ng/mmol) | 37.8 ± 26.7 | 30.8 ± 17.3 | 32.7 ± 15.6 | 0.867 |
| u-Na (mmol/24 h) | 108 ± 34 | 121 ± 27 | 106 ± 37 | 0.263 |
| u-K (mmol/24 h) | 80 ± 20 | 64 ± 21 | 60 ± 19 | 0.002 |
| bSBP (mmHg) | 119 ± 6 | 114 ± 7 | 116 ± 7 | 0.213 |
| bDBP (mmHg) | 72 ± 4 | 69 ± 3 | 70 ± 4 | 0.034 |
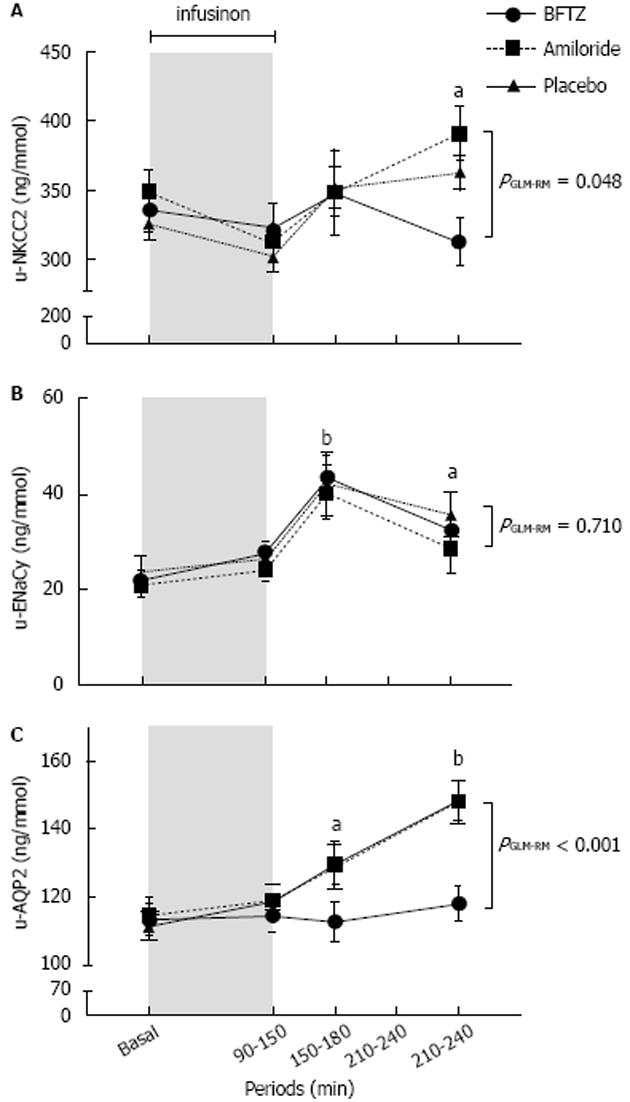
Effects of BFTZ and amiloride on u-NKCC2, u-ENaCγand u-AQP2
Figure 2 shows the changes in urinary excretion of AQP2, NKCC2 and ENaCγ during basal, infusion and post-infusion periods.
At baseline, u-NKCC2 did not differ between groups. U-NKCC2 decreased during the infusion period and increased during the first post infusion period in all three treatments. U-NKCC2 increased further during amiloride (6% ± 34%; P = 0.081) and placebo (17% ± 24%; P = 0.010), whereas u-NKCC2 declined in the BFTZ treated group (-7% ± 28%; P = 0.257), during the two last post infusion periods. By the end of the examination day there was a significant difference between BFTZ vs amiloride (P < 0.001) and vs placebo (P = 0.033). There was no significant difference between amiloride and placebo groups (P = 0.407).
At baseline, u-ENaCγ was similar. In response to 3% saline, u-ENaCγ increased significant to a maximum after the first post infusion period. Although u-ENaCγ tended to be lower during amiloride treatment, there was no statistical difference between the three treatment groups throughout the examination day.
There was no significant difference in u-AQP2 at baseline. In response to 3% saline, u-AQP2 increased significantly and similarly during amiloride (31% ± 22%; P < 0.001) and placebo treatment (34 ± 27%; P < 0.001), but did not change during BFTZ (5% ± 16%; P = 0.261). By the end of the examination day there was a significant difference between BFTZ vs amiloride and placebo (P < 0.001), but there was no difference between amiloride and placebo.
Divided by gender, the creatinine adjusted excretion of u-AQP2, u-NKCC2 and u-ENaCγ tended to be higher in females compared to males in all three treatment groups, but the difference is attributed to a lower urinary excretion of creatinine in females (data not shown).
Table 3 shows the absolute values of CH2O, UO, FENa, u-Na, FEK, u-K and 51Cr-EDTA clearance.
| Periods | Baseline | Infusion | Post infusion | PGLM RM | ||
| 0-90 min | 90-150 min | 150-180 min | 180-210 min | 210-240 min | ||
| CH2O < 0.001 | ||||||
| BFTZ | 3.1 ± 1.6a | -0.2 ± 0.5d | -1.6 ± 0.6d | -1.6 ± 0.7d | -0.6 ± 1.6d | |
| Amiloride | 3.7 ± 0.9 | -0.6 ± 0.7d | -2.1 ± 0.6d | -2.4 ± 0.8d | -1.4 ± 1.7d | |
| Placebo | 4.4 ± 1.1 | -0.6 ± 0.6d | 1.8 ± 0.7d | -2.5 ± 1.0d | -2.0 ± 0.5d | |
| PGLM between subjects 0.061 | ||||||
| PANOVA | 0.006 | NS | NS | 0.001 | 0.007 | |
| UO (mL/min) 0.029 | ||||||
| BFTZ | 6.1 ± 1.6 | 2.6 ± 0.6d | 1.4 ± 0.5d | 1.7 ± 1.0d | 2.7 ± 1.9d | |
| Amiloride | 6.8 ± 1.3 | 2.5 ± 0.6d | 1.6 ± 0.5d | 2.0 ± 0.7d | 3.2 ± 1.2d | |
| Placebo | 7.3 ± 1.2 | 2.3 ± 0.9d | 1.7 ± 0.8d | 2.2 ± 1.2d | 2.4 ± 1.1d | |
| PGLM between subjects 0.245 | ||||||
| PANOVA | 0.019 | NS | NS | NS | NS | |
| u-Na (mmol/min) 0.001 | ||||||
| BFTZ | 1.4 ± 0.4 | 1.7 ± 0.4b | 1.9 ± 0.7b | 2.1 ± 0.6d | 2.0 ± 0.6b | |
| Amiloride | 1.6 ± 0.5 | 2.0 ± 0.7 | 2.5 ± 1.0b | 2.9 ± 1.2d | 3.0 ± 1.0d | |
| Placebo | 1.3 ± 0.3 | 1.9 ± 1.1a | 2.5 ± 1.3b | 3.3 ± 1.7d | 3.0 ± 1.0d | |
| PGLM between subjects 0.020 | ||||||
| PANOVA | 0.028 | NS | NS | 0.007 | <0.001 | |
| FENa (%) < 0.001 | ||||||
| BFTZ | 1.5 ± 0.4 | 1.8 ± 0.5a | 2.0 ± 0.6b | 2.2 ±0.6d | 2.1 ± 0.6d | |
| Amiloride | 1.8 ± 0.6 | 2.1 ± 0.6a | 2.6 ± 1.0b | 2.9 ± 1.2d | 3.0 ± 1.1d | |
| Placebo | 1.4 ± 0.4 | 2.1 ± 1.0b | 2.7 ± 1.2d | 3.0 ± 1.1d | 3.1 ± 1.1d | |
| PGLM between subjects 0.036 | ||||||
| PANOVA | 0.022 | NS | NS | 0.019 | 0.001 | |
| u-K (mmol/min) | ||||||
| BFTZ | 20.3 ± 6.7 | 17.7 ± 5.3 | 15.8 ± 4.4b | 14.2 ± 5.6b | 13.3 ± 6.3b | < 0.001 |
| Amiloride | 18.5 ± 8.4 | 15.7 ± 9.3 | 18.6 ± 11.1 | 22.4 ± 12.0 | 23.3 ± 10.2a | |
| Placebo | 22.3 ± 9.3 | 15.6 ± 6.9d | 16.4 ± 8.5a | 22.3 ± 12.3 | 20.4 ± 7.9 | |
| PGLM between subjects 0.255 | ||||||
| PANOVA | NS | NS | NS | 0.015 | 0.001 | |
| FEK (%) | ||||||
| BFTZ | 21.7 ± 7.4 | 20.0 ± 6.6 | 16.1 ± 4.8b | 15.0 ± 5.9b | 14.4 ± 6.9b | < 0.001 |
| Amiloride | 20.8 ± 9.8 | 18.7 ± 10.8 | 20.5 ± 12.0 | 23.6 ± 12.4 | 25.0 ± 11.0a | |
| Placebo | 23.7 ± 9.3 | 18.1 ± 8.2b | 17.5 ± 8.9a | 20.6 ± 9.9 | 21.4 ± 8.8 | |
| PGLM between subjects 0.254 | ||||||
| PANOVA | NS | NS | NS | 0.018 | 0.001 | |
| 51Cr-EDTA (mL/min per 1.73m2) 0.271 | ||||||
| BFTZ | 92.1 ± 10.8 | 91.3 ± 11.9 | 96.0 ± 16.7 | 96.9 ± 16.8 | 96.5 ± 21.2 | |
| Amiloride | 92.7 ± 13.7 | 93.2 ± 12.8 | 94.1 ± 12.1 | 96.7 ± 14.1 | 100.2 ± 15.6 | |
| Placebo | 96.5 ± 9.5 | 90.0 ± 13.3 | 94.7 ± 15.1 | 102.4 ± 14.3 | 98.1 ± 15.9 | |
| PGLM between subjects 0.887 | ||||||
CH2O and UO decreased significantly in all three treatments. At baseline, CH2O was lower during BFTZ and showed an attenuated decrease at post infusion period 210-240 min compared to amiloride (P = 0.207) and placebo (P = 0.005).
At baseline, FENa and u-Na were higher during amiloride compared to BFTZ and placebo. After 3% saline infusion there was a significant increase in u-Na and FENa in all three treatments, but less pronounced during BFTZ (P = 0.001).
There was no difference in u-K at baseline. In response to 3% saline, u-K and FEK decreased during BFTZ and increased during amiloride compared to placebo. There was a significant difference between all three treatments (P = 0.001). GFR did not change significantly.
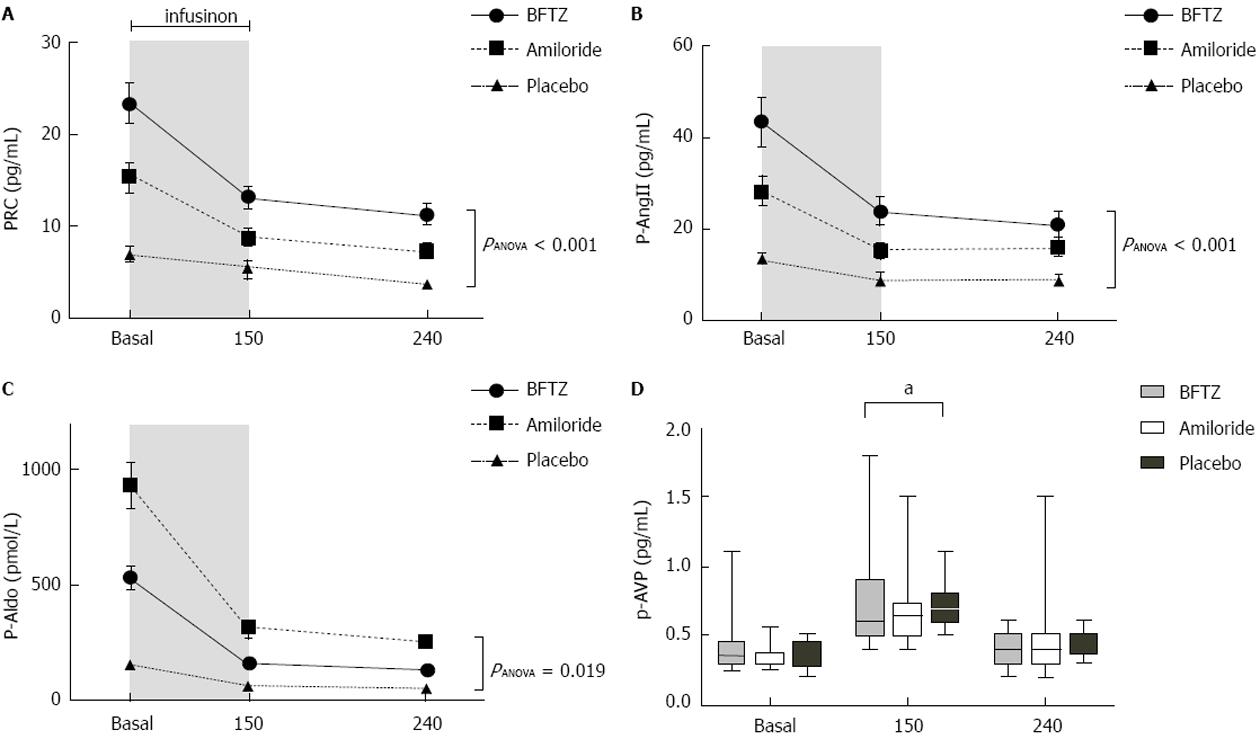
Figure 3 shows the changes in PRC, AngII, p-Aldo and p-AVP during the examination day. PRC, AngII and p-Aldo were significantly increased during active treatment compared to placebo. PRC and p-AngII were highest during BFTZ treatment (P < 0.001), whereas p-Aldo was highest during amiloride treatment (P < 0.001). PRC, AngII and p-Aldo declined significantly in response to 3% saline, in all three treatments, with no relative differences between treatments.
P-AVP was similar at baseline. P-AVP increased in all three groups, in response to 3% saline. Although, p-AVP was lower during BFTZ at 150 min (P = 0.048), the relative increase in p-AVP, after 3% saline, was not significantly different between BFTZ vs placebo (82% ± 100% vs 116% ± 67%; P = 0.072)
Table 4 shows the absolute values of p-Na, p-K, p-Osm and p-Alb during basal-, infusion- and post infusion periods. During baseline conditions p-osm and p-Na were significantly lower during BFTZ and amiloride compared to placebo. P-K was higher in the amiloride group compared to placebo and BFTZ, and p-K was lower during BFTZ compared to placebo.
| Time | Baseline | Infusion | Post infusion | PGLM-RM | ||
| 0-90 min | 150 min | 180 min | 210 min | 240 min | ||
| p-Na | 0.281 | |||||
| BFTZ | 137 ± 2 | 141 ± 2d | 141 ± 2d | 139 ± 2d | 139 ± 2d | |
| Amilorid | 137 ± 2 | 141 ± 2d | 141 ± 2d | 140 ± 2d | 139 ± 2d | |
| Placebo | 139 ± 1 | 43 ± 2d | 142 ± 1d | 141 ± 1d | 140 ± 1d | |
| PGLM between subjects 0.003 | ||||||
| PANOVA | 0.001 | 0.019 | 0.004 | 0.007 | 0.005 | |
| p-K | 0.001 | |||||
| BFTZ | 3.35 ± 0.22 | 3.32 ± 0.23 | 3.43 ± 0.26 | 3.42 ± 0.21 | 3.40 ± 0.20 | |
| Amilorid | 4.32 ± 0.30 | 4.17 ± 0.23d | 4.26 ± 0.27 | 4.27 ± 0.25 | 4.20 ± 0.20a | |
| Placebo | 3.89 ± 0.18 | 3.83 ± 0.22 | 3.97 ± 0.23 | 3.94 ± 0.22 | 3.92 ± 0.20 | |
| PGLM between subjects < 0.0001 | ||||||
| PANOVA | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| p-Osm | 0.600 | |||||
| BFTZ | 281 ± 5 | 288 ± 4d | 288 ± 6d | 286 ± 5d | 284 ± 4d | |
| Amilorid | 283 ± 4 | 290 ± 4d | 290 ± 3d | 287 ± 3d | 285 ± 3a | |
| Placebo | 286 ± 3 | 294 ± 4d | 292 ± 4d | 290 ± 4d | 289 ± 3b | |
| PGLM between subjects < 0.0001 | ||||||
| PANOVA | < 0.001 | < 0.001 | 0.005 | 0.002 | < 0.001 | |
| p-Alb (g/L) | 0.007 | |||||
| BFTZ | 40.7 ± 3.1 | 35.5 ± 2.2d | 35.9 ± 2.7d | 36.0 ± 2.8d | 36.0 ± 2.7d | |
| Amilorid | 40.9 ± 2.9 | 35.5 ± 2.3d | 36.4 ± 2.6d | 36.4 ± 2.7d | 36.3 ± 2.5d | |
| Placebo | 39.0 ± 2.4 | 34.5 ± 2.0d | 35.1 ± 2.2d | 35.1 ± 2.3d | 35.3 ± 2.4d | |
| PGLM between subjects 0.203 | ||||||
In response to 3% saline infusion, p-Na and p-osm increased to the same extent in all three treatments, but remained highest in the placebo group. P-K decreased significant in the amiloride group compared to BFTZ and placebo. P-alb decreased significantly in all three treatments, in response to 3% saline.
Table 5 shows the absolute values of bSBP, bDBP, pulse rate, cSBP, cDBP, PWV and AIx. At baseline there was no difference in bSBP or bDBP. In response to 3% hypertonic saline, bSBP increased and bDBP decreased. At the end of the day the decrease in bDBP was more pronounced during BFTZ (-6% ± 6%) compared to amiloride (-2% ± 6%; P = 0.030) and placebo (-2% ± 5%; P = 0.021).
| Periods | Baseline | Infusion | Post infusion | PGLM RM | ||
| 0-90 min | 150 min | 180 min | 210 min | 240 min | ||
| bSBP | ||||||
| BFTZ | 112 ± 9 | 118 ± 9d | 116 ± 10 | 116 ± 11 | 117 ± 13 | 0.825 |
| Amilorid | 110 ± 8 | 115 ± 9d | 114 ± 10d | 114 ± 10b | 114 ± 10b | |
| Placebo | 113 ± 10 | 117 ± 10d | 115 ± 9 | 115 ± 8 | 115 ± 10 | |
| PGLM between subjects | 0.679 | |||||
| bDBP | ||||||
| BFTZ | 66 ± 7 | 63 ± 7b | 62 ± 6d | 62 ± 7d | 62 ± 7b | 0.055 |
| Amilorid | 64 ± 4 | 62 ± 5 | 60 ± 5b | 61 ± 5 | 62 ± 6 | |
| Placebo | 64 ± 6 | 63 ± 5 | 63 ± 5 | 62 ± 6 | 63 ± 6 | |
| PGLM between subjects | 0.695 | |||||
| Pulse Rate | ||||||
| BFTZ | 58 ± 10 | 61 ± 11a | 61 ± 11b | 62 ± 10b | 62 ± 10b | 0.782 |
| Amilorid | 57 ± 10 | 61 ± 11d | 60 ± 10d | 61 ± 11d | 61 ± 11d | |
| Placebo | 55 ± 10 | 59 ± 12a | 59 ± 12b | 59 ± 12b | 59 ± 13a | |
| PGLM between subjects | 0.712 | |||||
| cSBP | ||||||
| BFTZ | 99 ± 7 | 98 ± 7 | NS | |||
| Amilorid | 96 ± 5 | 97 ± 7 | NS | |||
| Placebo | 98 ± 6 | 98 ± 8 | NS | |||
| PANOVA | NS | NS | ||||
| cDBP | ||||||
| BFTZ | 67 ± 5 | 63 ± 6 | < 0.001 | |||
| Amilorid | 65 ± 5 | 65 ± 6 | NS | |||
| Placebo | 65 ± 5 | 64 ± 5 | NS | |||
| PANOVA | NS | NS | ||||
| PWV | ||||||
| BFTZ | 5.5 ± 0.6 | 5.3 ± 0.4 | 0.055 | |||
| Amilorid | 5.3 ± 0.5 | 5.3 ± 0.5 | NS | |||
| Placebo | 5.3 ± 0.7 | 5.3 ± 0.6 | NS | |||
| PANOVA | NS | NS | ||||
| AI | ||||||
| BFTZ | -2.2 ± 14.6 | -5.9 ±17.7 | NS | |||
| Amilorid | 0.4 ± 12.5 | -1.4 ± 13.4 | NS | |||
| Placebo | -1.6 ± 14.6 | -4.9 ± 18.6 | 0.034 | |||
| PANOVA | NS | NS | ||||
There was no difference in cSBP at baseline or in response to 3% saline between treatments. At baseline cDBP was the same in all three treatments, however cDBP decreased significant in the BFTZ group (P < 0.001) but not during amiloride and placebo. PWV followed the same pattern, however the decrease during BFTZ treatment was not significant.
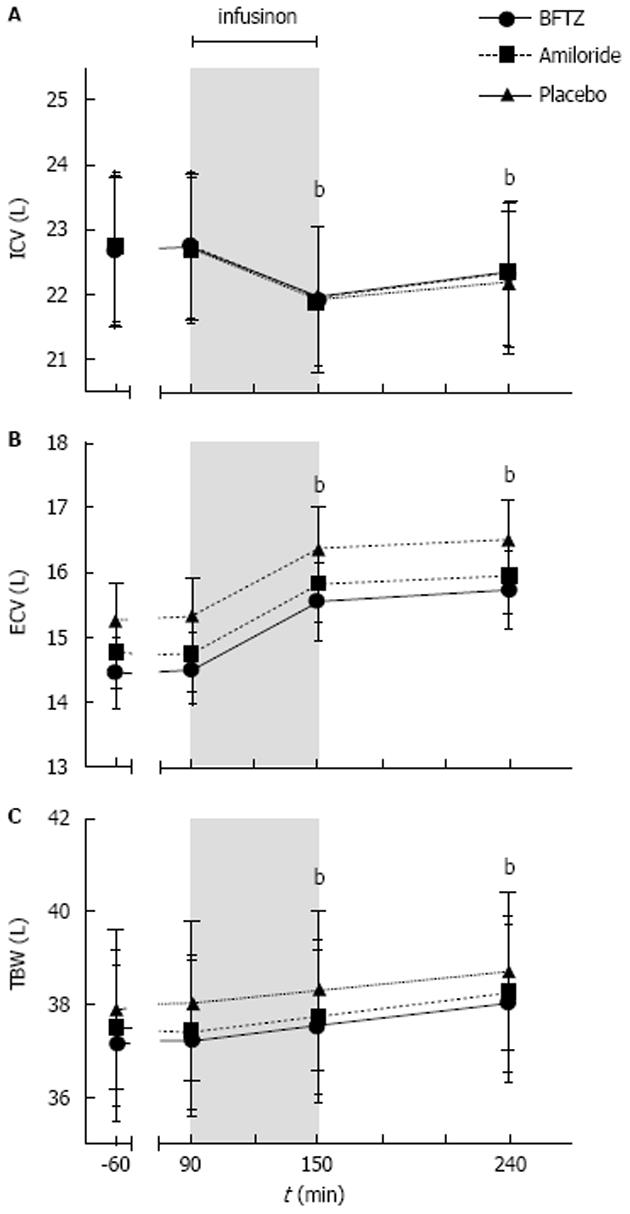
Figure 4 shows the changes in ICV, ECV and TBW during the examination day.
At baseline, ECV and TBW tended to be lower during amiloride (P = 0.515) and BFTZ (P = 0.951) compared to placebo. However, it did not reach statistical significance. ICV did not differ between treatments. As expected, after administering 3% saline, ICV decreased while ECV and TBW increased reaching a maximum at the end of the study day. Although there was a tendency towards a lower ECV and TBW in the two diuretic groups there were no statistically significant differences in volume status between the three treatments.
In the present study, the aim was to investigate the effect of five days BFTZ and amiloride treatment on the urinary excretion of NKCC2, ENaCγ and AQP2 during baseline conditions and after an acute intravenous volume load of 3% hypertonic saline in healthy subjects. To our knowledge, this study is the first randomized, placebo-controlled trial that measured the changes in u-NKCC2, u-ENaCγ and u-AQP2 during inhibition of the NCC cotransporter with BFTZ and ENaC with amiloride in humans.
This study showed that, in response to 3% saline, u-NKCC2, u-ENaCγ and u-AQP2 increased to the same extent during amiloride and placebo treatment, but neither u-NKCC2 nor u-AQP2 changed significantly during BFTZ.
Thiazides predominantly inhibit NCC along the distal convoluted tubules[2]. Animal studies have shown that when thiazide was administered chronically, urinary sodium returned to normal within 2-3 d[19]. This is in accordance with our findings in 24 h urine collection. Further, a study documented that longer term NCC inhibition might cause a structural adaption, which will activate ENaC and cause increased distal sodium reabsorption and kaliuresis[20]. Twenty-four hours urine collections, demonstrated a small, but significantly increased u-K, and increased u-ENaCγ during BFTZ compared to amiloride and placebo; which supports the theory of a compensatory increase in sodium reabsorption via ENaC during longer-term thiazide treatment.
In this present study, 3% hypertonic saline induced an increase in u-NKCC2 when subjects were treated with amiloride and placebo. It was probably related to a counter regulatory mechanism to compensate for temporarily impaired lower fractional sodium reabsorption in proximal tubules during volume expansion[21-23]. It has previously been described in healthy humans that u-NKCC2 decreased after 3% saline[12]. However, the subjects’ average age was approximately 35 years older in the aforementioned study. Tian et al[24] showed a blunting in the up regulation of sodium transport proteins in response to water restriction in aged rats, which seemed to be particularly apparent with regards to NKCC2. The age difference might explain the discrepancy in the u-NKCC2 response between the two studies.
During BFTZ treatment, u-NKCC2 ceased to increase. In rats, chronic thiazide treatment produces a compensatory fractional increased reabsorption of sodium in proximal tubules[19], which might explain why u-NKCC2 ceased to increase in the late post infusion periods during BFTZ. Thus, during BFTZ, there was no need of a compensatory reabsorption via NKCC, which is supported by the relative lower increase in FENa during BFTZ compared to both amiloride and placebo, in response to 3% saline. In animals, AVP has been demonstrated to increase NKCC2 activity, mediated by V2 receptors via adenylate-cyclase-6 to facilitate phosphorylation and trafficking of NKCC2 to the apical membrane[25,26]. As p-AVP was lower during BTFZ treatment, it cannot be excluded that the decline in u-NKCC2 might also reflect a lack of stimulation from AVP.
Thus, our findings reflect a more profound change in glomerular tubular balance during BFTZ treatment, than the more distal acting diuretic, amiloride.
In the collecting ducts, sodium transport occurs via the ENaC located in the luminal membrane of principal cells[27,28]. ENaC can be regulated by aldosterone[29,30], but is also regulated by AVP, that binds to the V2 receptors and induces a rapid change in channel activity via ENaC opening[31-36]. Recently our group demonstrated an increased u-ENaCγ after hypertonic saline infusion in healthy young subjects. The increase in u-ENaCγ was explained by an increased sodium load to the distal tubules caused by a decrease in renal sodium absorption in the proximal tubules[11,21,22]. In the present study, we measured a similar increase during all three treatments. As amiloride inhibits ENaC, we expected to find a decrease in u-ENaCγ both at baseline and in response to 3% saline treatment during amiloride treatment, especially as we had also found an increased FENa at baseline. There are several possible explanations: Firstly, as ENaC only controls as little as 2%-5% of sodium reabsorption, perhaps a small decrease in the fractional reabsorption of sodium via ENaC would cause a significant rise in excretion of sodium. Secondly, p-Aldo was highest during amiloride treatment and its stimulation on the principal cells might have increased the amount of ENaC within the apical membrane, and thus antagonized the effect of amiloride. Thirdly, amiloride treatment has been shown to increase whole cell channel abundance caused by an intracellular sodium feedback mechanism[37,38]. These intracellular counter regulatory mechanisms might also be involved.
As expected, during the acute hypertonic sodium load, PRC, p-AngII and p-Aldo decreased and urinary sodium excretion increased[9,11,39]. The increase in urinary sodium excretion is preceded by a decrease in ENaC expression and activity[40]. Meanwhile, p-AVP increased due to increased p-osm, and likely caused ENaC channels to be inserted in the apical membrane and thus increased reabsorption of sodium[41].
Thus, despite increased FENa at baseline during amiloride treatment, u-ENaCγ did not differ significantly between treatments neither at baseline nor after 3% saline. These findings do not appear to be dependent on aldosterone, but more to reflect a compensatory role of ENaC to adjust for the decreased reabsorption of sodium in proximal parts of the nephron during an acute sodium load.
Vasopressin (AVP) regulates AQP2 function by binding to V2 receptors in the basolateral membrane of principal cells, increasing the delivery of intracellular vesicles containing AQP2 to the apical membrane and thus increasing water reabsorption[42,43]. AQP2 is also excreted into urine[5-9,44-46]. Volume expansion with 3% hypertonic saline increases plasma osmolality, p-AVP, reabsorption of water and u-AQP2[11,14,47]. In the present study, there was an increase in u-AQP2, in response to 3% saline, during amiloride and placebo treatment, but not during BFTZ. The changes in u-AQP2 during BFTZ correspond to the attenuated decrease in CH2O. Different explanations include: (1) an increased water load to the collecting tubules due to inhibition of NCC in distal collecting ducts, resulting in higher water excretion; (2) Decreased p-osm and p-AVP during BFTZ treatment and thus a reduced effect on V2R in the collecting ducts; and (3) A reduced need for at counter regulatory water reabsorption in the collecting ducts due to the lower reabsorption of sodium via NKCC2.
Potassium is freely excreted in glomerulus and it is reabsorbed and secreted across the nephron[48]. Intracellular signalling networks, volume status, p-K status and aldosterone tightly regulate the balance of potassium excretion[49,50]. Thiazides do not affect potassium transport directly, but induce adaption primarily along the connecting and collecting tubules where enhanced sodium reabsorption stimulates potassium secretion via renal outer medullary K+ (ROMK) and large-conductance K+ (BK) channels[20,51].
It has recently been shown that angiotensinII directly inhibits ROMK in potassium-depleted animals, and thereby contributes to potassium conservation[52,53]. In this present study, p-Ang II was highest during BFTZ treatment, and may have inhibited ROMK, and explains the increased sodium reabsorption during 3% saline while potassium secretion decreased.
During amiloride treatment there was a decrease in u-K at baseline. Amiloride exerts a direct effect on potassium excretion due to the blocking of ENaC. If the influx of sodium does not occur, there will be no lumen negative potential to drive potassium excretion[48]. In response to 3% hypertonic saline however, the excretion of potassium increased most during amiloride. This phenomenon might have been due to prolonged effect of aldosterone to increase sodium reabsorption via ENaC and secretion via ROMK, despite current amiloride blockage.
Thiazide decreases ECV and peripheral vascular resistance[2,54]. Amiloride is an antihypertensiva that exhibits its effects by significant natriuresis[55]. In this study the lack of difference in 24 h ambulatory blood pressure might partly be explained by the fact that the subjects were young and healthy with normal BP before entering the study. Moreover, BFTZ and amiloride are relatively weak antihypertensives and a very negligible blood pressure lowering effect was expected in these normohypertensive subjects.
Data showed that bDBP decreased significant in all three treatments, but bDBP decreased relatively more during BFTZ treatment compared to amiloride and placebo. As brachial office BP was also used to calibrate the SphygmoCor cDBP this may explain the reduction in cDBP during BFTZ. A negative augmentation index (AIx) has been reported in healthy young subjects, but is of limited use due to normal cardiovascular elasticity in this age group[56].
The determination of body fluid volumes via bioimpedance spectroscopy (BIS) is an accurate method for estimating total body water and the distribution of water between the intracellular and extracellular spaces[57]. In this present study, we measured no statistical difference between the groups, but as expected TBV was lower during both diuretic treatments compared to placebo, due to a decrease in ECV followed by sodium deficit. This reduction in ECV, during BFTZ treatment, is in agreement with current knowledge[2]. We did not expect a major decrease in ECV after amiloride, being a weak diuretic agent[55]. However the decrease in ECV was very similar to BFTZ. A significant difference in body fluid volumes between treatment groups was not detected in the present study, possibly due to the small number of subjects in each group.
The major strength of this study was the design as a randomized, placebo controlled, double-blinded crossover study with a homogenous group of healthy young men and women. The test conditions were very well defined regarding diet, sodium and fluid intake. Thus, the results are not confounded by differences in sodium or water intake. However, as the study group was healthy humans the conclusions is limited to this population group and may not be extracted to patients with disturbances in water and sodium balance. Also the excretion of NCC was not measured, which would have provided us with even more information on renal handling of sodium.
In this study of healthy humans, amiloride and placebo clearly increased u-NKCC2, u-ENaCγ and u-AQP2 in response to 3% hypertonic saline, while u-NKCC2 and u-AQP2 were unchanged during BFTZ. In contrast to amiloride, BFTZ treatment seemed to have changed glomerular-tubular balance, which caused the absence of a compensatory reabsorption of sodium via NKCC2 after hypertonic saline. It is possible that the lower p-AVP during BFTZ treatment resulted in a relatively less stimulation of NKCC2 and AQP2, with subsequent reduced transport of sodium and water via the transporters. During all three treatments, the increase in u-ENaCγ might reflect a compensatory reabsorption to adjust for the decreased reabsorption of sodium in the proximal part of the nephron.
The authors greatly acknowledge the skilful assistance of our laboratory technicians: Inger-Merete Paulsen, Anne Mette Ravn, Kirsten Nygaard, Henriette Vorup Simonsen and Susan Milton Rasmussen.
The discovery that urine contains proteins from renal epithelia of proximal tubule, Henle’s loop, distal convoluted tubule and the collecting ducts has provided us with urinary biomarkers as a tool to investigate physiological and pathophysiological processes in renal sodium and water homeostasis.
Urinary excretion of the aquaporin2 water channel (u-AQP2) is a biomarker that has been investigated in numerous studies of various water-balance disorders. It has also been demonstrated that the urinary excretion of epithelial sodium channels (u-ENaC) can be used as biomarkers of sodium transport via excretion of epithelial sodium channels (ENaC). However the exact physiological mechanisms are still unknown, and studies are needed to address the complete physiological handling of sodium and water in humans.
In animals, volume expansion and diuretics changes proximal water and sodium reabsorption and the expression of AQP2 and sodium transporters along the nephron. In addition, changes in transport activity of the sodium-potassium-2chloride cotransporter (NKCC2); ENaC and AQP2 may also be involved in the abnormal tubular function in patients with chronic kidney disease. However, it has never been studied to what extent the function of NKCC2, ENaC and AQP2 is simultaneously affected in response to amiloride and bendroflumethiazide (BFTZ) in humans. In the present study, the u-NKCC2 and u-AQP2 increased during amiloride and placebo, while u-NKCC2 and u-AQP2 remained unchanged during BFTZ, in response to infusion of 3% saline.
Thus, measurements of water- and sodium transporters in urine, as biomarkers of water-and sodium transport via NKCC2, ENaC and AQP2 may provide important information of the mechanisms involved in water and sodium balance in the kidney.
AQP2, NKCC2 and ENaC are transporters in the nephron that play essential roles in regulating water and sodium homeostasis, extracellular volume and controlling blood pressure by reabsorbing water and sodium. BFTZ is a diuretic that inhibit the sodium-chloride co-transporter and amiloride is a diuretic that block the ENaC channels. These diuretics, which were developed empirically to treat patients with edema and hypertension, can be used as tools to characterize sodium transport pathways.
This is an interesting paper.
P- Reviewer: McNally RJQ, Su M S- Editor: Ji FF L- Editor: A E- Editor: Yan JL
| 1. | Esteva-Font C, Ballarin J, Fernández-Llama P. Molecular biology of water and salt regulation in the kidney. Cell Mol Life Sci. 2012;69:683-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Duarte JD, Cooper-DeHoff RM. Mechanisms for blood pressure lowering and metabolic effects of thiazide and thiazide-like diuretics. Expert Rev Cardiovasc Ther. 2010;8:793-802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 3. | Kleyman TR, Cragoe EJ. Amiloride and its analogs as tools in the study of ion transport. J Membr Biol. 1988;105:1-21. [PubMed] |
| 4. | Nielsen S, Agre P. The aquaporin family of water channels in kidney. Kidney Int. 1995;48:1057-1068. [PubMed] |
| 5. | Kanno K, Sasaki S, Hirata Y, Ishikawa S, Fushimi K, Nakanishi S, Bichet DG, Marumo F. Urinary excretion of aquaporin-2 in patients with diabetes insipidus. N Engl J Med. 1995;332:1540-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 191] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 6. | Rai T, Sekine K, Kanno K, Hata K, Miura M, Mizushima A, Marumo F, Sasaki S. Urinary excretion of aquaporin-2 water channel protein in human and rat. J Am Soc Nephrol. 1997;8:1357-1362. [PubMed] |
| 7. | Elliot S, Goldsmith P, Knepper M, Haughey M, Olson B. Urinary excretion of aquaporin-2 in humans: a potential marker of collecting duct responsiveness to vasopressin. J Am Soc Nephrol. 1996;7:403-409. [PubMed] |
| 8. | Saito T, Ishikawa SE, Sasaki S, Nakamura T, Rokkaku K, Kawakami A, Honda K, Marumo F, Saito T. Urinary excretion of aquaporin-2 in the diagnosis of central diabetes insipidus. J Clin Endocrinol Metab. 1997;82:1823-1827. [PubMed] |
| 9. | Pedersen RS, Bentzen H, Bech JN, Pedersen EB. Effect of water deprivation and hypertonic saline infusion on urinary AQP2 excretion in healthy humans. Am J Physiol Renal Physiol. 2001;280:F860-F867. [PubMed] |
| 10. | Lauridsen TG, Vase H, Starklint J, Graffe CC, Bech JN, Nielsen S, Pedersen EB. Increased renal sodium absorption by inhibition of prostaglandin synthesis during fasting in healthy man. A possible role of the epithelial sodium channels. BMC Nephrol. 2010;11:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Jensen JM, Mose FH, Bech JN, Nielsen S, Pedersen EB. Effect of volume expansion with hypertonic- and isotonic saline and isotonic glucose on sodium and water transport in the principal cells in the kidney. BMC Nephrol. 2013;14:202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Jensen JM, Mose FH, Kulik AE, Bech JN, Fenton RA, Pedersen EB. Abnormal urinary excretion of NKCC2 and AQP2 in response to hypertonic saline in chronic kidney disease: an intervention study in patients with chronic kidney disease and healthy controls. BMC Nephrol. 2014;15:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Pedersen EB, Eiskjaer H, Madsen B, Danielsen H, Egeblad M, Nielsen CB. Effect of captopril on renal extraction of renin, angiotensin II, atrial natriuretic peptide and vasopressin, and renal vein renin ratio in patients with arterial hypertension and unilateral renal artery disease. Nephrol Dial Transplant. 1993;8:1064-1070. [PubMed] |
| 14. | Graffe CC, Bech JN, Pedersen EB. Effect of high and low sodium intake on urinary aquaporin-2 excretion in healthy humans. Am J Physiol Renal Physiol. 2012;302:F264-F275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Lauridsen TG, Vase H, Starklint J, Bech JN, Pedersen EB. Protein-enriched diet increases water absorption via the aquaporin-2 water channels in healthy humans. Nephrol Dial Transplant. 2010;25:2502-2510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Matthesen SK, Larsen T, Vase H, Lauridsen TG, Jensen JM, Pedersen EB. Effect of amiloride and spironolactone on renal tubular function and central blood pressure in patients with arterial hypertension during baseline conditions and after furosemide: a double-blinded, randomized, placebo-controlled crossover trial. Clin Exp Hypertens. 2013;35:313-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Hager H, Kwon TH, Vinnikova AK, Masilamani S, Brooks HL, Frøkiaer J, Knepper MA, Nielsen S. Immunocytochemical and immunoelectron microscopic localization of alpha-, beta-, and gamma-ENaC in rat kidney. Am J Physiol Renal Physiol. 2001;280:F1093-F1106. [PubMed] |
| 18. | Matthesen SK, Larsen T, Vase H, Lauridsen TG, Pedersen EB. Effect of potassium supplementation on renal tubular function, ambulatory blood pressure and pulse wave velocity in healthy humans. Scand J Clin Lab Invest. 2012;72:78-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Walter SJ, Shirley DG. The effect of chronic hydrochlorothiazide administration on renal function in the rat. Clin Sci (Lond). 1986;70:379-387. [PubMed] |
| 20. | Hunter RW, Craigie E, Homer NZ, Mullins JJ, Bailey MA. Acute inhibition of NCC does not activate distal electrogenic Na+ reabsorption or kaliuresis. Am J Physiol Renal Physiol. 2014;306:F457-F467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Dirks JH, Cirksena WJ, Berliner RW. The effects of saline infusion on sodium reabsorption by the proximal tubule of the dog. J Clin Invest. 1965;44:1160-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 220] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | Svensén CH, Waldrop KS, Edsberg L, Hahn RG. Natriuresis and the extracellular volume expansion by hypertonic saline. J Surg Res. 2003;113:6-12. [PubMed] |
| 23. | Häberle DA, von Baeyer H. Characteristics of glomerulotubular balance. Am J Physiol. 1983;244:F355-F366. [PubMed] |
| 24. | Tian Y, Riazi S, Khan O, Klein JD, Sugimura Y, Verbalis JG, Ecelbarger CA. Renal ENaC subunit, Na-K-2Cl and Na-Cl cotransporter abundances in aged, water-restricted F344 x Brown Norway rats. Kidney Int. 2006;69:304-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Rieg T, Tang T, Uchida S, Hammond HK, Fenton RA, Vallon V. Adenylyl cyclase 6 enhances NKCC2 expression and mediates vasopressin-induced phosphorylation of NKCC2 and NCC. Am J Pathol. 2013;182:96-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Giménez I, Forbush B. Short-term stimulation of the renal Na-K-Cl cotransporter (NKCC2) by vasopressin involves phosphorylation and membrane translocation of the protein. J Biol Chem. 2003;278:26946-26951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 172] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 27. | Edinger RS, Bertrand CA, Rondandino C, Apodaca GA, Johnson JP, Butterworth MB. The epithelial sodium channel (ENaC) establishes a trafficking vesicle pool responsible for its regulation. PLoS One. 2012;7:e46593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Loffing J, Korbmacher C. Regulated sodium transport in the renal connecting tubule (CNT) via the epithelial sodium channel (ENaC). Pflugers Arch. 2009;458:111-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 129] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 29. | Garty H, Palmer LG. Epithelial sodium channels: function, structure, and regulation. Physiol Rev. 1997;77:359-396. [PubMed] |
| 30. | Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest. 1999;104:R19-R23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 593] [Cited by in RCA: 598] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 31. | Bankir L, Fernandes S, Bardoux P, Bouby N, Bichet DG. Vasopressin-V2 receptor stimulation reduces sodium excretion in healthy humans. J Am Soc Nephrol. 2005;16:1920-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 32. | Ecelbarger CA, Kim GH, Wade JB, Knepper MA. Regulation of the abundance of renal sodium transporters and channels by vasopressin. Exp Neurol. 2001;171:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 104] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Bugaj V, Pochynyuk O, Stockand JD. Activation of the epithelial Na+ channel in the collecting duct by vasopressin contributes to water reabsorption. Am J Physiol Renal Physiol. 2009;297:F1411-F1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Ecelbarger CA, Kim GH, Terris J, Masilamani S, Mitchell C, Reyes I, Verbalis JG, Knepper MA. Vasopressin-mediated regulation of epithelial sodium channel abundance in rat kidney. Am J Physiol Renal Physiol. 2000;279:F46-F53. [PubMed] |
| 35. | Perucca J, Bichet DG, Bardoux P, Bouby N, Bankir L. Sodium excretion in response to vasopressin and selective vasopressin receptor antagonists. J Am Soc Nephrol. 2008;19:1721-1731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 36. | Stockand JD. Vasopressin regulation of renal sodium excretion. Kidney Int. 2010;78:849-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 37. | Patel AB, Frindt G, Palmer LG. Feedback inhibition of ENaC during acute sodium loading in vivo. Am J Physiol Renal Physiol. 2013;304:F222-F232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Frindt G, Silver RB, Windhager EE, Palmer LG. Feedback regulation of Na channels in rat CCT. II. Effects of inhibition of Na entry. Am J Physiol. 1993;264:F565-F574. [PubMed] |
| 39. | Andersen LJ, Jensen TU, Bestle MH, Bie P. Isotonic and hypertonic sodium loading in supine humans. Acta Physiol Scand. 1999;166:23-30. [PubMed] |
| 40. | Pácha J, Frindt G, Antonian L, Silver RB, Palmer LG. Regulation of Na channels of the rat cortical collecting tubule by aldosterone. J Gen Physiol. 1993;102:25-42. [PubMed] |
| 41. | Butterworth MB. Regulation of the epithelial sodium channel (ENaC) by membrane trafficking. Biochim Biophys Acta. 2010;1802:1166-1177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 42. | DiGiovanni SR, Nielsen S, Christensen EI, Knepper MA. Regulation of collecting duct water channel expression by vasopressin in Brattleboro rat. Proc Natl Acad Sci USA. 1994;91:8984-8988. [PubMed] |
| 43. | Nielsen S, Chou CL, Marples D, Christensen EI, Kishore BK, Knepper MA. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci USA. 1995;92:1013-1017. [PubMed] |
| 44. | Wen H, Frokiaer J, Kwon TH, Nielsen S. Urinary excretion of aquaporin-2 in rat is mediated by a vasopressin-dependent apical pathway. J Am Soc Nephrol. 1999;10:1416-1429. [PubMed] |
| 45. | Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA. 2004;101:13368-13373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1494] [Cited by in RCA: 1690] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 46. | Pedersen RS, Bentzen H, Bech JN, Nyvad O, Pedersen EB. Urinary aquaporin-2 in healthy humans and patients with liver cirrhosis and chronic heart failure during baseline conditions and after acute water load. Kidney Int. 2003;63:1417-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 47. | Starklint J, Bech JN, Pedersen EB. Down-regulation of urinary AQP2 and unaffected response to hypertonic saline after 24 hours of fasting in humans. Kidney Int. 2005;67:1010-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 48. | Castañeda-Bueno M, Arroyo JP, Gamba G. Independent regulation of Na+ and K+ balance by the kidney. Med Princ Pract. 2012;21:101-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 49. | Arroyo JP, Ronzaud C, Lagnaz D, Staub O, Gamba G. Aldosterone paradox: differential regulation of ion transport in distal nephron. Physiology (Bethesda). 2011;26:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 50. | Hoorn EJ, Ellison DH. WNK kinases and the kidney. Exp Cell Res. 2012;318:1020-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 51. | Ellison DH, Loffing J. Thiazide effects and adverse effects: insights from molecular genetics. Hypertension. 2009;54:196-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 52. | Wei Y, Zavilowitz B, Satlin LM, Wang WH. Angiotensin II inhibits the ROMK-like small conductance K channel in renal cortical collecting duct during dietary potassium restriction. J Biol Chem. 2007;282:6455-6462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 53. | Yue P, Sun P, Lin DH, Pan C, Xing W, Wang W. Angiotensin II diminishes the effect of SGK1 on the WNK4-mediated inhibition of ROMK1 channels. Kidney Int. 2011;79:423-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 54. | Jessup JA, Brosnihan KB, Gallagher PE, Chappell MC, Ferrario CM. Differential effect of low dose thiazides on the Renin Angiotensin system in genetically hypertensive and normotensive rats. J Am Soc Hypertens. 2008;2:106-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 55. | Bull MB, Laragh JH. Amiloride. A potassium-sparing natriuretic agent. Circulation. 1968;37:45-53. [PubMed] |
| 56. | Hughes AD, Park C, Davies J, Francis D, McG Thom SA, Mayet J, Parker KH. Limitations of augmentation index in the assessment of wave reflection in normotensive healthy individuals. PLoS One. 2013;8:e59371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 57. | Moissl UM, Wabel P, Chamney PW, Bosaeus I, Levin NW, Bosy-Westphal A, Korth O, Müller MJ, Ellegård L, Malmros V. Body fluid volume determination via body composition spectroscopy in health and disease. Physiol Meas. 2006;27:921-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 439] [Article Influence: 23.1] [Reference Citation Analysis (0)] |