Published online Nov 12, 2016. doi: 10.5501/wjv.v5.i4.135
Peer-review started: April 12, 2016
First decision: June 12, 2016
Revised: June 29, 2016
Accepted: August 11, 2016
Article in press: August 15, 2016
Published online: November 12, 2016
Processing time: 214 Days and 15.6 Hours
Zika virus (ZIKV) is a flavivirus (Flaviviridae family) transmitted mainly by Aedes mosquitoes. The virus was restricted to the African continent until its spread to south-east Asia in the 1980’s, the Micronesia in 2007, the French Polynesia in 2013 and, more recently in the Americas in 2015, where, up to date, the World Health Organization (WHO) has estimated about 3-4 million total cases of ZIKV infection. During outbreaks in the French Polynesia and Brazil in 2013 and 2015, respectively, national health authorities reported potential neurological complications of ZIKV disease, chiefly an upsurge in Guillain-Barré syndrome, which coincided with ZIKV outbreaks. On the other hand, the emergence of ZIKV in Brazil has been associated with a striking increase in the number of reported cases of microcephaly in fetus and newborns, twenty times higher than in that reported in previous years. While investigations are currently assessing whether there is an actual association between neurological complications and ZIKV infections, the evidence was enough worrisome for WHO to declare a public health emergency of international concern. Here we present an updated review addressing what is currently known about the possible association between ZIKV infection and the development of severe neurological disorders.
Core tip: Zika virus (ZIKV), a mosquito-borne flavivirus, was restricted to Africa until its spread to south-east Asia, the Pacific, and, finally, to the Americas, where an estimated 4 million cases of ZIKV infection have been recorded, and where a worrisome possible association of ZIKV with the development of severe neurological disorders, such as Guillain-Barré Syndrome and microcephaly, have been reported. In this contribution we present an updated review addressing what is currently known about the possible association between ZIKV infection and the development of severe neurological disorders, remarking the urgent need for further investigations to clearly resolve this point.
- Citation: Blázquez AB, Saiz JC. Neurological manifestations of Zika virus infection. World J Virol 2016; 5(4): 135-143
- URL: https://www.wjgnet.com/2220-3249/full/v5/i4/135.htm
- DOI: https://dx.doi.org/10.5501/wjv.v5.i4.135
Zika virus (ZIKV) is a mosquito-borne Flavivirus classified into the Flaviviridae family. It is closely related to other important pathogens that affect human and animal health such as Japanese encephalitis virus, dengue virus (DENV), yellow fever virus (YFV), West Nile virus (WNV) or St. Louis encephalitis virus[1]. ZIKV was first isolated in 1947 from the serum of a febrile sentinel rhesus monkey in the Zika Forest (Uganda) during the investigations performed to study the enzootic cycle of YFV. The virus was isolated for the second time from Aedes africanus mosquitoes collected at the same site one year later. In both cases, the virus was isolated by intracranial inoculation into infant mice[2].
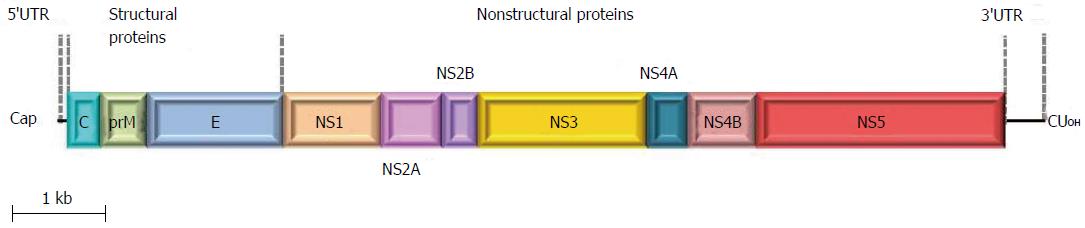
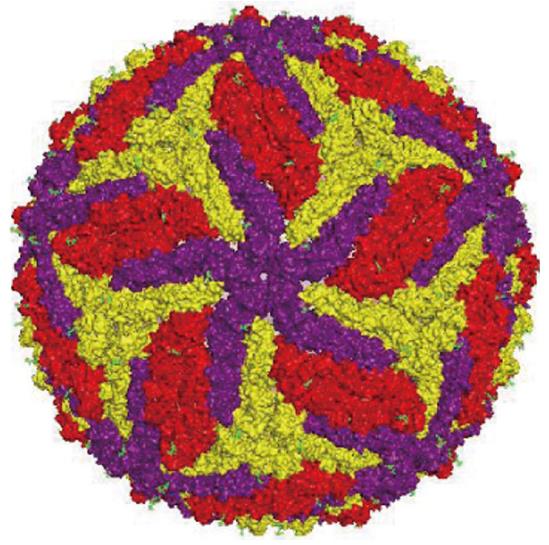
ZIKV genome is constituted by a positive polarity RNA molecule of about 11 kb in length, comprising two untranslated regions flanking an open reading frame coding for a polyprotein of about 3420 amino acids. Similar to other flaviviruses, the ZIKV single polyprotein is expected to be post-translationally cleaved by host and viral proteases into three structural proteins [capsid (C), pre-membrane (prM), and envelope (E)] and seven non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A NS4B, and NS5)[3] (Figure 1). The structure of mature ZIKV particle has been recently described[4] (Figure 2), and the virus particle has been observed to be structurally stable even at 40 °C[5].
Phylogenetic analyses of the virus confirm its inclusion within the mosquito-borne flavivirus cluster with the presence of two major lineages: One includes the African strains, which is divided into two groups, the East and the West African clusters, and the other gathers the Asian and American strains[1]. ZIKV life cycle, as any other arbovirus, has several barriers to accumulate mutations as a consequence of the intrinsic constraints associated with dual replication in mammalian and invertebrate hosts, thus driving to a relatively slow fixation of mutations[1]. For instance, ZIKV strains collected over a few years interval in Central African Republic show minimal changes on their sequences[6].
Even though ZIKV strains from different continents and outbreaks showed up to 99% identity[1], nonsynonymous nucleotide differences have been described among them that, in other flaviviruses, have been implicated in viral infectivity. For instance, a full-length ZIKV genome amplified from fetal tissues obtained during the Brazilian outbreak presented five nonsynonymous mutations when compared with the French Polynesian isolate[7]. Three of these amino acid changes were found in NS1, implicated in immune evasion in the case of DENV[8], one in NS4B, related to the inhibition of type I interferon signaling in other flaviviruses[9,10], and one in a NS5 domain which has been shown to mask the viral RNAs from host recognition in the case of WNV[11,12]. In this line, it has been hypothesized the possible adaptation of the ZIKV virus to the human host by changes in non-structural proteins[13]. Thereby, Asian strains of ZIKV differ significantly from the African ones in codon usage in the NS1 region of the genome[14]. Codon usage by the pandemic strain is optimized for adaptation to human housekeeping cells, which could facilitate viral replication in human cells. In fact, codon optimization could result in higher viral titers and increased infectivity for mosquito vectors, as seen in other viruses[15].
Analysis of the polyprotein sequence predicted the presence of potential N-glycosylation sites in the ZIKV proteins prM, E and NS1[4,16-18]. Noteworthy, a 4 amino acid deletion corresponding to the envelope protein 154 glycosylation motif was found in several ZIKV strains, in a similar way to many other flaviviruses, such as West Nile virus strains[6]. Glycosylation has been associated in some instances with virulence[19,20], even though the functional importance of the N-glycosylations is not clear in related flaviviruses, since flaviviruses presenting or not this N-glycosylation can maintain the same antigenicity[21]. Additionally, glycosylation could play a role in replication and maturation[22]. In fact, it has been suggested that extensive mouse brain or cell culture passage could lead to the deletion of the potential glycosylation site, since there are differences on this site even between ZIKV isolates with different passage history, such as those of the prototypic strain ZIKV MR766[23,24]. Even more, it has been suggested that ZIKV may have experienced recombination in nature and that a loss of the N154 glycosylation site in the envelope protein was a possible adaptive response to the vector[25]. Therefore, a detailed analysis of whether and how these differences are directly related to virulence and pathogenicity has to be clearly elucidated for a better control of ZIKV infection.
ZIKV is transmitted by mosquitoes of the genus Aedes, mainly of Aedes aegypti and Aedes albopictus, although the virus has been isolated from other genus such as Anopheles, Culex, and Mansonia spp[1]. Both Ae. aegypti and Ae. albopictus have a history of global expansion associated with trade and travel and are widely distributed[26].
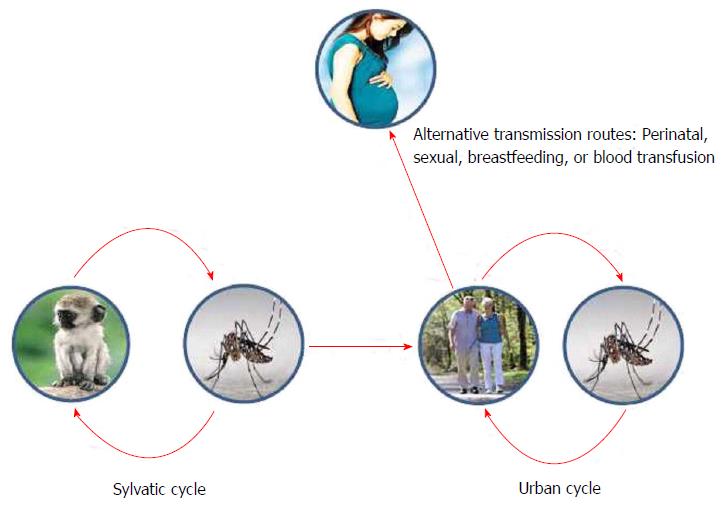
Non-human primates are considered to serve as reservoir hosts for ZIKV, although the primary species have not been identified. ZIKV natural transmission cycle has been described to involve Cercopithecus aethiops and Erythrocebus patas monkeys in Africa[27], while ZIKV antibodies have been found among semi-captive and wild orangutans in Asia[28] (Figure 3). There is no current evidence of other animals than humans and non-human primates acting as amplifying hosts for ZIKV[29]. However, antibodies against ZIKV have been found in many other vertebrate species, such as sheep, goats, cattle birds, rodents and even reptiles[1].
Even though mosquito transmission is the main cause of ZIKV outbreaks, other additional routes of transmission have been proposed: Breastfeeding, perinatal, sexual or by blood transfusion (Figure 3).
The potential for viral transmission through blood transfusion was first suggested during the French Polynesia outbreak. Almost 3% of blood donors, who were asymptomatic at the time of donation, were found positive for acute ZIKV infection by specific reverse transcriptase polymerase chain reaction (RT-PCR)[30]. Moreover, in a very recent prospective study carried out in 72 pregnant women in Brazil, 26 tested positive for ZIKV RNA in blood samples[31]. These data point to the need for implementation of measures to prevent this way of infection in endemic areas, and, in other zones free of ZIKV, to advice people coming back from affected areas to delay blood donations[1].
Besides blood transfusion, sexual activity could be another risk factor for horizontal transmission. In this regard, ZIKV RNA and replicative virus have been found in semen[32-34]. In 2008, a case of sexual transmission was suspected to occur from an American scientist, who contracted ZIKV infection in Senegal, to his wife. Even though she had not left the United States during the previous year, she also developed clinical symptoms related to ZIKV infection. Even though, ZIKV was not investigated in the semen of the patient, virus infection was serologically confirmed in both[35]. A recent retrospective study in Italy detected ZIKV specific neutralizing antibodies in the sera of a couple with a suspected DENV infection, of which the female had not travelled to tropical areas during the previous year[36]. Later on, in early February 2016, the case of a ZIKV infected person after sexual contact in the United States has been reported[37]. In this line of investigations, the CDC received reports of 14 cases of suspected sexual transmission of ZIKV during February 2016, of which only two were laboratory-confirmed and four classified as probable cases of Zika disease. All reported cases belonged to women which only known risk factor was to have had sexual intercourse with symptomatic partners recently returned from an area with ongoing ZIKV circulation[38]. Up to date, and according to WHO, five countries have reported locally acquired infection in the absence of any known mosquito vectors, probably through sexual transmission (Argentina, France, Italy, New Zealand and the United States). Additionally, ZIKV RNA and infectious ZIKV in urine[39] and saliva[40] have been reported. All these data suggest that sexual transmission could play a role on ZIKV infection and transmission, even though this route seems unlikely to play a major role in ZIKV spread. In any case, the CDC have considered that ZIKV sexual transmission is of particular concern and, consequently, have published an interim guideline for prevention of sexual transmission of ZIKV[41].
ZIKV RNA in breast milk was first detected during the outbreak in the French Polynesia[42] and, more recently, the presence of infective ZIKV particles, with substantial viral loads, in breast milk has also been described[43]. Nevertheless, since there is no evidence supporting viral transmission to babies by lactation, the CDC encourage mothers to breastfeed their children, arguing that the benefits of it outweigh the risk of transmission (http://www.cdc.gov/zika/transmission/), as so do the Pan American Health Organization (PAHO/WHO) (http://www.paho.org), and several national health authorities. However, it should be noted that breast milk transmission has been previously documented in humans and experimentation animal models in other flaviviruses, such as DENV or WNV[44,45].
In any case, the most worrying aspect of recent ZIKV outbreaks is the increasing evidence pointing to mother-to-child viral transmission, which can lead to infants neurological disorders. As mentioned early, perinatal transmission was documented for the first time during the French Polynesia outbreak[42]. Sera from two mothers and their newborns were RT-PCR tested positive for ZIKV, although contamination during delivery could not be discarded. Later on, during the outbreak in Brazil, RT-PCR detection and histopathologic findings in tissue samples from two newborns with microcephaly who died within 20 h of birth and two miscarriages showed the presence of ZIKV. All four mothers had clinical signs of ZIKV infection during the first trimester of pregnancy, but not at the time of delivery or miscarriage[46]. Further reports in Brazil have described the presence of ZIKV RNA in fetuses and amniotic fluids[31,47,48]. Even though sporadic vertical transmission in humans has been previously reported in other members of the Flaviviridae family, such DENV[49] or YFV[50], the surprisingly high number of infants born with microcephaly in Brazil during the current outbreak, which could probably be the result of a possible vertical transmission, has urged the WHO to publish some advice for women who are pregnant, or planning to become pregnant, to take extra care to protect themselves from the bites of the mosquitoes that transmits ZIKV (http://www.who.int/features/qa/zika-pregnancy/en/).
ZIKV infection has been described to be symptomatic only in around 18% of the cases[51], causing a mild, self-limiting illness with an incubation period of up to 10 d[52]. Signs and symptoms generally include an onset of fever, maculopapular rash, arthralgia, myalgia, and conjunctivitis, and can be often mistaken with other arboviral infections, like dengue or chikungunya (Table 1). However, severe disease with hospitalization has not been commonly needed until now[1]. However, and even though a causal link has not been yet established, there seem to be growing evidences linking ZIKV infection to Guillain-Barré syndrome (GBS) and microcephaly in newborns. So that, due to this unexpectedly upsurge of severe neuronal complications, a case definition for ZIKV disease has been established by the WHO (http://www.who.int/csr/disease/zika/case-definition/en/) for the purpose of providing global standardization for classification and reporting of ZIKV cases. These interim guidelines distinguish between suspected cases, probable cases, and confirmed cases of ZIKV disease, showing the essential requirements for each of them[12] (Table 2).
| Mild symptoms | Other complications of the disease |
| Fever | Guillain-Barré syndrome |
| Rash | Microcephaly in fetuses and newborns |
| Joint pain | |
| Conjunctivitis | |
| Muscle pain | |
| Headache |
| Suspected case | Probable case | Confirmed case |
| A person presenting with rash and/or fever and at least one of the following signs or symptoms: Arthralgia, or Arthritis, or Conjunctivitis (non-purulent/ hyperaemic) | A suspected case with presence of: IgM antibody against Zika virus (with no evidence of infection with other flaviviruses) and An epidemiological link (contact with a confirmed case, or a history of residing in or travelling to an area with local transmission of Zika virus within 2 wk prior to onset of symptoms) | A person with laboratory confirmation of recent Zika virus infection: Presence of Zika virus RNA or antigen in serum or other samples, or IgM antibody against Zika virus positive and PRNT90 for Zika virus with titre ≥ 20 and Zika virus PRNT90 titre ratio ≥ 4 compared to other tested flaviviruses, and Exclusion of other flaviviruses |
GBS is a clinical syndrome of multiple autoimmune etiologies, which involve idiopathic peripheral neuropathy manifested as a progressive paralysis over 1-3 wk, with a 5% death rate and up to 20% of patients left with a significant disability[53-55]. Severe manifestation of GBS with respiratory failure affects 20%-30% of cases[56]. GBS is the most common and severe acute paralytic neuropathy, with en estimate incidence ranging 0.8-1.9 cases per 100000 people per year, with a 70% of these cases associated with previous infectious diseases. The syndrome was also first associated with ZIKV infection during the French Polynesian outbreak in 2013[57], where the incidence rate of GBS cases was about 20-fold higher than expected[58]. Likewise, in Colombia, during the ongoing outbreak, a three times higher number of GBS cases than the averaged expected cases during the 6 previous years has been reported. An association between the increase of GBS cases and ZIKV infection has also been reported in Venezuela (http://www.who.int/csr/don/12-february-2016-gbs-colombia-venezuela/en/). Very recently, two cases of GBS with confirmed ZIKV infection have been notified from the United States to the PAHO/WHO (http://www.who.int/csr/don/21-march-2016-gbs-usa/en/). According to the WHO, and in the context of ZIKV circulation, twelve countries or territories have reported an increased incidence of GBS and/or laboratory confirmation of a ZIKV infection among GBS cases (http://www.who.int/emergencies/zika-virus/situation-report/17-march-2016/en/). These data point to an alarming increase in the potential clinical severity of ZIKV infection[59].
In a case-control study performed during the French Polynesia outbreak, 42 patients were diagnosed with GBS at the Centre Hospitalier de Polynésie Française (Papeete, Tahiti, French Polynesia). Study control cohorts were age-matched, sex-matched, and residence-matched patients who presented at the hospital with a non-febrile illness (control group 1; n = 98) and age-matched patients with acute ZIKV disease and no neurological symptoms (control group 2; n = 70). Up to 98% of the patients with GBS had anti-ZIKV IgM or IgG, compared with 56% in control group 1[60]. Even though in this study a history of past dengue virus infection seemed not to differ significantly between patients with GBS and those in the two control groups, other reports have suggested that the simultaneous increase in dengue and chikungunya infections in the region may have contribute to the registered increase in GBS incidence[61]. The 42 GBS cases reported in the French Polynesia between November 2013 and February 2014 contrasted with the less than ten cases per year recorded during the previous four years (http://ecdc.europa.eu/en/publications/Publications/Zika-virus-French-Polynesia-rapid-risk-assessment.pdf), and suggests a possible association between ZIKV and GBS[60]. GBS was also the first important ZIKV-associated condition documented in Brazil, with 121 cases during the first half of 2015 (http://portalsaude.saude.gov.br/index.php/o-ministerio/principal/secretarias/svs/noticias-svs/19139-evento-desaude-publica-relacionado-aos-casos-de-febre-do-zika).
Even though GBS has also been associated to other arboviral infections, such as DENV[62,63], WNV[64], or CHIKV[65], it is believed to be a rare event. The onset of GBS presumably involves an autoimmune process[66], and although the possible factors determining the association of GBS and ZIKV have not yet been established, it has been suggested that sequential arbovirus infections may exacerbate the immune response and trigger an immunopathogenic process attacking peripheral nerves, and thus leading to the onset of GBS[58].
No matter what, the most concerning manifestation of ZIKV infection is the dramatic increase of reported cases of microcephaly in Brazil. Microcephaly is a head size smaller than expected for age, and is associated to different genetic factors, maternal malnutrition, intrauterine infection (including toxoplasmosis, cytomegalovirus, or rubella), and exposure to toxins during gestation (http://www.cdc.gov/ncbddd/birthdefects/microcephaly.html). Microcephaly is defined as an occipitofrontal head circumference below the third centile, or more than 2 standard deviations (SD) below the mean for sex, age, and ethnicity[67]. Anyway, the possible link of microcephaly with ZIKV is not still clear among researchers. The Latin American Collaborative Study of Congenital Malformations (ECLAMC) suggested that this increase in reported cases of microcephaly might largely be due to the intense search for cases of the birth defect, and to misdiagnoses, that arose from heightened awareness in the wake of the possible link with ZIKV; and the WHO had also stated that the causal relation of these disorders with ZIKV infection had not yet been scientifically proven[68].
According to the WHO data, between October 2015 and January 2016, Brazil reported 4783 cases of microcephaly and/or central nervous system malformation, while during the fifteen previous years the average number of cases reported in the country was 163 per year[69]. Although most of the Brazilian cases have not yet been confirmed, as only a few studies have investigated in detail the possible link between ZIKV infection and fetus cerebral damage, an increase in microcephaly and other fetal malformations has been widely reported in Brazil[31,70,71] and the French Polynesia[72]. In a retrospective analysis of data performed from the ZIKV outbreak in French Polynesia, eight cases of microcephaly were identified between September 2013 and July 2015. Seven of them occurred in a 4-mo period around the end of the ZIKV outbreak. With the development of a mathematical model, the study estimated a prevalence of risk of microcephaly associated with ZIKV infection in the first trimester of pregnancy of 95 out of 10000 infected women (around 1%) vs a baseline prevalence of microcephaly of 2 out of 10000[73]. Two additional cases, linked to a stay in Brazil, were detected in the United States[74] and Slovenia[7]. Even though no such a high increase has been observed in ZIKV Brazil endemic neighboring countries, a very recent report has diagnosed, for the first time in Colombia, one newborn with microcephaly and two with congenital brain abnormalities, which tested positive for ZIKV[75], and Panama has recently reported to the WHO a newborn with microcephaly and occipital encephalocoele who died a few hours after birth and also tested positive for ZIKV by RT-PCR.
It is also noteworthy to mention that first experimental studies with ZIKV infection in two mouse model revealed that virus replication is mainly performed in brain cells, such as neurons and astroglial cells[76,77], which would be in line with a possible physiological mechanism linking ZIKV infection with microcephaly. Otherwise, a very recent study have showed that ZIKV infection of human cortical neural progenitors cells derived from induced pluripotent stem cell produced an attenuation of their growth, pointing to a possible mechanistic link between ZIKV and microcephaly[78]. On the other hand, it has been hypothesized that infection could damage the fetus either by evading the natural immunoprotective response of the placenta by direct transmission of the virus to the early embryo or fetus, or by the placenta itself provoking a response to the exposure, and thus contributing to, or causing, the brain defects[79]. In any case, the mechanism by which ZIKV may cause fetal microcephaly is still unknown and, thus, this point need to be clearly established.
As in most flaviviral infections, there is no current specific antiviral treatment, vaccine or prophylaxis available for ZIKV. Treatment is generally symptomatic and based on analgesics, antipyretics, and antihistamines. This lack of specific measures against the virus emphasizes the importance of vector control strategies (Table 3). ZIKV is principally spread by mosquitoes, and not by person-to-person contact, although a limited number of cases of sexual transmission has been reported. Accordingly, vector control measures are analogous to those suggested in other mosquito-transmitted diseases[3], such as removing sources of standing water, insecticide application, avoidance of mosquito exposure, and implementation of accurate mosquito control programs. Besides these vector control approaches, development of effective ZIKV vaccines, and search for specific antiviral drugs are current challenges for Zika disease.
| Vector control measures | Personal preventive measures |
| Removal of sources of standing water | Avoidance of mosquito exposure Insecticide application |
| Implementation of accurate mosquito control programs | Prevention of sexual transmission by use of preventive measures Travelling avoidance to risk countries during pregnancy |
Since the WHO declared a public health emergency of international concern on the 1st of February of 2016, a list of preventive guidelines has been assessed, particularly during pregnancy. Recommendations for pregnant women considering travel to an area with ZIKV circulation and recommendations for screening, testing, and management of pregnant returning travelers are included in the CDC interim guidelines[80]. However, it should be taken into account that, even though ZIKV has been identified in a few cases in fetuses with microcephaly, this association does not demonstrate causality, and it will be necessary careful assessment to find the causal link between ZIKV infection and microcephaly[1,81]. Furthermore, in the case of newborns with microcephaly, the lack of data on short or long-term outcomes of neonatal or infant infection makes it difficult to take into consideration more subtle effects of ZIKV infection in the brain until later stages of childhood. Therefore, systematic and longer-term follow-up is mandatory to assess this point and to determine whether there are more fetal effects.
On the other hand, Zika’s association with other viral infections in humans, such as dengue and chikungunya, has raised questions about the potential roles of these other viruses as cofactors for the more serious complications of ZIKV infection[82]. As the current ZIKV expansion is occurring in regions where dengue is endemic, pre-existing dengue immunity can cause increased ZIKV replication in patients, resulting in increased viremia and increased infectivity. In this sense, the possibility of immune enhancement by pre-existing heterologous anti-flavivirus antibodies, like DENV, has been hypothesized to increase viral replication[13]. Immune enhancement has been reported to play a major role in the pathogenesis of severe dengue infections[83]. In fact, ZIKV replication in cell culture were shown to be enhanced by heterologous flavivirus antibodies[4]. In any case, the potential role of this immune enhancement by previous infection with other flaviviruses as cofactors for the more serious complications associated with ZIKV should be addressed in future research.
Beyond the considerable efforts exerted by the scientific community and the national and international health authorities focused on improving the knowledge on ZIKV infection, sufficient resources should be allocated to provide the necessary tools for assessing the potential mechanisms of ZIKV association to severe neurological diseases, such as GBS or microcephaly, as well as the development of more systematic diagnostic tools, vaccines, and design of antiviral therapies.
Manuscript source: Invited manuscript
Specialty type: Virology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chen CJ, Chen SS, Lin CW S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
| 1. | Saiz JC, Vázquez-Calvo Á, Blázquez AB, Merino-Ramos T, Escribano-Romero E, Martín-Acebes MA. Zika Virus: the Latest Newcomer. Front Microbiol. 2016;7:496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 2. | Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509-520. [PubMed] |
| 3. | Martín-Acebes MA, Saiz JC. West Nile virus: A re-emerging pathogen revisited. World J Virol. 2012;1:51-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (5)] |
| 4. | Sirohi D, Chen Z, Sun L, Klose T, Pierson TC, Rossmann MG, Kuhn RJ. The 3.8 Å resolution cryo-EM structure of Zika virus. Science. 2016;352:467-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 579] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 5. | Kostyuchenko VA, Lim EX, Zhang S, Fibriansah G, Ng TS, Ooi JS, Shi J, Lok SM. Structure of the thermally stable Zika virus. Nature. 2016;533:425-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 376] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 6. | Berthet N, Nakouné E, Kamgang B, Selekon B, Descorps-Declère S, Gessain A, Manuguerra JC, Kazanji M. Molecular characterization of three Zika flaviviruses obtained from sylvatic mosquitoes in the Central African Republic. Vector Borne Zoonotic Dis. 2014;14:862-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 7. | Mlakar J, Korva M, Tul N, Popović M, Poljšak-Prijatelj M, Mraz J, Kolenc M, Resman Rus K, Vesnaver Vipotnik T, Fabjan Vodušek V. Zika Virus Associated with Microcephaly. N Engl J Med. 2016;374:951-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2157] [Cited by in RCA: 1918] [Article Influence: 213.1] [Reference Citation Analysis (0)] |
| 8. | Scaturro P, Cortese M, Chatel-Chaix L, Fischl W, Bartenschlager R. Dengue Virus Non-structural Protein 1 Modulates Infectious Particle Production via Interaction with the Structural Proteins. PLoS Pathog. 2015;11:e1005277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 176] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 9. | Morrison J, Aguirre S, Fernandez-Sesma A. Innate immunity evasion by Dengue virus. Viruses. 2012;4:397-413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 10. | Nitta S, Sakamoto N, Nakagawa M, Kakinuma S, Mishima K, Kusano-Kitazume A, Kiyohashi K, Murakawa M, Nishimura-Sakurai Y, Azuma S. Hepatitis C virus NS4B protein targets STING and abrogates RIG-I-mediated type I interferon-dependent innate immunity. Hepatology. 2013;57:46-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 11. | Daffis S, Szretter KJ, Schriewer J, Li J, Youn S, Errett J, Lin TY, Schneller S, Zust R, Dong H. 2’-O methylation of the viral mRNA cap evades host restriction by IFIT family members. Nature. 2010;468:452-456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 756] [Cited by in RCA: 701] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 12. | Weaver SC, Costa F, Garcia-Blanco MA, Ko AI, Ribeiro GS, Saade G, Shi PY, Vasilakis N. Zika virus: History, emergence, biology, and prospects for control. Antiviral Res. 2016;130:69-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 521] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 13. | Russell PK. The Zika Pandemic - A Perfect Storm? PLoS Negl Trop Dis. 2016;10:e0004589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Freire CC, Iamarino A, Neto DF, Sall AA, Zanotto PM. Spread of the pandemic Zika virus lineage is associated with NS1 codon usage adaptation in humans. BioRxiv. 2016;. |
| 15. | Andersen KG, Shapiro BJ, Matranga CB, Sealfon R, Lin AE, Moses LM, Folarin OA, Goba A, Odia I, Ehiane PE. Clinical Sequencing Uncovers Origins and Evolution of Lassa Virus. Cell. 2015;162:738-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 212] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 16. | Baronti C, Piorkowski G, Charrel RN, Boubis L, Leparc-Goffart I, de Lamballerie X. Complete coding sequence of zika virus from a French polynesia outbreak in 2013. Genome Announc. 2014;2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 189] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 17. | Kuno G, Chang GJ. Full-length sequencing and genomic characterization of Bagaza, Kedougou, and Zika viruses. Arch Virol. 2007;152:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 389] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 18. | Fagbami AH, Halstead SB, Marchette NJ, Larsen K. Cross-infection enhancement among African flaviviruses by immune mouse ascitic fluids. Cytobios. 1987;49:49-55. [PubMed] |
| 19. | Mondotte JA, Lozach PY, Amara A, Gamarnik AV. Essential role of dengue virus envelope protein N glycosylation at asparagine-67 during viral propagation. J Virol. 2007;81:7136-7148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 158] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 20. | Shirato K, Miyoshi H, Goto A, Ako Y, Ueki T, Kariwa H, Takashima I. Viral envelope protein glycosylation is a molecular determinant of the neuroinvasiveness of the New York strain of West Nile virus. J Gen Virol. 2004;85:3637-3645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 135] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 21. | Winkler G, Heinz FX, Kunz C. Studies on the glycosylation of flavivirus E proteins and the role of carbohydrate in antigenic structure. Virology. 1987;159:237-243. [PubMed] |
| 22. | Li J, Bhuvanakantham R, Howe J, Ng ML. The glycosylation site in the envelope protein of West Nile virus (Sarafend) plays an important role in replication and maturation processes. J Gen Virol. 2006;87:613-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Adams SC, Broom AK, Sammels LM, Hartnett AC, Howard MJ, Coelen RJ, Mackenzie JS, Hall RA. Glycosylation and antigenic variation among Kunjin virus isolates. Virology. 1995;206:49-56. [PubMed] |
| 24. | Haddow AD, Schuh AJ, Yasuda CY, Kasper MR, Heang V, Huy R, Guzman H, Tesh RB, Weaver SC. Genetic characterization of Zika virus strains: geographic expansion of the Asian lineage. PLoS Negl Trop Dis. 2012;6:e1477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 534] [Cited by in RCA: 523] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 25. | Faye O, Freire CC, Iamarino A, Faye O, de Oliveira JV, Diallo M, Zanotto PM, Sall AA. Molecular evolution of Zika virus during its emergence in the 20(th) century. PLoS Negl Trop Dis. 2014;8:e2636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 524] [Cited by in RCA: 553] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 26. | Kraemer MU, Sinka ME, Duda KA, Mylne AQ, Shearer FM, Barker CM, Moore CG, Carvalho RG, Coelho GE, Van Bortel W. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife. 2015;4:e08347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1330] [Cited by in RCA: 1273] [Article Influence: 127.3] [Reference Citation Analysis (0)] |
| 27. | Faye O, Faye O, Diallo D, Diallo M, Weidmann M, Sall AA. Quantitative real-time PCR detection of Zika virus and evaluation with field-caught mosquitoes. Virol J. 2013;10:311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 287] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 28. | Wolfe ND, Kilbourn AM, Karesh WB, Rahman HA, Bosi EJ, Cropp BC, Andau M, Spielman A, Gubler DJ. Sylvatic transmission of arboviruses among Bornean orangutans. Am J Trop Med Hyg. 2001;64:310-316. [PubMed] |
| 29. | Lazear HM, Diamond MS. Zika Virus: New Clinical Syndromes and Its Emergence in the Western Hemisphere. J Virol. 2016;90:4864-4875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 371] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 30. | Musso D, Nhan T, Robin E, Roche C, Bierlaire D, Zisou K, Shan Yan A, Cao-Lormeau VM, Broult J. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Euro Surveill. 2014;19:pii: 20761. [PubMed] |
| 31. | Brasil P, Pereira JP, Raja Gabaglia C, Damasceno L, Wakimoto M, Ribeiro Nogueira RM, Carvalho de Sequeira P, Machado Siqueira A, Abreu de Carvalho LM, Cotrim da Cunha D. Zika Virus Infection in Pregnant Women in Rio de Janeiro - Preliminary Report. N Engl J Med. 2016; Mar 4; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1575] [Cited by in RCA: 1447] [Article Influence: 160.8] [Reference Citation Analysis (0)] |
| 32. | Atkinson B, Hearn P, Afrough B, Lumley S, Carter D, Aarons EJ, Simpson AJ, Brooks TJ, Hewson R. Detection of Zika Virus in Semen. Emerg Infect Dis. 2016;22:940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 244] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 33. | Mansuy JM, Dutertre M, Mengelle C, Fourcade C, Marchou B, Delobel P, Izopet J, Martin-Blondel G. Zika virus: high infectious viral load in semen, a new sexually transmitted pathogen? Lancet Infect Dis. 2016;16:405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 276] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 34. | Musso D. Zika Virus Transmission from French Polynesia to Brazil. Emerg Infect Dis. 2015;21:1887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 224] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 35. | Foy BD, Kobylinski KC, Chilson Foy JL, Blitvich BJ, Travassos da Rosa A, Haddow AD, Lanciotti RS, Tesh RB. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17:880-882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 906] [Cited by in RCA: 795] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 36. | Venturi G, Zammarchi L, Fortuna C, Remoli ME, Benedetti E, Fiorentini C, Trotta M, Rizzo C, Mantella A, Rezza G. An autochthonous case of Zika due to possible sexual transmission, Florence, Italy, 2014. Euro Surveill. 2016;21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 150] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 37. | McCarthy M. Zika virus was transmitted by sexual contact in Texas, health officials report. BMJ. 2016;352:i720. [PubMed] |
| 38. | Hills SL, Russell K, Hennessey M, Williams C, Oster AM, Fischer M, Mead P. Transmission of Zika Virus Through Sexual Contact with Travelers to Areas of Ongoing Transmission - Continental United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:215-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 301] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 39. | Gourinat AC, O’Connor O, Calvez E, Goarant C, Dupont-Rouzeyrol M. Detection of Zika virus in urine. Emerg Infect Dis. 2015;21:84-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 570] [Cited by in RCA: 507] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 40. | Musso D, Roche C, Nhan TX, Robin E, Teissier A, Cao-Lormeau VM. Detection of Zika virus in saliva. J Clin Virol. 2015;68:53-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 347] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 41. | Oster AM, Russell K, Stryker JE, Friedman A, Kachur RE, Petersen EE, Jamieson DJ, Cohn AC, Brooks JT. Update: Interim Guidance for Prevention of Sexual Transmission of Zika Virus--United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:323-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 42. | Besnard M, Lastere S, Teissier A, Cao-Lormeau V, Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill. 2014;19:pii: 20751. [PubMed] |
| 43. | Dupont-Rouzeyrol M, Biron A, O’Connor O, Huguon E, Descloux E. Infectious Zika viral particles in breastmilk. Lancet. 2016;387:1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 151] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 44. | Barthel A, Gourinat AC, Cazorla C, Joubert C, Dupont-Rouzeyrol M, Descloux E. Breast milk as a possible route of vertical transmission of dengue virus? Clin Infect Dis. 2013;57:415-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 45. | Blázquez AB, Sáiz JC. West Nile virus (WNV) transmission routes in the murine model: intrauterine, by breastfeeding and after cannibal ingestion. Virus Res. 2010;151:240-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Martines RB, Bhatnagar J, Keating MK, Silva-Flannery L, Muehlenbachs A, Gary J, Goldsmith C, Hale G, Ritter J, Rollin D. Notes from the Field: Evidence of Zika Virus Infection in Brain and Placental Tissues from Two Congenitally Infected Newborns and Two Fetal Losses--Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:159-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 330] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 47. | de Oliveira CS, da Costa Vasconcelos PF. Microcephaly and Zika virus. J Pediatr (Rio J). 2016;92:103-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 48. | Sarno M, Sacramento GA, Khouri R, do Rosário MS, Costa F, Archanjo G, Santos LA, Nery N, Vasilakis N, Ko AI. Zika Virus Infection and Stillbirths: A Case of Hydrops Fetalis, Hydranencephaly and Fetal Demise. PLoS Negl Trop Dis. 2016;10:e0004517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 261] [Cited by in RCA: 247] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 49. | Ribeiro CF, Lopes VG, Brasil P, Coelho J, Muniz AG, Nogueira RM. Perinatal transmission of dengue: a report of 7 cases. J Pediatr. 2013;163:1514-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 50. | Bentlin MR, de Barros Almeida RA, Coelho KI, Ribeiro AF, Siciliano MM, Suzuki A, Fortaleza CM. Perinatal transmission of yellow fever, Brazil, 2009. Emerg Infect Dis. 2011;17:1779-1780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 51. | Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, Pretrick M, Marfel M, Holzbauer S, Dubray C. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360:2536-2543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2211] [Cited by in RCA: 2116] [Article Influence: 132.3] [Reference Citation Analysis (0)] |
| 52. | Macnamara FN. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg. 1954;48:139-145. [PubMed] |
| 53. | Domínguez-Moreno R, Tolosa-Tort P, Patiño-Tamez A, Quintero-Bauman A, Collado-Frías DK, Miranda-Rodríguez MG, Canela-Calderón OJ, Hurtado-Valadez P, de Gante-Castro R, Ortiz-Guillén KM. Mortality associated with a diagnosis of Guillain-Barré syndrome in adults of Mexican health institutions. Rev Neurol. 2014;58:4-10. [PubMed] |
| 54. | Smith DW, Mackenzie J. Zika virus and Guillain-Barré syndrome: another viral cause to add to the list. Lancet. 2016;387:1486-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 55. | van den Berg B, Bunschoten C, van Doorn PA, Jacobs BC. Mortality in Guillain-Barre syndrome. Neurology. 2013;80:1650-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 56. | Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barré syndrome. Lancet. 2016;388:717-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 902] [Article Influence: 100.2] [Reference Citation Analysis (0)] |
| 57. | Cao-Lormeau VM, Roche C, Teissier A, Robin E, Berry AL, Mallet HP, Sall AA, Musso D. Zika virus, French polynesia, South pacific, 2013. Emerg Infect Dis. 2014;20:1085-1086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 323] [Cited by in RCA: 560] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 58. | Oehler E, Watrin L, Larre P, Leparc-Goffart I, Lastere S, Valour F, Baudouin L, Mallet H, Musso D, Ghawche F. Zika virus infection complicated by Guillain-Barre syndrome--case report, French Polynesia, December 2013. Euro Surveill. 2014;19:pii: 20720. [PubMed] |
| 59. | Roth A, Mercier A, Lepers C, Hoy D, Duituturaga S, Benyon E, Guillaumot L, Souares Y. Concurrent outbreaks of dengue, chikungunya and Zika virus infections - an unprecedented epidemic wave of mosquito-borne viruses in the Pacific 2012-2014. Euro Surveill. 2014;19:pii: 20929. [PubMed] |
| 60. | Cao-Lormeau VM, Blake A, Mons S, Lastère S, Roche C, Vanhomwegen J, Dub T, Baudouin L, Teissier A, Larre P. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387:1531-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1606] [Cited by in RCA: 1664] [Article Influence: 184.9] [Reference Citation Analysis (0)] |
| 61. | Malone RW, Homan J, Callahan MV, Glasspool-Malone J, Damodaran L, Schneider Ade B, Zimler R, Talton J, Cobb RR, Ruzic I. Zika Virus: Medical Countermeasure Development Challenges. PLoS Negl Trop Dis. 2016;10:e0004530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 62. | Garg RK, Malhotra HS, Jain A, Malhotra KP. Dengue-associated neuromuscular complications. Neurol India. 2015;63:497-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 63. | Simon O, Billot S, Guyon D, Daures M, Descloux E, Gourinat AC, Molko N, Dupont-Rouzeyrol M. Early Guillain-Barré Syndrome associated with acute dengue fever. J Clin Virol. 2016;77:29-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 65. | Wielanek AC, Monredon JD, Amrani ME, Roger JC, Serveaux JP. Guillain-Barré syndrome complicating a Chikungunya virus infection. Neurology. 2007;69:2105-2107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 66. | Yuki N, Hartung HP. Guillain-Barré syndrome. N Engl J Med. 2012;366:2294-2304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 786] [Cited by in RCA: 775] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 67. | von der Hagen M, Pivarcsi M, Liebe J, von Bernuth H, Didonato N, Hennermann JB, Bührer C, Wieczorek D, Kaindl AM. Diagnostic approach to microcephaly in childhood: a two-center study and review of the literature. Dev Med Child Neurol. 2014;56:732-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 148] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 68. | Rodrigues LC. Microcephaly and Zika virus infection. Lancet. 2016;387:2070-2072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 69. | Zwizwai R. Infection disease surveillance update. Lancet Infect Dis. 2016;16:157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 70. | Calvet G, Aguiar RS, Melo AS, Sampaio SA, de Filippis I, Fabri A, Araujo ES, de Sequeira PC, de Mendonça MC, de Oliveira L. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis. 2016;16:653-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 767] [Cited by in RCA: 808] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 71. | Kleber de Oliveira W, Cortez-Escalante J, De Oliveira WT, do Carmo GM, Henriques CM, Coelho GE, Araújo de França GV. Increase in Reported Prevalence of Microcephaly in Infants Born to Women Living in Areas with Confirmed Zika Virus Transmission During the First Trimester of Pregnancy - Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:242-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 308] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 72. | Jouannic JM, Friszer S, Leparc-Goffart I, Garel C, Eyrolle-Guignot D. Zika virus infection in French Polynesia. Lancet. 2016;387:1051-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 73. | Cauchemez S, Besnard M, Bompard P, Dub T, Guillemette-Artur P, Eyrolle-Guignot D, Salje H, Van Kerkhove MD, Abadie V, Garel C. Association between Zika virus and microcephaly in French Polynesia, 2013-15: a retrospective study. Lancet. 2016;387:2125-2132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 690] [Cited by in RCA: 669] [Article Influence: 74.3] [Reference Citation Analysis (0)] |
| 74. | Driggers RW, Ho CY, Korhonen EM, Kuivanen S, Jääskeläinen AJ, Smura T, Rosenberg A, Hill DA, DeBiasi RL, Vezina G. Zika Virus Infection with Prolonged Maternal Viremia and Fetal Brain Abnormalities. N Engl J Med. 2016;374:2142-2151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 635] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 75. | Butler D. First Zika-linked birth defects detected in Colombia. Nature. 2016;531:153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 76. | Bell TM, Field EJ, Narang HK. Zika virus infection of the central nervous system of mice. Arch Gesamte Virusforsch. 1971;35:183-193. [PubMed] |
| 77. | Weinbren MP, Williams MC. Zika virus: further isolations in the Zika area, and some studies on the strains isolated. Trans R Soc Trop Med Hyg. 1958;52:263-268. [PubMed] |
| 78. | Tang H, Hammack C, Ogden SC, Wen Z, Qian X, Li Y, Yao B, Shin J, Zhang F, Lee EM. Zika Virus Infects Human Cortical Neural Progenitors and Attenuates Their Growth. Cell Stem Cell. 2016;18:587-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1093] [Cited by in RCA: 972] [Article Influence: 108.0] [Reference Citation Analysis (0)] |
| 79. | Adibi JJ, Marques ET, Cartus A, Beigi RH. Teratogenic effects of the Zika virus and the role of the placenta. Lancet. 2016;387:1587-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 80. | Petersen EE, Staples JE, Meaney-Delman D, Fischer M, Ellington SR, Callaghan WM, Jamieson DJ. Interim Guidelines for Pregnant Women During a Zika Virus Outbreak--United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:30-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 192] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 81. | Frank C, Faber M, Stark K. Causal or not: applying the Bradford Hill aspects of evidence to the association between Zika virus and microcephaly. EMBO Mol Med. 2016;8:305-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 82. | Nabel GJ, Zerhouni EA. Once and future epidemics: Zika virus emerging. Sci Transl Med. 2016;8:330ed2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 83. | Clyde K, Kyle JL, Harris E. Recent advances in deciphering viral and host determinants of dengue virus replication and pathogenesis. J Virol. 2006;80:11418-11431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 268] [Article Influence: 14.1] [Reference Citation Analysis (0)] |