Published online May 6, 2015. doi: 10.5527/wjn.v4.i2.148
Peer-review started: June 15, 2014
First decision: July 10, 2014
Revised: January 24, 2015
Accepted: January 30, 2015
Article in press: February 2, 2015
Published online: May 6, 2015
Processing time: 327 Days and 13 Hours
The number of patients reinitiating dialysis after a failed transplant increases over time and has more than doubled between the year 1988 and 2010 (an increase from 2463 to 5588). More importantly, patients returning to dialysis have been shown to have a greater than three-fold increase in the annual adjusted mortality rates compared with those with a functioning graft. Continuation of immunosuppression to preserve residual graft function has been implicated to be a contributing factor, seemingly due to immunosuppression-associated cardiovascular and infectious complications and malignancy risk, among others. Nonetheless, maintenance low-dose immunosuppression has been suggested to confer survival benefit in patients returning to peritoneal dialysis. Whether early vs late reinitiation of dialysis or whether transplantectomy has an impact on patient survival remains poorly defined. Consensus guidelines for the management of a failed allograft are lacking. In this article, we present a literature overview on the ideal timing of dialysis reinitiation after graft loss, the management of immunosuppression after graft failure, and the risks and benefits of transplantectomy. The authors’ perspectives on the management of this special patient population are also discussed.
Core tip: The number of patients with a failed allograft returning to dialysis increases over time. Studies suggest that such patients are at increased morbidity and mortality risks compared with their transplant-naïve, incident dialysis patients. This review provides a critical literature overview of the risks and benefits of early vs late dialysis re-initiation, immunosuppression weaning, and transplantectomy in patients with a failed allograft. Based on currently available literature, suggested guidelines for the management of this unique patient population are presented.
- Citation: Pham PT, Everly M, Faravardeh A, Pham PC. Management of patients with a failed kidney transplant: Dialysis reinitiation, immunosuppression weaning, and transplantectomy. World J Nephrol 2015; 4(2): 148-159
- URL: https://www.wjgnet.com/2220-6124/full/v4/i2/148.htm
- DOI: https://dx.doi.org/10.5527/wjn.v4.i2.148
Retrospective analysis of the United States Renal Data System (USRDS) database showed that mortality in patients reinitiating dialysis after graft failure was primarily due to cardiac (36%) or infectious complications (17%)[1]. Continuation of immunosuppression has been suggested to play a causative role. Nevertheless, immunosuppression cessation is not without morbidity. Similarly, although transplantectomy would permit immunosuppression withdrawal, it may lead to other unfavorable outcomes.
Clinicians caring for patients with a recently failed allograft are generally faced with three important decisions: timing of dialysis reinitiation, immunosuppression management, and whether to perform transplantectomy[2]. When the cause of graft loss is due to primary nonfunction, arterial or venous thrombosis, hyperacute or early refractory acute rejection, most treating physicians advocate transplantectomy and immunosuppression cessation. In these circumstances, graft rupture or hemorrhage may occur if the graft is left in situ. However, when the allograft has been in place for more than 1-2 years, it is common practice to leave the failed allograft in situ. Nonetheless, a retained failed transplant has been suggested to be a source of a chronic inflammatory state, potentially leading to unfavorable outcomes. Immunosuppression management in such patients can be challenging. Although maintenance low dose-immunosuppression may preserve residual kidney function, circumvent graft intolerance syndrome, minimize allosensitization, and avoid overt acute rejection, long-term maintenance immunosuppression is not without adverse effects (Table 1). These may include immunosuppression-related malignancy and cardiovascular and metabolic complications. It is also noteworthy that although the number of patients reinitiating dialysis after a failed transplant has more than doubled in the last two decades, studies evaluating the optimal timing of dialysis reinitiation are lacking. A literature overview on the timing of dialysis reinitiation after graft failure, the potential beneficial and adverse effects of low-dose maintenance immunosuppression, and the risks and benefits of transplantectomy are presented.
| Potential beneficial effects | Potential adverse effects |
| Preservation of residual kidney function | Metabolic complications (diabetes, hypertension, dyslipidemia) |
| Decreased incidence of graft intolerance syndrome and the need for allograft nephrectomy | Steroid-associated adverse effects (e.g., diabetes, cataracts, myopathy, and avascular necrosis among others) |
| Minimization of allosensitization | Cardiovascular complications |
| Avoidance of overt acute rejection | Increased susceptibility to infection |
| Prevention of adrenal insufficiency syndrome | Malignancy (especially skin cancers, Kaposi’s sarcoma, non-Hodgkin’s lymphoma, and lip cancers) |
| Prevention of reactivation of systemic disease (e.g., systemic lupus erythematosus, vasculitis) | Costs (particularly when data supporting continued immunosuppression are lacking) |
Early studies in the mid 1970s to 1980s suggested that initiation of dialysis in end-stage kidney disease (ESKD) patients with a higher estimated glomerular filtration rate (eGFR) was associated with lower mortality[3]. However, these studies were subsequently criticized for small sample sizes and potential confounding factors. Over the past decade, several observational studies failed to demonstrate the survival benefits of early commencement of dialysis and such practice may even be associated with increased mortality risk[4]. A recent multicenter randomized controlled trial (the Initiating Dialysis Early and Late study) showed comparable mortality rates among early vs late dialysis initiation. Eight hundred and twenty eight patients with progressive stage V chronic kidney disease (CKD) (including patients with a failed transplant) were randomized to either start dialysis at eGFR of 10.0 to 14.0 mL/min (early-start group) or to continue routine medical management and start dialysis when eGFR reached 5.0 to 7.0 mL/min (late-start group)[5]. During a median follow up period of 3.59 years, mortality occurred in 37.6% and 36.6% of patients in the early- and late-start groups, respectively (HR 1.04, P = 0.75). The frequency of cardiovascular events, infections, or dialysis complications was comparable between the two groups. However, it is noteworthy that in the late-start group, nearly 76% of patients were started on dialysis when the estimated GFR was above the target 7.0 mL/min due to symptomatic uremia. It was thus concluded that planned early dialysis initiation in patients with stage V CKD provided no benefits in terms of survival or clinical outcomes. Similarly, a retrospective USRDS database study (n = 310932 patients who were started on dialysis between 2006 and 2008) showed no harmful or beneficial effects of early dialysis initiation on mortality (HR 1.025 per 1 mL/min per 1.73 m2 for eGFR 5-14 1 mL/min per 1.73 m2 and 0.973 per 1 mL/min per 1.73 m2 for eGFR 14-20 mL/min per 1.73 m2)[6].
Studies on the optimal timing of dialysis reinitiation after a failed transplant are limited. Current guidelines for transplant naïve patients with progressive CKD advocate late-start dialysis (defined as dialysis initiation at an eGFR between 6-9 mL/min). Results of two large registry studies suggested that early compared with late dialysis reinitiation in patients with failed kidney transplants may adversely impact survival. The USRDS registry study (n = 4741 patients followed for a median of 15 ± 11 mo after dialysis initiation) demonstrated that nonsurvivors had a significantly higher eGFR at dialysis initiation than their survivor counterparts (9.7 ± 4.8 vs 8.0 ± 3.7 mL/min per 1.73 m2, respectively). Specifically, each 1 mL/min per m2 higher eGFR at the time of dialysis reinitiation was found to be associated with a 4% higher mortality risk after dialysis reinitiation (P < 0.01)[1]. Nonetheless, it is speculated that the sickest patients tended to require commencement of dialysis at higher levels of residual kidney function. This confounding by indication was subsequently addressed in an analysis of the SRTR registry study using propensity score analysis. The study cohort consists of 747 failed kidney transplant patients who had reinitiated dialysis with eGFR < 15 mL/min. A propensity score for early (eGFR > 10.5 mL/min per 1.73 m2) vs late dialysis reinitiation was fitted by logistic regression. Peripheral vascular disease, diabetes mellitus, and male gender were associated with higher odds of early reinitiation of dialysis. In an unadjusted model, each 1 mL/min per 1.73 m2 higher eGFR at dialysis reinitiation was associated with a 6% higher mortality risk. Such association was not observed in the fully adjusted model. However, there was a trend towards increased mortality risk in patients with a higher eGFR upon reinitiation of dialysis, particularly among the healthiest subgroups of patients identified by the propensity score, including female gender and younger subjects[7].
Whether early dialysis reinitiation in patients with failed transplants adversely impact outcomes is currently not known and warrants further studies. Based on available data, a number of investigators feel that reinitiation of dialysis based on eGFR alone is not justified and could be harmful in some cases[3]. Thus, as with transplant naïve patients, dialysis reinitiation in patients with graft failure may rely on eGFR as a rough guide that must be redefined by patients’ comorbidities, nutritional status, and overall wellness.
Consensus guidelines for the management of immunosuppression in patients with a failed allograft are lacking. Both continuation of low-dose immunosuppression and immunosuppression withdrawal have their inherent risks and benefits (Table 1)[2].
Preservation of residual kidney function: In the non-transplant settings, peritoneal dialysis (PD) and hemodialysis (HD) patients with preserved kidney function have been shown to have better survival rates compared with their oliguric or anuric counterparts[8,9]. Similar to the transplant naïve end stage kidney disease population, patient with a retained failed transplant and preserved residual graft function have been shown to have survival advantage over those who lost residual kidney function. A decision analytic model comparing continuation of immunosuppression with immunosuppressant withdrawal in patients returning to PD after graft failure suggests that continued immunosuppression may confer survival benefit over immunosuppressant cessation despite increased malignancy and infection risks (life expectancy: 5.8 years vs 5.3 years, respectively)[10]. The survival benefit was apparent even at marginal GFR (defined as an additional GFR of 1.48 mL/min), and incremental at increasing residual graft function. The study results suggest that the loss of residual kidney function may have an adverse impact on survival in patients reinitiating PD. Nevertheless, the study was not without shortcomings. The model hypothesized that continuation of maintenance immunosuppression would preserve residual kidney function, and the beneficial effects of residual graft function are similar to those of the native kidneys. Of interest, results of the USRDS registry analysis demonstrated that compared with hemodialysis, PD was associated with greater survival within the first year after dialysis initiation, but lower after 2 years[11]. It may be speculated that the early survival benefit of PD over HD was due to greater preservation of residual kidney function. Notably, the survival advantage of PD was not seen among patients who initiated PD at lower levels of eGFR. However, neither details on immunosuppression maintenance after graft failure nor data on differential rates of decline in residual kidney over time was provided. A case of well-preserved residual kidney function in a PD patient maintained on low dose dual immunosuppressive therapy after a failed allograft has been described. After return to PD, the patient continued to make 600-1200 cc of urine/day at one-year follow-up[12].
There is currently insufficient evidence to routinely recommend continued immunosuppression in patients returning to PD after graft loss.
Data for any potential survival benefits of continuation of maintenance immunosuppression among patients returning to HD are lacking.
Prevention of allosensitization: Immunosuppression withdrawal after kidney graft failure with or without transplantectomy has been shown to be an independent predictor of allosensitization[13,14]. In a single-center study consisting of 69 patients with confirmed alloantibody negative at the time of graft loss, more than half (38/69) became sensitized over the following months or years. De novo class I and/or class II anti HLA antibodies (primarily of donor specificity) were detected only in patients whose immunosuppressants were discontinued after graft loss regardless of whether they had a nephrectomy or blood transfusion. Four of fifteen patients without nephrectomy or transfusion developed antibodies after cessation of immunosuppression. In contrast, none of the eleven patients who continued immunosuppressants developed antibodies, seven of whom had an allograft nephrectomy or blood transfusion[14]. In another study, de novo donor-specific antibodies (DSAs) appeared in nearly 48% of patients when immunosuppressive therapy was discontinued after graft loss. None of these patients had an allograft nephrectomy[15]. Of interest, it has been shown that a short exposure to the allograft is sufficient to stimulate the immune system and to induce alloantibody production[16]. In a small series of 32 patients who required transplantectomy after early graft loss, DSAs and non-DSA anti-HLA antibodies developed in > 50% of patients whose immunosuppressants were discontinued after transplantectomy (median time between transplantation and transplantectomy was 2.5 d). Histological analysis of explanted allografts showed no features of cellular or humoral rejection. There was no significant difference in the incidence of DSAs among patients receiving transfusions and those who did not[16].
Given current evidence, albeit scant of potential increased allosensitization with cessation of immunosuppression, it may be suggested that patients who are re-allograft transplant candidates be considered for continuation of maintenance therapy, particularly when living donation is a possibility. Whether patients with early graft loss requiring transplantectomy (particularly those anticipated to have a short wait time after early relisting) benefit from continuation of immunosuppression to minimize allosensitization warrant further investigation. In addition, the duration and intensity of maintenance immunosuppression remain to be defined.
Prevention of graft intolerance syndrome and transplantectomy: Graft intolerance syndrome refers to an immunologic intolerance to a retained failed graft, and commonly develops within the first year of dialysis reinitiation. Clinically, patients may present with graft enlargement or tenderness, gross hematuria, fevers, malaise, flu-like symptoms, or any constellation of signs and symptoms thereof. Graft intolerance syndrome may develop in 30% to 50% of patients despite various immunosuppression withdrawal protocols. Although such syndrome may be treated with a short course of high dose corticosteroid, symptom recurrence following immunosuppression weaning generally necessitates transplantectomy. In one single center study, immunosuppression weaning commonly led to symptomatic rejection with fever mimicking infection (93 of 186 study subjects were African Americans). A nearly 7-fold risk (P = 0.017) for admission within six months of graft failure with fever in the absence of infection was observed among African Americans who were tapered from immunosuppression. The majority of these patients ultimately required transplantectomy due to symptomatic rejection or fever of unknown etiology. Notably, fever resolved in all patients after transplantectomy[17].
In a single-center study consisting of 41 patients with graft loss occurring more than 6 mo after transplantation, the need for transplantectomy following immunosuppression weaning was found to correlate with the number of previous acute rejection episodes. In patients who had zero, one, or two or more rejection episodes, transplantectomy was required in 30%, 53% and 83%, respectively. It is suggested that gradual immunosuppression weaning or indefinite low-dose maintenance immunosuppression may prevent the need for transplantectomy[18].
Rapid steroid withdrawal may result in overt adrenal insufficiency variously manifested as hypotension, weakness, fevers, malaise, and weight loss, among others. In severe steroid withdrawal, patients may experience frequent hypotensive episodes during dialysis despite having volume overload[19]. When the graft is left in situ for more than one to two years, gradual immunosuppression weaning with close monitoring for clinically overt adrenal insufficiency or acute allograft rejection is advisable.
Infectious, cardiovascular, and metabolic syndrome risks: While low-dose maintenance immunosuppression may be beneficial in preserving residual kidney function in patients who maintain good urine output, such practice is not without adverse effects. In a multi-center cohort study comprising 197 failed allografts in 177 transplant recipients whose allograft functioned for at least 3 mo, low-dose maintenance immunosuppression was associated with an increase in infectious- and cardiovascular disease-related morbidity and mortality[20]. The incidence of infectious complications per patient year was significantly higher in the immunosuppression continuation compared with that of immunosuppression withdrawal groups (1.7 vs 0.51, respectively, P < 0.0001). Similarly mortality associated with cardiovascular and infectious complications was higher among patients who continued immunosuppression compared with those whose immunosuppression was discontinued [Odd ratio (OR) of 4.9, 95%CI: 1.8-13.5 from cardiovascular disease and OR of 2.8, 95%CI: 1.1-7.0 from infectious complications]. Clinical acute rejection rates (graft tenderness and hematuria, in the absence or presence of non-infectious low-grade fever) were similar between the two groups (P = NS). Based on the study findings, the authors favored immunosuppression withdrawal over low-dose maintenance immunosuppression when patients returned to dialysis. In one single center study, an increase in infection-related complications was observed among patients whose immunosuppression was weaned over a prolonged period (mean 14 ± 2 mo) compared with those whose immunosuppression was weaned over a shorter (mean 3 ± 1 mo) period (1.34 vs 0.87) infections per year, respectively[21]. Furthermore, the longer taper had no advantage over the shorter taper group in forestalling the need for transplantectomy. Mortality associated with disseminated histoplasmosis in a hemodialysis patient maintained on low-dose steroid and azathioprine after graft failure has been described[22].
Similar to early reports, a recent study suggests that although immunosuppression weaning results in a higher risk of allosensitization, maintenance of immunosuppression other than low-dose steroid is associated with a greater incidence of infection and infection-related mortality[17]. In a single center consisting of 186 patients with failed kidney transplants, 44% were hospitalized with fever within six months of graft loss. The rates of hospitalization were comparable between patients who continued immunosuppression and those whose immunosuppression was tapered before hospitalization (45% vs 40%, respectively, P = NS). However, among febrile hospitalized patients, documented infections occurred in 88% of patients maintained on immunosuppressive therapy compared with 38% of those who had been weaned off of immunosuppression (defined as withdrawal of all immunosuppressive therapy with the exception of ≤ 10 mg of prednisone daily). Notably, mortality risk was significantly higher in patients with documented infection, with dialysis catheter being the most common infectious source in both groups[17].
Adverse effects associated with long-term steroid use: Well-established adverse effects associated with long-term steroid use include avascular necrosis, osteoporosis, hyperglycemia, cataracts, myopathy and increased susceptibility to infections among others. Nevertheless, it is occasionally necessary to continue steroid to prevent flares of systemic disease such as vasculitis or systemic lupus erythematosus.
Malignancy: Recipients of organ transplants are at increased risk for developing certain neoplasms compared to that of the general population. Kidney transplant recipients receiving low-dose cyclosporine (CSA) was shown to have a significantly lower overall frequency of cancers (P < 0.034) and a lower incidence of virus-associated cancers (P = 0.05) compared with their normal-dose CSA counterparts[23]. Both the duration and intensity of immunosuppressive agents and their ability to foster the replication of oncogenic viruses have been implicated to play contributory roles in the carcinogenic process[2].
Studies evaluating reversal of cancer risk in patients reinitiating dialysis after graft loss is limited. However, it is noteworthy that studies in ESKD patients who received dialysis or a kidney transplant, and in HIV/AIDS subjects suggest that cancers may be classified into those that are related to ESKD, immune deficiency, non-immune deficiency, or uncertain status (Table 2)[24,25]. Although it is conceivable that immunosuppression cessation has no impact on risk reversal of various “non-immune deficiency-related” cancers, most treating physicians advocate rapid immunosuppression weaning or withdrawal in patients who had a history of cancer, irrespective of malignancy types. In cancers associated with immunosuppression, the risks of continued immunosuppression probably outweigh its benefits.
| ESKD-related | Kidney |
| Urinary tract | |
| Thyroid | |
| Myeloma | |
| Immune-deficiency related | Hodgkin’s lymphoma |
| Non-Hodgkin’s lymphoma | |
| Leukemia | |
| Melanoma of skin | |
| Kaposi’s sarcoma | |
| Carcinoma of | |
| Lip | |
| Mouth, tongue, tonsil, oropharynx | |
| Esophagus | |
| Stomach | |
| Anus | |
| Liver | |
| Larynx | |
| Lung | |
| Cervix, uteri, vagina, vulva | |
| Penis | |
| Eye, squamous cell carcinoma only | |
| Not-related to | Rectum |
| immune deficiency | Breast |
| Ovary | |
| Prostate | |
| Of uncertain status | All other cancers |
The Australia and New Zealand Dialysis and Transplantation Registry analysis demonstrated that among all cancers that occur at increased rates in kidney transplant recipients, the pattern of incidence after allograft loss was highly variable[26]. Nonetheless, the incidence of Kaposi’s sarcoma and non-Hodgkin’s lymphoma decreased markedly upon dialysis reinitiation and cessation of immunosuppression. The study also showed a significant decline in melanoma and lip cancer incidence. Of interest, risk reversal was commonly seen among infection-associated malignancies such as Kaposi’s sarcoma with human herpes type 8 and non-Hodgkin’s lymphoma with Epstein-Barr virus. The exact cause of increased risk of lip cancer in transplant patients has not been well-established. However, human papillomavirus has been implicated to play a causative role. Although an infectious source has not been identified in transplant patients with melanoma, the association between immunosuppression and its development in kidney transplant recipients has been well described[24].
Costs: Following graft loss and reinstitution of dialysis, the cost of low-dose maintenance immunosuppression should not be overlooked, particularly since data supporting continued immunosuppression are lacking. A typical immunosuppressive regimen consisting of low-dose prednisone and cyclosporine or tacrolimus (or mTOR inhibitors) costs more than two thousand United States dollars annually.
While practices differ among centers, most advocate transplantectomy in patients whose allograft failed within one to two years posttransplantation. However, no consensus exists on the timing and indications for transplantectomy when graft loss occurs more than 1-2 years after transplant.
The USRDS registry study demonstrated that transplantectomy was nearly twice as common in patients with early (< 12 mo) compared with late graft loss (≥ 12 mo)[27]. However, whether transplantectomy was performed electively or for graft-related symptoms could not be determined from the study. A single-center study consisting of 34 pediatric kidney transplant recipients demonstrated that children with graft failure within one year of transplantation were four-fold more likely to require transplantectomy than those with graft loss after one year (P = 0.04)[28]. Fever, graft tenderness, and an elevated C-reactive protein were significantly more common in children who subsequently underwent transplantectomy than in those who did not. Of interest, one retrospective study suggested that transplantectomy may minimize allosensitization in patients with early (graft survival < 6 mo) but not late graft loss. Patients with early graft loss and nephrectomy demonstrated a decline in PRA at a median follow up of 47 mo (46% at the time of graft loss and 27% at last follow up, P = 0.02). In contrast, PRA remained elevated among those who had a nephrectomy after late graft loss[29]. It is suggested that the time of graft failure and subsequent allograft nephrectomy may play a contributory role in allosensitization.
In general, the decision to perform a failed graft nephrectomy requires careful consideration of potential risks and benefits.
A retained failed allograft has been suggested to serve as a focus for a chronic inflammatory state. In one single-center study, patients with failed kidney transplants returning to hemodialysis were shown to exhibit worse anemia, erythropoietin resistance, and hypoalbuminemia, as well as worse C reactive protein (CRP), erythrocyte sedimentation rate (ESR), and ferritin profiles compared with their transplant naïve hemodialysis counterparts. Furthermore, amelioration of both clinical and laboratory parameters of the chronic inflammatory state was observed following transplantectomy. Although symptomatic patients undergoing transplantectomy had lower baseline hemoglobin and higher CRP, ESR, and ferritin compared with those with a retained graft, the former group of patients had a better hematologic and biochemical profile at 6 mo after transplantectomy compared with the latter[30].
It is noteworthy that hypoalbuminemia and high CRP have been shown to be markers for increased cardiovascular and global morbidity and mortality both in the general population and in ESKD patients on hemodialysis. Some centers favor transplantectomy in patients with biochemical indicators of chronic inflammation before the onset of overt clinical manifestations[31,32].
Retrospective study using the USRDS database (n = 10951 patients returning to long-term dialysis after a failed transplant) demonstrated that transplantectomy was associated with a 32% lower relative risk for all-cause mortality (adjusted HR = 0.68; 95%CI: 0.63 to 0.74)[32]. However, the study was not without shortcomings. Patients who had graft nephrectomy (n = 3451) were younger and in better health condition than their non-nephrectomized counterparts. It is also noteworthy that despite adjustment for confounding factors and likelihood of undergoing transplantectomy, limitations intrinsic to retrospective registry studies remain. In addition, in patients with the failed allograft left in situ, it is not known whether low-dose maintenance immunosuppression might be independently associated with increased infectious- and cardiovascular disease-related mortality.
Of interest, in a large retrospective studies consisting of more than 19000 patients with graft failure, transplantectomy in patients reinitiating dialysis was found to be associated with increased mortality among those with early graft loss [graft survival < 12 mo, HR 1.13 (95%CI: 1.01-1.26)] whereas among those with late graft loss (graft survival > 12 mo), transplantectomy was associated with decreased mortality rates (0.89 95%CI: 0.83-0.95)[27]. It is speculated that the association of transplantectomy and mortality risk in patients with early graft loss was due to graft-related symptoms rather than the nephrectomy procedure per se. Further studies are needed to determine whether transplantectomy after late graft loss confers a survival advantage over leaving the graft in situ.
Leaving a failed allograft in situ may avoid potential morbidity and mortality associated with the surgical procedure. In addition, in patients with residual kidney function, a retained graft may allow more liberal fluid intake and improve patients’ quality of life. In most series reported, transplantectomy-associated morbidity occurred in 17%-60% and mortality in 1.5% to 14% of patients[33]. The wide variation in the mortality rates reported may be due in part to the timing of surgery, the indication for graft nephrectomy, the patients’ condition at the time of surgery, the surgical techniques, and individual centers’ practice and experiences[31]. Symptomatic patients who need urgent transplantectomy are more likely to have worse outcomes than those undergoing elective transplantectomy. In one study, patients who underwent graft nephrectomy under suboptimal medical conditions (severe rejection or graft sepsis, hemorrhage from anastomotic suture line), a mortality rate of up to 39% has been reported[34].
Allograft nephrectomy has been shown to be associated with allosensitization, potentially resulting in prolonged wait times for a crossmatch negative kidney in re-allograft candidates. It is speculated that a retained allograft may serve as an antibody sponge, or alternatively, rapid immunosuppression weaning after transplantectomy may promote antibody-mediated allosensitization against the allograft. In one single-center study, de novo donor-specific antibodies (DSAs, tested via Luminex single-antigen assay) were detected as soon as five days after transplantectomy, suggesting that the antibodies were preformed[15]. Furthermore, the median fluorescence intensity (MFI) of alloantibodies remained stable or declined during follow up. It was hypothesized that if DSAs had appeared because of injury caused by graft nephrectomy, the MFI would have increased during follow up. Whether the detection of preformed DSAs after graft nephrectomy may have important implications in identifying unacceptable antigens for patients awaiting a repeat transplant remains to be studied.
Although post allograft nephrectomy rise in PRAs or DSAs may reflect preformed antibodies, it is also tempted to speculate that transplantectomy may stimulate pro-inflammatory cytokine production and upregulation of HLA alloantibodies. Alternatively, sensitization may occur due to the persistence of antigen-presenting cells or residual donor tissues and vessels[16].
The mechanism(s) or predominant mechanism of de novo development of anti-HLA alloantibodies after graft nephrectomy is currently not fully understood. Nonetheless, there has been ample literature showing that transplantectomy leads to an increase in class I and class II PRA, and DSA and non-DSAs to variable extent[13,16,35-38]. Whether immunosuppression weaning over a prolonged period after graft nephrectomy may reduce the risk of de novo anti-HLA alloantibodies development is unknown and warrants further exploration. Prospective studies to assess the potential mechanism(s) of allosensitization after transplantectomy and the impact of such procedure on graft and patient survival as well as on acute rejection rates after a repeat transplant are needed.
The literature on the impact of transplantectomy on the outcomes of retransplantation have yielded variable and even contradictory results. Selected studies are discussed.
Studies indicating an adverse impact of transplantectomy on various clinical outcomes of a repeat transplant: Early single-center study demonstrated that transplantectomy was associated with a significant increase in PRA levels and a higher incidence of delayed graft function in a repeat transplant[39]. A trend for reduced graft survival was observed among patients whose first grafts failed within the first post-transplant year. However, transplant nephrectomy had no impact on the incidence of acute rejection or renal function of a repeat graft at 3-year follow-up.
In a retrospective study consisting of 192 recipients of a reallograft transplant, nephrectomy of the primary failed graft was shown to have an adverse impact on reallograft transplantation (P = 0.0003)[40]. Multivariate analysis demonstrated a significant relationship between survival of the primary allograft and repeat transplant outcomes. Subgroup analysis performed in patients whose graft functioned more than 6 mo (n = 90) similarly demonstrated that nephrectomy of the failed graft is a risk factor for worse retransplantation outcomes. Other identified risk factors included advanced donor age, longer time interval from transplantectomy to reallograft transplantation, and the lack of induction with Minnesota antilymphocyte globulin.
In a retrospective study comprising 121 patients who had a nephrectomy and 45 who did not undergo nephrectomy prior to repeat transplantation, pretransplant graft nephrectomy and panel reactive antibody levels greater than 70% were found to be independent risk factors for graft failure after a repeat transplant[41]. Subgroup analysis showed that pretransplant graft nephrectomy adversely affected survival of a subsequent graft among high risk patients defined as those with multiple transplants (≥ 2 transplants) and those who received an allograft from an older donor (> 65 years of age), as well as among European Senior Program patients. However, in the subgroup of patients without “high risk” factors, nephrectomy of a previous graft had no impact on delayed graft function, or graft or patient survival rates after a repeat transplant. Nonetheless, pretransplant nephrectomy was associated with increased rejection rates presumably due to elevated PRA levels.
Studies suggesting a neutral impact of transplantectomy on various outcomes of a repeat transplant: In a retrospective analysis to evaluate graft survival in patients who underwent transplantectomy prior to reallograft transplantation (n = 68) compared with those who did not (n = 21), nephrectomy of a failed graft was found to have no significant impact on the survival of a future allograft[42]. Five-year actuarial patient survival were 94.1% and 87.5%, respectively (P = 0.69). PRA levels at the time of retransplantation were comparable between the two groups (37% vs 29%, respectively). Multivariate analysis showed a negative impact of PRA levels on graft survival independent of transplantectomy (P = 0.04).
One single-center retrospective study demonstrated that dialysis time was significantly longer in patients who had a graft nephrectomy than those who did not, presumably due to higher PRA levels in the nephrectomy group, making it difficult to obtain a negative crossmatch donor kidney. Nonetheless, acute rejection episodes and one-, five-, and ten-year graft survival rates were not different between the nephrectomy and no nephrectomy group[43]. Univariate analysis demonstrated that PRA levels and the number of acute rejection episodes had no significant impact on graft or patient survival, whether or not the patient had transplantectomy (P = 0.3 for both).
Differential impact of transplantectomy on the outcomes of a future allograft: Retrospective study using the USRDS database (n = 19107 patients returning to dialysis after first graft failure) demonstrated that transplantectomy after early graft loss (graft survival of less than twelve months) was associated with a lower risk of repeat graft failure, whereas transplantectomy for late graft loss (graft survival of ≥ 12 mo) may be deleterious to repeat transplant outcomes[27]. However, further analysis demonstrated that the protective effect of transplantectomy among those with early graft loss was due to a decrease in death with a functioning graft rather than an improvement in death-censored graft survival. It is speculated that there is a complex association between a retained failed graft and cardiovascular disease. In contrast to early graft loss, leaving the graft in situ in patients with late graft loss was shown have some protective effect on a repeat transplant, possibly related to development of tolerance and acceptance of a repeat transplant in the presence of donor antigen. Alternatively, it is suggested that if symptomatic immunological responses prompted a transplantectomy, then primary graft nephrectomy is simply a marker of high immunological risk for repeat transplant failure.
The potential risks and benefits of nephrectomy of a failed graft and its impact on a repeat transplant are summarized in Table 3.
| Comments | |
| Potential benefits | |
| A failing graft is a focus of a chronic inflammatory state | |
| May reduce mortality rates | Variable results, further studies are needed |
| Potential adverse effects | |
| Residual kidney function may allow less stringent fluid restriction | |
| Surgery-related morbidity and mortality | Morbidity 17%-60% in most series reported |
| Mortality 1.5%-14% in most series reported | |
| Allosensitization and the potential for future prolonged wait-times for a compatible crossmatch kidney | |
| Impact on a repeat transplant | |
| Mixed reports due to potential confounding factors | |
| Differences among studies in: | |
| Immunosuppression withdrawal protocols | |
| Recipient and donor demographics | |
| Era of transplantation | |
| Indications for transplantectomy | |
| Time on dialysis prior to a repeat transplant | |
| Causes of prior graft loss | |
| Allosensitization associated with blood transfusion | |
| Pre-existing DSA with or without complement-fixing DSA (see text) | |
| HLA matching of subsequent graft | |
| Donor type (living vs deceased) | |
| Others |
Transplantectomy in patients with graft loss due to BK nephropathy: While some centers advocate graft nephrectomy prior to repeat transplant in patients with graft loss due to BK nephropathy (BKN), re-allograft transplant can be safely performed without original allograft nephrectomy but preferably following BK viral clearance[44]. Nonetheless, successful re-allograft transplant in the setting of severe viremia without concomitant nephrectomy of the allograft in a patient with graft failure due to BKN can be achieved. The patient is a 65-year-old woman who underwent urgent combined liver and repeat kidney allograft transplant due to fulminant hepatic failure and kidney graft failure due to BKN. She received no induction therapy and was maintained on low-dose tacrolimus and prednisone dual therapy. At the time of transplant, plasma BK PCR was 946000 copies/mL. Three months after transplant plasma BK was undetectable and remained undetectable at 15 mo follow-up (unpublished observation).
The variable and even conflicting results on the impact of transplantectomy on future reallograft transplantation may reflect a multitude of potential contributing factors including but not limited to institution dependent practice on indications for nephrectomy following a failed graft, differences in study design and immunosuppressive withdrawal protocols, donor and recipient demographics, recipient comorbid conditions, era of transplantation, time on dialysis prior to a repeat transplant, the causes of prior graft loss, donor type (living vs deceased), quality and HLA-matching of subsequent allograft, alllosensitization associated with blood transfusion, and pre-existing DSA with or without complement-fixing DSA at the time of transplantation, among others. Recent studies have shown that DSA with the ability to bind to C1q and activate complement are associated with greater risk of acute rejection and graft loss than non-complement fixing DSA[45].
While it remains unclear whether transplantectomy after late graft failure has a salutary or harmful effect on a repeat transplant, graft intolerance syndrome refractory to medical treatment is an indication for transplantectomy. In patients with multiple retained failed allografts, graft nephrectomy prior to retransplantation may also be inevitable. Monitoring PRA levels and HLA class I/II alloantibodies (using Luminex single-antigen assays) prior to and after graft nephrectomy as well as before retransplantation may be invaluable in guiding immunosuppression in re-allograft transplant recipients. In recent years various desensitization protocols have allowed highly sensitized patients to undergo successful retransplantation. Although no consensus exists, graft nephrectomy in patients with erythropoietin resistance and refractory anemia or hypoalbuminemia attributed to the failed allograft may be justifiable. Nonetheless, the decision to perform transplantectomy should be individualized. Effort to reduce cardiovascular and infectious complications undoubtedly improves clinical outcomes after reallograft transplantation whether or not nephrectomy is performed.
Clinical studies to support or refute early vs late reinitiation of dialysis in patients with a failed kidney transplant are currently lacking. In the authors’ opinion, reinitiation of dialysis should not be based solely on an absolute level of residual kidney function. Nonetheless, dialysis reinitiation when eGFR reaches 6-9 mL/min or less seems reasonable. In patients with higher level of residual kidney function, dialysis reinitiation should be based on clinical or laboratory parameters or both. Similar to the nontransplant settings, clinical indications may include symptomatic uremia, volume overload or hyperkalemia refractory to medical treatment, or malnourishment among others. In patients with a failed transplant and significant comorbid conditions such as long-standing diabetes with its associated micro- and macrovascular complications, or infectious or urological complications, weaning of immunosuppression and early return to dialysis seem justifiable.
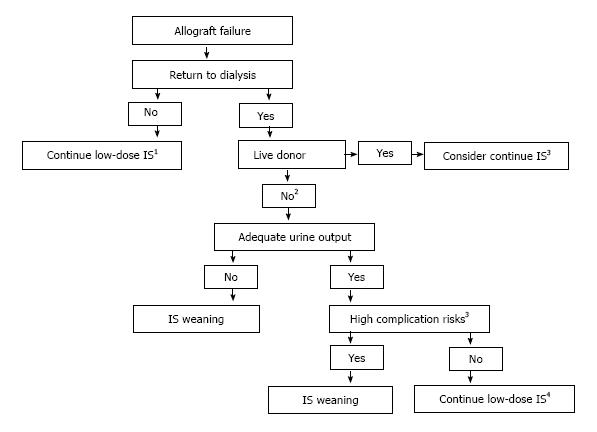
Although evidence-based recommendations are lacking, continuation of low-dose immunosuppression seems appropriate in pre-dialysis patients and in those with symptomatic rejection to serve as a bridge to allograft nephrectomy. Maintenance low-dose immunosuppression may also be beneficial in patients with anticipated living donor re-allograft transplant or those with residual urine output greater than 0.5 to 1 liter a day. Nevertheless, in the latter group, immunosuppression withdrawal should be considered in high risk patients or those with significant comorbid conditions. These include older age, obesity, diabetes mellitus, neurogenic bladder, recurrent episodes of urinary tract infections or urosepsis, or history of cancers, among others. Proposed algorithm for the management of immunosuppression after allograft loss is shown in Figure 1. Although immunosuppression withdrawal protocols differ among centers, most clinicians advocate immediate discontinuation of antimetabolite (mycophenolate mofetil/mycophenolic acid or azathioprine). Cyclosporine or tacrolimus is generally weaned over several weeks and prednisone over three to six months. At the authors’ institution, antimetabolite is discontinued upon return to dialysis, calcineurin inhibitors are weaned over four to six weeks, and prednisone dose is decreased by 1 mg/month until discontinued. Proposed immunosuppression weaning protocols are shown in Table 4.
| CNI + antimetabolitea + prednisone | CNI + mTOR inh + prednisone | mTOR inh + prednisone |
| Discontinue antimetabolite at initiation of dialysis | Discontinue mTOR inh at initiation of dialysis | Taper mTOR inh over 4-6 wkb |
| Taper CNI over | Taper CNI over | Maintain same steroid dose at initiation of dialysis x 2-4 wk, then taper by 1 mg/mo (starting from 5 mg daily) until off |
| 4-6 wkb | 4-6 wkb | |
| Maintain same steroid dose at initiation of dialysis x 2-4 wk, then taper by 1 mg/mo (starting from 5 mg daily) until off | Maintain same steroid dose at initiation of dialysis x 2-4 wk, then taper by 1 mg/mo (starting from 5 mg daily) until off |
Transplantectomy is usually performed and immunosuppression rapidly tapered when graft loss occurs within one year after transplant. Whether patients with early graft loss requiring transplantectomy (particularly those with a live donor and those anticipated to have a relatively short wait time after early relisting) benefit from continuation of immunosuppression to minimize allosensitization warrant further exploration. In addition, the duration and intensity of maintenance immunosuppression remain to be defined. At the authors’ institution, the graft is usually left in place when graft loss occurs more than one year after transplant. Transplantectomy is generally performed in patients with graft intolerance syndrome or those requiring space for retransplantation. In patients with clinical signs or symptoms suggestive of a chronic inflammatory state, transplantectomy may be considered at the discretion of the treating physician. More importantly, community nephrologists should remain vigilant to the early recognition of signs and symptoms of an infected or acutely rejecting allograft for early medical treatment and prevention of emergent transplantectomy given the increased morbidity and mortality associated with the latter. In patients with graft failure due to recent episodes of late acute rejection, gradual weaning of immunosupression is advisable to prevent graft intolerance syndrome and obviate the need to perform urgent transplantectomy. In patients with graft loss due to BK nephropathy, repeat transplant can be safely performed without prior graft nephrectomy but preferably following BK viral clearance. Suggested absolute and relative indications for graft nephrectomy are shown in Table 5.
| Absolute indications (commonly accepted) | Relative indications (controversial) |
| Primary nonfunction Hyperacute rejection Early recalcitrant acute rejection Early graft loss (generally defined as graft loss within the first year) Arterial or venous thrombosis Graft intolerance syndrome Recurrent urinary tract infections or sepsis/urosepsis Multiple retained failed transplants prior to a repeat transplant | The presence of hematologic or biochemical markers of the chronic inflammatory state Erythropoietin resistance anemia Elevated ferritin level Elevated C reactive protein Elevated erythrocyte sedimentation rate Low prealbumin/albumin Graft loss due to BK nephropathy and high level BK viremia (see text) |
P- Reviewer: Markic D, Martins LSS, Ravaioli M S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Gill JS, Abichandani R, Kausz AT, Pereira BJ. Mortality after kidney transplant failure: the impact of non-immunologic factors. Kidney Int. 2002;62:1875-1883. [PubMed] |
| 2. | Pham PT, Pham PC. Immunosuppressive management of dialysis patients with recently failed transplants. Semin Dial. 2011;24:307-313. [PubMed] |
| 3. | Molnar MZ, Ichii H, Lineen J, Foster CE, Mathe Z, Schiff J, Kim SJ, Pahl MV, Amin AN, Kalantar-Zadeh K. Timing of return to dialysis in patients with failing kidney transplants. Semin Dial. 2013;26:667-674. [PubMed] |
| 4. | Beddhu S, Samore MH, Roberts MS, Stoddard GJ, Ramkumar N, Pappas LM, Cheung AK. Impact of timing of initiation of dialysis on mortality. J Am Soc Nephrol. 2003;14:2305-2312. [PubMed] |
| 5. | Cooper BA, Branley P, Bulfone L, Collins JF, Craig JC, Fraenkel MB, Harris A, Johnson DW, Kesselhut J, Li JJ. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363:609-619. [PubMed] |
| 6. | Scialla JJ, Liu J, Crews DC, Guo H, Bandeen-Roche K, Ephraim PL, Tangri N, Sozio SM, Shafi T, Miskulin DC. An instrumental variable approach finds no associated harm or benefit with early dialysis initiation in the United States. Kidney Int. 2014;86:798-809. [PubMed] |
| 7. | Molnar MZ, Streja E, Kovesdy CP, Hoshino J, Hatamizadeh P, Glassock RJ, Ojo AO, Kalantar-Zadeh K. Estimated glomerular filtration rate at reinitiation of dialysis and mortality in failed kidney transplant recipients. Nephrol Dial Transplant. 2012;27:2913-2921. [PubMed] |
| 8. | Shemin D, Bostom AG, Laliberty P, Dworkin LD. Residual renal function and mortality risk in hemodialysis patients. Am J Kidney Dis. 2001;38:85-90. [PubMed] |
| 9. | Bargman JM, Thorpe KE, Churchill DN. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol. 2001;12:2158-2162. [PubMed] |
| 10. | Jassal SV, Lok CE, Walele A, Bargman JM. Continued transplant immunosuppression may prolong survival after return to peritoneal dialysis: results of a decision analysis. Am J Kidney Dis. 2002;40:178-183. [PubMed] |
| 11. | Perl J, Dong J, Rose C, Jassal SV, Gill JS. Is dialysis modality a factor in the survival of patients initiating dialysis after kidney transplant failure? Perit Dial Int. 2013;33:618-628. [PubMed] |
| 12. | Elmahi N, Csongrádi E, Kokko K, Lewin JR, Davison J, Fülöp T. Residual renal function in peritoneal dialysis with failed allograft and minimum immunosuppression. World J Transplant. 2013;3:26-29. [PubMed] |
| 13. | Scornik JC, Kriesche HU. Human leukocyte antigen sensitization after transplant loss: timing of antibody detection and implications for prevention. Hum Immunol. 2011;72:398-401. [PubMed] |
| 14. | Augustine JJ, Woodside KJ, Padiyar A, Sanchez EQ, Hricik DE, Schulak JA. Independent of nephrectomy, weaning immunosuppression leads to late sensitization after kidney transplant failure. Transplantation. 2012;94:738-743. [PubMed] |
| 15. | Del Bello A, Congy-Jolivet N, Sallusto F, Guilbeau-Frugier C, Cardeau-Desangles I, Fort M, Esposito L, Guitard J, Cointault O, Lavayssière L. Donor-specific antibodies after ceasing immunosuppressive therapy, with or without an allograft nephrectomy. Clin J Am Soc Nephrol. 2012;7:1310-1319. [PubMed] |
| 16. | Del Bello A, Congy N, Sallusto F, Cardeau-Desangles I, Fort M, Esposito L, Guitard J, Cointault O, Lavayssière L, Nogier MB. Anti-human leukocyte antigen immunization after early allograft nephrectomy. Transplantation. 2012;93:936-941. [PubMed] |
| 17. | Woodside KJ, Schirm ZW, Noon KA, Huml AM, Padiyar A, Sanchez EQ, Sarabu N, Hricik DE, Schulak JA, Augustine JJ. Fever, infection, and rejection after kidney transplant failure. Transplantation. 2014;97:648-653. [PubMed] |
| 18. | Madore F, Hébert MJ, Leblanc M, Girard R, Bastien E, Morin M, Beaudry C, Boucher A, Dandavino R. Determinants of late allograft nephrectomy. Clin Nephrol. 1995;44:284-289. [PubMed] |
| 19. | Verresen L, Vanrenterghem Y, Waer M, Hauglustaine D, Michielsen P. Corticosteroid withdrawal syndrome in dialysis patients. Nephrol Dial Transplant. 1988;3:476-477. [PubMed] |
| 20. | Smak Gregoor PJ, Zietse R, van Saase JL, op de Hoek CT, IJzermans JN, Lavrijssen AT, de Jong GM, Kramer P, Weimar W. Immunosuppression should be stopped in patients with renal allograft failure. Clin Transplant. 2001;15:397-401. [PubMed] |
| 21. | Kiberd BA, Belitsky P. The fate of the failed renal transplant. Transplantation. 1995;59:645-647. [PubMed] |
| 22. | Smak Gregoor PJ, van Saase JL, vd Ingh HF, Weimar W, Kramer P. Disseminated histoplasmosis in a haemodialysis patient on immunosuppression after graft failure. Nephrol Dial Transplant. 1996;11:542-544. [PubMed] |
| 23. | Dantal J, Hourmant M, Cantarovich D, Giral M, Blancho G, Dreno B, Soulillou JP. Effect of long-term immunosuppression in kidney-graft recipients on cancer incidence: randomised comparison of two cyclosporin regimens. Lancet. 1998;351:623-628. [PubMed] |
| 24. | Vajdic CM, van Leeuwen MT, Webster AC, McCredie MR, Stewart JH, Chapman JR, Amin J, McDonald SP, Grulich AE. Cutaneous melanoma is related to immune suppression in kidney transplant recipients. Cancer Epidemiol Biomarkers Prev. 2009;18:2297-2303. [PubMed] |
| 25. | Stewart JH, Vajdic CM, van Leeuwen MT, Amin J, Webster AC, Chapman JR, McDonald SP, Grulich AE, McCredie MR. The pattern of excess cancer in dialysis and transplantation. Nephrol Dial Transplant. 2009;24:3225-3231. [PubMed] |
| 26. | van Leeuwen MT, Webster AC, McCredie MR, Stewart JH, McDonald SP, Amin J, Kaldor JM, Chapman JR, Vajdic CM, Grulich AE. Effect of reduced immunosuppression after kidney transplant failure on risk of cancer: population based retrospective cohort study. BMJ. 2010;340:c570. [PubMed] |
| 27. | Johnston O, Rose C, Landsberg D, Gourlay WA, Gill JS. Nephrectomy after transplant failure: current practice and outcomes. Am J Transplant. 2007;7:1961-1967. [PubMed] |
| 28. | Minson S, Muñoz M, Vergara I, Mraz M, Vaughan R, Rees L, Olsburgh J, Calder F, Shroff R. Nephrectomy for the failed renal allograft in children: predictors and outcomes. Pediatr Nephrol. 2013;28:1299-1305. [PubMed] |
| 29. | Sener A, Khakhar AK, Nguan CY, House AA, Jevnikar AM, Luke PP. Early but not late allograft nephrectomy reduces allosensitization after transplant failure. Can Urol Assoc J. 2011;5:E142-E147. [PubMed] |
| 30. | López-Gómez JM, Pérez-Flores I, Jofré R, Carretero D, Rodríguez-Benitez P, Villaverde M, Pérez-García R, Nassar GM, Niembro E, Ayus JC. Presence of a failed kidney transplant in patients who are on hemodialysis is associated with chronic inflammatory state and erythropoietin resistance. J Am Soc Nephrol. 2004;15:2494-2501. [PubMed] |
| 31. | Ayus JC, Achinger SG. At the peril of dialysis patients: ignoring the failed transplant. Semin Dial. 2005;18:180-184. [PubMed] |
| 32. | Ayus JC, Achinger SG, Lee S, Sayegh MH, Go AS. Transplant nephrectomy improves survival following a failed renal allograft. J Am Soc Nephrol. 2010;21:374-380. [PubMed] |
| 33. | Akoh JA. Transplant nephrectomy. World J Transplant. 2011;1:4-12. [PubMed] |
| 34. | Sharma DK, Pandey AP, Nath V, Gopalakrishnan G. Allograft nephrectomy--a 16-year experience. Br J Urol. 1989;64:122-124. [PubMed] |
| 35. | Knight MG, Tiong HY, Li J, Pidwell D, Goldfarb D. Transplant nephrectomy after allograft failure is associated with allosensitization. Urology. 2011;78:314-318. [PubMed] |
| 36. | van den Berg-Loonen EM, Billen EV, Voorter CE, van Heurn LW, Claas FH, van Hooff JP, Christiaans MH. Clinical relevance of pretransplant donor-directed antibodies detected by single antigen beads in highly sensitized renal transplant patients. Transplantation. 2008;85:1086-1090. [PubMed] |
| 37. | Marrari M, Duquesnoy RJ. Detection of donor-specific HLA antibodies before and after removal of a rejected kidney transplant. Transpl Immunol. 2010;22:105-109. [PubMed] |
| 38. | Adeyi OA, Girnita AL, Howe J, Marrari M, Awadalla Y, Askar M, Martell J, Zeevi A, Shapiro R, Nalesnik M. Serum analysis after transplant nephrectomy reveals restricted antibody specificity patterns against structurally defined HLA class I mismatches. Transpl Immunol. 2005;14:53-62. [PubMed] |
| 39. | Sumrani N, Delaney V, Hong JH, Daskalakis P, Sommer BG. The influence of nephrectomy of the primary allograft on retransplant graft outcome in the cyclosporine era. Transplantation. 1992;53:52-55. [PubMed] |
| 40. | Abouljoud MS, Deierhoi MH, Hudson SL, Diethelm AG. Risk factors affecting second renal transplant outcome, with special reference to primary allograft nephrectomy. Transplantation. 1995;60:138-144. [PubMed] |
| 41. | Schleicher C, Wolters H, Kebschull L, Anthoni C, Suwelack B, Senninger N, Palmes D, Mersfeld B. Impact of failed allograft nephrectomy on initial function and graft survival after kidney retransplantation. Transpl Int. 2011;24:284-291. [PubMed] |
| 42. | Ahmad N, Ahmed K, Mamode N. Does nephrectomy of failed allograft influence graft survival after re-transplantation? Nephrol Dial Transplant. 2009;24:639-642. [PubMed] |
| 43. | Surga N, Viart L, Wetzstein M, Mazouz H, Collon S, Tillou X. Impact of renal graft nephrectomy on second kidney transplant survival. Int Urol Nephrol. 2013;45:87-92. [PubMed] |
| 44. | Geetha D, Sozio SM, Ghanta M, Josephson M, Shapiro R, Dadhania D, Hariharan S. Results of repeat renal transplantation after graft loss from BK virus nephropathy. Transplantation. 2011;92:781-786. [PubMed] |
| 45. | Sutherland SM, Chen G, Sequeira FA, Lou CD, Alexander SR, Tyan DB. Complement-fixing donor-specific antibodies identified by a novel C1q assay are associated with allograft loss. Pediatr Transplant. 2012;16:12-17. [PubMed] |