Peer-review started: August 11, 2015
First decision: September 22, 2015
Revised: October 14, 2015
Accepted: December 13, 2015
Article in press: December 15, 2015
Published online: February 12, 2016
Processing time: 178 Days and 2.2 Hours
AIM: To investigate the neuropathology of the brain in a rare case of remission following diagnosis of progressive multifocal leukoencephalopathy (PML).
METHODS: Consent from the family for an autopsy was obtained, clinical records and radiograms were retrieved. A complete autopsy was performed, with brain examination after fixation and coronal sectioning at 1 cm intervals. Fourteen regions were collected for paraffin embedding and staining for microscopic analysis. Histologic sections were stained with Luxol blue, hematoxylin/eosin, and immunostained for myelin basic protein, neurofilament, SV40 T antigen and p53. The biopsy material was also retrieved and sections were stained with hematoxylin/eosin and immunostained for SV40 and p53. Sections were examined by American Board of Pathology certified pathologists and images captured digitally.
RESULTS: Review of the clinical records was notable for a history of ulcerative colitis resulting in total colectomy in 1977 and a liver transplant in 1998 followed by immune-suppressive therapy. Neurological symptoms presented immediately, therefore a biopsy was obtained which was diagnosed as PML. Immunotherapy was adjusted and clinical improvement was noted. No subsequent progression was reported. Review of the biopsy demonstrated atypical astrocytes and enlarged hyperchromatic oligodendroglial cells consistent with JC virus infection. Strong SV40 and p53 staining was found in glial cells and regions of dense macrophage infiltration were present. On gross examination of the post-mortem brain, a lesion in the same site as the original biopsy in the cerebellum was identified but no other lesions in the brain were found. Microscopic analysis of this cerebellar lesion revealed a loss of myelin and axons, and evidence of axonal damage. This single burned-out lesion was equivocally positive for SV40 antigen with little p53 staining. Examination of thirteen other brain regions found no other occult sites.
CONCLUSION: Our study reveals residual damage, rare macrophages or other inflammation and minimal evidence of persistent virus. This case demonstrates the possibility of complete remission of PML.
Core tip: Progressive multifocal leukoencephalopathy after organ transplant is rapidly fatal in most cases, with an average time to death of 6.4 mo. We report a case with no clinical progression over 14 years despite on-going immunosuppressive therapy. At initial diagnosis the biopsy demonstrated classic histopathological features of JC virus. At autopsy, microscopic analysis of the cerebellar lesion revealed a residual loss of myelin and evidence of axonal damage without evidence of viral activity. These results suggest that JC virus can be kept in check even in a setting of immunosuppression, and argue for more investigation into the microbiome of the brain.
- Citation: SantaCruz KS, Roy G, Spigel J, Bearer EL. Neuropathology of JC virus infection in progressive multifocal leukoencephalopathy in remission. World J Virol 2016; 5(1): 31-37
- URL: https://www.wjgnet.com/2220-3249/full/v5/i1/31.htm
- DOI: https://dx.doi.org/10.5501/wjv.v5.i1.31
Progressive multifocal leukoencephalopathy (PML) is a demyelinating disease of the central nervous system caused by reactivation of latent JC virus in immuno-compromised individuals. Oligodendroglial cells are preferentially infected with consequent loss of myelin and coalescing demyelination plaques, sometimes leading to mild-to-moderate axonal loss associated with axonal spheroids. Long-term survival in patients with PML is increasingly common in human immunodeficiency virus (HIV)-infected people treated with highly active anti-retroviral therapy (HAART)[1-8]. In contrast, prolonged survival in patients with immunosuppression following solid organ transplant is unusual[9,10]. Here we present an unusual case in which there was a 14-year clinical remission after biopsy-proven PML, despite continued immuno-suppression after liver transplant.
PML had been universally fatal, usually within 6 mo, until the late 1990’s. Although no specific treatment has proven particularly effective, enhancing natural immunity by reducing the effects of HIV virus or by altering immunosuppressant therapy has been shown to improve survival[11]. One explanation for prolonged survival is improved cellular immune responses against JC virus (JCV) in long term survivors vs those with poor outcomes[12]. Detectable cytotoxic T lymphocytes specific for JCV -T or VP-1 have been shown to be a prognostic indicator of long-term survival in HIV patients[12]. Although long-term survival in immunosuppressed transplant patients has been described[13,14] these cases are unusual[9] and detailed neuropathologic descriptions of residual demyelinating plaques in patients in complete remission are few, possibly because they are very rare or not frequently examined post-mortem[15]. This study therefore fills an important gap in our knowledge of pathological processes that appear in long-term survival.
Consent for the autopsy from the family was obtained as approved by Presbyterian Hospital. According to Internal Review Board of University of New Mexico Health Sciences Center neither post-mortem material nor case reports require IRB approvals. Clinical records were retrieved, and the clinical history together with results of all brain imaging studies that had been performed at UNM (12/2006 and 4/2008) reviewed. The original imaging studies were not available, and, in the absence of neurological symptomatology, imaging and CSF sampling were not performed during the final hospitalization, nor was post-mortem brain imaging done.
A complete autopsy was performed with subsequent examination of the brain after fixation. Gross examination of the brain included coronal sectioning of the neocortex at 1 cm intervals, and sectioning of the cerebellum and brainstem at 0.5 cm intervals. The surface of each slice was examined. Thirteen brain regions were selected, slabs 1.5 cm × 1.5 cm × 0.1 cm dissected and these were submitted for paraffin embedding. Histologic sections were stained with Luxol blue, hematoxylin/eosin, and for myelin basic protein (Dako, polyclonal rabbit anti-human), neurofilament (Dako, Clone 2F11), SV40 T antigen (Calbiochem, Ab-2, PAb 416) or p53 (Dako, Clone DO7) by immunohistochemistry at TriCore Reference Laboratories, Albuquerque, NM. The SV40 T-antigen (Ab-2) antibody is a mouse monoclonal antibody with specific determinants unique to the SV40 large T antigen and non-reactive with the small T antigen. The antigenic epitope is between Ile83 and Lys128 of the SV40 large T antigen, a region highly homologous to the JC virus large T antigen. For each immunostain, positive and negative controls were run in parallel. The paraffin block containing the biopsy was retrieved together with its slides from archives, new sections were made and also stained for SV40 and p53. Sections were examined on an Olympus BX40 microscope using 4 ×, 10 ×, 20 × and 40 × objectives, and digital images captured on an Olympus DP26 camera using cellSens Standard software. Images were prepared for figures using Adobe Photoshop to resize, create multi-image panels, adjust levels and add lettering.
A 76-year-old woman with a history of ulcerative colitis had a total colectomy in 1977 and subsequently developed sclerosing cholangitis. She received an orthotopic liver transplant in 1998. In 1999 neurological symptoms occurred, primarily consisting of ataxia in a setting of immunosuppressive therapy for the liver transplant. She was found to have a white matter lesion involving cerebellar white matter with no other sites of involvement. Brain biopsy performed in March 1999 showed classic changes of PML. The dosage of immunosuppressive therapy with Tacrolimus and Sirolimus was subsequently adjusted to minimize progression of further neurological disease and her mild cerebellar symptoms stabilized.
Her medical history was also significant for right hip fracture in 2003, status post hip replacement complicated by infection and requiring long term antibiotic therapy, chronic renal insufficiency, due to congenital hypoplastic kidney, end stage renal disease on dialysis since 2008, cardiovascular disease with episodes of atrial fibrillation and rapid ventricular response, hypothyroidism, gout and recurrent infections.
She had multiple hospital admissions from February 2012 to March 2013 due to gastroenteritis with subsequent workup for stool pathogens that was negative. She developed pancreatic insufficiency with findings on ultrasound examination that showed an atrophic right kidney, small liver and pancreatic cysts. Due to recurrence of the gastrointestinal illness, and to ultrasound findings, there was concern for an intraductal papillary mucinous neoplasm of the pancreas with associated pancreatic insufficiency. Computed tomography scan of the abdomen showed diffuse dilatation of the pancreatic duct, as well as a liver abscess. She died six days following abdominal imaging studies on March 25, 2013.
Mortality was due to complications related to remote liver transplantation for primary sclerosing cholangitis. An intraductal papillary mucinous neoplasm of the pancreatic duct was identified at autopsy with associated chronic atrophic pancreatitis. Post mortem examination determined the immediate cause of death to be due to infection from the liver abscess, cardiac arrhythmia and cardiomegaly.
Neurologic and Radiographic studies: The patient was seen by a neurologist at UNM on 3/2003, 2/2005, 12/2006, 6/2007, 4/2008, 7/2009, 4/2010 and 9/2011. During this period symptoms were stable on the reduced immunosuppressant protocol.
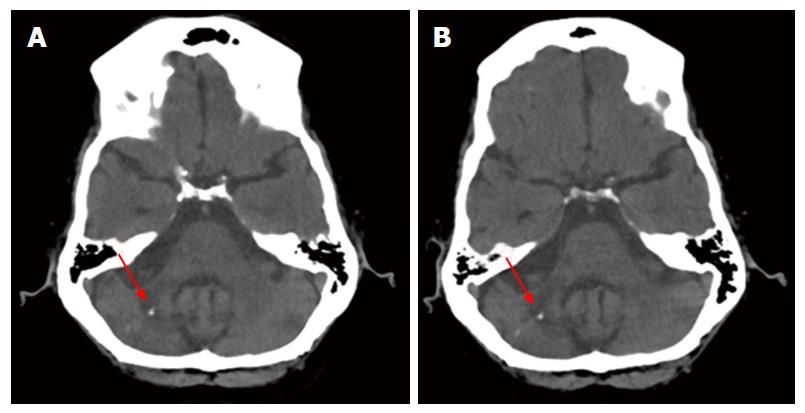
Imaging from 4/4/2008 was read as unchanged compared to the computed tomography (CT) done 12/12/2006 (Figure 1). Both images show diffuse cerebral as well as cerebellar atrophy. A region of greater volume loss and accompanying low attenuation appeared in the right cerebellar hemisphere and middle cerebellar peduncle. Small foci of calcification were noted. These were not significantly changed between the 2006 and 2008 images. No new areas of abnormal attenuation were identified within the brain. Vertebral and internal carotid artery calcifications were also noted.
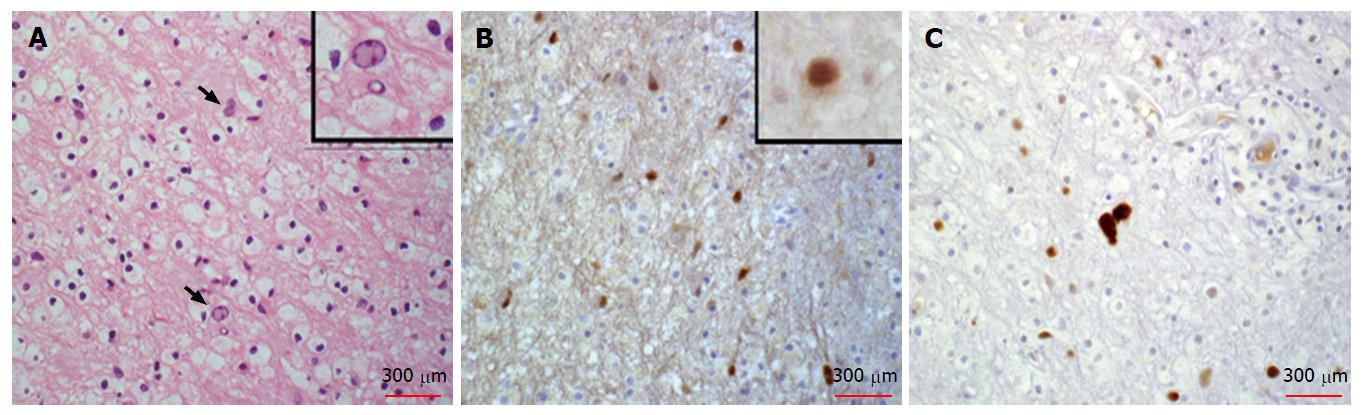
Histopathologic review of the hematoxilin and eosin stained slides from the cerebellar stereotactic biopsy specimen of 1999 showed the classic features of PML, including demyelination with abundant macrophages, no lymphocytes or granulocytes and relative preservation of axons. Occasional enlarged oligodendroglial cells with dense chromatin and atypical astrocytes were also present (Figure 2). To detect virus, SV40 T antigen immunostaining was used. JC virus is a papovavirus in the polyoma family. SV40 monoclonal antibody was raised against a short peptide from the large T antigen of Simian Virus 40, another member of this virus family. This antibody also recognizes the large T antigen from both JC and BK viruses. It does not recognize small T antigens. SV40 immunostaining highlighted nuclei of infected oligodendroglial cells and occasional bland-appearing astrocytes, but did not highlight atypical astrocytes in the biopsy. Staining for p53 was performed to detect secondary viral effects on glia as support for the diagnosis[16]. The p53 antibody stained the nuclei of atypical glial cells. Thus these atypical astrocytes were likely reactive rather than infected. This review of the biopsy confirmed the previous diagnosis of PML.
At autopsy, gross examination of the brain surface and of coronal sections at 1 cm intervals revealed no ventricular enlargement, and no periventricular, or other white matter abnormalities. The brain was thoroughly examined by coronal sectioning from forebrain to brain stem and no areas of softening or discoloration were found. PML frequently extends initially to periventricular regions, yet no cerebral lesions were found in this case. Sagittal sectioning of the cerebellum revealed a 1.0 cm × 0.8 cm × 0.5 cm focus of tissue softening just lateral to the vermis on the left, in the region of the original biopsy. This is the area of the lesion identified in the 2006 and 2008 CT brain scans. This area of softening included cerebellar white matter and the dentate nucleus.
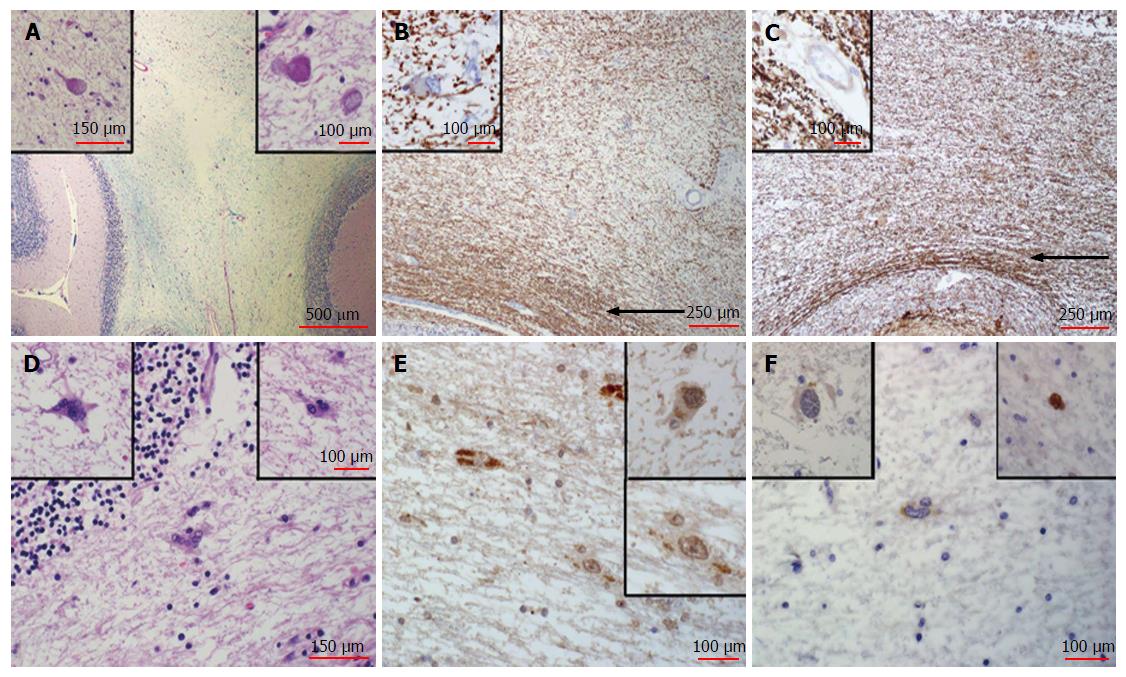
On histologic examination of the post-mortem brain sections from the original lesion in the cerebellar white matter were remarkable for white matter rarefaction, as evidenced by loss of myelin that was nearly proportional to axonal loss (Figure 3). Thus repair of the damage had not occurred. However, continuing damage was not detected. The heavy macrophage infiltration observed in the 1999 biopsy was absent, and macrophages were not apparent. Classical features of PML, such as enlarged oligodendroglial cells, were also mainly absent, atypical astrocytes were rare and only weak nuclear SV40 or p53 immunostaining was noted (Figure 3). We considered weak staining of the cytoplasm of glial cells to be non-specific since this low level of background staining was present in normal tissue within the section and also present in the negative control where no virus was present. A few rare cells displayed slightly more intense staining, which are shown in insets (Figure 3). Due to these rare cells, we cannot rule out residual viral antigens or continued low level of expression from latent virus in this burnt-out lesion.
There was minimal involvement of overlying cerebellar folia and minimal depletion of cerebellar granular cells, as determined by lack of detectable pathologic processes (Figure 3). Thirteen additional histologic sections from throughout the brain were dissected and processed according to the standard neuropathological brain examination procedure. Random sections of periventricular white matter, adjacent to the lateral ventricles and the aqueduct, where infection is most likely to spread, revealed no diagnostically significant abnormality. Sections from the pons were also stained for SV40 and p53 and no staining was detected.
Remarkably, fourteen years after diagnosis the lesion appeared confined to a single focus in the cerebellum, as in the original presentation. No additional foci throughout the brain, which are normally common in this multifocal disease, were found.
PML is a demyelinating disease of the brain caused by the polyomavirus, JCV in immunosuppressed individuals. Although long-term survival has been reported in PML, the histological appearance of a demyelinating plaque in complete remission has not been well described. Here we show that demyelination in the original lesion was not repaired despite 14 years of remission, while evidence of continuing acute infection was absent.
The lack of defined viral particles, absence of progressive lesions, and inflammatory processes suggests that the virus had either been cleared or was latent. Since polyomaviruses persist in cells in a latent form, either episomally or when integrated into cellular DNA[17,18], surviving glia in the lesion could harbor latent virus and continue to express viral antigens at low levels without producing sufficient infective particles to spread the virus.
The risk of PML is present throughout the post transplantation period with a higher case fatality and incidence than reported in HIV patients on HAART or multiple sclerosis patients treated with natalizum[9]. There is no cure for PML, but prolonged survival rates are becoming increasingly common; although in one series, patients with cerebellar lesions tended to have a worse clinical outcome[6]. Magnetic resonance imaging brain findings typically show leukomalacia with ventricular enlargement secondary to destruction of the white matter at the site of previous PML lesions, and focal areas of subcortical atrophy with preservation of the cortical ribbon[6,8].
Although this case illustrates the classic histological features of PML at initial presentation together with neurological symptoms, imaging findings in the cerebellum and JCV confirmed biopsy, the patient’s neurological symptoms were non-progressive despite continued immunosuppression. At autopsy, only residual damage in the location of the original lesion was observed, and histopathologic features of active infection in this region were absent, presumably indicating an effective cellular immune response against the virus. Serological workup of HIV cases has suggested a role for CD8+ cytotoxic T-lymphocytes against JCV[12], although in this current case no significant lymphocytic presence was detected in either the cerebellar biopsy or the post-mortem brain. Recent reports suggest findings of mutated JCV in CSF may correlate with slower or halted disease progression in HIV but no correlation was found in transplant recipients despite similarly mutated virus[2,19,20].
One of the earliest histopathological descriptions of patients with long term survival with PML revealed classical findings of progressive multifocal leukoencephalopathy, but with numerous eosinophils[21]. Viral particles were found in oligodendrocyte nuclei and cytoplasm with electron microscopy. Other cases of long term survival in non-HIV-infected patients are so rare as to be reportable, and include immunosuppressed patients for leukemia-lymphoma treatment[6,7,22] as well as solid organ transplant such as kidney[14] and liver[13]. The current case is unusual in that the neurological status was stable and at autopsy, gross evidence of multifocal pathology was absent, and histologic evidence of active viral infection was absent. Despite detection of low levels of viral antigen in the cerebellar region by immunostaining at autopsy this patient was clinically stable for fourteen years. No progression was detected symptomatically, neurologically, or radiographically. No evidence of progressive demyelination or spread of pathology beyond the original lesion was found in post-mortem evaluation of the brain.
A multicenter, retrospective cohort study of cases of PML was performed among transplant recipients at Mayo Clinic, Johns Hopkins University, Washington University, and Amsterdam Academic Medical Center[9].The incidence of PML was calculated at 1.24 per 1000 post transplantation person-years. In this study of 69 cases of PML associated with solid organ and bone marrow transplantation, median survival following symptom onset was 6.4 mo for solid organ vs 19.5 mo for bone marrow recipients; with survival beyond one year of only 55.7%[9]. Anti-retroviral treatment for HIV improves the immune system and is beneficial for those with progressive multifocal leukoencephalopathy[1]; however the only effective treatment for iatrogenically immunosuppressed patients appears withdrawal or re-configuration of life-saving immunosuppressive therapy and consequent enhancement of their natural immunity.
The mechanisms for reactivation of latent JCV in brain are poorly understood but thought to be related to immune competence. Viral and/or host genotypes may also play a role, since variation in human leukocyte antigens correlates with antibody response[23]. Other viruses latent in brain include herpes simplex virus (HSV). While HSV DNA is found in a large percentage of normal brains, little evidence exists as to whether HSV reactivates in brain[24]. Attempts to correlate HSV reactivation in the brain with the risk of neurodegenerative diseases such as Alzheimer’s are on-going[25,26]. How either HSV or JCV are kept in check in the immune-competent infected person remains a mystery.
Our study reveals residual damage, rare macrophages, a few reactive astrocytes and minimal evidence of persistent viral antigen expression with no evidence of viral replication and infective particle production. This case demonstrates the possibility of complete remission of PML with long-term survival in a patient after solid organ transplant who was maintained on immunosuppressive therapy.
The authors would like to acknowledge the additional neuropathological guidance and editorial contributions of neuropathologist, Bette Kleinschmidt-DeMasters MD, technical assistance of Kevin Reagan at UNM, histotech Karen Buehler at TriCore Reference Labs, and digital image editing of the first draft by Mike Grady.
JC virus (JCV) causes progressive multifocal leukoencephalopathy (PML), especially in immun-compromised patients. After solid organ transplant is typically rapidly progressive and fatal.
Here the authors present a case of PML in a patient who received a solid organ transplant that did not progress for over 14 years despite on-going immune-suppression. The diagnosis was validated by pathological analysis of brain biopsy.
Rare insight into the histopathology of quiescent JCV infection and residual damage, not repaired after 14 years, are presented. This is the first reported histopathology of a JCV-induced lesion in remission.
This report demonstrates that PML progression may be halted but the original lesion does not repair.
JCV is a polyoma genetically similar to SV40. “JC” stands for John Cunningham, the first patient in which the virus was discovered. It is very common in the general population but only causes overt disease in immune compromised hosts. PML thought to be caused by JCV, is a rare and usually fatal disease of the white matter in the brain.
JCV is a human polyomavirus that infects greater than 60% of the human population during childhood, and establishes a latent infection in healthy individuals. Replication of the neurotropic strain of JCV in glial cells causes the fatal demyelinating disease of the central nervous system, PML, which is seen in patients with underlying immunocompromised conditions. PML has also been described in patients with autoimmune diseases treated with immunomodulatory therapies. PML is a mortal disease and there is no specific therapy. Long-term survivors have been reported with no sign of viral reactivation and replication. There is little known about neuropathologic description of long-term survivals. In this manuscript, authors provided an interesting case report of a long-term PML survivor with immunohistological evaluation. These observations are interesting for the readers of the Journal.
P- Reviewer: Sariyer IK, Williamson EM S- Editor: Qiu S L- Editor: A E- Editor: Li D
| 1. | Engsig FN, Hansen AB, Omland LH, Kronborg G, Gerstoft J, Laursen AL, Pedersen C, Mogensen CB, Nielsen L, Obel N. Incidence, clinical presentation, and outcome of progressive multifocal leukoencephalopathy in HIV-infected patients during the highly active antiretroviral therapy era: a nationwide cohort study. J Infect Dis. 2009;199:77-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 2. | Ferenczy MW, Marshall LJ, Nelson CD, Atwood WJ, Nath A, Khalili K, Major EO. Molecular biology, epidemiology, and pathogenesis of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev. 2012;25:471-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 301] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 3. | Focosi D. Does contrast enhancement predict survival in progressive multifocal leukoencephalopathy? J Infect Dis. 2009;199:1410-1411; author reply 1411-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Gasnault J, Costagliola D, Hendel-Chavez H, Dulioust A, Pakianather S, Mazet AA, de Goer de Herve MG, Lancar R, Lascaux AS, Porte L. Improved survival of HIV-1-infected patients with progressive multifocal leukoencephalopathy receiving early 5-drug combination antiretroviral therapy. PLoS One. 2011;6:e20967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Gheuens S, Pierone G, Peeters P, Koralnik IJ. Progressive multifocal leukoencephalopathy in individuals with minimal or occult immunosuppression. J Neurol Neurosurg Psychiatry. 2010;81:247-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 6. | Lima MA, Bernal-Cano F, Clifford DB, Gandhi RT, Koralnik IJ. Clinical outcome of long-term survivors of progressive multifocal leukoencephalopathy. J Neurol Neurosurg Psychiatry. 2010;81:1288-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Stam FC. Multifocal leuko-encephalopathy with slow progression and very long survival. Psychiatr Neurol Neurochir. 1966;69:453-459. [PubMed] |
| 8. | Tavazzi E, White MK, Khalili K. Progressive multifocal leukoencephalopathy: clinical and molecular aspects. Rev Med Virol. 2012;22:18-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Mateen FJ, Muralidharan R, Carone M, van de Beek D, Harrison DM, Aksamit AJ, Gould MS, Clifford DB, Nath A. Progressive multifocal leukoencephalopathy in transplant recipients. Ann Neurol. 2011;70:305-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 10. | Shitrit D, Lev N, Bar-Gil-Shitrit A, Kramer MR. Progressive multifocal leukoencephalopathy in transplant recipients. Transpl Int. 2005;17:658-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Antinori A, Cingolani A, Lorenzini P, Giancola ML, Uccella I, Bossolasco S, Grisetti S, Moretti F, Vigo B, Bongiovanni M. Clinical epidemiology and survival of progressive multifocal leukoencephalopathy in the era of highly active antiretroviral therapy: data from the Italian Registry Investigative Neuro AIDS (IRINA). J Neurovirol. 2003;9 Suppl 1:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 94] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Du Pasquier RA. JCV-specific cellular immune response correlates with a favorable clinical outcome in HIV-infected individuals with progressive multifocal leukoencephalopathy. J Neurovirol. 2001;7:318-322. |
| 13. | Boulton-Jones JR, Fraser-Moodie C, Ryder SD. Long term survival from progressive multifocal leucoencephalopathy after liver transplantation. J Hepatol. 2001;35:828-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Crowder CD, Gyure KA, Drachenberg CB, Werner J, Morales RE, Hirsch HH, Ramos E. Successful outcome of progressive multifocal leukoencephalopathy in a renal transplant patient. Am J Transplant. 2005;5:1151-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Gheuens S, Wüthrich C, Koralnik IJ. Progressive multifocal leukoencephalopathy: why gray and white matter. Annu Rev Pathol. 2013;8:189-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 16. | Yang B, Prayson RA. Expression of Bax, Bcl-2, and P53 in progressive multifocal leukoencephalopathy. Mod Pathol. 2000;13:1115-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Coelho TR, Almeida L, Lazo PA. JC virus in the pathogenesis of colorectal cancer, an etiological agent or another component in a multistep process? Virol J. 2010;7:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Wold WS, Green M, Mackey JK, Martin JD, Padgett BL, Walker DL. Integration pattern of human JC virus sequences in two clones of a cell line established from a JC virus-induced hamster brain tumor. J Virol. 1980;33:1225-1228. [PubMed] |
| 19. | Jensen PN, Major EO. A classification scheme for human polyomavirus JCV variants based on the nucleotide sequence of the noncoding regulatory region. J Neurovirol. 2001;7:280-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Delbue S, Elia F, Carloni C, Tavazzi E, Marchioni E, Carluccio S, Signorini L, Novati S, Maserati R, Ferrante P. JC virus load in cerebrospinal fluid and transcriptional control region rearrangements may predict the clinical course of progressive multifocal leukoencephalopathy. J Cell Physiol. 2012;227:3511-3517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Kepes JJ, Chou SM, Price LW. Progressive multifocal leukoencephalopathy with 10-year survival in a patient with nontropical sprue. Report of a case with unusual light and electron microscopic features. Neurology. 1975;25:1006-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Demir E, Liebert UG, Söylemezoglu F, Yalaz K, Köse G, Anlar B. Childhood case of progressive multifocal leukoencephalopathy with improved clinical outcome. J Child Neurol. 2005;20:241-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Sundqvist E, Buck D, Warnke C, Albrecht E, Gieger C, Khademi M, Lima Bomfim I, Fogdell-Hahn A, Link J, Alfredsson L. JC polyomavirus infection is strongly controlled by human leucocyte antigen class II variants. PLoS Pathog. 2014;10:e1004084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Roizman B, Whitley RJ. An inquiry into the molecular basis of HSV latency and reactivation. Annu Rev Microbiol. 2013;67:355-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 25. | Bearer EL. HSV, axonal transport and Alzheimer’s disease: in vitro and in vivo evidence for causal relationships. Future Virol. 2012;7:885-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Itzhaki RF. Herpes simplex virus type 1 and Alzheimer’s disease: increasing evidence for a major role of the virus. Front Aging Neurosci. 2014;6:202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 156] [Article Influence: 14.2] [Reference Citation Analysis (0)] |