Published online Jun 25, 2025. doi: 10.5527/wjn.v14.i2.100117
Revised: December 2, 2024
Accepted: February 6, 2025
Published online: June 25, 2025
Processing time: 244 Days and 1.5 Hours
Hyperphosphatemia (HP) is a common complication in an advanced stage of chronic kidney disease (CKD) and is associated with cardiovascular issues, metabolic bone abnormalities and worsening of secondary hyperparathyroidism. Most patients on dialysis require phosphate binders to control HP. Sucroferric oxyhydroxide (SO) (Dynulta™) is a calcium-free, polynuclear iron (III) based oral phosphate binder, for the treatment of HP. In this phase IV, open-label, single-arm, multi-center, 12-week, SOLO CKD study evaluated efficacy and safety of Dynulta™ in Indian CKD patients undergoing hemodialysis.
To investigate the efficacy, safety and tolerability of SO Chewable Tablet (Dynulta™) in patients with CKD on hemodialysis.
Hyperphosphatemic patients on hemodialysis and fulfilling eligibility criteria were included in the study for at least 12 weeks and received SO 1500 mg chewable tablet per day. The key endpoint was change in mean serum phosphorus levels after 12 weeks. Data were analysed using analysis of variance, Paired test, Wilcoxon test, and post-hoc comparisons, with P < 0.05 considered statistically significant, using Graph Pad software.
A total of 114 patients were enrolled and 94 patients completed the study. The mean ± SD serum phosphorous level was reduced from 7.62 mg/dL ± 2.02 mg/dL at baseline to 5.13 mg/dL ± 1.88 mg/dL after 12 weeks of treatment. At each follow-up visit, the reduction in mean serum phosphorous levels was statistically significant (P value < 0.05) compared to baseline, confirming the efficacy of SO. A total of 33.33% of patients experienced adverse events (AEs). The most frequently reported AEs were pyrexia, nasopharyngitis and headache, which were considered unlikely to be related to the study drug treatment. No serious AEs was reported during the study period and no patients discontinued treatment due to AEs.
This first real-world study in Indian CKD patients on hemodialysis shows SO as a safe, and effective monotherapy for HP, though its small sample size limits generalizability.
Core Tip: This is the first research study on the use of Sucroferric oxyhydroxide (SO) in Indian patients and also marks the first such study from the Southeast Asia region. The study was a single-arm study, registered with the Clinical Trials Registry India. The positive results from this study will add to the growing body of evidence, primarily generated in the United States and European Union, supporting the efficacy of SO in the Asian population.
- Citation: Niranjan MR, Srinivasa S, Gupta V, Bhalla AK, Gaikwad A, Wangikar P, Suryawanshi S, Gajbe P. Sucroferric oxyhydroxide monotherapy for hyperphosphatemia in Indian chronic kidney disease patients undergoing hemodialysis: A phase IV, single-arm, open-label study. World J Nephrol 2025; 14(2): 100117
- URL: https://www.wjgnet.com/2220-6124/full/v14/i2/100117.htm
- DOI: https://dx.doi.org/10.5527/wjn.v14.i2.100117
Hyperphosphatemia (HP) is a frequent consequence of end stage renal disease (ESRD) caused by the inability of the kidney to excrete excess phosphate[1]. Regulation of phosphorus excretion by the kidney is the key mechanism of maintaining body’s phosphate balance[2].
Nearly 90% of hemodialysis patients need to take oral phosphate binders, and 40%–50% of them still have increased phosphate levels after treatment[3]. Available oral phosphate binders are associated with a range of limitations and side effects. Although effective, aluminium-based binders are no longer extensively utilized due to the slow accumulation of absorbed aluminium in tissues. Although affordable and efficient, calcium-based salts may contribute to the progression of vascular calcification. Sevelamer carbonate, a calcium free phosphate binder, is widely used to reduce serum phosphate levels in patients with advanced chronic kidney disease (CKD)[4]. Sucroferric oxyhydroxide (SO) had a numerically lower mean daily pill burden and better treatment adherence than sevelamer carbonate[5]. Pill burden is a particularly important consideration, because patients receiving dialysis are often required to take a large number of concomitant tablets each day. Indeed, lower pill burden is associated with increased adherence to phosphate binders, and high level of medication adherence is associated with increased control of serum phosphorus[6].
SO is an oral, iron-based, non-calcium phosphate binder, formulated as a chewable tablet (500 mg iron equivalent to 2500 mg SO). It is composed of sucrose, starches and the active moiety, polynuclear iron (III)-oxyhydroxide (pn-FeOOH). SO displays effective phosphate binding across a wide pH range in the gastrointestinal (GI) tract, with minimal systemic absorption and no evidence of iron accumulation even after long term use[6,7]. Phosphate binding occurs through ligand exchange between hydroxyl groups and/or water molecules and phosphate ions, maintaining efficacy throughout the physiological pH range of the GI tract[8]. In phase I clinical studies, SO was well tolerated and associated with minimal GI iron absorption[9,10]. A phase II study demonstrated that doses of 1.0−2.5 g/day (based on iron content) substantially reduced serum phosphorus concentrations and reaffirmed its tolerability profile[11]. In a phase III study conducted in multiple sites across Europe, the United States, Russia, Ukraine, and South Africa, the phosphorus-lowering effect of SO was shown by demonstrating its non-inferiority to sevelamer carbonate and superiority to an ineffective control[12]. An extension of the phase III study confirmed the long-term efficacy of SO which was maintained over 1 year[13]. SO is available as a treatment option for patients in United States, Europe, Japan, Australia and Canada. In India it was approved in 2020[7].
Studies from various parts of India provide some background, even though the precise prevalence of CKD and dialysis patients in particular regions is unknown. For example, a study conducted in Delhi found that the prevalence of CKD was 7852 per million people (pmp), but studies conducted in Chennai and Bhopal found that the prevalence at the community level was 8600 pmp, and the incidence of ESRD was 151 pmp. In India, there are about 700 dialysis facilities and 4000 dialysis machines, most of which are in the private sector and mostly found in cities. Currently, there are an estimated 20000 dialysis patients nationwide. These facts aid in placing the study's sampling in context by showing how CKD patients and dialysis recipients are distributed throughout India[14].
In the present study we aimed to evaluate the efficacy, safety and tolerability of SO chewable tablet in controlling serum phosphorus concentration after 12 weeks of treatment in patients with CKD on hemodialysis.
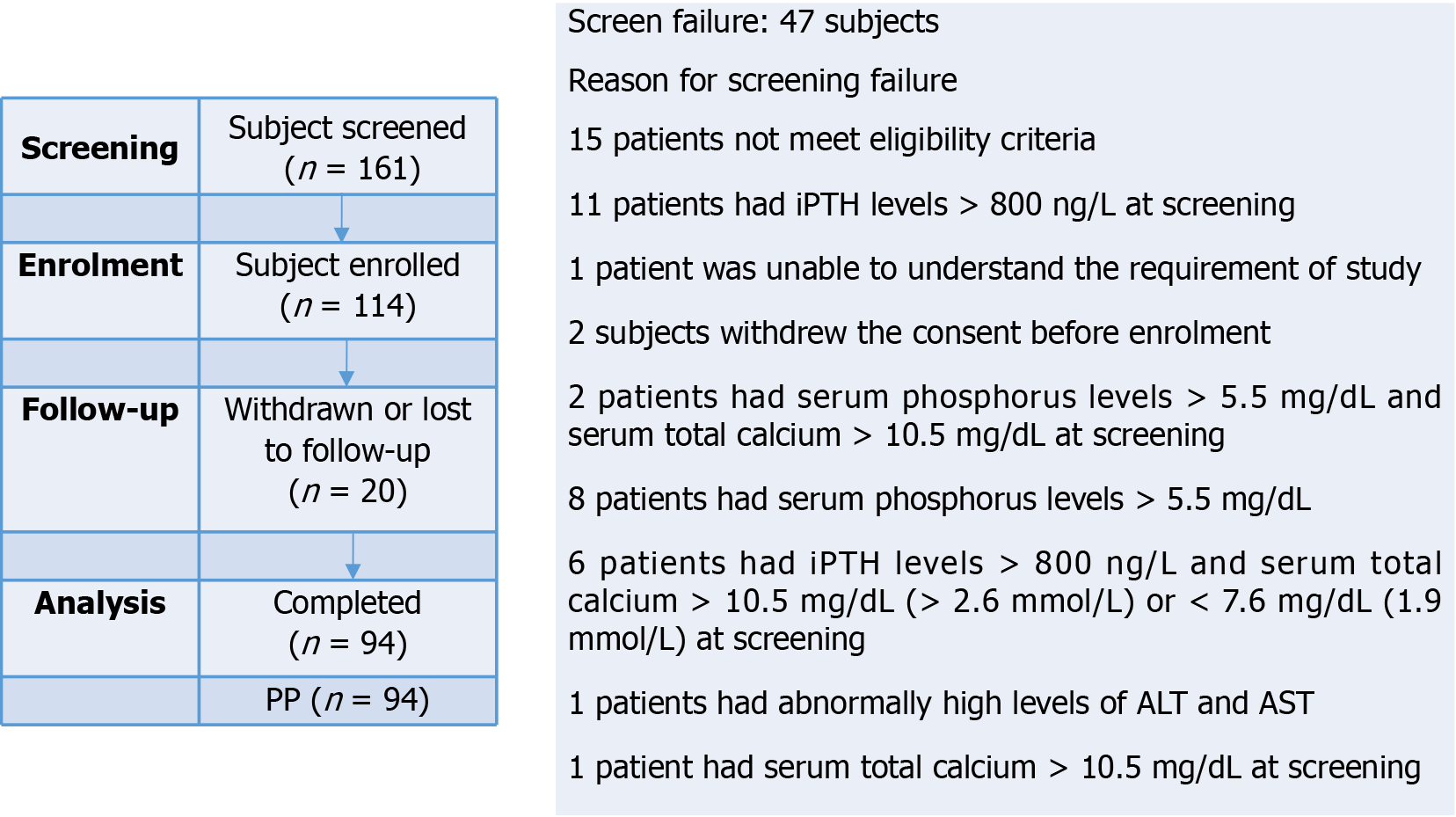
This was a phase IV, prospective, multi-center, single arm, open label study in CKD patients with HP who were on maintenance hemodialysis. The study was conducted at four clinical sites located in Bangalore, Mysore, Delhi and Agra, providing a diverse representation of urban and rural populations across India in accordance with regulatory and ethical guidelines (Declaration of Helsinki, ICH GCP, and New Drug and Clinical Trial Rule 2019). The study protocol and protocol related documents were approved by the institutional ethics committee before initiating the trial related activity and written informed consent was obtained from all individual participants included in the study. The study was registered with Clinical Trials Registry of India (Clinical Trials Registry India//2021/07/034812). Study flow chart is presented in Figure 1.
The potential participants were screened and the eligibility of the patients were determined on the basis of inclusion and exclusion criteria. A total of 114 adult patients of either sex with CKD aged > 18 years, providing informed consent, receiving maintenance hemodialysis for at least 12 weeks prior to screening, have a history of HP and serum phosphorus levels > 5.5 mg/dL (> 1.78 mmol/L) at screening constituted the study population. Enrolled patients were on a diet standardized by dietician throughout the study. All patients were dialyzed on high flux dialyzer and remained on same dialyzer throughout the entire study duration.
Patients were excluded who were taking any interfering medications like oral calcium supplements, any drugs/agents having a phosphate binding action that contain aluminium, magnesium or calcium (apart from antihyperkalaemic drugs), phosphate binders, sevelamer carbonate, nicotinamide, oral iron products, oral vitamins containing iron and other oral iron containing supplement or they stopped medication and screened after 1 week of washout. Dietary compliance was assessed at every visit post enrolment. Patients were excluded who had intact parathyroid hormone (iPTH) levels > 800 ng/L (> 800 pg/mL or 88 pmol/L) at screening, planned or expected parathyroidectomy within the next 6 months, serum total calcium > 10.5 mg/dL (> 2.6 mmol/L) or < 7.6 mg/Dl (< 1.9 mmol/L) at screening, any history of major GI surgery, clinically significant active GI disorders, swallowing difficulties/dysphagia, estimated life expectancy of less than 12 months, anticipated renal transplantation during study participation, history of haemochromatosis or other iron accumulation disorders that might lead to iron overload and raised alanine aminotransferase or aspartate aminotransferase > 3 times the upper limit of the normal range at screening.
In cases where the patient was already taking medications for HP, those medications were stopped at least 1 week before the administration of study drug. After the start of this study, patients were instructed to administer chewable SO tablets 500 mg of Emcure Pharmaceuticals Limited, India orally three times a day before meals. Dose increase or decrease of 500 mg/day (1 tablet/day) was permitted, provided a patient had received that dose for a minimum of 2-4 weeks until an acceptable serum phosphorus level reached, with regular monitoring for efficacy, safety or tolerability reasons at any time. Treatment compliance was assessed via daily diary recordings as well as tablet counts.
Total study duration for each enrolled patient was maximum 93 days (7 days for screening and 84 days ± 2 days of treatment). Patients reported to the study center four times for evaluation of study parameters: (1) During the screening (valid up to 7 days prior to day of enrolment); (2) Enrolment visit 1 (Day 1); (3) Visit 2 follow-up visit (week 4, Day 28 ± 2); (4) Visit 3 follow-up visit (week 8, Day 56 ± 2); and (5) The end-of-study (EOS) visit 4 (week 12, Day 84 ± 2).
Blood sampling was conducted four times to measure serum phosphorus levels. Other laboratory parameters were measured at the screening visit and at the EOS visit, including complete blood count (CBC), serum glutamic-oxaloacetic transaminase (SGOT), serum glutamic-pyruvic transaminase (SGPT), glucose, serum calcium, and iPTH.
Based on a power of 90% and a type I error rate of alpha = 0.05 (2-tailed), a sample size of at least 93 patients was required to detect a clinically acceptable difference of 0.3 mmol/L in mean change in serum phosphorus level from baseline after treatment with a SD of 0.63 mmol/L based on previously published study[15]. Considering dropout rate of 20%, adjusted sample size was 117 patients required to draw conclusion of study. Thus, total sample size including dropout rate was 117 patients.
The primary efficacy variable was change in mean serum phosphorus level from baseline to each visit. Using per protocol (PP) population principle, patients were analysed. The PP-population was defined as all patients who were enrolled as per entry criteria and completed the study in compliance with the protocol. Safety was evaluated on the basis of the number of adverse events (AEs), as well as their seriousness, severity, and causality. Additionally, at week 12, changes from baseline of other laboratory parameters (CBC, SGOT, SGPT, glucose, serum calcium, serum iPTH) and vital signs and physical findings were recorded. All data were expressed as the mean ± SD. Statistical analyses were performed with analysis of variance (ANOVA)/Paired test/Wilcoxon test based on the normality of data followed by post-hoc individual comparisons (for repeat measures ANOVA) vs baseline. P < 0.05 was considered to be statistically significant. Statistical analysis was performed using Graph Pad (version 9.4.1) software.
All 114 enrolled patients received SO, of whom 94 patients completed 12 weeks’ treatment (Figure 1). Age of patients who were enrolled in this study was found to be in a range of 23 years to 72 years with a mean age of 49.67 years ± 11.63 years, mean height and weight of patients were 162.91 ± 7.79 centimeters and 60.95 kg ± 10.08 kg respectively. The most common etiologies of CKD observed were hypertension (82.46%) and diabetes mellitus (31.57%) among the study patients presented in Table 1.
| Characteristics | |
| Gender | |
| Male | 62 (54.39) |
| Female | 52 (45.61) |
| Age (years) | |
| ≥ 65 | 9 (7.89) |
| ≤ 65 | 105 (92.11) |
| Body mass index (kg/m2) (mean ± SD) | 22.96 ± 3.37 |
| Asian race | 114 (100) |
| Dialysis history | |
| < 1 year | 38 (33.33) |
| ≥ 1 year and < 3 years | 39 (34.21) |
| ≥ 3 years | 37 (32.46) |
| Presence of complication | |
| Hypertension | 94 (82.46) |
| Diabetes mellitus | 36 (31.57) |
| Anemia | 1 (0.88) |
| Coronary artery disease | 3 (2.63) |
| Hyperthyroidism | 1 (0.88) |
| Thyroid | 1 (0.88) |
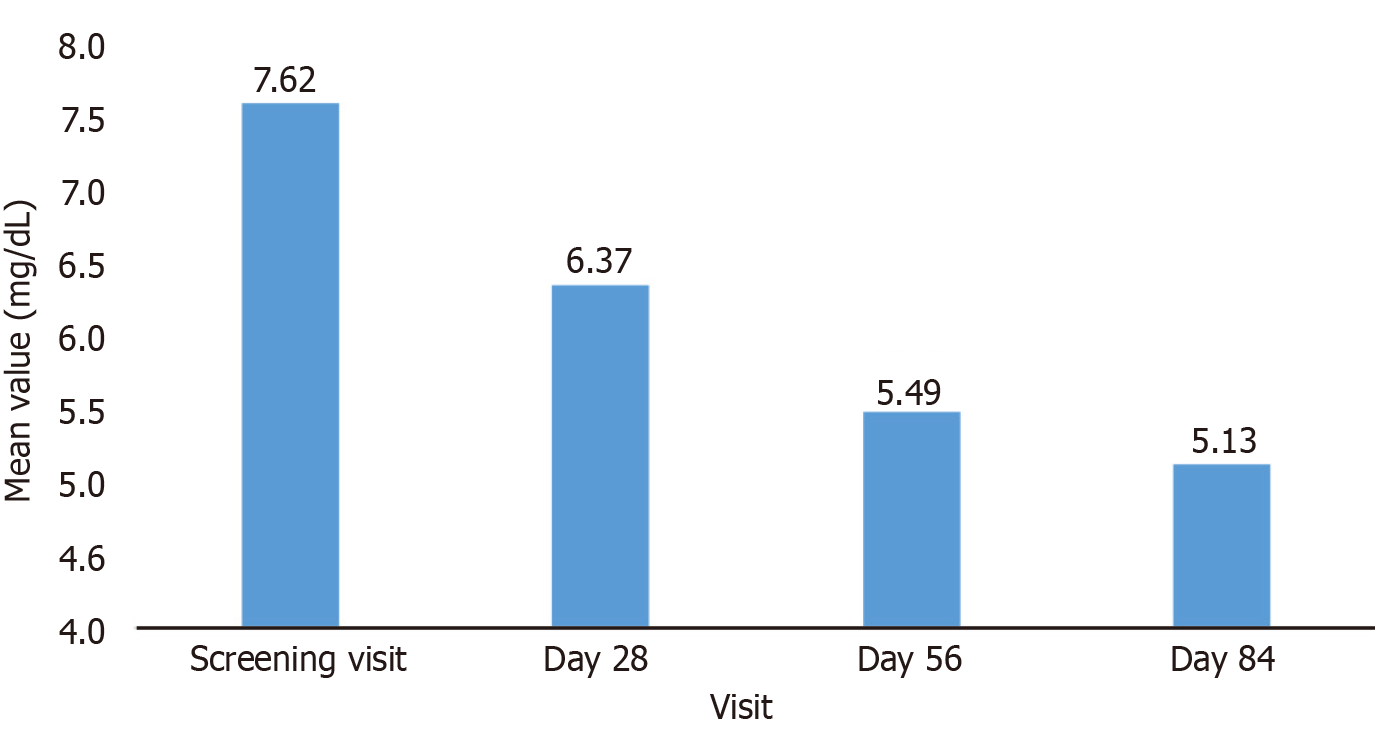
The decrease in mean serum phosphorus at each follow-up visit was statistically significant (P < 0.05) compared to baseline, reducing from 7.62 mg/dL at baseline to 5.13 mg/dL at the end of the study confirming, the efficacy of the SO treatment (Table 2). Serum phosphorous levels were significantly declined by day 28, with continued reductions observed at day 56 and day 84 (Figure 2). By the end of study treatment, a substantial proportion of patients achieved the target serum phosphorus level of < 5.5 mg/dL and a noteworthy percentage achieved levels < 4.5 mg/dL at week 12 (Table 3).
| Visit | mean ± SD | P value (compared to baseline) |
| Serum phosphorus (mg/dL) | ||
| Per protocol population (n = 94) | ||
| Screening visit: Day 7 to Day 1 | 7.62 ± 2.02 | - |
| Follow-up visit: Day 28 | 6.37 ± 2.33 | P < 0.05 |
| Follow-up visit: Day 56 | 5.49 ± 2.19 | P < 0.05 |
| End of study visit: Day 84 | 5.13 ± 1.88 | P < 0.05 |
| Change from baseline at Day 28 | 1.25 ± 1.55 | P < 0.05 |
| Change from baseline at Day 56 | 2.19 ± 2.07 | P < 0.05 |
| Change from baseline at Day 84 | 2.49 ± 2.13 | P < 0.05 |
| Visit | Percentage |
| Follow-up visit: Day 28 | 37 (39.36) |
| Follow-up visit: Day 56 | 50 (53.19) |
| End of study visit: Day 84 | 62 (65.96) |
A total of 83 adverse reactions occurred in 38 of 114 patients (33.33%), with the most frequent being pyrexia (23.68%), nasopharyngitis (14.91%) and headache (12.28%). AEs were classified as mild (40.61%) to moderate (59.32%) in severity with no events showing a definite causal relationship with SO. Only five AEs were considered possible/probably related to the study drug, including vomiting (n = 1) and diarrhea (n = 4), as shown in Table 4. No serious adverse event was found during the study and no patients discontinued the study due to a treatment emergent adverse event.
| Preferred term | Frequency (%) |
| Diarrhea | 3.51 |
| Vomiting | 0.88 |
| Total | 4.39 |
Vital signs (blood pressure, pulse, respiratory rate and oral body temperature) remained within clinically acceptable limits by the end of study period.
Regarding laboratory parameters, a minor and statistically non-significant change in serum calcium levels was observed after 12 weeks of treatment (P > 0.05), as summarized in Table 5. A similar trend was seen for serum iPTH levels, which decreased slightly from baseline, but this change was also not statistically significant (P > 0.05). Moreover, no statistically significant changes were observed in CBC and the biochemical parameter from baseline to end of the study visit suggesting that SO did not have any negative effect on patients’ general health condition.
| Parameter | Baseline (mean ± SD) | Day 84 (mean ± SD) | Change from baseline (mean ± SD) | P value (vs baseline) |
| Serum calcium (mg/dL) | 8.87 ± 0.67 | 8.75 ± 0.90 | 0.12 ± 0.92 | P > 0.05 |
| Serum intact parathyroid hormone (pg/mL) | 264.06 ± 196.70 | 236.89 ± 217.63 | 27.17 ± 150.89 | P > 0.05 |
| Hemoglobin (g/dL) | 11.16 ± 2.11 | 11.36 ± 2.15 | -0.21 ± 2.01 | P > 0.05 |
| Total red blood cell (million/cu.mm) | 3.91 ± 0.92 | 4.19 ± 0.95 | -0.28 ± 0.72 | P > 0.05 |
| Total white blood cell (cells/cu.mm) | 7057.34 ± 2027.30 | 7341.11 ± 2010.73 | -283.77 ± 2329.78 | P > 0.05 |
| Neutrophils | 60.03 (10.42) | 59.54 (11.38) | 0.49 (12.69) | P > 0.05 |
| Lymphocytes | 29.35 (10.37) | 29.57 (11.31) | -0.21 (10.81) | P > 0.05 |
| Eosinophils | 4.92 (3.75) | 5.02 (4.14) | -0.10 (5.24) | P > 0.05 |
| Monocytes | 4.93 (2.40) | 4.77 (3.06) | 0.16 (2.92) | P > 0.05 |
| Basophils | 0.77 (0.35) | 0.79 (0.43) | -0.01 (0.48) | P > 0.05 |
| Platelet (thou/mm³) | 2.38 ± 0.77 | 2.41 ± 0.84 | -0.03 ± 0.81 | P > 0.05 |
| Hematocrit | 34.44 (9.18) | 37.58 (22.40) | 3.14 (23.27) | P > 0.05 |
| Serum glutamic-oxaloacetic transaminase (U/L) | 23.97 ± 15.52 | 28.29 ± 18.32 | -3.85 ± 20.79 | P > 0.05 |
| Serum glutamic pyruvic transaminase (U/L) | 21.22 ± 14.84 | 28.21 ± 24.59 | -6.98 ± 25.58 | P < 0.05 |
| Glucose (mg/dL) | 138.38 ± 72.24 | 124.70 ± 53.52 | 13.68 ± 63.44 | P > 0.05 |
In this study, the use of SO as a monotherapy effectively reduced serum phosphorus levels in patients with CKD undergoing hemodialysis. A significant reduction (P < 0.05) in mean serum phosphorus levels was observed from baseline to end of the study and 65.96% of patients, achieved the target serum phosphorus level of < 5.5 mg/dL.
These results are consistent with those of Ramos et al[1] where a significant reduction in serum phosphate was observed during treatment with SO, with the proportion of patients achieving phosphate levels ≤ 5.5 mg/dL increasing from 41.3% to 56.2%–62.7% over 12 months. Similarly, in a United States database study involving 530 hemodialysis patients, the proportion of patients achieving target phosphate levels increased significantly from 17.7% at baseline to 36% after 1 year[1]. The same study also reported a 50% reduction in phosphate binder pill burden, from 8.5 pills per day to 4.0–4.3 pills per day.
Another 12 weeks’ phase-III study supported these findings, showing that patients switching from sevelamer hydrochloride to SO at a lower dose (814 mg/day) experienced effective phosphate control with reduced pill burden[16].
The present study assessed serum iPTH levels, which remained stable throughout the treatment period. This finding is consistent with other studies where no significant difference in plasma PTH levels was reported following SO treatment[1].
We did not observe any statistically significant changes in serum calcium levels at the end of the study treatment period, which is in contrast to Ramos et al[1] who observed a statistically significant decrease in serum calcium at the first quarter (Q1) of treatment. However, calcium levels remained stable in later treatment periods (Q2, Q3, Q4).
The safety profile of SO was favorable, with only 4.3% of AEs considered possible/probable related to the drug. The most common AE was diarrhoea, which aligns with previous studies that also identified diarrhea as the most frequently reported AE[16,17]. Importantly, no serious AEs occurred during the study.
Strengths of this study includes its prospective multi-center design, providing real-world evidence from an Indian population. The study was conducted over 84 days (approx 3 months) during which period, the dietary compliance and treatment adherence were closely monitored. Use of patient diary ensured treatment compliance as well recording AEs. Additionally, the study evaluated the impact of SO on various biochemical parameters, adding valuable data to the current literature.
However, there are several limitations to the study. The non-comparative single arm study design limits the ability to directly compare SO with other Phosphate binders/placebo. Furthermore, the relatively small sample size and regional focus may affect the generalizability of the findings, as dietary habits and population characteristics vary across different regions of India. Future research with larger sample sizes, longer follow-up, and comparisons to other phosphate binders is needed to confirm these findings and explore long-term outcomes.
In conclusion, this study establishes the efficacy of SO in reducing serum phosphorus levels in patients with CKD on hemodialysis while also establishing its safety and tolerability in Indian patients. These findings suggest that SO, as a monotherapy, can be an effective treatment option for managing HP in this patient population, with the added benefit of reduced pill burden.
While the results are promising more extensive research in different parts of India with larger sample sizes and comparator arms are required to generate more robust data and therefore a treatment's wider applicability in the Indian population. SO is positioned as a safe and effective monotherapy treatment option for CKD-associated HP due to its favorable safety profile and lower pill burden.
The authors gratefully thank clinical research organization, Ethicare clinical trial services for their help, support and cooperation in conduct of this study. We are thankful to all the patients who gave their consent to participate in this trial, without which this work was not possible.
| 1. | Ramos R, Chazot C, Ferreira A, Di Benedetto A, Gurevich K, Feuersenger A, Wolf M, Arens HJ, Walpen S, Stuard S. The real-world effectiveness of sucroferric oxyhydroxide in European hemodialysis patients: a 1-year retrospective database analysis. BMC Nephrol. 2020;21:530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Hruska KA, Mathew S, Lund R, Qiu P, Pratt R. Hyperphosphatemia of chronic kidney disease. Kidney Int. 2008;74:148-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 319] [Cited by in RCA: 304] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 3. | Chen JB, Chiang SS, Chen HC, Obayashi S, Nagasawa M, Hexham JM, Balfour A, Junge G, Akiba T, Fukagawa M. Efficacy and safety of SBR759, a novel calcium-free, iron(III)-based phosphate binder, in Asian patients undergoing hemodialysis: A 12-week, randomized, open-label, dose-titration study versus sevelamer hydrochloride. Nephrology (Carlton). 2011;16:743-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Ruggiero B, Trillini M, Tartaglione L, Rotondi S, Perticucci E, Tripepi R, Aparicio C, Lecchi V, Perna A, Peraro F, Villa D, Ferrari S, Cannata A, Mazzaferro S, Mallamaci F, Zoccali C, Bellasi A, Cozzolino M, Remuzzi G, Ruggenenti P, Kohan DE; ANSWER Study Organization. Effects of Sevelamer Carbonate in Patients With CKD and Proteinuria: The ANSWER Randomized Trial. Am J Kidney Dis. 2019;74:338-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Greig SL, Plosker GL. Sucroferric oxyhydroxide: a review in hyperphosphataemia in chronic kidney disease patients undergoing dialysis. Drugs. 2015;75:533-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Sprague SM, Marcuccilli M, Rakov V. Clinical rationale of sucroferric oxyhydroxide for controlling hyperphosphatemia in patients with chronic kidney disease. Clin Invest. 2015;5:9-21. |
| 7. | Sprague SM, Floege J. Sucroferric oxyhydroxide for the treatment of hyperphosphatemia. Expert Opin Pharmacother. 2018;19:1137-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Product Monograph Including Patient Medication Information of Velphoro® (Sucroferric Oxyhydroxide Chewable Tablet) 500 mg iron (equivalent to 2500 mg sucroferric oxyhydroxide) Phosphate Binder. Switzerland: Vifor Fresenius Medical Care Renal Pharma Ltd., 2018. Available from: https://pdf.hres.ca/dpd_pm/00043058.PDF. |
| 9. | Geisser P, Philipp E. PA21: a novel phosphate binder for the treatment of hyperphosphatemia in chronic kidney disease. Clin Nephrol. 2010;74:4-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Hergesell O, Ritz E. Stabilized polynuclear iron hydroxide is an efficient oral phosphate binder in uraemic patients. Nephrol Dial Transplant. 1999;14:863-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Wüthrich RP, Chonchol M, Covic A, Gaillard S, Chong E, Tumlin JA. Randomized clinical trial of the iron-based phosphate binder PA21 in hemodialysis patients. Clin J Am Soc Nephrol. 2013;8:280-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Koiwa F, Terao A. Dose-response efficacy and safety of PA21 in Japanese hemodialysis patients with hyperphosphatemia: a randomized, placebo-controlled, double-blind, Phase II study. Clin Exp Nephrol. 2017;21:513-522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Floege J, Covic AC, Ketteler M, Mann JF, Rastogi A, Spinowitz B, Chong EM, Gaillard S, Lisk LJ, Sprague SM; Sucroferric Oxyhydroxide Study Group. Long-term effects of the iron-based phosphate binder, sucroferric oxyhydroxide, in dialysis patients. Nephrol Dial Transplant. 2015;30:1037-1046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 14. | Khanna U. The economics of dialysis in India. Indian J Nephrol. 2009;19:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Floege J, Covic AC, Ketteler M, Rastogi A, Chong EM, Gaillard S, Lisk LJ, Sprague SM; PA21 Study Group. A phase III study of the efficacy and safety of a novel iron-based phosphate binder in dialysis patients. Kidney Int. 2014;86:638-647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 16. | Koiwa F, Yokoyama K, Fukagawa M, Akizawa T. Efficacy and Safety of Sucroferric Oxyhydroxide and Calcium Carbonate in Hemodialysis Patients. Kidney Int Rep. 2018;3:185-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Vervloet MG, Boletis IN, de Francisco ALM, Kalra PA, Ketteler M, Messa P, Stauss-Grabo M, Derlet A, Walpen S, Perrin A, Ficociello LH, Rottembourg J, Wanner C, Cannata-Andía JB, Fouque D. Real-world safety and effectiveness of sucroferric oxyhydroxide for treatment of hyperphosphataemia in dialysis patients: a prospective observational study. Clin Kidney J. 2021;14:1770-1779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |