Published online Sep 10, 2018. doi: 10.5500/wjt.v8.i5.167
Peer-review started: April 7, 2018
First decision: May 16, 2018
Revised: July 23, 2018
Accepted: August 3, 2018
Article in press: August 4, 2018
Published online: September 10, 2018
Processing time: 155 Days and 13.7 Hours
To compare trends in donor/recipient characteristics and outcomes using four period cohorts of liver transplant recipients from 1990 to 2009.
Seventy thousand three hundred and seventy-seven adult first-time recipients of whole-organ deceased-donor liver grafts from 1990 to 2009 were followed up until September 2013. Four periods based on transplantation dates were considered to account for developments in transplantation. Descriptive statistics were used to describe donor/recipient characteristics and transplant outcomes. Statistical comparisons between periods were performed using χ2/Fischer’s exact test (categorical variables) and t-tests/Mann-Whitney U test (continuous variables). Univariate descriptive statistics/survival data were generated using Kaplan-Meier curves. Cox Proportional Hazards models were used for regression analyses of patient and graft survival.
Mean age (years), body mass index (kg/m2), and the proportion of males were, respectively, 39.1 (± 17.4), 25.9 (± 5.7) and 60.3 for donors, and 51.3 (± 10.5), 27.7 (± 5.6), and 64.4 for recipients. Donor and transplantation rates differed between racial/ethnic groups. Median (Q1-Q3) cold and warm ischemia, waitlist, and hospital stay times were 8 (6.0-10.0) h and 45 (35-59) min, 93 (21-278) d, and 12 (8-20) d. Total functional assistance was required by 8% of recipients at wait-listing and 13.4% at transplantation. Overall survival at 1, 3, 5, 10, 15, and 20 years was 87.3%, 79.4%, 73.6%, 59.8%, 46.7%, and 35.9%, respectively. The 2005-2009 cohort had better patient and graft survival than the 1990-1994 cohort overall [HR 0.67 (0.62-0.72) and 0.66 (0.62-0.71)] and at five years [HR 0.73 (0.66-0.80) and 0.71 (0.65-0.77)].
Despite changes in donor quality, recipient characteristics, and declining functional status among transplant recipients, overall patient survival is superior and post-transplant outcomes continue to improve.
Core tip: The objective of this study was to compare trends in liver transplant donor/recipient characteristics and outcomes using four period cohorts of adult, first-time whole-organ deceased donor recipients from 1990-2009 using historical data from the OPTN/UNOS database. The landscape of donors and recipients undergoing liver transplantation (LT) in the United States has changed. Donor age, body mass index, and the contribution of racial minorities have increased. Transplant recipients are older, more deconditioned and obese, and with changing causes of cirrhosis. Despite this, the long-term patient survival has improved over time. This paper provides an overview of the landscape of LT in the United States.
- Citation: Ayloo S, Pentakota SR, Molinari M. Trends of characteristics and outcomes of donors and recipients of deceased donor liver transplantation in the United States: 1990 to 2013. World J Transplantation 2018; 8(5): 167-177
- URL: https://www.wjgnet.com/2220-3230/full/v8/i5/167.htm
- DOI: https://dx.doi.org/10.5500/wjt.v8.i5.167
Liver transplantation (LT) is a life-saving surgical option for many people with end-stage liver disease. According to annual data from the OPTN, 5710 deceased donor and 211 living donor LT were performed in 139 centers across the United States in 2013[1]. Although several short-term studies have analyzed the OPTN/UNOS database, few have evaluated LT over an extended period[2-7], leading to uncertainty regarding the long-term course of LT.
Numerous advances have occurred in LT management over the last several decades, including advancements in surgical techniques, anesthesia, and perioperative care in intensive care units, evolution of immunosuppressive medications and regimens[8,9], changes in organ allocation policies, institution of the Model for End-stage Liver Disease score (MELD)[10-12] to prioritize transplant candidates, improvements in tissue and organ preservation[13,14], and refinements in histocompatibility matching[15]. Therefore, we hypothesize that overall patient survival during this time has improved. However, transplant programs have extended their acceptance of grafts from donors who are older, higher risk, and have increased comorbidities to alleviate the paucity of available organs. The objective of this study was to compare donor and recipient characteristics and outcomes among four cohorts of LT recipients from 1990 to 2009.
Historical data from the OPTN/UNOS database were obtained for all LT performed in the United States from 1989 to 2013. The primary objective was to evaluate post-transplant patient survival (1, 3, 5, 10, and 20 years), and the secondary objective was to evaluate transplant outcomes, including cold ischemia time (CIT) and warm ischemia time (WIT), hospital length of stay (LOS), waitlist time (WL), MELD, re-transplantation, rejection of graft, graft failure, reasons for graft failure, and post-transplant causes of death.
Data were provided by OPTN/UNOS as Standard Transplant and Research files. The study did not require approval by the ethics review board of our institution because it was conducted and reported per STROBE statement recommendations[16-18]. Analyses were limited to first-time, adult, whole-organ LT from a deceased donor from January 1st, 1990 to December 31st, 2009. Patients with missing data on liver type, donor type, previous LT, with multiple records, or who underwent multi-organ transplantation or re-transplantation were excluded from the study. Study subjects were grouped arbitrarily into four cohorts representing five-year intervals (1990-1994; 1995-1999; 2000-2004; 2005-2009) by transplant date. Study follow-up extended from transplant date until re-transplant, death, or September 06, 2013 (the last follow-up date recorded in the UNOS database), whichever occurred first. Data were updated with the date of death listed in the Social Security Death Master File for patients marked as “alive” or “lost to follow-up.”
Demographic and clinical variables analyzed for both donors and recipients included: age, gender, highest education level, race/ethnicity, and body mass index (BMI). The World Health Organization classification was used to categorize the weight status of donors and recipients as follows: underweight (BMI < 18.5 kg/m2), normal weight (BMI = 18.5-24.9 kg/m2), overweight (BMI = 25.0-29.9 kg/m2), class I obesity (BMI = 30.0-34.9 kg/m2), class II obesity (BMI = 35.0-39.9 kg/m2), and class III obesity (BMI ≥ 40.0 kg/m2). The donor cause of death was also analyzed.
Several recipient-specific variables were included in the analyses. These variables were related to transplant (CIT, WIT, LOS, and WL), recipient comorbidities including hypertension (HTN: no, yes, unknown), chronic obstructive pulmonary disease (COPD: no, yes, unknown), diabetes [no, type 1 (insulin-dependent diabetes mellitus), type 2 (non-insulin-dependent diabetes mellitus or other types of diabetes), unknown, angina (no, yes, unknown), dialysis in the week prior to LT, recipient functional status (no, some, or total assistance for activities of daily living), and recipient medical condition (admitted to ICU, hospitalized, not hospitalized). Individuals with coronary artery disease since 2004 were included in the angina group, whereas no such categorization was available prior to 2004.
Functional status was classified into three simple, clinically-useful categories. Patients requiring “total assistance” carried out 50% or less of daily activity functions and needed frequent medical care, or were severely disabled or moribund. Patients required “some assistance” if they were able to carry out 60%-80% of their daily functional activities and care for themselves, with some disease-related symptoms affecting daily activities. Patients requiring “no assistance” could perform 90%-100% of daily activities without substantial disease-related limitations.
Descriptive statistics were used to describe donor/recipient characteristics and transplant outcomes for the overall and the four period cohorts. Categorical variables were described using counts and proportions. Continuous variables were described using means and standard deviation, or with medians and interquartile ranges when skewed. Statistical comparisons of donor/recipient characteristics and transplant outcomes between period 1 (1990-1994) and period 4 (2004-2009) were performed using χ2 and Fischer’s Exact test as appropriate (categorical variables), t-tests (normally distributed continuous variables), and Mann-Whitney U test for skewed continuous variables. Univariate descriptive statistics and survival data on patient survival, both overall and by the four period cohorts, were generated using Kaplan-Meier curves. Cox Proportional Hazards models were used for regression analyses of patient and graft survival data, which was analyzed for overall and five year survival. Unadjusted and adjusted Cox Proportional Hazards regression models were run for patient and graft survival with “period” as the main exposure variable. In addition to period, the adjusted models included donor characteristics (age, gender, race/ethnicity, BMI, and cause of death) and recipient characteristics (age, gender, race/ethnicity, BMI, cause of liver failure, wait-list time, angina, diabetes, HTN, COPD, CIT, and functional and medical status). Given the numerous statistical tests performed, the level of statistical significance for interpretation of statistical results was assumed to be 1% (a two-sided alpha of < 0.01) instead of the traditional cut-off value of 5%. All analyses were performed using SAS version 9.4 (SAS, Cary, NC) and SPSS version 24 (IBM Corp., Armonk, NY).
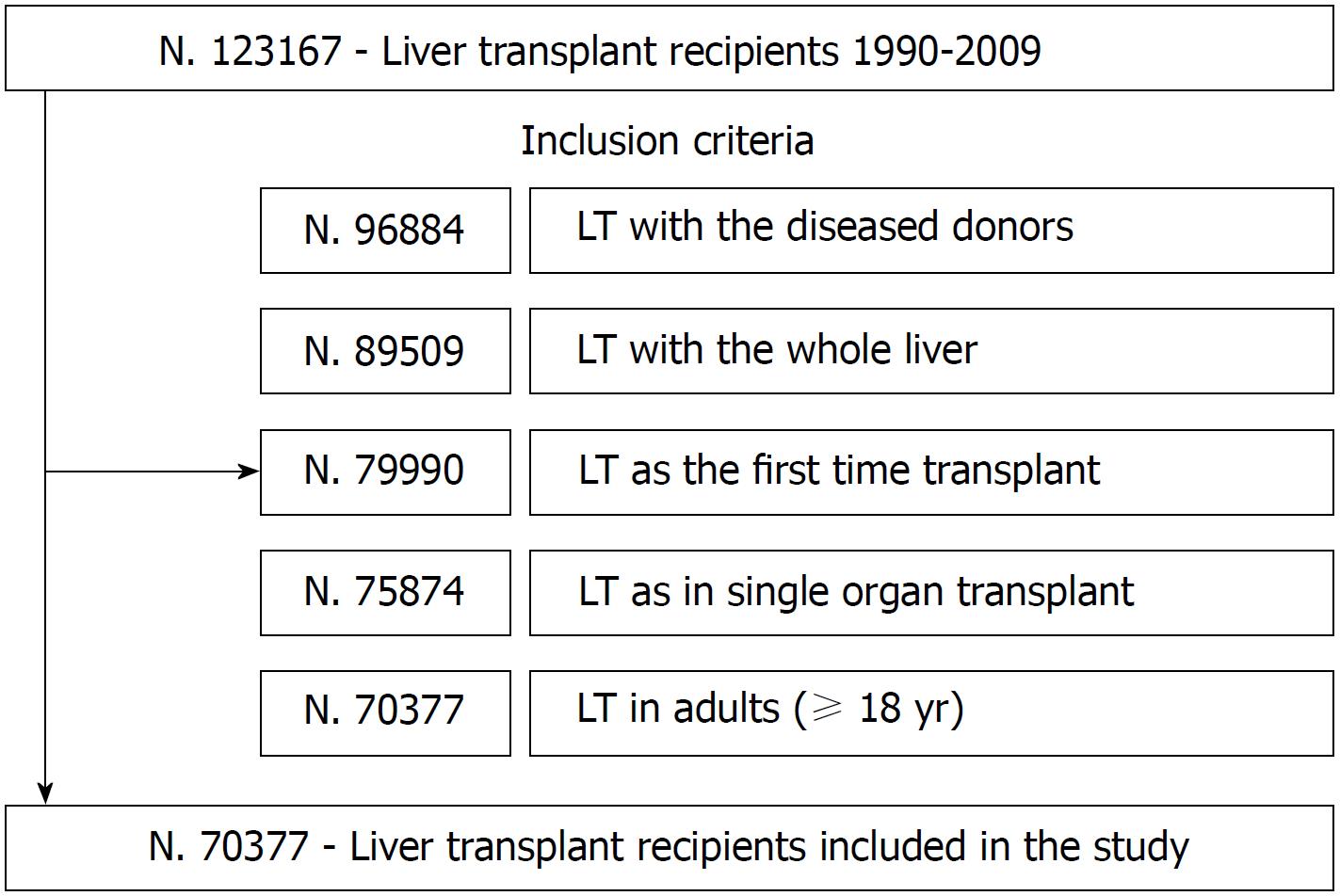
A total of 70,377 LT met the inclusion criteria (Figure 1). Transplants were mostly performed in OPTN/UNOS Region five (14.7%) and three (14.5%). The mean age of donors was 39.1 ± 17.4 years, 60.4% were men, and the majority (73.3%) were white. The mean (± SD) BMI was 25.9 kg/m2 (± 5.7), and 40.3% of donors had a normal body weight. The leading primary causes of donor deaths were cardiovascular adverse events (42.3%) and head traumas (39.9%) (Table 1).
| Donor characteristics | Total | 5 yr periods | P-value1 | |||
| Period 1 | Period 2 | Period 3 | Period 4 | |||
| 1990-1994 | 1995-1999 | 2000-2004 | 2005-2009 | |||
| Age, mean (SD) | 39.1 (17.4) | 32.3 (15.2) | 37.0 (17.4) | 40.6 (17.6) | 42.4 (17.2) | < 0.001 |
| Gender | < 0.001 | |||||
| Female | 27884 (39.6) | 3831 (35.4) | 6397 (40.1) | 8077 (41.0) | 9579 (40.1) | |
| Male | 42492 (60.4) | 6998 (64.6) | 9573 (59.9) | 11625 (59.0) | 14296 (59.9) | |
| BMI, mean (SD) | 25.9 (5.7) | 23.8 (4.6) | 24.8 (5.2) | 26.0 (5.6) | 27.0 (6.0) | < 0.001 |
| BMI | < 0.001 | |||||
| Underweight | 3310 (4.7) | 502 (4.6) | 1077 (6.7) | 910 (4.6) | 821 (3.4) | |
| Normal | 29093 (41.3) | 3655 (33.8) | 7698 (48.2) | 8730 (44.3) | 9010 (37.7) | |
| Overweight | 20441 (29.1) | 1582 (14.6) | 4461 (27.9) | 6345 (32.2) | 8053 (33.7) | |
| Obese - Class I | 7921 (11.3) | 384 (3.6) | 1369 (8.6) | 2459 (12.5) | 3709 (15.5) | |
| Obese - Class II | 2722 (3.9) | 84 (0.8) | 367 (2.3) | 801 (4.1) | 1470 (6.2) | |
| Obese - Class III | 1496 (2.1) | 47 (0.4) | 196 (1.2) | 446 (2.3) | 807 (3.4) | |
| Unknown | 5394 (7.7) | 4575 (42.3) | 802 (5.0) | 12 (0.1) | 5 (0.0) | |
| Ethnicity | < 0.001 | |||||
| White | 51594 (73.3) | 8747 (80.8) | 12334 (77.2) | 14444 (73.3) | 16069 (67.3) | |
| Black | 9195 (13.1) | 1044 (9.6) | 1741 (10.9) | 2481 (12.6) | 3929 (16.5) | |
| Hispanic | 7460 (10.6) | 812 (7.5) | 1396 (8.7) | 2144 (10.9) | 3108 (13.0) | |
| Asian | 1327 (1.9) | 135 (1.3) | 255 (1.6) | 395 (2.0) | 542 (2.3) | |
| Other | 662 (0.9) | 47 (0.4) | 164 (1.0) | 224 (1.1) | 227 (1.0) | |
| Unknown | 139 (0.2) | 44 (0.4) | 80 (0.5) | 15 (0.1) | ||
| Causes of death | < 0.001 | |||||
| Anoxia | 7848 (11.2) | 483 (4.5) | 1256 (7.9) | 2028 (10.3) | 4081 (17.1) | |
| Cerebrovascular/stroke | 29788 (42.3) | 3778 (34.9) | 6645 (41.6) | 8929 (45.3) | 10436 (43.7) | |
| Head trauma | 28087 (39.9) | 3576 (33.0) | 7592 (47.5) | 8171 (41.5) | 8748 (36.6) | |
In the subset analyses, mean donor age and BMI were significantly higher in period four than in period one. Donors with normal BMI dropped from 47.4% to 36.47% in Periods two to four, while the overweight donor group steadily increased from 14.5% to 33.3%. The percent of livers retrieved from obese donors more than tripled in period four compared with period one.
The mean age of recipients was 51.3 ± 10.5 years, and 64.4% were men (Table 2). The majority (76%) of recipients were white. The mean (± SD) BMI was 27.7 kg/m2 (± 5.6), and 31% of recipients were normal body weight. Overall, 30.2% of recipients were either high school graduates or received a general education diploma. The leading primary causes for liver failure were hepatitis C (25%) followed by alcoholic cirrhosis (14%) (Table 3). The median (Q1-Q3) MELD at listing and transplant were 16 (12-24) and 18 (14-28), respectively. The median (Q1-Q3) wait-list time including days inactive on the list was 93 (21-278). The median (Q1-Q3) CIT in hours, WIT in minutes, and LOS during index transplant surgery were 8.0 (6.0-10.0), 45.0 (35-59), and 12.0 (8-20) days, respectively (Table 3).
| Recipient characteristics | Total | 5 yr periods | P-value1 | |||
| Period 1 | Period 2 | Period 3 | Period 4 | |||
| 1990-1994 | 1995-1999 | 2000-2004 | 2005-2009 | |||
| Age, mean (SD) | 51.3 (10.5) | 48.2 (11.4) | 49.8 (10.5) | 51.5 (9.7) | 53.5 (9.9) | < 0.001 |
| Gender | < 0.001 | |||||
| Female | 25073 (35.6) | 4724 (43.6) | 6272 (39.3) | 6544 (33.2) | 7533 (31.6) | |
| Male | 45304 (64.4) | 6105 (56.4) | 9698 (60.7) | 13159 (66.8) | 16342 (68.5) | |
| BMI, mean (SD) | 27.75 (5.6) | 26.26 (5.3) | 27.47 (5.6) | 28.03 (5.6) | 28.35 (5.6) | < 0.001 |
| BMI | < 0.001 | |||||
| Underweight | 1458 (2.1) | 371 (3.4) | 319 (2.0) | 364 (1.9) | 404 (1.7) | |
| Normal | 22533 (32.0) | 4580 (42.3) | 5395 (33.8) | 5868 (29.8) | 6690 (28.0) | |
| Overweight | 24550 (34.9) | 3436 (31.7) | 5494 (34.4) | 7077 (35.9) | 8543 (35.8) | |
| Obese - Class I | 13417 (19.1) | 1488 (13.7) | 2771 (17.4) | 3991 (20.3) | 5167 (21.6) | |
| Obese - Class II | 5583 (7.9) | 527 (4.9) | 1166 (7.3) | 1623 (8.2) | 2267 (9.5) | |
| Obese - Class III | 2084 (3.0) | 203 (1.9) | 452 (2.8) | 629 (3.2) | 800 (3.4) | |
| Unknown | 752 (1.1) | 224 (2.1) | 373 (2.3) | 151 (0.8) | 4 (0.0) | |
| Ethnicity | < 0.001 | |||||
| White | 53474 (76.0) | 8839 (81.6) | 12501 (78.3) | 14844 (75.3) | 17290 (72.4) | |
| Black | 5448 (7.7) | 631 (5.8) | 1097 (6.9) | 1565 (7.9) | 2155 (9.0) | |
| Hispanic | 7907 (11.2) | 901 (8.3) | 1655 (10.4) | 2294 (11.6) | 3057 (12.8) | |
| Asian | 2785 (4.0) | 317 (2.9) | 555 (3.5) | 785 (4.0) | 1128 (4.7) | |
| Other | 719 (1.0) | 99 (0.9) | 160 (1.0) | 215 (1.1) | 245 (1.0) | |
| Unknown | 44 (0.1) | 42 (0.4) | 2 (0.0) | |||
| Highest education level | 0.2 | |||||
| Unknown | 26282 (37.3) | 9872 (91.2) | 5735 (35.9) | 5897 (29.9) | 4778 (20.0) | |
| Less than high school | 2335 (3.3) | 55 (0.5) | 513 (3.2) | 704 (3.6) | 1063 (4.5) | |
| High school (9-12) or GED | 21249 (30.2) | 438 (4.0) | 4846 (30.3) | 6835 (34.7) | 9130 (38.2) | |
| College less than graduate | 17559 (25.0) | 384 (3.6) | 4183 (26.2) | 5359 (27.2) | 7633 (32.0) | |
| Graduate | 2952 (4.2) | 80 (0.7) | 693 (4.3) | 908 (4.6) | 1271 (5.3) | |
| Causes of liver failure | < 0.001 | |||||
| Alcoholic cirrhosis | 9857 (14.0) | 2165 (20.0) | 2366 (14.8) | 2497 (12.7) | 2829 (11.9) | |
| Alcoholic cirrhosis with hepatitis C | 4467 (6.4) | 302 (2.8) | 1373 (8.6) | 1244 (6.3) | 1548 (6.5) | |
| Cirrhosis: Autoimmune | 2486 (3.5) | 568 (5.3) | 696 (4.4) | 629 (3.2) | 593 (2.5) | |
| Cirrhosis: Cryptogenic (Idiopathic) | 5918 (8.4) | 1397 (12.9) | 1565 (9.8) | 1515 (7.7) | 1441 (6.0) | |
| Cirrhosis: Fatty liver (NASH) | 1442 (2.1) | 9 (0.1) | 173 (0.9) | 1260 (5.3) | ||
| Cirrhosis: Hepatitis type B (HBSAG+) | 2367 (3.4) | 509 (4.7) | 675 (4.2) | 694 (3.5) | 489 (2.1) | |
| Cirrhosis: Hepatitis type C | 17611 (25.0) | 1849 (17.1) | 4024 (25.2) | 6058 (30.8) | 5680 (23.8) | |
| Other | 13851 (19.7) | 2344 (21.7) | 3195 (20.0) | 4196 (21.3) | 4116 (17.2) | |
| PLM: Hepatoma (HCC) and cirrhosis | 4960 (7.1) | 143 (1.3) | 272 (1.7) | 984 (5.0) | 3561 (14.9) | |
| PLM: Hepatoma - HCC | 1954 (2.8) | 173 (1.6) | 141 (0.9) | 423 (2.2) | 1217 (5.1) | |
| Primary biliary cirrhosis (PBC) | 3762 (5.4) | 1122 (10.4) | 1105 (6.9) | 814 (4.1) | 721 (3.0) | |
| PSC: Ulcerative colitis | 1702 (2.4) | 257 (2.4) | 549 (3.4) | 476 (2.4) | 420 (1.8) | |
| Recipient characteristics | Total | 5 yr periods | P-value1 | |||
| Period 1 | Period 2 | Period 3 | Period 4 | |||
| 1990-1994 | 1995-1999 | 2000-2004 | 2005-2009 | |||
| MELD (median) (Q1-Q3) | ||||||
| Listing | 16 (12-23) | NA | NA | 16 (12-23) | 16 (12-23) | |
| Transplant | 18 (13-26) | NA | NA | 18 (13-25) | 19 (14-27) | |
| CIT (median hours) (Q1-Q3) | 8 (6.0-10.0) | 10.3 (8.0-13.2) | 8.5 (6.5-10.9) | 7.3 (5.7-9.5) | 7 (5.1-8.7) | < 0.001 |
| WIT(median Minutes) (Q1-Q3) | 45 (35.0-59.0) | 58 (45.0-75.0) | 48 (38.0-60.0) | 40 (31.0-50.0) | 40 (31.0-49.0) | < 0.001 |
| Waiting list/inactive (median days) (Q1-Q3) | 93 (21-278) | 53 (14-31) | 151 (45-332) | 124 (27-386) | 68 (15-235) | < 0.001 |
| Hospital stay (median days) (Q1-Q3) | 12 (08-20) | 20 (14-31) | 13 (09-21) | 10 (07-17) | 10 (07-16) | < 0.001 |
In the subset analyses, mean recipient age and BMI were significantly higher in the later period. Significant decrease in transplanting normal weight recipients was observed with a rise in transplanting obese liver failure patients. Significant differences were noted in the recipient utilization of livers among different ethnicities and trends over different periods. Furthermore, recipients in the later period had higher education then period one. In terms of recipient functional status, the most common adult daily living functional status was the “no assistance” group at both wait-listing and transplantation. Similarly, 68.3% of recipients were not hospitalized for their medical condition at the time of their transplantation (Table 4). In terms of recipient comorbidities, diabetes was the most common medical comorbidity, followed by HTN. Approximately 4.3% of recipients were receiving dialysis before their transplantation (Table 5).
| Recipient characteristics | Total | 5 yr periods | P-value1 | |||
| Period 1 | Period 2 | Period 3 | Period 4 | |||
| 1990-1994 | 1995-1999 | 2000-2004 | 2005-2009 | |||
| Functional status - listing | < 0.001 | |||||
| Unknown | 16999 (24.2) | 7131 (65.9) | 3916 (24.5) | 4279 (21.7) | 1673 (7.0) | |
| ADL with no assistance | 30882 (43.9) | 1527 (14.1) | 8019 (50.2) | 11006 (55.9) | 10330 (43.3) | |
| ADL with some assistance | 16803 (23.9) | 2096 (19.4) | 3772 (23.6) | 4101 (20.8) | 6834 (28.6) | |
| ADL with total assistance | 5693 (8.1) | 75 (0.7) | 263 (1.7) | 317 (1.6) | 5038 (21.1) | |
| Functional status - transplant | < 0.001 | |||||
| Unknown | 22251 (31.6) | 7686 (71) | 6695 (41.9) | 6722 (34.1) | 1148 (4.8) | |
| ADL with no assistance | 23277 (33.1) | 1338 (12.4) | 5959 (37.3) | 8363 (42.5) | 7617 (31.9) | |
| ADL with some assistance | 15434 (21.9) | 1686 (15.6) | 2876 (18.0) | 3986 (20.2) | 6886 (28.8) | |
| ADL with total assistance | 9415 (13.4) | 119 (1.1) | 440 (2.8) | 632 (3.2) | 8224 (34.5) | |
| Medical condition - listing | < 0.001 | |||||
| Unknown | 14394 (20.5) | 83 (0.8) | 76 (0.5) | 5 (0.0) | 14230 (59.6) | |
| ICU | 4549 (6.5) | 1354 (12.5) | 1208 (7.6) | 1339 (6.8) | 648 (2.7) | |
| Hospitalized not in ICU | 5949 (8.5) | 1447 (13.4) | 1615 (10.1) | 1819 (9.2) | 1068 (4.5) | |
| Not Hospitalized | 45485 (64.6) | 7945 (73.4) | 13071 (81.9) | 16540 (84.0) | 7929 (33.2) | |
| Medical condition - transplant | < 0.001 | |||||
| Unknown | 28 (0.0) | 2 (0.0) | 26 (0.2) | |||
| ICU | 10220 (14.5) | 1883 (17.4) | 2824 (17.7) | 2946 (15.0) | 2567 (10.8) | |
| Hospitalized not in ICU | 12076 (17.2) | 2219 (20.5) | 3467 (21.7) | 2613 (13.3) | 3777 (15.8) | |
| Not Hospitalized | 48053 (68.3) | 6725 (62.1) | 9653 (60.4) | 14144 (71.8) | 17531 (73.4) | |
| Recipient characteristics | Total | 5 yr periods | P-value1 | |||
| Period 1 | Period 2 | Period 3 | Period 4 | |||
| 1990-1994 | 1995-1999 | 2000-2004 | 2005-2009 | |||
| Diabetes | < 0.001 | |||||
| Unknown | 11392 (16.2) | 9331 (86.2) | 848 (5.3) | 714 (3.6) | 499 (2.1) | |
| No DM | 47401 (67.4) | 1310 (12.1) | 12792 (80.1) | 15326 (77.8) | 17973 (75.3) | |
| Type 1 DM | 702 (1.0) | 63 (0.3) | 639 (2.7) | |||
| Type 2 DM | 10882 (15.5) | 188 (1.7) | 2330 (14.6) | 3600 (18.3) | 4764 (20.0) | |
| COPD | 0.4 | |||||
| Unknown | 26589 (37.8) | 9359 (86.4) | 1387 (8.7) | 1159 (5.9) | 14684 (61.5) | |
| No | 43172 (61.3) | 1449 (13.4) | 14412 (90.2) | 18280 (92.8) | 9031 (37.8) | |
| Yes | 616 (0.9) | 21 (0.2) | 171 (1.1) | 264 (1.3) | 160 (0.7) | |
| Hypertension | < 0.001 | |||||
| Unknown | 26387 (37.5) | 9412 (86.9) | 1115 (7.0) | 1159 (5.9) | 14701 (61.6) | |
| No | 37629 (53.5) | 1288 (11.9) | 13356 (83.6) | 15664 (79.5) | 7321 (30.7) | |
| Yes | 6361 (9.0) | 129 (1.2) | 1499 (9.4) | 2880 (14.6) | 1853 (7.8) | |
| Angina | 0.5 | |||||
| Unknown | 28259 (40.2) | 9365 (86.5) | 1019 (6.4) | 2103 (10.7) | 15772 (66.1) | |
| No angina | 40926 (58.2) | 1416 (13.1) | 14567 (91.2) | 17081 (86.7) | 7862 (32.9) | |
| Angina | 1192 (1.7) | 48 (0.4) | 384 (2.4) | 519 (2.6) | 241 (1.0) | |
| Dialysis | < 0.001 | |||||
| Unknown | 9682 (13.8) | 8532 (78.8) | 629 (3.9) | 471 (2.4) | 50 (0.2) | |
| No | 57690 (82.0) | 2234 (20.6) | 14789 (92.6) | 18282 (92.8) | 22385 (93.8) | |
| Yes | 3005 (4.3) | 63 (0.6) | 552 (3.5) | 950 (4.8) | 1440 (6.0) | |
Analysis by different periods showed the WL for LT decreased from a median (Q1-Q3) of 151 (45-332) days in period two (1995-2000) to 68 (15-235) days in period four (2005-2009). Similarly, significant factors that affect transplant outcomes of median CIT and WIT decreased in later periods vs early periods of transplantation.
Rejection was treated in 9.5% of patients within 12 months post-transplantation. Primary graft failure (9.3%) and recurrence of hepatitis (9.1%) were the leading identifiable causes of graft failure (Table 6), with 8.2% of LT patients undergoing re-transplantation.
| Recipient characteristics | Total | 5 yr periods | P-value1 | |||
| Period 1 | Period 2 | Period 3 | Period 4 | |||
| 1990-1994 | 1995-1999 | 2000-2004 | 2005-2009 | |||
| Graft status | < 0.0001 | |||||
| Not Failed | 35460 (50.4) | 2879 (26.6) | 6221 (39) | 10361 (52.6) | 15999 (67) | |
| Failed | 34917 (49.6) | 7950 (73.4) | 9749 (61.1) | 9342 (47.4) | 7876 (33) | |
| Treated for rejection ≤ 12 mo | < 0.0001 | |||||
| Unknown | 37869 (53.8) | 10081 (93.1) | 12610 (79) | 8066 (40.9) | 7112 (29.8) | |
| No | 25835 (36.7) | 145 (1.3) | 2000 (12.5) | 9392 (47.7) | 14298 (59.9) | |
| Yes | 6673 (9.5) | 603 (5.6) | 1360 (8.5) | 2245 (11.4) | 2465 (10.3) | |
| Causes of graft failure | ||||||
| Biliary | < 0.001 | |||||
| Unknown | 23875 (68.4) | 6299 (79.2) | 6558 (62.3) | 5989 (64.1) | 5029 (63.9) | |
| No | 10098 (28.9) | 1514 (19) | 2981 (30.6) | 3112 (33.3) | 2491 (31.6) | |
| Yes | 944 (2.7) | 137 (1.7) | 210 (2.1) | 241 (2.6) | 356 (4.5) | |
| Hep de novo | 0.0006 | |||||
| Unknown | 23854 (68.32) | 6329 (79.61) | 6551 (67.2) | 5971 (63.92) | 5003 (63.52) | |
| No | 10968 (31.41) | 1591 (20.01) | 3168 (32.5) | 3356 (35.92) | 2853 (36.22) | |
| Yes | 95 (0.27) | 30 (0.38) | 30 (0.31) | 15 (0.16) | 20 (0.25) | |
| Hep recurrence | 0.9 | |||||
| Unknown | 23670 (67.79) | 6230 (78.36) | 6523 (66.91) | 5929 (63.47) | 4988 (63.33) | |
| No | 8086 (23.16) | 1232 (15.5) | 2387 (24.48) | 2403 (25.72) | 2064 (26.21) | |
| Yes | 3161 (9.05) | 488 (6.14) | 839 (8.61) | 1010 (10.81) | 824 (10.46) | |
| Infection | <0.001 | |||||
| Unknown | 23794 (68.14) | 6213 (78.15) | 6564 (67.33) | 5986 (64.08) | 5031 (63.88) | |
| No | 9429 (27) | 1333 (16.77) | 2690 (27.59) | 2897 (31.01) | 2509 (31.86) | |
| Yes | 1694 (4.85) | 404 (5.08) | 495 (5.08) | 459 (4.91) | 336 (4.27) | |
| Primary graft failure | 0.0013 | |||||
| Unknown | 23289 (66.7) | 5921 (74.48) | 6475 (66.42) | 5901 (63.17) | 4992 (63.38) | |
| No | 8392 (24.03) | 1369 (17.22) | 2432 (24.95) | 2521 (26.99) | 2070 (26.28) | |
| Yes | 3236 (9.27) | 660 (8.3) | 842 (8.64) | 920 (9.85) | 814 (10.34) | |
| Recurrent disease | 0.3 | |||||
| Unknown | 23686 (67.84) | 6177 (77.7) | 6536 (67.04) | 5965 (63.85) | 5008 (63.59) | |
| No | 9548 (27.34) | 1464 (18.42) | 2862 (29.36) | 2890 (30.94) | 2332 (29.61) | |
| Yes | 1683 (4.82) | 309 (3.89) | 351 (3.6) | 487 (5.21) | 536 (6.81) | |
| Acute rejection | 0.6 | |||||
| Unknown | 23854 (68.32) | 6318 (79.47) | 6546 (67.15) | 5969 (63.89) | 5021 (63.75) | |
| No | 10374 (29.71) | 1530 (19.25) | 2999 (30.76) | 3180 (34.04) | 2665 (33.84) | |
| Yes | 689 (1.97) | 102 (1.28) | 204 (2.09) | 193 (2.07) | 190 (2.41) | |
| Chronic rejection | <0.001 | |||||
| Unknown | 26635 (76.28) | 6507 (81.85) | 7441 (76.33) | 6927 (74.15) | 5760 (73.13) | |
| No | 7018 (20.1) | 1124 (14.14) | 1949 (19.99) | 2128 (22.78) | 1817 (23.07) | |
| Yes | 1264 (3.62) | 319 (4.01) | 359 (3.68) | 287 (3.07) | 299 (3.8) | |
| Vascular thrombosis | 0.3 | |||||
| Unknown | 23750 (68.02) | 6231 (78.38) | 6535 (67.03) | 5970 (63.9) | 5014 (63.66) | |
| No | 9635 (27.59) | 1473 (18.53) | 2780 (28.52) | 2964 (31.73) | 2418 (30.7) | |
| Yes | 1532 (4.39) | 246 (3.09) | 434 (4.45) | 408 (4.37) | 444 (5.64) | |
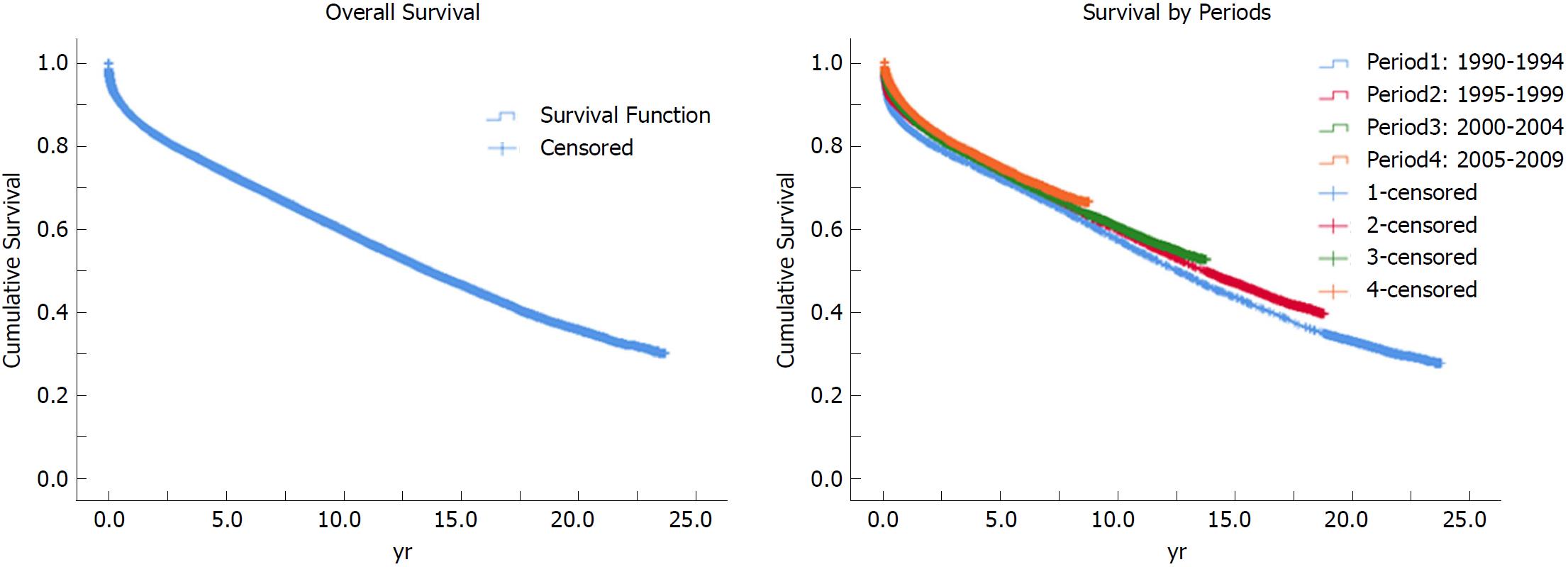
Percent cumulative patient survival at 1, 3, 5, 10, 15 and 20 years is 87.3, 79.4, 73.6, 59.8, 46.7 and 35.9, respectively (Figure 2). Of the identifiable causes, infection and malignancy were the leading causes of death in recipients, accounting for 13% and 12% of deaths, respectively (Table 7).
| Recipient characteristics | Total | 5 yr periods | P-value1 | |||
| Period 1 | Period 2 | Period 3 | Period 4 | |||
| 1990-1994 | 1995-1999 | 2000-2004 | 2005-2009 | |||
| Re-transplantation | < 0.001 | |||||
| No | 64588 (91.8) | 9586 (88.5) | 14350 (89.9) | 18180 (92.3) | 22472 (94.1) | |
| Yes | 5789 (8.2) | 1243 (11.5) | 1620 (10.1) | 1523 (7.7) | 1403 (5.9) | |
| Causes of death | < 0.001 | |||||
| Cardiovascular/cardio | 2893 (9.9) | 718 (10.7) | 783 (9.6) | 735 (9.4) | 657 (10.2) | |
| Cerebrovascular | 647 (2.2) | 177 (2.6) | 191 (2.4) | 146 (1.9) | 133 (2.1) | |
| Graft Failure | 3363 (11.6) | 677 (10.1) | 895 (11) | 948 (12.1) | 843 (13) | |
| Hemorrhage | 825 (2.8) | 237 (3.5) | 213 (2.6) | 222 (2.8) | 153 (2.4) | |
| Infection | 3794 (13) | 1032 (15.4) | 1011 (12.4) | 893 (11.4) | 858 (13.3) | |
| Malignancy | 3477 (12) | 704 (10.5) | 847 (10.4) | 931 (11.9) | 995 (15.4) | |
| Multiorgan failure | 2192 (7.5) | 349 (5.2) | 536 (6.6) | 669 (8.6) | 638 (9.9) | |
| Other | 3378 (11.6) | 629 (9.4) | 919 (11.3) | 1004 (12.9) | 826 (12.8) | |
| Pulmonary | 965 (3.3) | 187 (2.8) | 260 (3.2) | 269 (3.4) | 249 (3.9) | |
| Renal failure | 708 (2.4) | 208 (3.1) | 237 (2.9) | 167 (2.1) | 96 (1.5) | |
| Unknown | 6861 (23.6) | 1788 (26.7) | 2232 (27.5) | 1825 (23.4) | 1016 (15.7) | |
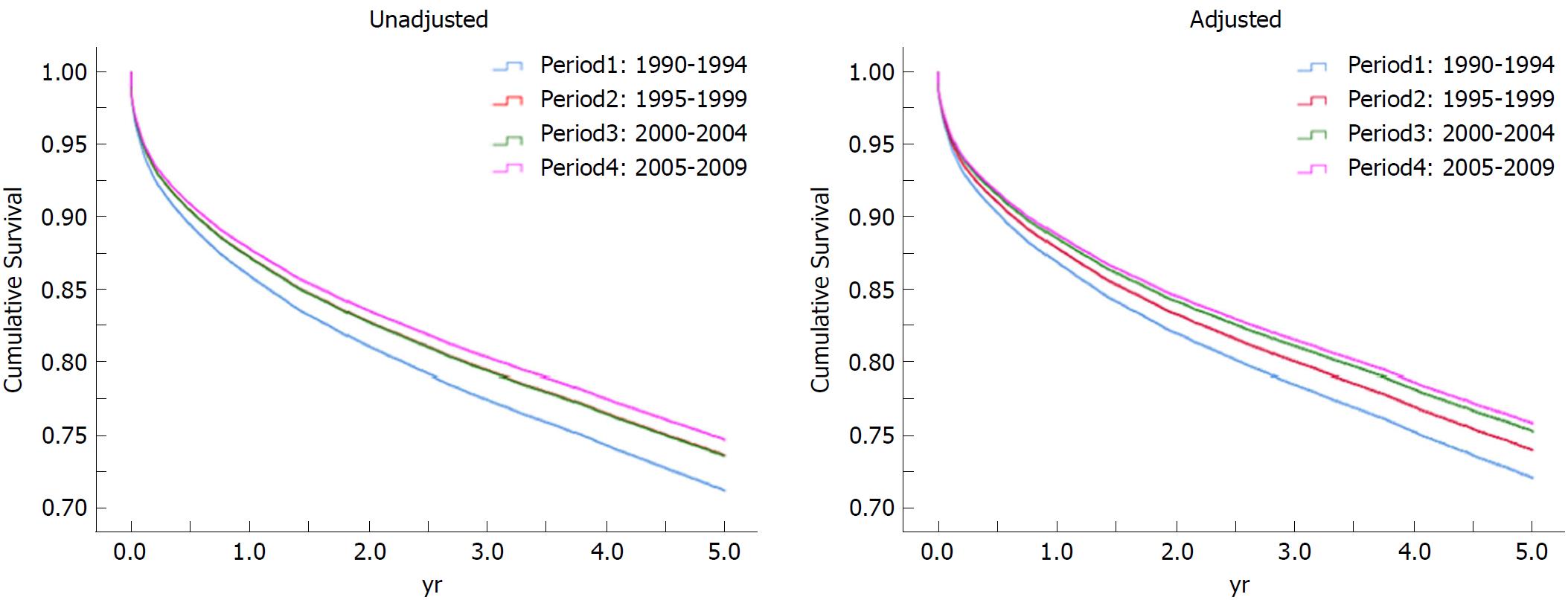
When adjusted for donor age, gender, BMI, ethnicity, causes of death and recipient age, gender, BMI, causes of liver failure, ethnicity/race, functional status, medical condition, CIT, WL, comorbidities of diabetes, COPD, HTN, angina and dialysis, the adjusted hazard ratio of patient and graft survival in period four in comparison to period one was 0.67 (0.62-0.72) and 0.66 (0.62-0.71), respectively. When the analysis was limited to five years of follow-up, the adjusted hazard ratios of patient and graft survival were 0.73 (0.66-0.80) and 0.71 (0.65-0.77), respectively (Figure 3 and Table 8).
| Unadjusted HR (95%CI) | Adjusted HR (95%CI)1 | |
| Over all patient survival | ||
| Period 2 (1995-1999 vs 1990-1994) | 0.90 (0.87-0.93) | 0.90 (0.84-0.94) |
| Period 3 (2000-2004 vs 1990-1994) | 0.87 (0.84-0.90) | 0.76 (0.72-0.81) |
| Period 4 (2005-2009 vs 1990-1994) | 0.83 (0.80-0.86) | 0.67 (0.62-0.72) |
| 5 yr patient survival | ||
| Period 2 (1995-1999 vs 1990-1994) | 0.90 (0.86-0.95) | 0.90 (0.82-0.98) |
| Period 3 (2000-2004 vs 1990-1994) | 0.90 (0.86-0.95) | 0.80 (0.73-0.88) |
| Period 4 (2005-2009 vs 1990-1994) | 0.86 (0.82-0.90) | 0.73 (0.66-0.80) |
| Over all graft survival | ||
| Period 2 (1995-1999 vs 1990-1994) | 0.90 (0.87-0.93) | 0.88 (0.83-0.93) |
| Period 3 (2000-2004 vs 1990-1994) | 0.84 (0.82-0.87) | 0.74 (0.70-0.79) |
| Period 4 (2005-2009 vs 1990-1994) | 0.80 (0.76-0.81) | 0.66 (0.62-0.71) |
| 5 yr graft survival | ||
| Period 2 (1995-1999 vs 1990-1994) | 0.91 (0.87-0.95) | 0.89 (0.82-0.96) |
| Period 3 (2000-2004 vs 1990-1994) | 0.87 (0.84-0.91) | 0.77 (0.71-0.84) |
| Period 4 (2005-2009 vs 1990-1994) | 0.81 (0.76-0.84) | 0.71 (0.65-0.77) |
This study describes the landscape of LT in the United States over a period of 20 years. It is important to understand the impact of changes that have occurred in the United States over this period of time on LT outcomes. Therefore, we analyzed UNOS data on LT performed from 1990 to 2009, followed up to September 2013. Cox proportional hazards regression analysis highlights an interesting fact; over the 20-year period, the graft loss has decreased by 34% and patient survival has improved by 33% after adjusting for donor and recipient age, gender, BMI, ethnicity, CIT, donor cause of death, recipient cause of liver failure, WL, comorbidities of diabetes, chronic obstructive pulmonary disease, hypertension, angina, on dialysis, functional status and medical condition.
In terms of race/ethnicity, white patients were the most common transplant donors and recipients, however our study showed that the contribution from this group has been decreasing while that of other racial/ethnic groups is growing. Hispanic (10.6%) and Asian (1.9%) individuals were the lowest contributors to the liver organ donation pool but were recipients more often (11.2% and 3.9%, respectively). Black donors and recipients showed a different distribution, constituting 13.1% of donors but only 7.7% of recipients. The discrepancy may be at least partly attributable to the higher mortality of blacks candidates while on the LT waitlist relative to that of Hispanic and Asian candidates[1].
Hepatitis C was the foremost identified cause of liver failure in our study, with a 25.0% incidence over the 20 year time period. This underscores the importance of efforts to intensively treat hepatitis C in order to prevent both end-stage liver disease and graft failure after transplantation. Recurrence of hepatitis was the leading cause of graft failure (9.1%) in our study. However, it is important to note that our results mostly reflect patients treated in the era of low-efficacy treatment options for hepatitis C. With the advent of direct-acting antiviral agents[19], we suspect that these trends will change in the future[20,21].
Consistent with the worldwide obesity epidemic, cirrhosis due to non-alcoholic steatohepatitis (NASH) has risen as an indication for LT from 1.2% in 2001 to 9.7% in 2009. Currently, NASH is the third-most common cause for LT in the United States, and it has been projected to become the leading cause by 2025[22]. Our results showed a similar trend, with NASH cirrhosis increasing substantially from 0.06% in 1995-1999 to 5.3% in 2005-2009, coinciding with the increasing obesity rates in the United States and improved understanding of NASH. When we evaluated the causes of end-stage liver disease from 2009 to 2013, the latest available data in the dataset, NASH cirrhosis constituted 8.2%. In this period, NASH remained the third leading cause of liver failure following hepatitis C (22.0%), cirrhosis with HCC (18.9%), and alcoholic cirrhosis (12.3%). NASH-associated liver failure had been the least prevalent identifiable etiology of liver failure in the early 1990s (Table 3), highlighting its significant growth[23].
While the two leading causes of liver failure (hepatitis C and alcoholic cirrhosis) decreased in the second decade of our study, the rates of primary liver malignancy, both alone and in combination with cirrhosis, rose substantially from 1990-1999 to 2000-2009. This increase likely reflects the 2002 UNOS allocation policy assigning exceptional (additional) MELD score points for HCC.
OPTN annual data from 2013 reported that of the 15,027 patients placed on the wait-list, 1,767 (11.8%) died while on the wait-list and 1,223 (8.1%) were too sick to undergo transplantation[1]. With a median WL of 93 d, it is not surprising that we observed a decrease in functional status between the time liver transplant candidates were placed on the list and the time of transplantation. The percentage of transplant candidates requiring no assistance in daily functioning decreased by approximately 10% from the time of listing to the time of transplantation, whereas the percentage of candidates requiring total assistance increased. A similar study by Orman et al[24], using data from the OPTN/UNOS database from 2005 to 2015, likewise reported that the proportion of patients with Karnofsky performance status A (able to carry out normal activity or work) decreased, whereas the proportion with a status of B and C (unable to work plus able (B) or not able (C) to carry out personal care) increased. In patients with cirrhosis, worsening of performance status was associated with increased risk of mortality. Several other studies have previously reported functional status as a predictor of WL and post-transplant mortality[25-27].
Despite recipients’ deteriorating functional status at the time of transplantation, the median LOS for LT in our study was 12 d, which is relatively short considering the complexity of, and complications associated with, the procedure. We also noted a decrease in LOS by about 10 d from the earliest to the latest period. This may reflect improvements in perioperative care, growth in follow-up management experience, ease in outpatient management of immunosuppressive medications, and the recent trend of encouraging earlier hospital discharge.
About 9.5% of transplants experienced rejection within one year of transplantation. Primary graft failure and hepatitis recurrence were the leading causes of graft failure. About 8.2% of patients in this dataset underwent re-transplantation. The percentage of re-transplantations improved over the different time periods, from 11.5% to 5.9%, which probably reflects multifactorial improvement in every aspect of transplantation. The leading causes of mortality in transplant recipients were infection and malignancy, suggesting that aggressive screening for post-transplant malignancies and prompt treatment of infections may be important ways to improve future survival. Since the leading cause of graft failure is the recurrence of hepatitis, we anticipate that implementation of new anti-viral therapeutic regimens before and after transplantation may improve graft survival rates. Reducing obesity is another strategy to potentially improve survival. Not only is obesity a modifiable risk factor for cardiovascular adverse events, which accounted for 9.9% of deaths in our study, but it is also a major contributor to NASH, which is becoming an increasingly common indication of LT. In addition to lifestyle changes and medically-supervised weight loss, the role of metabolic surgery needs to be explored very early in the course of liver failure[28,29].
Although this study was restricted to adults undergoing first-time single whole-organ deceased donor LT, with multi-organ and re-transplanted recipients excluded to improve homogeneity and adjusted for broad changes, there is an intrinsic drawback of using data from a 20 year period. Many advances in LT occurred over this extended period, which likely affected the findings. Dividing the time period arbitrarily into four epochs provided insight into the potential impact of these advances. In order to maintain the homogeneity, we have excluded donation after cardiac death, split liver and living donor recipients, who were directly related to advancements in the field of transplantation at the study period. It is also significant to note that there are a high number of recipients in the ‘unknown’ category, especially in the function condition category, which makes it difficult to draw a confident conclusion. This study also did not address the impact of introducing new immunosuppressive medications on graft and patient survival.
In conclusion, this paper provides an overview of the landscape of LT in the United States from 1990 to 2009 in adults receiving first-time, deceased donor whole-organ LT. The landscape of donors and recipients undergoing transplantations in the United States has changed. Donor age and BMI, and the contribution of racial minorities, have increased. Recipient characteristics have also changed; we are transplanting recipients who are older, more deconditioned, more obese, and with changing causes of cirrhosis. Despite this, the long-term patient survival has improved over time. There is a potential for further improvement by understanding the leading causes of patient death and graft failure in the post-transplant period.
The long-term impacts of clinical advancements and policy interventions over the past two decades on liver transplant outcomes have been poorly studied.
The motivation for such a study is the vast amount of large data that are mandatorily reported from 1989 by all transplant institutions in the United States, from which key observations could be made for future policy changes in transplantation.
The objective of this study was to compare trends in donor/recipient characteristics and outcomes over time. Subjects included 70,377 adult first-time recipients of whole-organ deceased donor liver grafts between 1990 and 2009 who were followed up until September 2013.
Descriptive statistics were used to describe donor/recipient characteristics and transplant outcomes. Statistical comparisons between periods were performed using χ2/Fischer’s Exact test (categorical variables) and t-tests/Mann-Whitney U test (continuous variables). Univariate descriptive statistics/survival data were generated using Kaplan-Meier curves. Cox Proportional Hazards models were used for regression analyses of patient and graft survival.
Mean age (years), body mass index (BMI) (kg/m2), and proportion males were, respectively, 39.1 (± 17.4), 25.9 (± 5.7) and 60.3 for donors, and 51.3 (± 10.5), 27.7 (± 5.6), and 64.4 for recipients. Donor and transplantation rates differed between racial/ethnic groups. Overall survival at 1, 3, 5, 10, 15, and 20 years was 87.3%, 79.4%, 73.6%, 59.8%, 46.7%, and 35.9%, respectively. The 2005-2009 cohort had better patient and graft survival than the 1990-1994 cohort overall [HR 0.67 (0.62-0.72) and 0.66 (0.62-0.71)] and at five years [HR 0.73 (0.66-0.80) and 0.71 (0.65-0.77)].
The key findings were that despite changes in donor quality, recipient characteristics, and declining functional status among transplant recipients, overall patient survival is superior and post-transplant outcomes continue to improve. The long duration that this study encompassed involving the entire United States transplant institutions data has not been previously evaluated.
This is the first study to show that over time, despite transplanting high-risk recipients and utilizing high-risk deceased donors, transplant outcomes are getting better with the accumulation of experience. Future studies involving more specified liver transplant groups (such as transplant for hepatitis vs non-alcoholic steatohepatitis vs Laennec cirrhosis) would give insight into long-term outcomes within the category of end-stage liver disease.
Manuscript source: Unsolicited manuscript
Specialty type: Transplantation
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Tzamaloukas AHH, Quarleri J, Qi S S- Editor: Ji FF L- Editor: Filipodia E- Editor: Yin SY
| 1. | Kim WR, Lake JR, Smith JM, Skeans MA, Schladt DP, Edwards EB, Harper AM, Wainright JL, Snyder JJ, Israni AK. OPTN/SRTR 2013 Annual Data Report: liver. Am J Transplant. 2015;15 Suppl 2:1-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 250] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 2. | Rana A, Gruessner A, Agopian VG, Khalpey Z, Riaz IB, Kaplan B, Halazun KJ, Busuttil RW, Gruessner RW. Survival benefit of solid-organ transplant in the United States. JAMA Surg. 2015;150:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 357] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 3. | Hanto DW, Fishbein TM, Pinson CW, Olthoff KM, Shiffman ML, Punch JD, Goodrich NP. Liver and intestine transplantation: summary analysis, 1994-2003. Am J Transplant. 2005;5:916-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Kim WR, Lake JR, Smith JM, Skeans MA, Schladt DP, Edwards EB, Harper AM, Wainright JL, Snyder JJ, Israni AK. OPTN/SRTR 2015 Annual Data Report: Liver. Am J Transplant. 2017;17 Suppl 1:174-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 234] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 5. | Kim WR, Smith JM, Skeans MA, Schladt DP, Schnitzler MA, Edwards EB, Harper AM, Wainright JL, Snyder JJ, Israni AK. OPTN/SRTR 2012 Annual Data Report: liver. Am J Transplant. 2014;14 Suppl 1:69-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 170] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 6. | Kim WR, Stock PG, Smith JM, Heimbach JK, Skeans MA, Edwards EB, Harper AM, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2011 Annual Data Report: liver. Am J Transplant. 2013;13 Suppl 1:73-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 7. | Jain A, Reyes J, Kashyap R, Dodson SF, Demetris AJ, Ruppert K, Abu-Elmagd K, Marsh W, Madariaga J, Mazariegos G. Long-term survival after liver transplantation in 4,000 consecutive patients at a single center. Ann Surg. 2000;232:490-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 409] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 8. | Mukherjee S, Botha JF, Mukherjee U. Immunosuppression in liver transplantation. Curr Drug Targets. 2009;10:557-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Fritsche L, Dragun D, Neumayer HH, Budde K. Impact of cyclosporine on the development of immunosuppressive therapy. Transplant Proc. 2004;36:130S-134S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Elwir S, Lake J. Current Status of Liver Allocation in the United States. Gastroenterol Hepatol (NY). 2016;12:166-170. [PubMed] |
| 11. | Jurado-García J, Muñoz García-Borruel M, Rodríguez-Perálvarez ML, Ruíz-Cuesta P, Poyato-González A, Barrera-Baena P, Fraga-Rivas E, Costán-Rodero G, Briceño-Delgado J, Montero-Álvarez JL. Impact of MELD Allocation System on Waiting List and Early Post-Liver Transplant Mortality. PLoS One. 2016;11:e0155822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Kwong AJ, Goel A, Mannalithara A, Kim WR. Improved posttransplant mortality after share 35 for liver transplantation. Hepatology. 2018;67:273-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Ravikumar R, Leuvenink H, Friend PJ. Normothermic liver preservation: a new paradigm? Transpl Int. 2015;28:690-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 14. | van Rijn R, Karimian N, Matton APM, Burlage LC, Westerkamp AC, van den Berg AP, de Kleine RHJ, de Boer MT, Lisman T, Porte RJ. Dual hypothermic oxygenated machine perfusion in liver transplants donated after circulatory death. Br J Surg. 2017;104:907-917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 195] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 15. | Wesson RN, Etchill EW, Garonzik-Wang J. Application and interpretation of histocompatibility data in liver transplantation. Curr Opin Organ Transplant. 2017;22:499-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Vandenbroucke JP. Analytic approaches to observational studies with treatment selection bias. JAMA. 2007;297:2077-2078; auhor reply 2078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12:1500-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1101] [Cited by in RCA: 1857] [Article Influence: 168.8] [Reference Citation Analysis (0)] |
| 18. | von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3667] [Cited by in RCA: 6863] [Article Influence: 623.9] [Reference Citation Analysis (0)] |
| 19. | Chen T, Terrault NA. Perspectives on treating hepatitis C infection in the liver transplantation setting. Curr Opin Organ Transplant. 2016;21:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Liao HT, Tan P, Huang JW, Yuan KF. Ledipasvir + Sofosbuvir for Liver Transplant Recipients With Recurrent Hepatitis C: A Systematic Review and Meta-analysis. Transplant Proc. 2017;49:1855-1863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Charlton M, Everson GT, Flamm SL, Kumar P, Landis C, Brown RS Jr, Fried MW, Terrault NA, O’Leary JG, Vargas HE, Kuo A, Schiff E, Sulkowski MS, Gilroy R, Watt KD, Brown K, Kwo P, Pungpapong S, Korenblat KM, Muir AJ, Teperman L, Fontana RJ, Denning J, Arterburn S, Dvory-Sobol H, Brandt-Sarif T, Pang PS, McHutchison JG, Reddy KR, Afdhal N; SOLAR-1 Investigators. Ledipasvir and Sofosbuvir Plus Ribavirin for Treatment of HCV Infection in Patients With Advanced Liver Disease. Gastroenterology. 2015;149:649-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 637] [Cited by in RCA: 633] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 22. | Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 835] [Cited by in RCA: 855] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 23. | Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1211] [Cited by in RCA: 1382] [Article Influence: 138.2] [Reference Citation Analysis (1)] |
| 24. | Orman ES, Ghabril M, Chalasani N. Poor Performance Status Is Associated With Increased Mortality in Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2016;14:1189-1195.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 25. | Lai JC, Feng S, Terrault NA, Lizaola B, Hayssen H, Covinsky K. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant. 2014;14:1870-1879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 384] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 26. | Lai JC, Dodge JL, Sen S, Covinsky K, Feng S. Functional decline in patients with cirrhosis awaiting liver transplantation: Results from the functional assessment in liver transplantation (FrAILT) study. Hepatology. 2016;63:574-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 27. | Jacob M, Copley LP, Lewsey JD, Gimson A, Rela M, van der Meulen JH; UK and Ireland Liver Transplant Audit. Functional status of patients before liver transplantation as a predictor of posttransplant mortality. Transplantation. 2005;80:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Ayloo S, Armstrong J, Hurton S, Molinari M. Obesity and liver transplantation. World J Transplant. 2015;5:95-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (2)] |
| 29. | Heimbach JK, Watt KD, Poterucha JJ, Ziller NF, Cecco SD, Charlton MR, Hay JE, Wiesner RH, Sanchez W, Rosen CB. Combined liver transplantation and gastric sleeve resection for patients with medically complicated obesity and end-stage liver disease. Am J Transplant. 2013;13:363-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 195] [Article Influence: 16.3] [Reference Citation Analysis (0)] |