Published online Nov 6, 2013. doi: 10.5527/wjn.v2.i4.111
Revised: June 11, 2013
Accepted: October 18, 2013
Published online: November 6, 2013
Processing time: 172 Days and 22.2 Hours
Diabetes mellitus (DM) is associated with increased oxidative stress due to elevated glucose levels in the plasma. Glucose promotes glycosylation of both plasma and cellular proteins with increased risk for vascular events. Diabetic patients suffer from a higher incidence of cardiovascular complications such as diabetic nephropathy. Haptoglobin (Hp) is an antioxidant plasma protein which binds free hemoglobin, thus preventing heme-iron mediated oxidation. Two alleles exist at the Hp gene locus (1 and 2) encoding three possible Hp genotypes that differ in their antioxidant ability, and may respond differently to vitamin E treatment. Several clinical studies to have shown that Hp 1-1 genotype is a superior antioxidant to the Hp 2-2 genotype and Hp 2-2 genotype is associated with a higher incidence of cardiovascular disease. Vitamin E was found to have beneficial effect in patient and mice with Hp 2-2 genotype. In this review we have summarized the results of our studies in patients with diabetic nephropathy treated with vitamin E and in diabetic mice with different haptoglobin genotypes.
Core tip: In diabetes mellitus there is an increase in oxygen radical formation due to glucose auto oxidation, the formation of advanced glycosylation end products, and metabolic stress. Epidemiologic studies suggest that vitamin E supplementation might decrease the risk of developing cardiovascular disease, others showed increased risk of cardiac death with the vitamin E treatment. To the contradictory results in the literature regarding the beneficial role of vitamin E in protecting against cardiovascular complications, high dose vitamin E supplementation has not been recommended by the medical community. In fact, a meta-analysis of over 135000 individuals treated with vitamin E concluded that high dose vitamin E (greater than 400 mg/d) slightly increases the risk of mortality. However, recent investigations into the polymorphic serum protein haptoglobin (Hp) indicate that vitamin E may be beneficial in a genetically defined subgroup of patients, namely, diabetic patients of the Hp 2-2 genotype. The role of Hp as an antioxidant, its importance in diabetes, and the therapeutic role of vitamin E will be discussed in this review.
- Citation: Farid N, Inbal D, Nakhoul N, Evgeny F, Miller-Lotan R, Levy AP, Rabea A. Vitamin E and diabetic nephropathy in mice model and humans. World J Nephrol 2013; 2(4): 111-124
- URL: https://www.wjgnet.com/2220-6124/full/v2/i4/111.htm
- DOI: https://dx.doi.org/10.5527/wjn.v2.i4.111
Diabetic Nephropathy (DN) is the leading cause of end stage renal disease and accounts for approximately 40% of all patients who require replacement therapy. The well known risk factors for DN are uncontrolled diabetes mellitus and genetic factors[1,2]. The inter-individual variability in the probability for developing DN and its clustering within families, suggest a substantial genetic predisposition. Reactive oxygen species, particularly those derived from iron, have been implicated in the progression of DN and other vascular complications of diabetes. Therefore, polymorphic genetic loci, encoding variants in enzymes protecting against iron-induced oxidative stress, serve as potential susceptibility determinants for the development of DN[3,4]. Diabetes is accompanied by severe oxidative stress (especially lipid per-oxidation) which is caused by increased oxygen free radical production. Toxic oxygen free radicals have been implicated in the pathogenesis of diabetes mellitus, and its micro- and macro vascular complications. An imbalance resulting from the increased production and/or reduced scavenging of these free radicals, leads to a metabolic state of oxidative stress, which consequently leads to tissue damage.
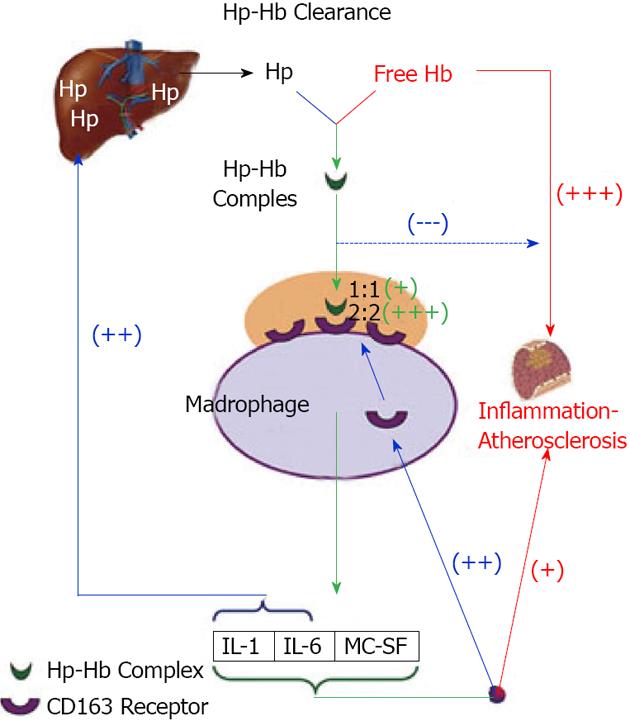
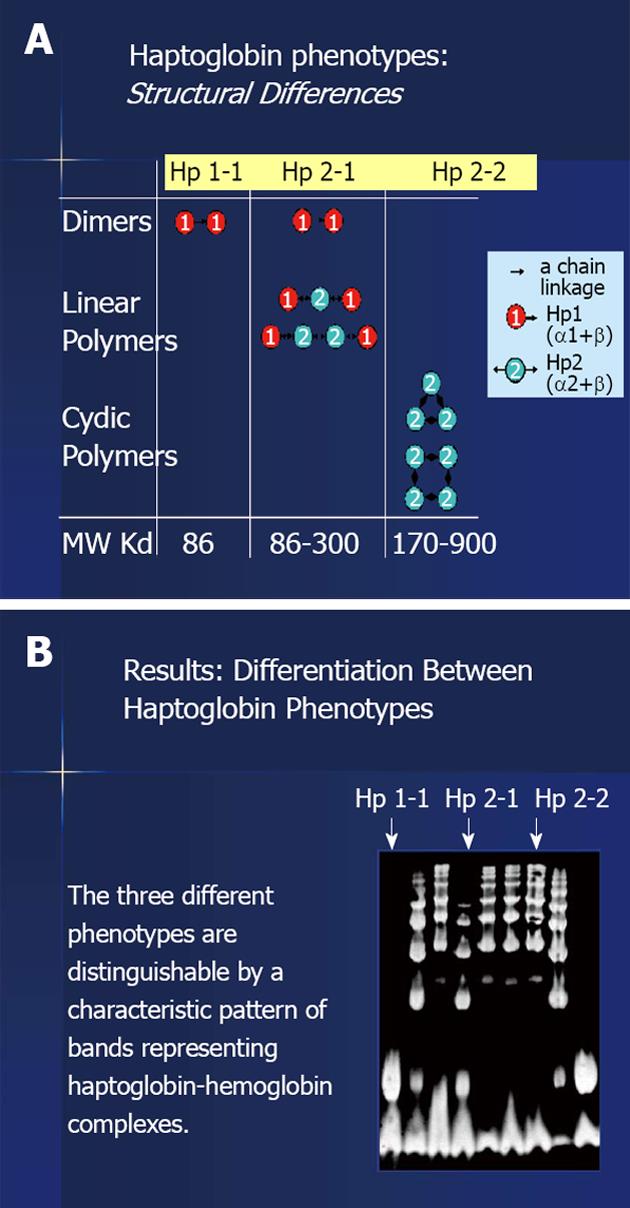
One of these protecting factors is the haptoglobin (Hp). Hp is an acute phase protein synthesized in the liver by the hepatocytes. It acts as an antioxidant by virtue of its ability to prevent hemoglobin (Hb) induced oxidative tissue damage[4-6]. Its synthesis is rapidly and dramatically increased in response to numerous inflammation stimuli due to a transcriptional activation of the Hp gene. Whenever Hb is released into the circulation, its binds immediately to Hp to form an Hp-Hb complex and this complex is rapidly removed predominately by the monocyte/macrophage CD 163 Hp-Hb receptor expressed on Kupfer cells in the liver (Figure 1). When Hp is depleted, as a result of hemolysis or in Hp Knockout mice, Hb accumulates in the kidney and us secreted in the urine. Therefore, a major role of Hp is to prevent renal damage[6-9]. Two classes of Hp alleles are known in humans (1 and 2) with homozygous (1-1 or 2-2) and heterozygous (2-1) possible genotypes. The Hp 1 allele contains 5 exons and is found in all animal species while the Hp 2 allele contains 7 exons and exists only in humans, with polymorphic expression using the two classes of alleles. Our group has revealed profound differences in the antioxidant capacity of the protein product of the two Hp alleles and has demonstrated that these differences are exaggerated in the diabetic state. Studies, both in vivo and in vitro, have shown that the Hp 1 protein has superior antioxidant capacity compared to the Hp 2 protein[9,10] (Figure 2).
Vitamin E is a fat-soluble vitamin with antioxidant properties. Vitamin E exists in eight different forms (isomers): alpha-, beta-, gamma-, and delta-tocopherol; and alpha-, beta-, gamma-, and delta-tocotrienol. Alpha-tocopherol is the most active form in humans. Dosing and daily allowance recommendations for vitamin E are often provided in alpha-tocopherol equivalents to account for the different biological activities of the various forms of vitamin E, or in international units (IU), which food and supplement labels may use. Due to its antioxidant properties, Vitamin E has been proposed to have a role in preventing or treating numerous health conditions, often by its antioxidant properties. When highly-reactive species attack the membranes lipids or the lipoproteins, it sets off a chain reaction of lipid per oxidation. Vitamin E halts this chain reaction, e.g., it thereby acting as a chain breaking inhibitor of lipid per oxidation[11,12].
In patients with type 1 diabetes mellitus (DM), the most important renal structure changes occur in the glomeruli: In these patients, diabetic glomerulopathy is characterized by increased glomerular basement membrane (GBM) width and mesangial expansion with reduction in the glomerular filtration surface area. Concomitantly, the renal arterioles, tubules and interstitium also develop lesions. Early stage of diabetic nephropathy is associated with the development of glomerular hyperfiltration, hyperalbuminuria, thickening of the GBM, mesangial expansion, and progressive decline in glomerular filtration rate. Fioretto et al[13] also described proximal tubular basement thickening with atubular glomerular junction. When renal insufficiency ensues with proteinuria and hypertension, glomerulosclerosis and fibrosis develops. Although animal models with diabetes mellitus type 1 and 2 are exist, no single animal model develops glomerular and tubular changes identical to those seen in humans. While the field of diabetic nephropathy has made much progress in understanding the disease process and its progression, only limited success has been attained. One reason for this is the inability to develop a murine model of diabetic nephropathy with the spectrum of micro- and macrovascular complications similar to human disease.
Antioxidants have been shown to play a beneficial role in the prevention of the diabetic complications. Diabetes is a good model of chronic oxidative damage and it is a particularly suitable disease for antioxidant supplementation. It was found that there is a significant correlation between the increased blood sugar levels and the depletion of the antioxidants has been found. This depletion was a major risk factor for developing diabetic complications, and antioxidant supplementation (vitamin E, C) could decrease this risk. Nevertheless, only a few studies have shown the impact of the antioxidant therapy in diabetic patients. According to these facts, the present review will evaluate the role of antioxidant supplementation along with the standard diabetic therapy in the prevention of diabetic complications[14-16].
Aims of the review: (1) to evaluate the role of vitamin E therapy in preventing the development of complications in diabetic patients (primary prophylaxis); (2) to evaluate the role of the vitamin E supplementation in controlling the progression of the complications in diabetic patients (secondary intervention); and (3) to evaluate the role of the vitamin E supplementation in controlling the progression of diabetic nephropathy in diabetic mice with different Hp genotype.
Diabetes mellitus is associated with increased oxygen radical formation due to glucose auto-oxidation, the formation of advanced glycosylation end products and metabolic stress. Therefore the influence of different antioxidants has been the subject of many studies over the years. Due to the involvement of low-density lipoprotein (LDL) oxidation in the pathology of atherosclerosis, numerous studies have been made carried out on vitamin E, as an antioxidant that has the ability to react with lipid peroxyl radicals and terminate the propagation of free radicals. Several observational epidemiologic studies suggest that vitamin E supplementation might decrease the risk of developing cardiovascular disease (CVD). These studies have been divided into two categories. The first includes primary prevention studies involving healthy people, and the second category comprises secondary prevention studies involving people with known cardiovascular complications[17,18].
Extensive preclinical and observational studies have shown the apparent benefit of vitamin E supplementation in preventing cardiovascular events, created an atmosphere in which more than 40% of cardiologists were routinely prescribing high doses of vitamin E[19,20]. Over the past 10 years, several prospective randomized clinical trials including the first randomized controlled trial (RCT) published by Virtamo et al[21], have investigated whether vitamin E supplementation provides cardiovascular protection. The overwhelming consensus from these studies was that vitamin E supplementation does not provide cardiovascular benefit.
In the physicians health study, 14641 males over age 50 years were randomized to receive vitamin E (400 IU/d) and C for 8 years. In this study, no effect was found on CV death, nonfatal stroke or CVD[22]. The meta-analysis of these studies suggests that high doses of vitamin E and C supplementation may increase mortality, and several opinion articles have called for a moratorium on the prescription of high dose vitamin E supplements. A possible explanation for the failure of these studies in spite of solid preclinical data is the inadequate nature of patient selection in these studies.
High-dose antioxidant therapy may provide benefit only to individuals who suffer from particularly high levels of oxidative stress. Hence, the Hp genotype may help identify patients with high levels of oxidative stress that may benefit from antioxidant therapy with vitamin E. The Hp gene is polymorphic with 2 common classes of alleles denoted 1 and 2. We and others have demonstrated that the Hp 2 allele protein product is an inferior antioxidant compared with the Hp 1 allele protein product. These differences in antioxidant protection are profoundly accentuated in the diabetic state resulting in a marked relative increase in oxidative stress in Hp 2 individuals with DM (the distribution of the 3 Hp genotypes in Western societies is approximately 16% Hp 1-1, 36% Hp 2-2, and 48% Hp 2-1)[23-27].
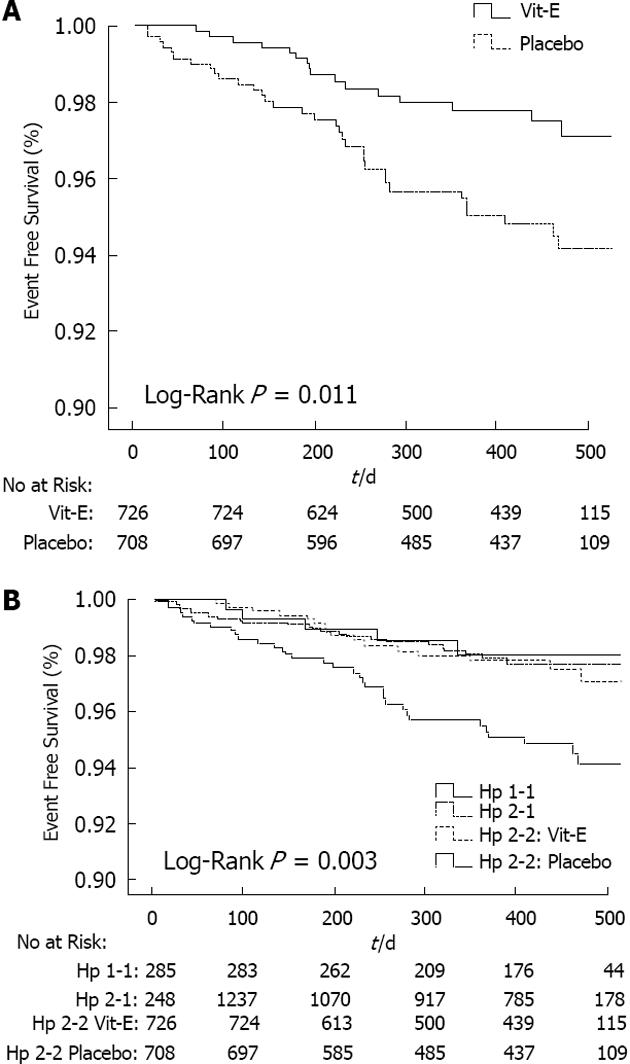
Our groups, at the Technion-Faculty of Medicine, have demonstrated an interaction between the Hp genotype and DM with regard to the development of cardiovascular events. In multiple longitudinal studies Hp 2-2 DM individuals have shown 2- to 5-fold increase in cardiovascular events as compared with Hp 1-1 and Hp 2-1 DM individuals. According to our data we next examined whether antioxidant therapy with vitamin E may reduce cardiovascular events in Hp 2-2 DM individuals in the heart and outcome prevention evaluation (HOPE) study. For this purpose we have assessed the Hp genotype in stored blood samples from HOPE and found that in Hp 2-2 DM individual’s vitamin E significantly reduced myocardial infarction and cardiovascular death by 43% and 55%, respectively. However, these data were interpreted with considerable caution because of the retrospective nature of this analysis, as well as the inability to demonstrate a statistical interaction between vitamin E and Hp genotype for either the HOPE composite outcome (stroke, CVD death, myocardial infarction, MI) or any of its components. Then, we sought to test the validity of these findings in Hp 2-2 DM individuals in a prospective, double-blind, placebo-controlled trial of vitamin E[28-33] (Figure 3).
According to our results vitamin E provides cardiovascular protection to individuals with diabetes and the haptoglobin 2-2 genotype but appears to increase cardiovascular risk in individuals with diabetes and the haptoglobin 2-1 genotype. We have previously demonstrated that the haptoglobin protein is associated with high-density lipoprotein (HDL) and HDL function and its oxidative modification are haptoglobin genotype dependent. Hence, we set out to test the hypothesis that the pharmacogenetic interaction between the haptoglobin genotype on cardiovascular risk might be secondary to a parallel interaction between the haptoglobin genotype and vitamin E on HDL function.
Oxidative modification has been proposed to be the mechanism by which HDL is rendered dysfunctional, and antioxidant therapy appears to restore HDL functionality. We therefore sought to determine whether the interaction between vitamin E and Hp genotype on RCT can be explained by a differential effect of vitamin E on HDL oxidative modification in Hp 2-1 and Hp 2-2. We have found that vitamin E supplementation resulted in a 50% reduction in HDL associated lipid peroxides in Hp 2-2 (0.55 ± 0.10 nmol vitamin E vs 1.07 ± 0.19 nmol placebo; P = 0.003) but had no effect in Hp 2-1.
In order to determine why vitamin E reduced HDL lipid peroxidation in Hp 2-2 but not Hp 2-1 we have investigated the effect of vitamin E on the mass or activity of the antioxidant proteins glutathione peroxidase and paraoxonase known to be associated with HDL, as well as the amount of redox-active non-transferrin-bound iron which has previously been implicated in the oxidation of HDL. While the effect of vitamin E did not reach statistical significance for any of these measurements it was associated with an approximately 50% increase in HDL associated glutathione peroxidase and a 25% reduction in redox active iron in Hp 2-2, while there was a 3-4 fold decrease in HDL associated glutathione peroxidase (P = 0.06) and no change in redox active iron in Hp 2-1[34-39].
The increase in redox-active iron in Hp 2-2 DM has been attributed to the impaired clearance of Hp 2-2-Hb by the CD163 Hp-Hb receptor. The surface expression of CD163 is regulated by oxidative stress and hyperglycemia. Thus we sought to determine whether the decrease in redox-active iron in Hp 2-2 DM individuals who received vitamin E, may be associated with an increase in CD163 expression on peripheral blood mononuclear cells (PBMs). We have observed a trend showing a greater than 50% increase in CD163 expression in PBMs of Hp 2-2 individuals receiving vitamin E. Vitamin E appeared to be associated with a 50% reduction in CD163 expression in Hp 2-1 individuals. In addition to functioning in the promotion of RCT and preventing the oxidation of LDL, HDL has been described as having an anti-inflammatory function. However, pro-inflammatory biomarkers such as C3 have been associated with dysfunctional HDL in individuals with CVD. We next sought to determine whether vitamin E would decrease the association of C3 with HDL in Hp 2-2. We have found that vitamin E treatment was associated with a borderline significant decrease in C3 associated HDL in Hp 2-2 individuals (1.07 ± 0.09 vitamin E vs 1.34 ± 0.20 placebo; n = 25; P = 0.09) but had no effect on the association of C3 with HDL in Hp 2-1 (1.08 ± 0.07 vs 1.08 ± 0.07; n = 30; P = 0.99). This effect of vitamin E on a HDL associated inflammatory marker was not associated with an overall change in serum markers of inflammation such as CRP and adiponectin.
Collectively, we have provided a plausible mechanism for the divergent effects of vitamin E therapy on cardiovascular risk in DM individuals with the Hp 2-1 and Hp 2-2 genotypes. While vitamin E improves HDL function in Hp 2-2 DM individuals it decreases HDL function in Hp 2-1 DM individuals. Structural analysis of HDL in study participants suggested a similar interaction in which vitamin E appeared to result in a favorable although non-statistically significant change was found in a number of HDL associated oxidative and inflammatory markers in Hp 2-2 individuals (the decrease in HDL associated lipid peroxides was statistically significant) while it was produced no beneficial effect on these markers in Hp 2-1 individuals. Therefore, this study supports the concept that there is a pharmacogenetic interaction between the Hp genotype and vitamin E in individuals with DM.
The favorable effects of vitamin E on HDL structure and lipid peroxidation described here are most likely due to the inhibition of oxidative modifications mediated by Hp 2-2-Hb associated with apolipoprotein A-I (ApoA1). Hp has been demonstrated to binds ApoA1 and this Hp can tether Hb to HDL. Hp 2-2 is inefficient in blocking the redox activity of Hb derived iron and therefore in Hp 2-2 individuals, HDL becomes the carrier of a cargo which is pro-oxidative. The detrimental effects of vitamin E on HDL structure in Hp 2-1 individuals may be due to an overshoot in the suppression of oxidative stress by vitamin E. Excessive suppression of oxidative stress may be deleterious. Several groups have demonstrated, both in animals and in humans, a down-regulation of protective antioxidant enzymes in response to high dose antioxidant supplementation. This down-regulation may paradoxically increase the susceptibility of these individuals to acute increases in oxidative stress (as with wide swings in hyperglycemia). Therefore, it may be possible to demonstrate that in Hp 2-1 individuals a lower dose of vitamin E may have beneficial effects on CVD.
The public health and economic implications of the pharmacogenetic interaction between the Hp type and vitamin E on CVD are profound. Implementation of a pharmacogenetic algorithm for DM patients in which all individuals with DM and the Hp 2-2 genotype would receive vitamin E cannot be achieved without an additional clinical trial testing this hypothesis. We hope that the mechanistic data presented here will help to increase the interest for such a trial. The notion of pharmacogenomics is that not all individuals with a given disease may benefit from the same drug treatment. We have demonstrated that vitamin E provides renal protection to Hp 2-2 DM mice but does not have any effect on Hp 1-1 DM mice. The pharmacogenomic implications of these findings are significant. Large-scale clinical trials of vitamin E to prevent macrovascular complications of diabetes have failed to show that vitamin E provided any clinical benefit. Studies assessing the effect of vitamin E on the progression of DN in humans with DM have yielded inconsistent findings. Moreover, recent meta-analysis suggested that there is an increased risk of all cause mortality with high-dose vitamin E supplementation. One explanation for the failure of vitamin E to provide benefit in human studies may be due to the inadequate nature of patient selection in these studies. We have recently provided concrete evidence in humans for a pharmacogenomic interaction between the Hp genotype and vitamin E supplementation in relation to the development of atherosclerotic cardiovascular disease. We have found by analyzing stored blood samples from the HOPE study that individuals with DM and the Hp 2-2 genotype received significant clinical benefit from vitamin E. Moreover, we have recently demonstrated in a prospective double blind clinical trial that vitamin E dramatically reduces cardiovascular disease in Hp 2-2 DM individuals. The ability of vitamin E to reduce features of renal disease characteristic of early human DN in Hp 2-2 DM mice but not in Hp 1-1 DM mice, suggests that there may also be an interaction between Hp genotype and vitamin E therapy in diabetic renal disease.
In the other arm of the studies of secondary prevention, we can mention the Gruppo Italiano per lo Studio della Sopravvivenzane II’Infartomiocardico-Prevenzione study, in which 11324 patients who had a recent MI were randomized to vitamin E ( 300 mg/d), polyunsaturated fatty acids, both or neither of them. They were followed for 3.5 years after which no effect of treatment with vitamin E was observed. In the Cambridge heart antioxidant study, treatment with vitamin E decreased the risk of developing non-fatal MI, but increased the risk of CV death. Similar results were obtained from the HOPE in 2000. The patients, men and women, were followed for about 4.5 years during which they received Vitamin E (400 mg/d) or angiotensin converting enzyme (ACE) inhibitor or a placebo. The results showed that vitamin E did not influence the risk for developing CVD. In 2005 an extension of this study was performed (HOPE-TOO study). In this analysis the incidence of CVD, cancer and cancer death was investigated in patients who received vitamin E or a placebo. Patients who received vitamin E had a higher risk of heart failure and required hospitalization.
As we mentioned above, hyperglycemia is accompanied by severe oxidative stress (especially lipid per-oxidation) which is caused by increased oxygen free radical production. Toxic oxygen free radicals have been implicated in the pathogenesis of diabetes mellitus, and its micro and macro vascular complications. An imbalance resulting from the increased production and/or reduced scavenging of these free radicals leads to a metabolic state of oxidative stress, which consequently leads to tissue damage. Auto glycosylation reactions, alterations in the sorbitol pathway and hyperglycemia have been proposed as some of the mechanisms which are responsible for this increased oxidative stress.
Antioxidants have been shown to play a beneficial role in preventing diabetic complications. Diabetes is a good model for chronic oxidative damage and it is a particularly suitable disease for antioxidant supplementation. It was found that there is a significant correlation has been found between the increased blood sugar levels and the depletion of the antioxidants. This depletion was a major risk factor for developing diabetes complications, and antioxidant supplementation (such as vitamin E) could decrease this risk.
Genetically modified mice offer the most direct means to demonstrate a gene-disease association. Such mice are inbred allowing one to study the effect of a change in one single gene. The Hp 2 allele is found only in humans. All other animals including higher primates have only the Hp 1 allele and therefore the Hp 1-1 genotype. One approach to mimic the Hp polymorphism in mice is to introduce the human Hp 2 allele as a transgene. Human Hp 2 transgenic mice in an Hp knockout background have been used to study mice only expressing the Hp 2 allelic protein product[41,42]. The wild-type murine C57Bl/6 Hp gene is a type 1 Hp allele with over 90% homology to the human Hp 1 allele. The construction of C57Bl/6 mice with targeted insertion of a murine Hp 2 allele has generated mice with the Hp 2-2 genotype. It was created by genetically engineering an Hp 2 allele by duplication of exons 3 and 4 in the genomic sequence of the murine Hp 1 allele. Then this murine Hp 2 allele was inserted at the endogenous Hp locus using a targeting strategy that is specifically selected for a homologous recombination event between the murine Hp 2 allele and the endogenous murine Hp 1 allele.
Streptozotocin was administered at 6 wk of age in a low-dose 5-d protocol as recently described by the National Institutes of Health sponsored Diabetes Consortium (50 mg/kg for 5 d). For all studies, a group of littermates who were not injected with streptozotocin was followed in parallel so that the only difference between the groups was the presence or absence of DM. Therefore, the parameters described below were measured for four groups of animals: Hp 1-1, Hp 1-1 DM and Hp 2-2, Hp 2-2 DM. There was no difference in spot glucose or HbA1c between Hp 1-1 and Hp 2-2 DM mice. For all analysis measurements were made when mice were 4 mo of age.
Vitamin E was administered in the drinking water, for 6 wk, beginning 1 mo after onset of DM until the mice were killed at 4 mo of age. We used vitamin E from Merck which is water miscible as documented by the manufacturer (Merck cat. No. 500862). This is DL alpha tocophorol acetate which enters easily into water. We made up a stock solution of vitamin E 1 mL in 50 mL of water and then used 5 mL of this stock solution in a 250-mL bottle of water for the mice. Each DM mouse received 600 mg/kg per day during the course of treatment.
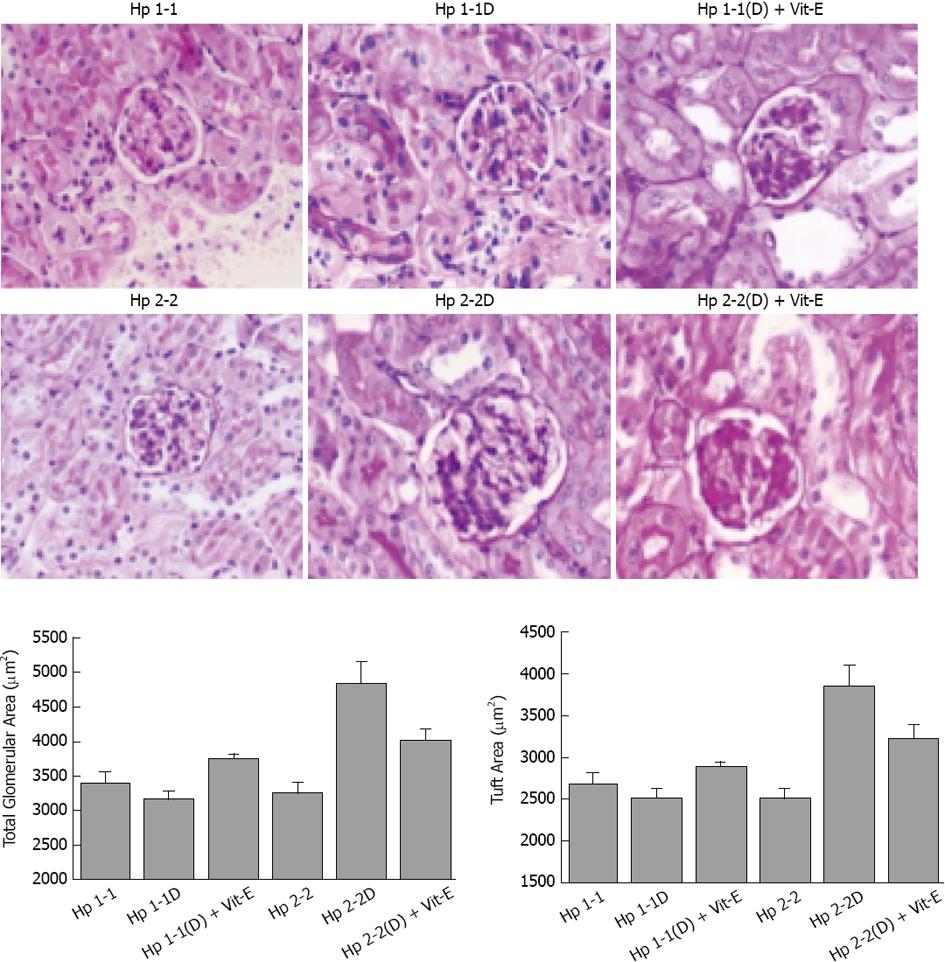
Gross kidney size which expressed as the kidney index (kidney mass/body mass) was significantly elevated in Hp 2-2 DM mice compared with their non-DM littermates and with Hp 1-1 DM mice (15.5 ± 0.97 g/kg for Hp 2-2 DM vs 11.9 ± 1.1 g/kg for Hp 1-1 DM and 10.1 ± 0.4 g/kg for Hp 2-2 non-DM; P < 0.05 comparing Hp 2-2 DM mice with Hp 1-1 DM or Hp 2-2 non-DM mice). There was a significant increase in both total glomerular area and proximal tubule area in Hp 2-2 DM mice compared with Hp 1-1 DM mice glomerular area: 4852.9 ± 308.7 for Hp 2-2 DM vs 3176.8 ± 99.3 for Hp 1-1 DM, P < 0.001 (Figure 4); Proximal tubular area: 1152.6 ± 42.4 for Hp 2-2 DM vs 818.0 ± 7.2 for Hp 1-1 DM, P < 0.05. We observed no significant difference in the cellularity of Hp 1-1 vs Hp 2-2 glomeruli or tubules suggesting that the glomerular expansion seen in Hp 2-2 DM mice was more likely to be due to hypertrophy than hyperplasia. There was a significant decrease in total glomerular area in Hp 2-2 DM with vitamin E (P < 0.05)[40-42].
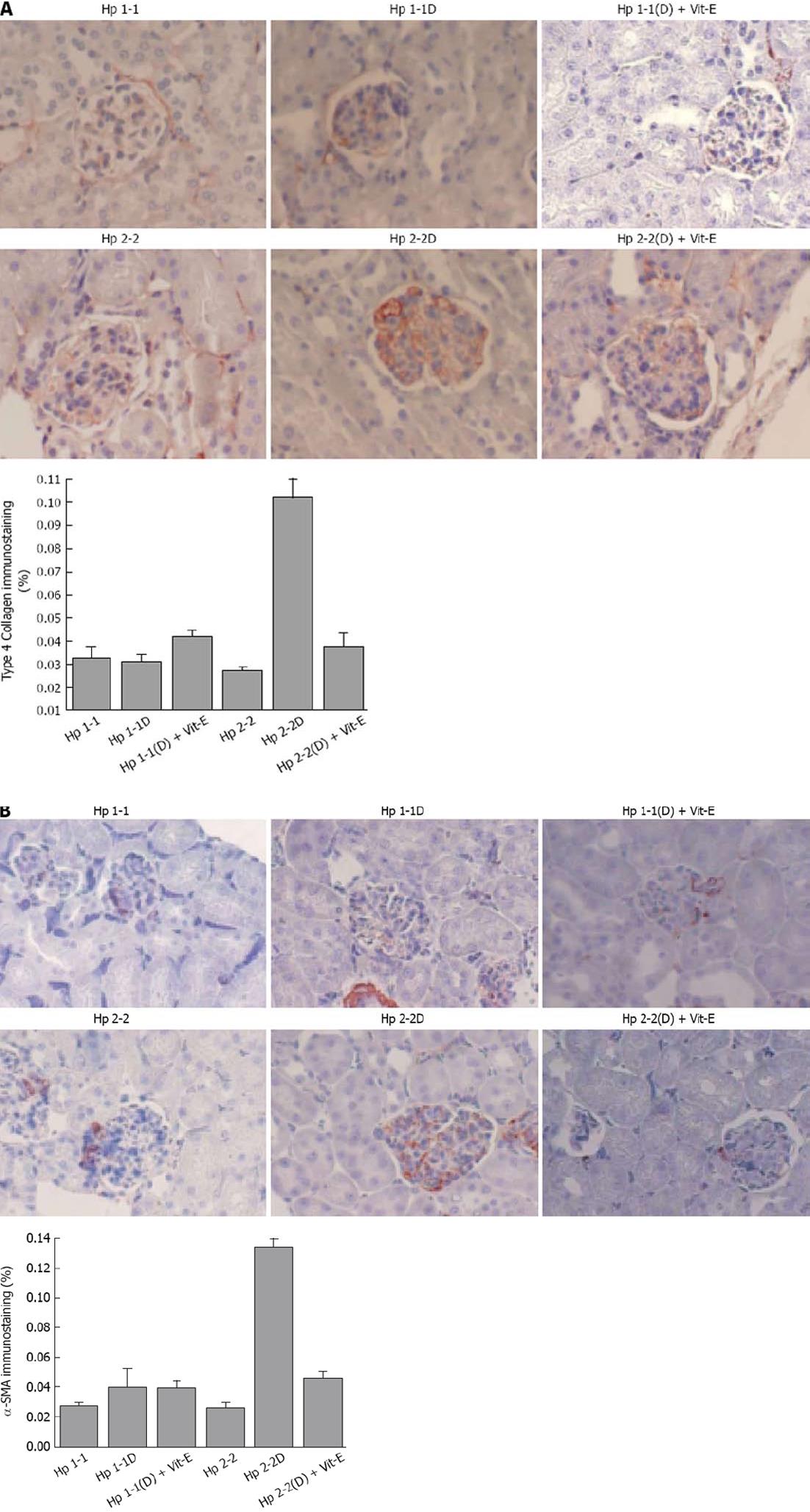
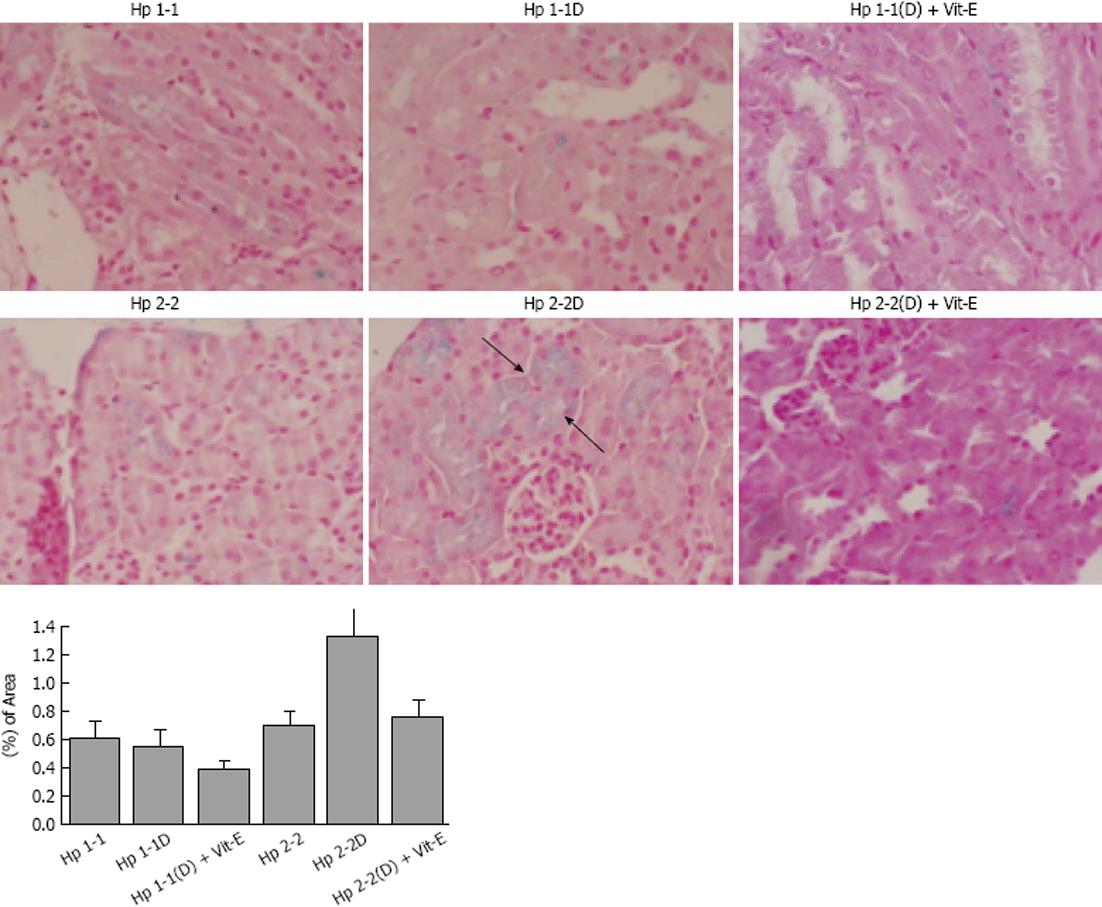
Collagen type IV (Figure 5A) and smooth muscle cell actin (Figure 5B), which are proteins known to be increased in human DN glomeruli, were significantly increased in Hp 2-2 DM mice (0.10 ± 0.07 for Hp 2-2 DM and 0.030 ± 0.003 for Hp 1-1 DM, P < 0.001 and 0.14 ± 0.01 for Hp 2-2 DM and 0.04 ± 0.01 for 1-1 DM, P < 0.001 respectively). There was a significant decrease in collagen IV immunostaining area and actin staining in Hp 2-2 DM mice with Vit-E (P < 0.05, P < 0.001 respectively). Furthermore, significantly greater amounts of iron were found in the renal tissue (localized to the proximal tubular cells) of Hp 2-2 DM mice compared with Hp 1-1 DM mice(1.34 ± 0.19 for Hp 2-2 DM and 0.56 ± 0.12 for Hp 1-1 DM, P < 0.01).
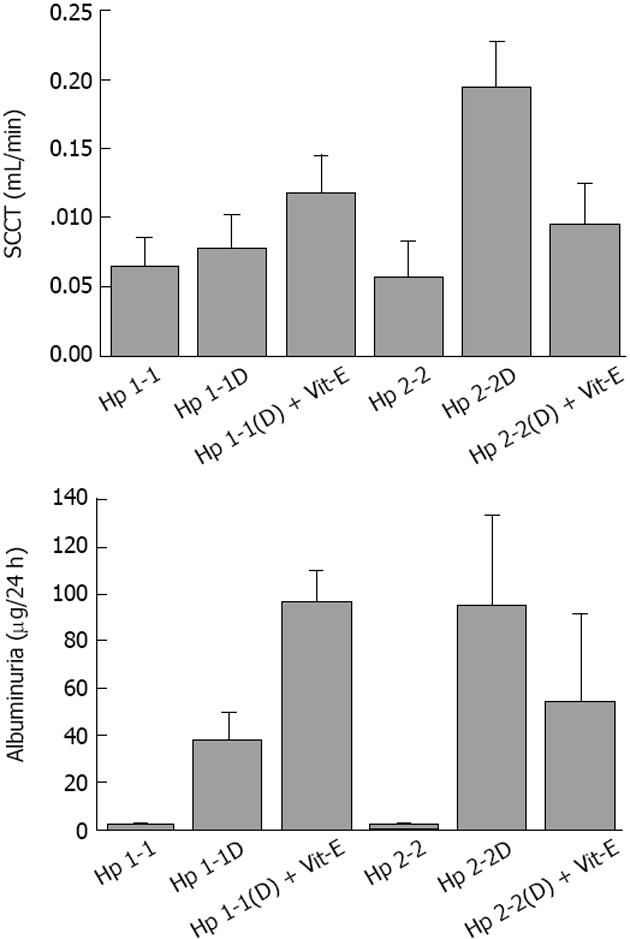
As we mentioned above, the Hp 2-2 mice have increased renal hypertrophy together with increased levels of Collagen, Smooth Muscle Actin, and Iron. We found in the Hp 2-2 DM mice which received vitamin E, a significant reduction in total glomerular area (P < 0.05), proximal tubule area (P < 0.05), glomerular collagen content (P < 0.001), glomerular actin content (P < 0.001). Creatinine clearance (CCT) and albuminuria are increased in Hp 2-2 DM mice (Figure 6). We have found a significant increase in CCT in Hp 2-2 DM mice compared with Hp 1-1 DM mice (P < 0.05) and Hp 2-2 non-DM mice (P < 0.05). There was a significant decrease in CCT in Hp 2-2 DM mice treated with vitamin E (P < 0.05). We have also found a non significant reduction in albuminuria in Hp 2-2 DM mice receiving vitamin E (18.5 ± 7.2 vs 95.3 ± 38.0; P < 0.16). Vitamin E supplementation to Hp 2-2 DM mice also resulted in a significant 50% reduction in global oxidative stress in renal tissue slices assessed as lipid peroxidation (P < 0.01). In contrast, in Hp 1-1 DM mice, vitamin E did not affect any morphometric or functional parameter as demonstrated in Figures 4-7.
In our last publication[43], we have studied the protective effect of vitamin E against the toxic effects of free radicals in diabetic mice with Hp 2-2 phenotype. The primary objective of this study was to determine the intracellular localization of this iron in the proximal tubule cells and to assess its potential toxicity. Transmission electron microscopy demonstrated a marked accumulation of electron-dense deposits in the lysosomes of proximal tubules cells in Hp 2-2 DM mice. Energy-dispersive X-ray spectroscopy and electron energy loss spectroscopy were used to perform elemental analysis of these deposits and demonstrated that these deposits were iron rich. These deposits were associated with lysosomal membrane lipid peroxidation and loss of lysosomal membrane integrity. Vitamin E administration to Hp 2-2 DM mice resulted in a significant decrease in both intralysosomal iron-induced oxidation and lysosomal destabilization. Therefore, Iron-induced renal tubular injury may play a major role in the development of diabetic nephropathy and may be a target for slowing the progression of renal disease.
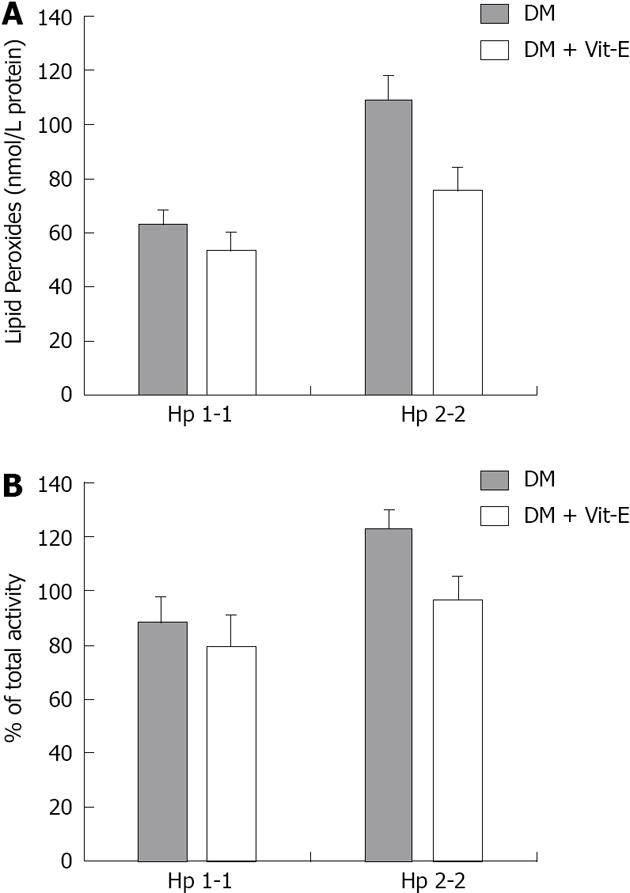
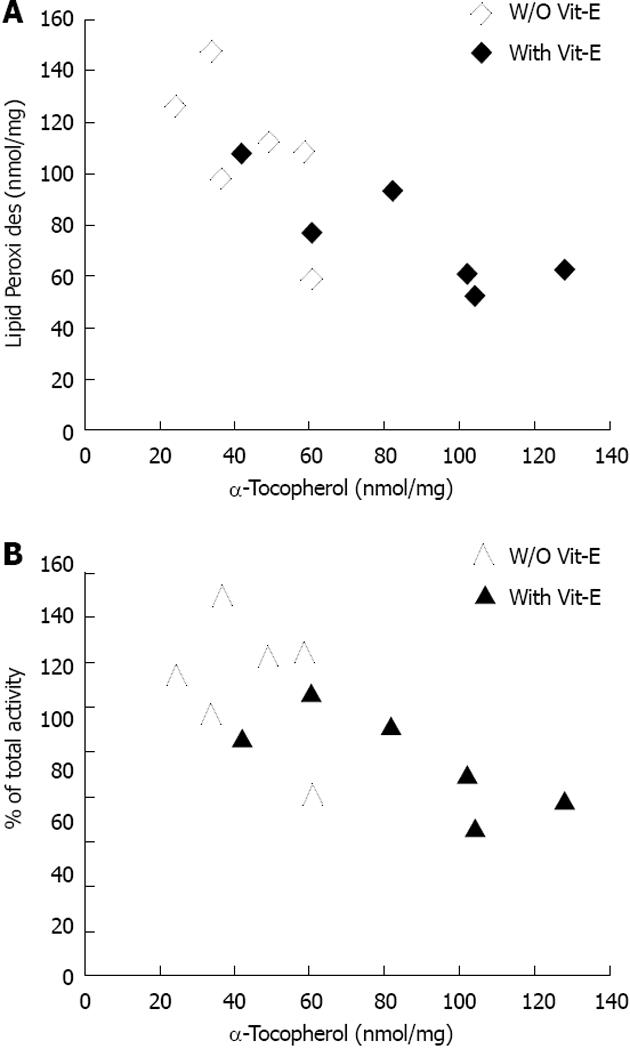
We sought to evaluate the role of lipid peroxidation in the maintenance of lysosomal membrane integrity by showing that chronic administration of the lipid-soluble antioxidant, vitamin E, could decrease lysosomal membrane oxidation and maintain lysosomal membrane integrity. Vitamin E supplementation resulted in a significant 45% reduction in lysosomal redox-active iron in Hp 2-2 DM mice (P < 0.05) with no significant effect on lysosomal redox-active iron in Hp 1-1 DM mice. Moreover, we have found that vitamin E supplementation significantly decreased lysosomal lipid peroxides in Hp 2-2 DM kidneys as compared with lysosomal preparations of Hp 2-2 DM mice treated with placebo (75.7 ± 8.7 nmol lipid peroxides/mg protein for Hp 2-2 DM with vitamin E vs 109.2 ± 8.8 nmol lipid peroxides/mg protein for Hp 2-2 DM without vitamin E; P = 0.03). There was no significant reduction in lysosomal lipid peroxides in Hp 1-1 DM mice treated with vitamin E (Figure 8). Moreover, there was a significant correlation between lysosome membrane α-tocopherol concentrations and the degree of lysosomal membrane oxidation in Hp 2-2 DM mice but not in Hp 1-1 DM mice. Finally, we have found a significant reduction in the loss of lysosomal membrane integrity in lysosomes purified from kidneys of Hp 2-2 DM mice treated with vitamin E as compared with those treated with placebo (24.1% ± 2.3% for vitamin E group vs 30.7% ± 1.7% for placebo group; n = 6 per group; P = 0.03). No significant differences in lysosomal membrane integrity were found after vitamin E administration to Hp 1-1 DM mice as compared to those treated with placebo (19.9% ± 2.7% for vitamin E group vs 22.1% ± 2.3% for placebo group; n = 6; P = 0.24) . There was a significant correlation in Hp 2-2 DM mice, but not in Hp 1-1 DM mice, between the concentration of vitamin E in the lysosomal membrane and the lysosomal membrane integrity (Figure 9).
An early morphological characteristic of the microangiopathy seen in diabetic retinal disease is retinal capillary basement membrane (RCBM) thickening. RCBM thickness as assessed by electron microscopy was performed on a total of 12 eyes taken from three mice in each of the four study groups (three eyes from C57Bl/6 Hp 1 and C57Bl/6 Hp 2 mice with and without streptozotocin-induced diabetes). Diabetes was produced by intraperitoneal injection at 6 wk of age with streptozotocin at a concentration of 200 mg/kg dissolved in 50 mmol/L citrate buffer, pH 4.5. Glucose levels were monitored with a glucometer. Animals were sacrificed at 6 mo of age. For these studies involving diabetes, a group of non-diabetic mice was followed in parallel so that the only difference between the groups was the presence or absence of diabetes. We found no difference in the degree of glucose control between mice with the different Hp genotypes.
Electron microscopy was performed on a total of 12 eyes from the four groups (three eyes from Hp 1 and Hp 2 animals with and without diabetes) for the determination of the retinal basement membrane thickness. Mice were sacrificed with intraperitoneal injection of pentabarbitone sodium. The eyes were enucleated, opened at the equator, fixed in 3.5% glutaraldehyde in 0.1 mol/L cacodylate buffer (pH 7.4) for 1 h, and then post-fixed in 2% osmium tetroxide. Semithin sections (1 μm) were stained with toluidine for orientation and identification of the capillary. Thin sections (60 nm) were produced with a diamond knife, placed on 300-mesh copper grids, and stained with uranyl acetate and lead citrate. The sections were viewed and photographed with a JEOL JEM 100SX electron microscope.
Sections mounted on copper grids and treated with the tannic acid solution prepared as described above were analysed using Image Pro software analysis. Basement membrane thickness was measured on five distinct capillaries for each eye and 5-10 measurements were taken per capillary with a minimum of 40 independent measurements from each eye. One reader scored all eyes in the study and was blinded to the genotype of the mice.
Retinal capillary basement membrane thickness was assessed from electron microscope photographs from three different mice with either Hp 1 or Hp 2 with or without streptozotocin-induced diabetes. For each animal, a minimum of 40 separate measurements of the RCBM thickness were obtained. This analysis demonstrated that there was no significant difference in retinal basement membrane thickness between non-diabetic Hp 1 and non-diabetic Hp 2 mice (Mann-Whitney P = 0.70; difference in median 2.6 nm). Diabetic Hp 1 mice did not demonstrate a significant increase in basement membrane thickness as compared to non-diabetic Hp 1 mice (Mann-Whitney P = 0.42; difference in median 5.2 nm). However, induction of diabetes resulted in a marked increase in basement membrane thickness in Hp 2 mice compared to non-diabetic Hp 2 mice (Mann-Whitney P = 0.0004; difference in median 32.1 nm), and to diabetic Hp 1 mice (Mann-Whitney P = 0.0005; difference in median 24.3 nm). Thus, the effect of diabetes in increasing basement membrane thickness occurred only in the Hp 2 group[43].
Fardoun et al[17] described the protective effect of vitamin E in patients with diabetic retinopathy in a prospective clinical study. Diabetic patients of either sex, above the age 45 years old, with or without diabetic complications were studied. The recruited patients were categorized into two groups: the primary and the secondary prevention groups. Type I group were divided in two groups, which consisted of the patients who received insulin and the vitamin E supplementation and the patients who received only insulin. The type II patients were further divided into the test and the control groups which consisted of those who received oral hypoglycemic and the vitamin E supplementation and those who were on oral hypoglycemic only. The number of the patients who developed cardiovascular complications and diabetic retinopathy in the test group (vitamin E) was significantly low in both type I and type II DM, as compared to those in the control groups. This study suggests that a long term vitamin E supplementation was beneficial for the cardiovascular complications.
The pharmacogenomic implications of these findings are significant. Large-scale clinical trials of vitamin E to prevent macrovascular complications of diabetes, have failed to show that vitamin E provided any clinical benefit. Studies assessing the effect of vitamin E on the progression of DN in humans with DM have yielded inconsistent findings. Moreover, recent meta-analysis has suggested that there is an increased risk of all causes of mortality with high-dose vitamin E supplementation. One explanation for the failure of vitamin E in providing benefit in human studies may be due to the inadequate nature of patient selection in these studies. We have recently provided concrete evidence in humans for a pharmacogenomic interaction between the Hp genotype and vitamin E supplementation in relation to development of atherosclerotic cardiovascular disease. We have found by analyzing stored blood samples from the HOPE study that individuals with DM and the Hp 2-2 genotype showed significant clinical benefit from vitamin E[17]. Moreover, we recently demonstrated in a prospective double blind clinical trial that vitamin E dramatically reduces cardiovascular disease in Hp 2-2DM individuals[24]. The ability of vitamin E to reduce features of renal disease characteristic of early human DN in Hp 2-2 DM mice but not in Hp 1-1 DM mice, suggests that there may also be an interaction between Hp genotype and vitamin E therapy in diabetic renal disease.
Different studies have shown that the vitamin E supplemented diabetics had a lesser incidence (a 25% lower risk) of the cardiovascular complications after 24 mo. This suggested that a long term vitamin E supplementation was beneficial for the cardiovascular complications. This is in accordance with the findings of the Cambridge Heart Antioxidant Study[9-11]. The Cambridge Heart Antioxidant Study showed that tocopherol treatment significantly reduced the risk of cardiovascular death and nonfatal myocardial infarction after 1 year of the treatment. An improvement was observed in the retinopathy in the test group treated with vitamin E. There were no significant differences between antioxidant vitamin supplementation and placebo in the relative risk for major cardiovascular outcome. In our last study[44], we have demonstrated that increased lysosomal redox-active iron results in lysosomal membrane injury in renal cells of Hp 2-2 DM mice. Therefore, this data provide a novel pathophysiological mechanism explaining why the progression to end-stage renal disease is increased in DM individuals with the Hp 2-2 DM genotype. Moreover, the interaction between the vitamin E and the Hp genotype on lysosomal injury suggests that a pharmacogenomic paradigm of selective administration of vitamin E to Hp 2-2 DM individuals may offer considerable renal protection similar to that recently demonstrated for cardiovascular disease.
Multiple studies blocking the course of diabetic retinopathy and nephropathy based on studies in rodents found the blocking agent under trial to be without value in humans, especially blockers of advanced glycation end-products. Diabetes induces the formation of advanced glycation end products (AGEs), which can alter the function of proteins and stimulate pathological cellular responses via AGE receptors. Increasing levels of AGEs, and their deposition in diabetic kidneys, correlate with the development of DN. Of the pathophysiologic mechanisms that have been identified in the development and progression of DN, oxidative stress is of major importance.
Pyridoxamine was introduced as an inhibitor of AGE formation from Amadori products[39-41]. The effects of pyridoxamine include: (1) the inhibition of AGE formation by blocking the oxidative degradation of the Amadori intermediate of the Millard reaction; (2) the scavenging of toxic carbonyl products of glucose and lipid degradation; and (3) the trapping of reactive oxygen species[42]. We demonstrated that pyridoxamine (K-163) ameliorates the levels of urinary albumin creatinine ratio (ACR) and serum 3-deoxyglucosone (3DG) in KK-Ay mice without changing systemic blood pressure. Furthermore, pyridoxamine prevented accumulations of Nq-(carboxymethyl)-lysine (CML), nitrotyrosine, transforming growth factor-β (TGF-β1), and laminin-β1 in the kidney tissues[41]. AGEs and oxidative stress might activate autocrine Ang II signaling and subsequently induce TGF-β1-Smad signaling in mesangial cells[24,43]. Our findings suggested that the amelioration of urinary ACR was related to the improvement of TGF-β1 and laminin-β1 expressions in the kidney because CML and nitrotyrosine accumulations were improved and the levels of serum 3DG were reduced by anti-AGE and/or the antioxidant effects of pyridoxamine.
Despite the successful use of lifestyle changes, metabolic control, and blood pressure control, including ACE inhibitors and angiotensin receptor blocker therapy, residual renal risk remains very high, leaving the diabetic population with a clear unmet need for novel treatment options. As outlined in this review, various drugs are in development. It is anticipated that some of the newer agents that are currently the focus of clinical trials will ultimately lead to improvements in slowing the progression and eventually improving the prognosis of this devastating disease.
Abutboul Family in memory of Daniel Abutboul.
P- Reviewer: Friedman EA S- Editor: Gou SX L- Editor: A E- Editor: Yan JL
| 1. | Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3804] [Cited by in RCA: 3689] [Article Influence: 153.7] [Reference Citation Analysis (0)] |
| 2. | Pezzolesi MG, Skupien J, Mychaleckyj JC, Warram JH, Krolewski AS. Insights to the genetics of diabetic nephropathy through a genome-wide association study of the GoKinD collection. Semin Nephrol. 2010;30:126-140. [PubMed] |
| 3. | Conway BR, Maxwell AP. Genetics of diabetic nephropathy: are there clues to the understanding of common kidney diseases? Nephron Clin Pract. 2009;112:c213-c221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Makuc J, Petrovič D. A review of oxidative stress related genes and new antioxidant therapy in diabetic nephropathy. Cardiovasc Hematol Agents Med Chem. 2011;9:253-261. [PubMed] |
| 5. | Costacou T, Ferrell RE, Orchard TJ. Haptoglobin genotype: a determinant of cardiovascular complication risk in type 1 diabetes. Diabetes. 2008;57:1702-1706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Levy AP, Purushothaman KR, Levy NS, Purushothaman M, Strauss M, Asleh R, Marsh S, Cohen O, Moestrup SK, Moller HJ. Downregulation of the hemoglobin scavenger receptor in individuals with diabetes and the Hp 2-2 genotype: implications for the response to intraplaque hemorrhage and plaque vulnerability. Circ Res. 2007;101:106-110. [PubMed] |
| 7. | Timmermann M, Högger P. Oxidative stress and 8-iso-prostaglandin F(2alpha) induce ectodomain shedding of CD163 and release of tumor necrosis factor-alpha from human monocytes. Free Radic Biol Med. 2005;39:98-107. [PubMed] |
| 8. | Melamed-Frank M, Lache O, Enav BI, Szafranek T, Levy NS, Ricklis RM, Levy AP. Structure-function analysis of the antioxidant properties of haptoglobin. Blood. 2001;98:3693-3698. [PubMed] |
| 9. | Asleh R, Guetta J, Kalet-Litman S, Miller-Lotan R, Levy AP. Haptoglobin genotype- and diabetes-dependent differences in iron-mediated oxidative stress in vitro and in vivo. Circ Res. 2005;96:435-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Asleh R, Marsh S, Shilkrut M, Binah O, Guetta J, Lejbkowicz F, Enav B, Shehadeh N, Kanter Y, Lache O. Genetically determined heterogeneity in hemoglobin scavenging and susceptibility to diabetic cardiovascular disease. Circ Res. 2003;92:1193-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 209] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 11. | Levy AP, Hochberg I, Jablonski K, Resnick HE, Lee ET, Best L, Howard BV. Haptoglobin phenotype is an independent risk factor for cardiovascular disease in individuals with diabetes: The Strong Heart Study. J Am Coll Cardiol. 2002;40:1984-1990. [PubMed] |
| 12. | Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet. 2003;361:2017-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 729] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 13. | Fioretto P, Mauer M. Diabetic nephropathy: diabetic nephropathy-challenges in pathologic classification. Nat Rev Nephrol. 2010;6:508-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Levy AP. Application of pharmacogenomics in the prevention of diabetic cardiovascular disease: mechanistic basis and clinical evidence for utilization of the haptoglobin genotype in determining benefit from antioxidant therapy. Pharmacol Ther. 2006;112:501-512. [PubMed] |
| 15. | Baburao Jain A, Anand Jain V. Vitamin E, Its Beneficial Role in Diabetes Mellitus (DM) and Its Complications. J Clin Diagn Res. 2012;6:1624-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Pazdro R, Burgess JR. The role of vitamin E and oxidative stress in diabetes complications. Mech Ageing Dev. 2010;131:276-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 142] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 17. | Fardoun RZ. The use of vitamin E in type 2 diabetes mellitus. Clin Exp Hypertens. 2007;29:135-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Ye Y, Li J, Yuan Z. Effect of antioxidant vitamin supplementation on cardiovascular outcomes: a meta-analysis of randomized controlled trials. PLoS One. 2013;8:e56803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 19. | Miller ER, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1820] [Cited by in RCA: 1627] [Article Influence: 81.4] [Reference Citation Analysis (0)] |
| 20. | Giannini C, Lombardo F, Currò F, Pomilio M, Bucciarelli T, Chiarelli F, Mohn A. Effects of high-dose vitamin E supplementation on oxidative stress and microalbuminuria in young adult patients with childhood onset type 1 diabetes mellitus. Diabetes Metab Res Rev. 2007;23:539-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Virtamo J, Rapola JM, Ripatti S, Heinonen OP, Taylor PR, Albanes D, Huttunen JK. Effect of vitamin E and beta carotene on the incidence of primary nonfatal myocardial infarction and fatal coronary heart disease. Arch Intern Med. 1998;158:668-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 160] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 22. | Christen WG, Gaziano JM, Hennekens CH. Design of Physicians’ Health Study II--a randomized trial of beta-carotene, vitamins E and C, and multivitamins, in prevention of cancer, cardiovascular disease, and eye disease, and review of results of completed trials. Ann Epidemiol. 2000;10:125-134. [PubMed] |
| 23. | Levy AP, Gerstein HC, Miller-Lotan R, Ratner R, McQueen M, Lonn E, Pogue J. The effect of vitamin E supplementation on cardiovascular risk in diabetic individuals with different haptoglobin phenotypes. Diabetes Care. 2004;27:2767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Milman U, Blum S, Shapira C, Aronson D, Miller-Lotan R, Anbinder Y, Alshiek J, Bennett L, Kostenko M, Landau M. Vitamin E supplementation reduces cardiovascular events in a subgroup of middle-aged individuals with both type 2 diabetes mellitus and the haptoglobin 2-2 genotype: a prospective double-blinded clinical trial. Arterioscler Thromb Vasc Biol. 2008;28:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 202] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 25. | Gaede P, Poulsen HE, Parving HH, Pedersen O. Double-blind, randomised study of the effect of combined treatment with vitamin C and E on albuminuria in Type 2 diabetic patients. Diabet Med. 2001;18:756-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Nakhoul FM, Zoabi R, Kanter Y, Zoabi M, Skorecki K, Hochberg I, Leibu R, Miller B, Levy AP. Haptoglobin phenotype and diabetic nephropathy. Diabetologia. 2001;44:602-604. [PubMed] |
| 27. | Blum S, Vardi M, Levy NS, Miller-Lotan R, Levy AP. The effect of vitamin E supplementation on cardiovascular risk in diabetic individuals with different haptoglobin phenotypes. Atherosclerosis. 2010;211:25-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:154-160. [PubMed] |
| 29. | Flores-Mateo G, Carrillo-Santisteve P, Elosua R, Guallar E, Marrugat J, Bleys J, Covas MI. Antioxidant enzyme activity and coronary heart disease: meta-analyses of observational studies. Am J Epidemiol. 2009;170:135-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Roguin A, Koch W, Kastrati A, Aronson D, Schomig A, Levy AP. Haptoglobin genotype is predictive of major adverse cardiac events in the 1-year period after percutaneous transluminal coronary angioplasty in individuals with diabetes. Diabetes Care. 2003;26:2628-2631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 100] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Suleiman M, Aronson D, Asleh R, Kapeliovich MR, Roguin A, Meisel SR, Shochat M, Sulieman A, Reisner SA, Markiewicz W. Haptoglobin polymorphism predicts 30-day mortality and heart failure in patients with diabetes and acute myocardial infarction. Diabetes. 2005;54:2802-2806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 106] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | Blum S, Vardi M, Brown JB, Russell A, Milman U, Shapira C, Levy NS, Miller-Lotan R, Asleh R, Levy AP. Vitamin E reduces cardiovascular disease in individuals with diabetes mellitus and the haptoglobin 2-2 genotype. Pharmacogenomics. 2010;11:675-684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 33. | Rainwater DL, Mahaney MC, VandeBerg JL, Wang XL. Vitamin E dietary supplementation significantly affects multiple risk factors for cardiovascular disease in baboons. Am J Clin Nutr. 2007;86:597-603. [PubMed] |
| 34. | Farbstein D, Blum S, Pollak M, Asaf R, Viener HL, Lache O, Asleh R, Miller-Lotan R, Barkay I, Star M. Vitamin E therapy results in a reduction in HDL function in individuals with diabetes and the haptoglobin 2-1 genotype. Atherosclerosis. 2011;219:240-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Asleh R, Blum S, Kalet-Litman S, Alshiek J, Miller-Lotan R, Asaf R, Rock W, Aviram M, Milman U, Shapira C. Correction of HDL dysfunction in individuals with diabetes and the haptoglobin 2-2 genotype. Diabetes. 2008;57:2794-2800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 36. | Asleh R, Miller-Lotan R, Aviram M, Hayek T, Yulish M, Levy JE, Miller B, Blum S, Milman U, Shapira C. Haptoglobin genotype is a regulator of reverse cholesterol transport in diabetes in vitro and in vivo. Circ Res. 2006;99:1419-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 37. | Nasser NJ, Kaplan M, Nevo E, Aviram M. Lipid profile and serum characteristics of the blind subterranean mole rat, Spalax. PLoS One. 2009;4:e4528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 38. | Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1646] [Cited by in RCA: 1590] [Article Influence: 113.6] [Reference Citation Analysis (0)] |
| 39. | Spagnuolo MS, Cigliano L, D’Andrea LD, Pedone C, Abrescia P. Assignment of the binding site for haptoglobin on apolipoprotein A-I. J Biol Chem. 2005;280:1193-1198. [PubMed] |
| 40. | Nakhoul FM, Miller-Lotan R, Awad H, Asleh R, Jad K, Nakhoul N, Asaf R, Abu-Saleh N, Levy AP. Pharmacogenomic effect of vitamin E on kidney structure and function in transgenic mice with the haptoglobin 2-2 genotype and diabetes mellitus. Am J Physiol Renal Physiol. 2009;296:F830-F838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 41. | Miller-Lotan R, Herskowitz Y, Kalet-Litman S, Nakhoul F, Aronson D, Zoabi R, Asaf R, Ben-Izhak O, Sabo E, Lim SK. Increased renal hypertrophy in diabetic mice genetically modified at the haptoglobin locus. Diabetes Metab Res Rev. 2005;21:332-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Asleh R, Nakhoul FM, Miller-Lotan R, Awad H, Farbstein D, Levy NS, Nakhoul N, Iancu TC, Manov I, Laue M. Poor lysosomal membrane integrity in proximal tubule cells of haptoglobin 2-2 genotype mice with diabetes mellitus. Free Radic Biol Med. 2012;53:779-786. |
| 43. | Miller-Lotan R, Miller B, Nakhoul F, Aronson D, Asaf R, Levy AP. Retinal capillary basement membrane thickness in diabetic mice genetically modified at the haptoglobin locus. Diabetes Metab Res Rev. 2007;23:152-156. [PubMed] |
| 44. | Nakhoul F, Nakhoul N, Asleh R, Miller-Lotan R, Levy AP. Is the Hp 2-2 diabetic mouse model a good model to study diabetic nephropathy? Diabetes Res Clin Pract. 2013;100:289-297. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |