Published online Mar 18, 2023. doi: 10.5500/wjt.v13.i3.58
Peer-review started: September 15, 2022
First decision: October 27, 2022
Revised: November 11, 2022
Accepted: February 17, 2023
Article in press: February 17, 2023
Published online: March 18, 2023
Processing time: 181 Days and 22.1 Hours
Lung transplantation is the treatment of choice for patients with end-stage lung disease. Currently, just under 5000 lung transplants are performed worldwide annually. However, a major scourge leading to 90-d and 1-year mortality remains primary graft dysfunction. It is a spectrum of lung injury ranging from mild to severe depending on the level of hypoxaemia and lung injury post-transplant. This review aims to provide an in-depth analysis of the epidemiology, patho
Core Tip: Primary graft dysfunction is spectrum of lung injury ranging from mild to severe depending on the level of hypoxaemia and lung injury post-transplant. It has significant bearings on short and long term mortality and morbidity with chronic lung allograft dysfunction playing a major part. While the pathophysiology remains uncertain, it is felt to be a result of ischaemic reperfusion injury. The con
- Citation: Avtaar Singh SS, Das De S, Al-Adhami A, Singh R, Hopkins PM, Curry PA. Primary graft dysfunction following lung transplantation: From pathogenesis to future frontiers. World J Transplant 2023; 13(3): 58-85
- URL: https://www.wjgnet.com/2220-3230/full/v13/i3/58.htm
- DOI: https://dx.doi.org/10.5500/wjt.v13.i3.58
Lung transplantation remains the only definitive treatment for patients with end-stage lung disease due to obstructive lung disease, fibrotic lung disease, vascular lung disease, and other causes (e.g., infection). In highly selective conditions, it may also be used in pulmonary malignancy[1]. The first successful lung transplant procedure was performed in Toronto, 19 years after the first heart transplantation[2]. In the current era, just under 5000 adult lung transplants are performed annually worldwide[3]. Primary graft dysfunction (PGD) after lung transplantation represents a spectrum of lung injury ranging from mild to severe depending on the level of hypoxaemia and lung injury post-transplant[4]. It is characterised by radiographic findings of non-specific pulmonary infiltrates and hypoxaemia. It represents the leading cause of early mortality post-transplantation and phenotypically resembles acute respiratory distress syndrome (ARDS)[4]. Prior to 2005, there was no unified definition for PGD, making it difficult to ascertain a true incidence. Different terms were also used to define the syndrome, indicative of possible pathogeneses, such as ischaemic-reperfusion lung injury, primary non-function of the lung, early graft dysfunction, reperfusion oedema, re-implantation oedema, primary graft failure, post-transplant acute respiratory distress syndrome, acute lung injury and non-cardiogenic pulmonary oedema[5]. There were also significantly variable rates of associated risk factors and mortality indicating the need for a consensus definition[6-8].
It is a major cause of early morbidity and mortality at 90-d (up to 23%) and 1 year (up to 34%)[9]. Diagnosis initially relied on the degree of hypoxaemia but has since been updated to primarily be reliant on chest radiograph findings and the degree of support required[10].
In 2005, the International Society of Heart and Lung Transplantation (ISHLT) published a standardised definition of PGD. In summary, the assessment was performed by evaluating the PaO2/FiO2 (P/F) ratio and the presence of bilateral infiltrates on a chest radiograph. These assessments are carried out at 6 h, 24 h, 48 h, and 72 h post-operatively and should be measured on FiO2 of 1.0 with positive end-expiratory pressure (PEEP) of 5 cmH2O.
Radiographic findings of PGD are relatively non-specific including perihilar ground glass opacities, reticular interstitial, and parenchymal opacities, alongside perivascular thickening.
In 2016, an updated ISHLT statement specified a ‘start’ time for the PGD clock beginning after the removal of the PA cross-clamp of the second lung. The commencement of reperfusion is noted to be T0 with assessments performed at T24, T48, and T72 h as per the 2005 edition. However, the absence of pulmonary oedema on radiographic imaging should be classified as grade 0 regardless of the PaO2/FiO2 ratio. This is highlighted in Table 1 below.
| PGD stage | PaO2/FiO2 ratio (mmHg) | Chest X-ray findings | 2016 update |
| 0 | > 300 | Normal | Any P/F ratio |
| 1 | > 300 | Diffuse allograft infiltration/pulmonary oedema | No changes |
| 2 | 200-300 | Diffuse allograft infiltration/pulmonary oedema | No changes |
| 3 | < 200 | Diffuse allograft infiltration/pulmonary oedema | No changes |
Another pertinent detail is the inclusion of an adjunct for the PaO2/FiO2 ratio due to the high incidence of missing data from the lack of partial pressure of arterial oxygen (PaO2) measurement using oxygen saturations instead (SaO2/FiO2), with different cutoffs of 235 and 315[10].
Despite this, the consensus statement remains a work in progress with several areas requiring further evaluation. For instance, subjects on mechanical ventilation with FiO2 > 50% or requiring inhaled nitric oxide (iNO) beyond T48 are classified as grade 3 PGD alongside patients on mechanical circulatory support. In addition, the definition has not taken into account the debate of single versus double lung transplantation. In an era of increasing austerity in organ allocation, the need to compare outcomes is of utmost importance and has been highlighted. Oto et al[11] identified an increased rate of PGD grade 3 in recipients of single lung transplants compared to bilateral transplants although the authors noted several differences such as protective ventilation strategies in the single lung transplant cohort and earlier extubation times which would invariably affect the PaO2/FiO2 ratio. Their study shed some light on the applicability of the current definition, especially in the single lung transplant cohort. Other potential mechanisms for the higher incidence of PGD in single lung transplant recipients include the admixture of poorly oxygenated blood associated with shunting in the native lung, higher cardiac output via the relatively lower pulmonary vascular resistance of the graft vasculature, and increased relevance of changes in the unilateral transplanted lung. The use of MCS may also be misleading as the role of ‘prophylactic’ extracorporeal membranous oxygenation (ECMO) institutions in high-risk recipients may lead to an overestimation of the true incidence of PGD, which raises questions regarding an interventional-based severity grade[12].
Early reports following the 2005 consensus definition indicated an incidence of around 30% early post-transplant and reduced to just under 20% at T72[8]. However, 10%-20% of these patients contract the severe form, PGD grade 3[8,9]. The higher incidence of PGD reported early on is probably due to the clinical and pathological similarities it shares with reversible pulmonary oedema, ARDS, and transfusion-related acute lung injury (TRALI). Analysis of the United Network for Organ Sharing (UNOS)/ISHLT database (1994-2000) noted a significantly higher all-cause mortality in all PGD vs non-PGD comparisons at 30 d (42.1% vs 6.1%, P < 0.001) and 1 year (64.9% vs 20.4% P < 0.001)[13]. A subsequent study by Diamond et al using the 2005 definition highlighted an overall PGD grade 3 rate of 30.8%, reducing to 16.8% after the exclusion of PGD Grade 3 classifications before T48[9]. Christie et al[4] noted increasing mortality with each grade of PGD. This ordinal pattern was present at all time points following the transplant (T24, T48, T72). PGD Grade 3 had the highest mortality which was also replicated in an analysis of biomarkers of insult severity. Many studies have used PGD grade 3 as a dichotomous outcome when discriminating between PGD and non-PGD due to the more distinct features i.e., prolonged mechanical circulatory support, mechanical ventilation with FiO2 > 50%, iNO or iEPO usage, and PaO2/FiO2 ratio < 200, leaving little room for ambiguity.
Given the heterogeneity in the locoregional donor, recipient, and procedural characteristics, ascertaining the true epidemiology of the lesser grades of PGD is slightly more challenging and remains variable. Another important consideration for PGD is the link with chronic lung allograft dysfunction (CLAD). Table 2 shows the phenotypes of CLAD.
| Phenotypes | Spirometry changes | CT opacities |
| CLAD | Persistent ≥ 20% decline in FEV1 (based on 2 FEV1 values ≥3 wk apart) compared to baseline | |
| BOS | CLAD and obstruction (FEV1/FVC < 0.7) | No |
| RAS | CLAD and restriction (≥ 10% decline in TLC from baseline) | Yes |
| Mixed phenotype | CLAD with obstruction and restriction | Yes |
| Undefined phenotype | CLAD with obstruction and/or restriction | Yes/No |
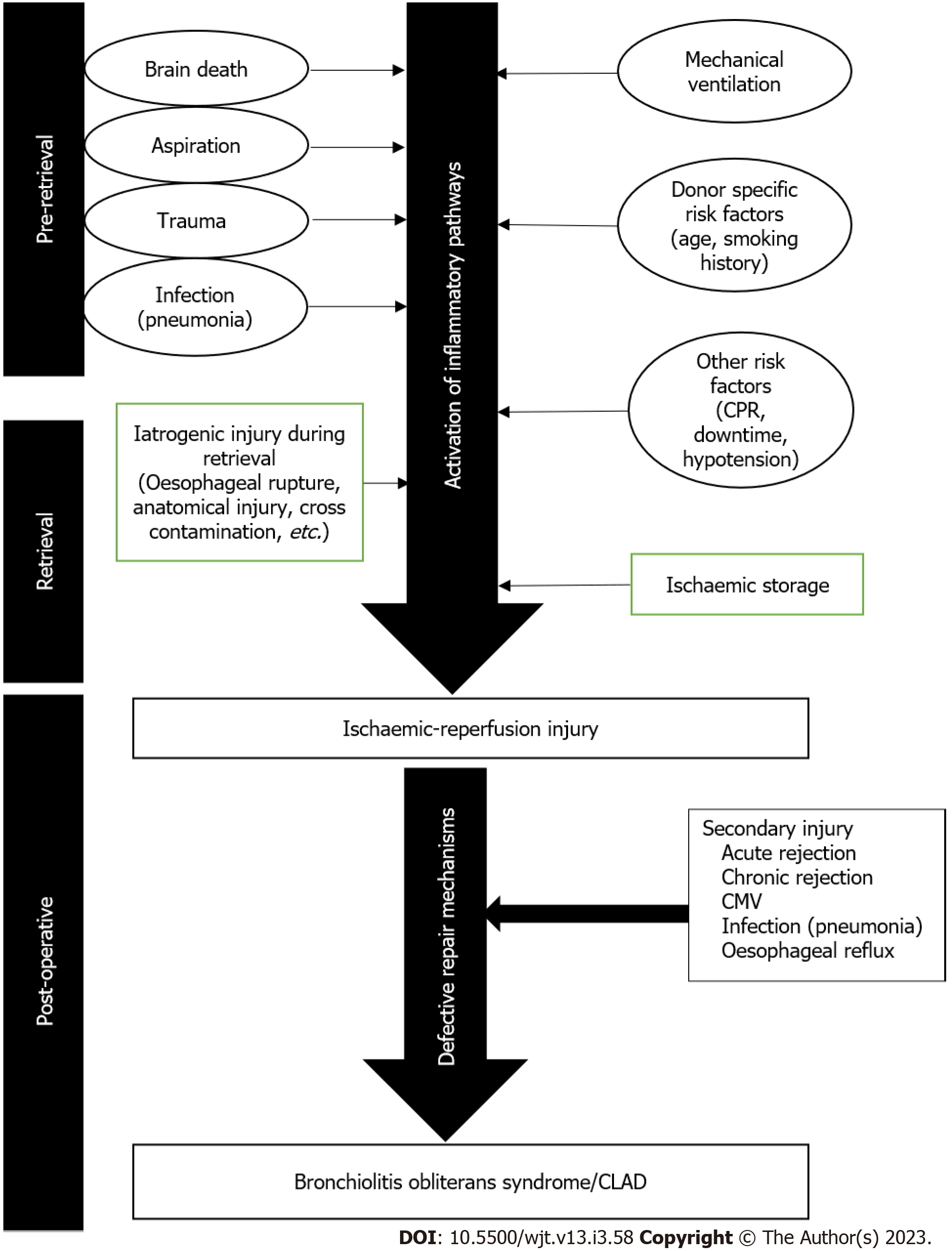
Bronchiolitis Obliterans syndrome was the term initially coined to describe allograft dysfunction occurring after lung transplantation. Ischaemic reperfusion injury plays a major part in the pathophysiology as highlighted in Figure 1. This syndrome has since undergone several revisions and refinements with the most recent definition using the umbrella term CLAD highlighting a series of phenotypes post transplantation[14].
According to the 2019 consensus statement, CLAD is defined as a substantial and persistent decline [≥ 20% in measured forced expiratory volume in one second (FEV1)] in the baseline. This is broadly classified into obstructive ventilatory pattern, restrictive, or mixed[14].
The tipping point is the drop in FEV1 with/without a change in the forced vital capacity. Secondary causes should be ruled out such as surgical complications, infections, rejection, and mechanical obstructions (effusions, stenosis, tumours, etc)[14]. After investigating, managing, and ruling out the secondary causes, there should be at least 3 wk between the first and 2nd FEV1 readings that indicate the reduction (≥ 20%). The staging of CLAD is also shown in Table 3.
| CLAD stage | Spirometric values |
| CLAD 0 | Current FEV1 > 80% baseline |
| CLAD 1 | Current FEV1 > 65%-80% baseline |
| CLAD 2 | Current FEV1 > 50%-65% baseline |
| CLAD 3 | Current FEV1> 35%-50% baseline |
| CLAD 4 | Current FEV1 ≤ 35% baseline |
The exact pathophysiology behind PGD remains unclear but is thought to be a summation of multiple insults that occur during the procurement, storage, and implantation of the lung. Ischaemic-reperfusion injury (IRI) is thought to be a major contributor to the pathophysiology. Native lungs have a dual vascular supply via bronchial vessels and pulmonary circulation, alongside available oxygen from alveolar ventilation. The pathogenic mechanism of IRI in the lungs, therefore, differs from other end organs which are often rendered ischaemic on cessation of blood flow[15]. From an anatomical perspective, there is a change in the vasculature of the lungs post-transplantation. In the native lungs, the airways are supplied by a dual circulation derived from the bronchial arteries and the pulmonary artery[16]. The post-transplant lung has the pulmonary artery circulation surgically restored but the bronchial anastomosis and distal airways may be exquisitely susceptible to further ischemia and hypoxic injury due to the loss of these bronchial arteries.
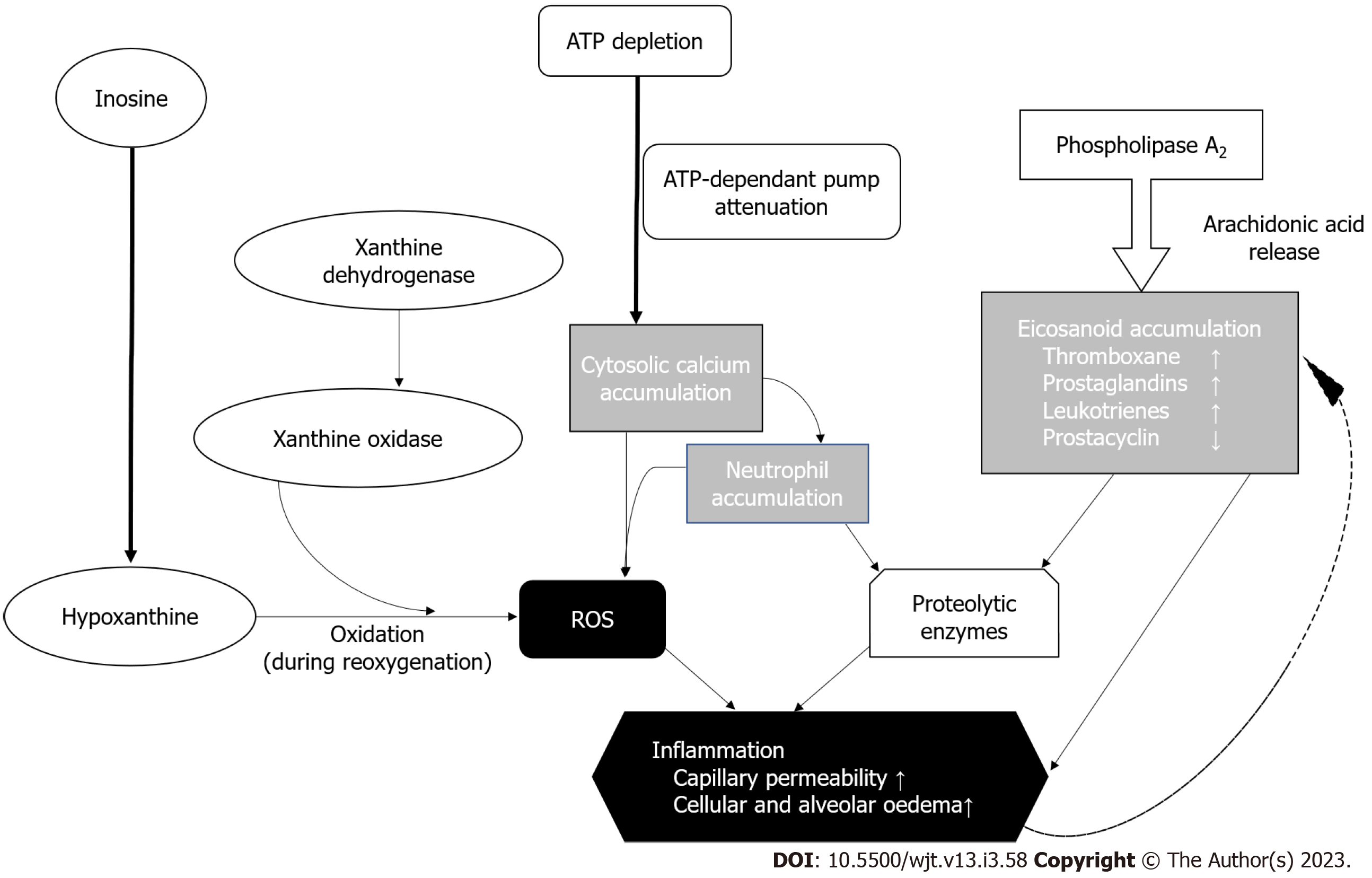
On a molecular level, IRI is driven but the formation of reactive oxygen species (ROS) (Figure 2). Traditionally, the lung is hypothermically stored to reduce the metabolic oxygen demand - hence reducing the rate of biochemical reactions which results in attenuated degradation of cellular components[17]. Adenosine triphosphate (ATP) stores however continuously deplete, which inactivates the ATP-dependent membrane pumps[15]. This causes accumulation of cytosolic calcium alongside activation of inflammatory pathways causing eicosanoid formation and ROS generation - eventually leading to more inflammation and spiraling escalation of inflammation leading to cell death[18]. IRI also induces necroptosis and apoptosis in laboratory-based studies, which also contribute to ROS formulation via accumulation of cytosolic calcium leading to further necrosis of pulmonary epithelial cells[19,20].
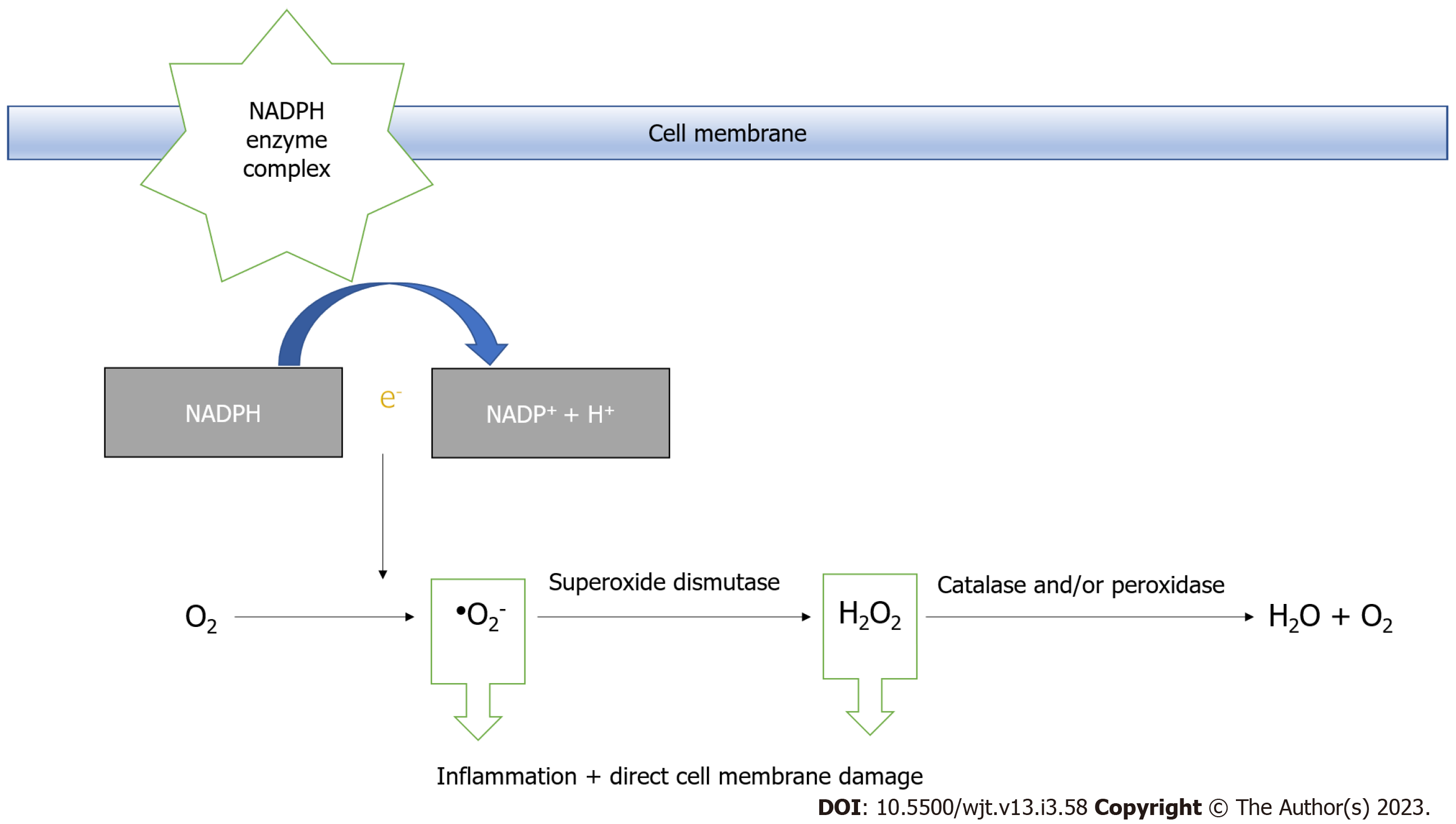
During aerobic metabolism, ATP is converted to urea and xanthine by xanthine dehydrogenase. Xanthine dehydrogenase either undergoes reversible sulfhydryl oxidation or irreversible proteolytic modification to form xanthine oxidase[21,22]. This irreversible modification occurs in IRI and breaks down hypoxanthine to ROS during rapid reoxygenation. In addition to the pathway illustrated in Figure 2, ROS is also generated by the NADPH (Nicotinamide Adenine Dinucleotide Phosphate) oxidase system. It is present on the membrane surfaces of phagocytic cells, where NADPH is oxidised to NADP+, releasing an electron into the phagocytic vacuole, where oxygen is reduced to superoxide anions in large quantities[21]. It plays a major role in pathogen killing but may also be aggravated inflammatory diseases[21]. The superoxide anion, hydrogen peroxide, and hydroxyl radical form part of the family of ROS, which damage cellular membranes by lipid peroxidation[22] as shown in Figure 3.
The inflammatory pathway is activated by ROS generation which causes a release of proinflammatory cytokines by macrophages. Neutrophils and lymphocytes are therefore recruited to the lung and extravasate into tissues due to increased vascular permeability as a sequalae of the acute inflammatory response[23]. The macrophages and recruited neutrophils generate more ROS alongside non-specific lysis proteins like proteolytic enzymes, lysozymes, and lactoferrin which contribute to cell damage[23]. Leukocytes can also mediate IRI through the elaboration of elastases and proteases, production of inflammatory cytokines, and neutrophil aggregation causing plugging of capillaries and no-reflow phenomena with endothelial damage[24].
In addition to the activation of inflammatory pathways via neutrophil activation, the pulmonary endothelium also contributes to the pathophysiology of PGD. TNF-α and IL-1β are non-specific pro-inflammatory cytokines that promote molecule adhesion to the endothelial surface. Several processes have been implicated in the propagation of this chemotaxis including upregulation of receptors for advanced glycation end products[25], platelet aggregation[26], and increased levels of intercellular adhesion molecule-1 (ICAM-1)[27]. These promote the migration of macrophages and polymorphonuclear cells into the air spaces in the lungs.
During LIRI, the processes above occur resulting in the extravascular displacement of leukocytes through chemotaxis-induced migration. The reperfusion causes cell depolarization which disrupts the homeostatic mechanisms within the endothelium. Pulmonary artery pressure during reperfusion was shown to have a significant effect on the endothelial wall with an increased likelihood of developing Grade 3 PGD in patients with higher PA pressures in a cohort of patients with idiopathic pulmonary fibrosis (38.5 ± 16.3 mmHg vs 29.6 ± 11.5 mmHg for patients without PGD [mean difference, 8.9 mmHg (95%CI: 3.6 to 14.2); P = 0.001][28]. Recipient leukocyte depletion attenuated this effect, implicating the circulating host leukocytes in the pathophysiology as demonstrated by Schnickel et al[24]. In addition, some studies have identified the role of donor innate lymphoid cell subsets with some protective against PGD and some associated with PGD[29].
Other studies have also shown that increased left-sided cardiac pressures have a detrimental effect and increase the risk of PGD development, further inculpating the role of endothelial integrity in the pathophysiology. Porteous et al[30] noted that preoperative diastolic dysfunction measured by echocardiography increased the risk of Grade 3 PGD. These patients had significantly higher mPAP and pulmonary vascular resistance. Li et al[31] noted a similar finding using LVEDP and mPCWP as a surrogate for increased left-sided pressures. The Toronto group highlighted a higher incidence of ECLS use post-transplant in patients with preoperative signs of diastolic dysfunction[32].
Multiple risk factors have been identified through various studies since the PGD consensus definition. We have identified donor, recipient, and procedural variables as follows.
Donor history of cigarette smoking is perhaps the best-reported risk factor of PGD. It has been associated with PGD in multiple studies[8,9,33]. In a large North American multicenter study, a positive donor smoking history was independently associated with PGD[9]. In addition to PGD, the United Kingdom national data revealed inferior post-transplantation survival showed inferior survival by donor history of smoking at 30 and 90 d after transplantation and sustained for up to 3 years post-transplant[34]. In addition, these patients also had a significantly higher perioperative morbidity with longer ICU and in-hospital length of stay[34]. Despite no mention of PGD in the study, one can infer that a high proportion of early morbidity and mortality would be directly related to PGD. The authors however did note that the survival benefit to recipients of smokers’ lungs compared to those remaining on the waitlist and waiting for a non-smoking donor[34].
The exact role of smoking in the pathophysiology of PGD remains elusive. Ware et al noted that smo
Oxidative injury with the generation of ROS may be potentiated by donor exposure to cigarette smoke[37]. Lipid peroxidation is a ROS-mediated chain of reactions that, once initiated, results in an oxidative deterioration of polyunsaturated lipids[38]. Biomarkers of lipid peroxidation such as thiobarbituric acid reacting substances are increased in bronchoalveolar lavage fluid of smokers (both acute and chronic) compared to non-smoking controls[39]. It is therefore conceivable that the lungs of smokers are more susceptible to IRI due to the increased accumulation of the byproducts of lipid peroxidation[37].
Chronic alcohol consumption has been linked with an increased risk of developing ARDS based on a metanalysis of 17 case-controlled and cohort studies with a total of 177674 patients[40]. Extending this to lungs for transplantation was reproducible in one study which showed 8.7 times greater odds of developing severe PGD following lung transplant compared to recipients whose donors did not consume alcohol after controlling for other variables (P = 0.0190)[41]. However, there was no difference in risk of developing PGD in recipients of donors with moderate alcohol consumption as compared to donors with no alcohol use[41]. The same study also showed worse acute lung injury post-transplant with poorer gas exchange and a trend of poorer survival following transplantation when lungs from heavy alcohol consumers were implanted[41]. Heavy alcohol use in donors was also related to a higher rate of PGD in another retrospective single-center study[42]. Heavy intake of alcohol is also linked with other high-risk behaviors including road traffic accidents, violence[43,44], and self-injuries including suicide attempts[45], thus, at risk of trauma and subsequent brain death, representing a significant proportion of donors. The exact pathophysiology of this is speculative but is probably a combination of poor mucociliary clearance, a degree of epithelial dysfunction, and impaired immune responses in the alveolar spaces which increases the susceptibility to oxidative stressors[41]. There is also evidence that chronic alcohol abuse impairs surfactant production in pneumocytes, impairing the epithelial barrier function[46]. There is also some evidence of glutathione depletion which increases susceptibility to oxidative stressors such as reperfusion injury post-cold ischaemic storage[47]. Animal models have been utilized to demonstrate the effects of modulating ischaemia-reperfusion. In a rat tracheal transplant model, alcohol intake increased inflammatory signaling with transforming growth factor-beta (TGF-β) and interleukin 13 (IL-13), which may induce fibrosis[48].
Donor age is a significant risk factor for PGD in other organs such as the heart[49], kidney, liver, and pancreas[50]. Analyzing the American Organ Procurement and Transplantation Network database, Baldwin et al[51] noted that there was a higher risk of graft failure at 1 year when using lungs from donors < 18 years old and > 65 years, and echoed by De Perrot[52]. This was not reproduced in other studies by the Pittsburgh group[53] and the Hannover group[54]. The 2016 ISHLT Working Group on Primary Lung Graft Dysfunction Report noted that recent data suggest the age-related risk of PGD is lower than previously believed and restricted to the extremes of ages[8].
In the 1990s, 2 case reports were published describing the impact of donor-related pulmonary emboli diagnosed by lung biopsy after transplantation from fat emboli following multiple fractures in a donor for an RTA[55] and cerebral emboli[56] respectively. Oto et al showed a significant association between donor pulmonary embolism and PGD in lungs[57]. On multivariate analysis, PGD rates following lung transplantation were 20.6-fold (P = 0.0002) higher with fat emboli and 4.8-fold (P = 0.02) with pulmonary embolism compared with those who received lungs without pulmonary embolism[57]. In a subsequent study by the same authors, emboli were diagnosed using retrograde flushing of the pulmonary arteries at the time of retrieval. They noted that donor death due to trauma with fractures and a smoking history of more than 20 pack-years were significant risk factors for pulmonary embolism[58]. The pathophysiology suggested by the authors is the failure to correct the ventilation-perfusion mismatch by the denervated lung in addition to a localized inflammatory response which may aggravate the IRI. They noted increased interstitial infiltration and opacities on chest radiographs in up to 93% of lungs post-transplantation. However, studies examining donor causes of death have shown no differences between donors with traumatic causes of death vs non-traumatic[59].
A likely issue that may account for PGD includes lung size mismatch, or specifically undersizing[60]. This is thought to be due to the changes in the pulmonary vasculature and potentially detrimental tidal volumes during mechanical ventilation. The undersized pulmonary vasculature provides increased resistance and thus a higher pulmonary artery pressure at reperfusion which is thought to be a contributing factor. The Lung Transplant Outcome Group noted that donor undersizing (Donor Lung < Recipient Lung) rather than donor sex or race conferred an increased risk as male lungs were generally larger than females[8,60]. A quotient called the predicted donor-recipient total lung capacity ratio (donor pTLC/recipient pTLC) of < 1 was noted as a risk factor for PGD post-bilateral lung trans
The causative pathology of lung disease has also been implicated as a risk factor for PGD. Diamond et al[9] noted that patients with pre-operative sarcoidosis (OR: 2.5; 95%CI: 1.1 to 5.6; P = 0.03) or pulmonary arterial hypertension (OR: 3.5; 95%CI: 1.6 to 7.7; P = 0.002) were at an increased risk of developing PGD. Pulmonary hypertension is linked to right ventricular dysfunction and the sudden peripheral vascular resistance reduction in the transplanted lungs may result in endothelial shear stressors, further worsening the reperfusion injury. However, similar findings were not noted in patients with cystic fibrosis regardless of pulmonary hypertension based on ISHLT database findings, indicating a disease process rather than the presence of elevated pulmonary pressures may be causative[62]. Fang et al[28] noted that secondary pulmonary hypertension in patients with idiopathic pulmonary fibrosis (IPF) was independently associated with PGD development. This could be explained by the progressive nature of the disease and implicate the role of vasoactive mediators (e.g., Endothelin-1, fibroblast growth factor) which have been linked with the pathogenesis of IPF[63]. The restrictive pattern of lung disease may also result in smaller-than-predicted total lung capacity. This causes progressive changes to the chest wall which may result in poorer graft function due to the irreversible mechanics of remodeling within the recipient’s chest wall to accommodate the ‘shrinking’ lung[64,65].
A raised body mass index (BMI) was previously shown to be a risk factor for prolonged ICU length of stay and mortality in lung transplant recipients[66,67]. Diamond et al[9] also conducted a large cohort study and showed a direct relationship between a raised BMI and PGD. Specifically, the odds ratio for developing PGD increased to 1.8 for BMI 25-30 and 2.3 for BMI > 30.
This may be directly related to the technical surgical challenges in obese recipients alongside the inflammatory milieu produced during IR. Leptin is a protein encoded by the obese gene located on human chromosome 7[68]. It is classically noted to be a hormone due to its effects in regulating food intake and energy exposure. In addition, it is also a member of the type 1 cytokine family. Other conditions such as type II diabetes are associated with hyperleptinemia and acquired resistance to signaling through the leptin receptors[69]. Serum levels of leptin are directly correlated to BMI and are increased in sepsis and ARDS, suggesting a pathogenic contribution[70]. A study by Jain et al[71] showed that lung leptin levels were increased in mice with acute lung injury. The Lung Transplant Outcomes Group then published data on over 500 patients who underwent transplantation for either COPD or ILD indicating a higher risk for PGD in patients with higher plasma leptin levels. In their study, the graphical depiction showed a stark increase in the risk of PGD with an inflection point noted just above 10 ng/mL. The associations between leptin and PGD were stronger when the cardiopulmonary bypass was not used[72]. The role of other modulators such as resistin and adiponectin have been postulated in the past from work done in animal models but have yet to be translated into clinical practice[73].
In humans, two pleurae (visceral and parietal) line the thoracic cavity and lungs. The pleurae are serous membranes that fold back onto themselves to form a two-layered membranous structure with a small amount of pleural fluid which plays a role in transmitting movements of the chest wall to the lungs during respiration[74]. Pleural symphysis or pleurodesis is a commonly performed procedure for the treatment of pneumothoraces and effusions. The team at the University of Pittsburgh noted that patients with prior pleurodesis had the highest incidence of severe PGD, alongside other early post-operative complications such as re-exploration for bleeding, phrenic nerve injury, chylothorax, and respiratory complications[75]. Although the post-operative rate of other complications was also increased in patients with prior thoracic surgery, the increased rate of PGD was specific to pleurodesis alone. On multivariate analysis, they noted an increased risk of death post-transplant in patients with prolonged cardiopulmonary bypass (CPB) time, chemical pleurodesis, and high transfusion requirements (> 20 units). The technical challenges intraoperatively due to the multiple adhesions post pleurodesis, combined with heparinization during CPB contribute to the high transfusion requirements, making elucidation of the exact pathophysiological mechanism difficult. The Harefield group noted that recipients with pleural disease defined as pleural thickening, plaque, or fibrosis either confirmed on a computed tomographic scan during assessment for transplantation or detected intraoperatively at the time of the transplantation, had worse 3-mo mortality and a trend toward poorer 30-d mortality despite similar CPB usage, albeit with significantly higher transfusion rates[76].
The use of extracorporeal life support (ECLS) preoperatively increases the risk of PGD postoperatively[77,78]. While the use of ECMO for treatment for severe PGD is well established, its use preoperatively as a bridging strategy to transplantation has been condemned in the past due to poor outcomes[79]. Analysis of the UNOS database in 2012 identified that only 1.3% of patients transplanted were bridged with ECMO[80]. Unadjusted survival of these patients was significantly worse compared to non-bridged recipients[81]. The increased acuity and deconditioning of these recipients due to the inability to mobilize and multiple pre-transplant interventions potentially creates a hostile environment for the donor graft[82]. It should be noted that the current devices used for ECLS last longer and are less prone to malfunctioning (improved oxygenators and circuits)[83]. Center-specific outcomes also denote improved survival with ECLS pre-transplant in larger, more experienced centers with similar survival outcomes to non-bridged patients reported, supporting its use in the current era[84,85].
Lung transplantation can be performed with or without ECLS. CPB was the traditional method for intraoperative cardiopulmonary support to permit low-pressure reperfusion in cases of severe pulmonary arterial hypertension, failing oxygenation, poor hemodynamic tolerance, or acute bleeding from major vessels[86]. Caveats of the use of CPB include its proinflammatory effects and the associated complications of full systemic heparinization[86]. Other techniques that are now more commonly used to avoid the use of CPB include transplantations with the use of ECMO support or transplantations with single lung ventilation without ECLS[86].
In most institutions worldwide, ECLS is reserved for patients who cannot tolerate single-lung ventilation, complex patients, or those who become haemodynamically unstable during the procedure. ECMO has, by and large, replaced CPB in this setting. CPB has been linked with the activation of cytokines, leukocytes, and the complement cascade alongside a higher transfusion requirement and postoperative coagulopathy probably secondary to the higher levels of anticoagulation required[62,86]. Several reasons have been cited such as the increased blood-activating surfaces present in the CPB tubing, venous reservoir, oxygenator, and cardiotomy compared to ECMO circuits which are closed circuits, without venous reservoirs or additional cardiotomy suction thereby eliminating the air-liquid interface and avoiding blood being washed and returned[87]. Newer ECMO systems have increased biocompatibility by using heparin-coated tubing with polymer-coated centrifugal pumps and oxygenators, permitting prolonged usage pre and post-transplant with limited metabolic derangement[87]. When CPB was used, patients had longer intubation times, higher rates of PGD, and reduced survival[62]. However, it should be noted that the use of CPB is also often linked with adverse donor-recipient characteristics, which may not directly infer causality. A metanalysis comparing the use of ECMO and CPB highlighted that CPB was more likely used in patients with severe pulmonary hypertension, increased risk of intraoperative bleeding, and combined cardiac defects[86].
Moreover, in a recent Austrian study, Hoetzenecker et al[88] compared bilateral lung transplantations performed without ECLS with those performed with the routine use of intraoperative ECMO support. The authors favoured central venoarterial ECMO intraoperatively and converted it to peripheral femoral venoarterial cannulation postoperatively if this was deemed necessary. They reported improved 1-, 3-, and 5-year survival compared to non-ECMO patients (91% vs 82%, 85% vs 76%, and 80% vs 74%; log-rank P = 0.041) along with a trend towards a reduced incidence of grade 2 and 3 PGD in the intraoperative ECMO group. The authors attribute these findings to the ability to ventilate patients with low tidal volumes and low ventilation pressures (protective ventilation conditions) during the implantation of the second lung, hemodynamic stability even with extensive heart manipulation, and shorter operation times by reducing unnecessary hands-off periods.
Ischaemic time has been shown to be a risk factor for PGD in multiple studies for other solid organs[89-91]. The link between ischaemic time and PGD in the lungs, however, is slightly less prominent. Multiple studies have published conflicting findings in this respect[92-95]. One reason for this could be due to the variability in reporting ischaemic times across the different centers[96]. Thabut et al[92] noted that prolonged ischaemic time led to increased tissue oedema and poorer 30-d survival. Snell et al[97] noted that in their series in the early 90s, ischaemic times exceeding 5 h led to poorer survival (P = 0.02, hazard ratio: 3.44, 95%CI: 1.12 to 9.8). Gammie showed no differences in early survival and late (3-year) survival with increased ischaemic time[98]. A recent Swedish study noted that there were early differences in outcomes with increasing ischaemic times alongside increased mortality of up to 24% within 5 years for every 2 h of ischaemic time increment[99].
The lungs also have shown to be less affected by ischaemia compared to the other organs as noted by the good outcomes of lungs procured in donation after circulatory death (DCD)[100]. There is also a probable threshold ischaemic time for the development of ischaemic reperfusion injury which is dependent on both warm and cold ischaemic times. Conflicting results may therefore be attributed to the variable definitions of ischaemic time which is even more variable in the DCD cohort[101]. Although DCD donors (Maastricht Category III) are not affected by the catecholamine surge and inflammatory milieu after brainstem death, they are exposed to several different ischaemic insults. After the withdrawal of life-sustaining therapy (WLST) during the process of procurement, the donor becomes hypoxemic and hypercarbic. The functional warm ischemia time ensues after WLST when the systolic blood pressure is less than 50 mmHg, with some centers also utilizing the oxygen saturations < 70% as a cut-off. There is then a universal stand-off period of 5 min before organ retrieval commencement[102]. This warm ischemia is associated with intracellular acidosis, activation of the Na+/H+ exchanger causing accumulation of intracellular Ca2+ worsening IRI. Reducing warm ischemia remains challenging during DCD lung procurement.
A bronchoscopic examination is usually performed and any aspiration is suctioned while the retrieval is happening. As the perfusion fluid is delivered, cyclic ventilation is performed to evenly distribute the preservation fluid followed by inflation to about 50%-75% of the lung capacity and a retrograde flush. Rat model studies have shown that inflation of the lungs with oxygenated air ensures the integrity of pulmonary surfactant alongside improved epithelial fluid transport[103,104]. Healey et al[105] recently published a case series of uncontrolled DCD donors and noted no PGD in their cohort, probably signifying a degree of tolerance towards ischaemia. Another study showed an increased incidence of PGD early on, but similar rates at 6 h onwards between DCD and DBD lungs[106]. The Harefield group had a similar finding with an increased PGD incidence in their propensity-matched analysis in the DCD group[107]. One reason to explain this could be the lack of assessments and optimization in DCD donors compared to DBD donors[108]. A metanalysis from 2015 showed similar outcomes from DCD and DBD lung donations[109]. It should be noted that DCD lungs remain an underused resource and are still growing with preservation techniques for other organs such as the thoracoabdominal normothermic regional perfusion and ex-vivo lung perfusion (EVLP) postulated to increase its use[110].
A metanalysis highlighted polytransfusion of blood products to be a risk factor for PGD[62]. Although the exact relationship remains unclear, it is probably a combination of increased technical difficulty which invariably results in increased transfusion requirement[111], a degree of TRALI[112], a result of a significant IRI resulting in an ARDS type presentation or systemic inflammatory response (SIRS)[25]. Each of these has been implicated and may play a role in accentuating the severity of IRI. In addition, the need for transfusion shares some co-linearity with other risk factors such as primary pulmonary hypertension and the use of CPB[62]. Therefore, it is difficult to elucidate whether this occurs due to causality or as a response to the abovementioned insults. The transfusion of red blood cells (RBC) alone is associated with increased mortality in a study in Zurich[113]. RBC transfusion is also associated with an increased amount of soluble receptors for advanced glycation end products (sRAGE), which is a marker of alveolar epithelial injury[25]. On the other hand, intraoperative use of fresh frozen plasma has been shown to increase mortality[113], albeit these were more often used in sicker patients[114].
In 2018, a multicenter group published a unique study that highlighted an increased risk of PGD in lungs reperfused between 0400-0759[115]. Following the pilot study of 25 patients, a larger retrospective cohort study of 563 patients revealed a significantly increased risk of PGD (OR: 1.12, 95%CI: 1.03 to 1.21; P = 0.01) on univariate binary logistic regression and OR: 1.299, 95%CI: 1.004 to 1.681; P = 0.046 on multivariable binomial logistic regression. There were no differences in ischaemic times or operation lengths although they could not directly account for operator fatigue. The authors attributed the results to ‘internal desynchrony between donor and recipient as a result of organ preservation’ The circadian clock oscillations were thought to play a part in this and using a mouse model, the authors showed delayed oscillation for lungs that were kept in cold storage compared to lungs maintained at 37 ℃. The authors highlighted the role of the clock protein, REV-ERBα which has previously been studied for its role in regulating neuroinflammation[116], and poorer outcomes following cardiac surgery[117]. We currently know that the nuclear receptor REV-ERBα is involved in the cell-autonomous mammalian circadian transcriptional/translational feedback loops as transcriptional repressors and hence indirectly mediates regulation of metabolic, neuronal, and inflammatory functions including bile acid metabolism, lipid metabolism, and production of inflammatory cytokines[118]. In an animal model, Cunningham et al[115] noted that in a panel of PGD biomarker gene expression was repressed by a synthetic REV-ERBα ligand. Conversely, 6 out of 7 biomarkers in this panel however demonstrated increased expression of macrophages in REV-ERBα knockout mice[115]. Though it is of interest, its current role remains limited to experimental research, although the introduction of normothermic ex-vivo perfusion devices may change the timing of the surgical aspect of transplantation.
The association between PGD and re-transplantation is not well established with some centers highlighting no differences in the rates of PGD compared to primary transplants[119,120], but others generally highlighting poorer survival outcomes especially if used in patients with severe PGD at the primary transplant[119,121,122]. Outcomes were also similar in single or bilateral lung transplantation during the re-transplant irrespective of the primary transplant[123]. The true incidence of PGD in the re-transplant setting is perhaps under-represented due to the higher incidence of secondary causes which include chest wall bleeding due to significant adhesions, coagulopathy, and infections, all of which preclude a diagnosis of PGD[119].
Treatment of PGD is primarily supportive with the exception of severe PGD which requires circulatory support (ECMO). The challenge with PGD is usually establishing a diagnosis as mild and moderate PGD (Grade II and III) may often be mistaken for other conditions such as pulmonary oedema, TRALI, or superimposed infection. Treatment management is therefore directed at managing the ARDS-type picture.
The target tidal volume for low tidal volume ventilation (LTVV) is usually 4-8 mL/kg calculated based on predicted body weight (PBW)[124]. This is calculated using the following formula for
PBW males = 50 + 0.91 (centimeters of height - 152.4)
PBW females = 45.5 + 0.91 (centimeters of height - 152.4)
It is performed using a volume-limited assist control mode and the plateau pressure is usually kept below 30 cmH2O with PEEP which usually starts at 5 cmH2O and is titrated upwards. The ventilation should be set at ≤ 35 breaths/min to mimic the baseline minute. It is important to reassess the patient and increase or decrease tidal volume based on the plateau pressure. A summary of the Acute Respiratory Distress Syndrome Network trial on LTVV settings is included in Table 4.
| Arterial oxygenation and PEEP | |
| Target Oxygenation PaO2 = 55-80 mmHg (7.35-10.7 kPa)or O2 saturations (SpO2) = 88%-95% | |
| FiO2/PEEP combinations | |
| FiO2 (%) | PEEP (H2O) |
| 30 | 5 |
| 40 | 5-8 |
| 50 | 8-10 |
| 60 | 10 |
| 70 | 10-14 |
| 80 | 14 |
| 90 | 14-18 |
| 100 | 18-24 |
The reasoning behind LTVV is lower tidal volumes may attenuate some of the alveolar overdis
During reperfusion, the ischaemic lung is suddenly exposed to the recipient’s circulation whereby there is rapid recruitment of neutrophils and ongoing propagation of ROS which as described above, results in a cascade of events heralding a viscous cycle of oxidative stress with increased vascular permeability and pulmonary hypertension[18]. Rapid reperfusion is also associated with mechanical stress failure of the alveolar/capillary barrier[129].
Hence altering the reperfusion process is a strategy to enervate PGD. The UCLA group utilized a modified reperfusion strategy by using buffered leukocyte-depleted blood and reperfused at the pressure of < 20 mmHg before cross-clamp release and noted a reduction in IRI in their cohort[130]. Leukocyte depletion has since been utilized with EVLP in several animal models with similar outcomes[131,132].
Diamond et al[9] noted that the odds ratio for PGD increased by 10% for every 10% increase in FiO2 at the time of reperfusion. However, it should be noted that this may have been a response to poor oxygenation during reperfusion, hence implying an association rather than causality. However, to date, no studies have been conducted examining FiO2 at reperfusion as a risk factor for PGD.
NO has been investigated as a therapeutic option for the prevention or treatment of PGD due to its pulmonary vasodilatory effects[133]. Given the effect of pulmonary hypertension, NO is believed to attenuate the effects of reperfusion. There is however a lack of randomized studies showing the survival benefit of the ubiquitous use of NO in treating PGD. Benefits noted from studies with ARDS did not result in improved mortality[134]. Moreno et al[135] published the only clinical study which elucidated a reduction in PGD by inhaled NO administration. The authors noted a significantly lower incidence of PGD in the iNO group vs the control group (17.2% vs 45%) (P < 0.035) alongside significant reductions in IL-6 (in blood at 12 h), IL-8 (in blood and BAL at 12 and 24 h), and IL-10 (in blood at 12 and 24 h and BAL at 24 h)[135]. In Moreno’s study, iNO was commenced at the beginning of surgery and continued for 48 h postoperatively. Similar findings were noted by Yerebakan in their cohort with a similar length of iNO administration[136]. Other studies have not shown similar outcomes with the caveat that iNO was used either before reperfusion or at the beginning of the case and not as prolonged as Moreno’s group[137]. The beneficial effects of NO may be transient and therefore not result in reductions in mortality[138]. Current evidence does not support the widespread routine use of NO for PGD prevention, although these studies were underpowered to detect minuscule differences in outcomes[12].
Prostaglandin E1 (PGE1) causes similar vasodilatory effects on pulmonary circulation and may therefore improve oxygenation. In addition, it may also play a role in attenuating IRI by reducing the expression of certain mediators such as IL-12 and TNF-α and increasing the expression of IL-10, an anti-inflammatory mediator[139]. The use of PGE1 has been proven in ARDS with improvements in oxygenation and reduction in PA pressures[12]. Hypotension was also a key feature in the metanalysis. Several studies have noted the benefits of using injected PGE1 during organ retrieval in transplantation. The Pittsburgh group noted an improvement in long-term survival following a change in their protocol in 1994 to include PGE1 addition to the graft preservation fluid[140]. Similarly, a group from Taipei noted that the addition of PGE1 to preservation fluid during pulmonary artery flushing resulted in the increased attenuation of IRI[141].
As with PGE1, iloprost is a prostacyclin analogue that primarily functions as a pulmonary vasodilator. In addition to the benefits of PGE1, PGI2 also plays a role in inhibiting neutrophil adherence to improve endothelial integrity[142] and in reducing platelet aggregation[143]. Animal model studies have also shown some benefit in ameliorating IRI[144,145]. However, its use in the treatment and prevention of PGD is limited to a single retrospective study by Lee et al[143]. In a propensity-matched analysis, patients who were administered inhaled iloprost immediately after reperfusion of the grafted lung had significantly lower severity of pulmonary infiltration on postoperative days (PODs) 1 to 3 compared to the non-intervention arm. The PaO2/FiO2 ratio was also significantly higher in the treatment group compared to the control group (318.2 ± 74.2 mmHg vs 275.9 ± 65.3 mmHg, P = 0.022 on POD 1; 351.4 ± 58.2 mmHg vs 295.8 ± 53.7 mmHg, P = 0.017 on POD 2; and 378.8 ± 51.9 mmHg vs 320.2 ± 66.2 mmHg, P = 0.013 on POD 3, respectively). Finally, PGD3 prevalence was significantly lower on POD1 [2 (6.7%) vs 9 (30%), P = 0.042], POD2 [1 (3.3%) vs 8 (26.7%), P = 0.026] and POD3 [0 (0) vs 6 (20%), P = 0.024]. It should be noted however there was a higher rate of PGD1 (mild) in the iloprost arm of the study on all 3 d[143]. Larger prospective studies are needed to validate the above findings.
The surfactant depletion theory is another potential pathogenetic mechanism of PGD. In a prospective open-label randomized study, a study based in Israel investigated the role of surfactant therapy post bronchial anastomosis[146]. The authors noted that patients who received surfactant had improved mean PaO2/FiO2 (418.8 ± 123.8 mmHg vs 277.9 ± 165 mmHg, P = 0.004) post-operatively, lower PGD grades (0.66 vs 1.86, P = 0.005), fewer cases of severe PGD (1 vs 12, P < 0.05)[146]. The same group then published a case series of 5 patients with severe PGD treated with surfactant instead of ECMO[147]. They noted a significant improvement in PaO2/FiO2 ratios within h of treatment (pretreatment mean PaO2/FiO2vs post-treatment PaO2/FiO2 (98.8 ± 21.7 mmHg vs 236.8 ± 52.3 mmHg, P = 0.0006) who were still alive 6 mo post-treatment.
ECMO is usually reserved for PGD grade 3 refractory to medical treatment. Outcomes post-ECMO are better with early initiation (< 24 h)[12,148]. The use of Veno-Venous ECMO (VV-ECMO) is currently preferred over Veno-Arterial ECMO (VA-ECMO) if the patient is haemodynamically stable due to the complications attributed to VA-ECMO[12]. The Pittsburgh group noted survival after both VV-ECMO and VA-ECMO were similar at 30 d, 1 year, and 5 years (58% vs 55%, P = 0.7; 42% vs 39%, P = 0.8; 29% vs 22%, P = 0.6)[149]. The Duke group noted that their VV-ECMO patients had better survival and medium-term outcomes, with all VV ECMO patients were successfully weaned from ECMO support, but only 50% (7 of 14) of the VA ECMO survived weaning (P = 0.02). The 30-d graft and patient survival for the VV ECMO group was 87.5% vs 0 in the VA-ECMO group[150]. In a subsequent study, the same group noted their VV-ECMO survival to be 82% at 30 d, 64% at 1 year, and 49% at 5 years which is arguably better compared to the previous report[151]. The poor outcomes for VA-ECMO are probably multifactorial, ranging from the severity of the SIRS response which necessitated VA-ECMO in the first place to the genuine complications attributed to VA-ECMO. Peripheral VA-ECMO has significant limb ischaemia complications reported to be around 16.9% (95%CI: 12.5% to 22.6%) with a 10.3% risk of fasciotomy or compartment syndrome (95%CI: 7.3% to 14.5%) and lower limb amputation rate of 4.7% (95%CI: 2.3% to 9.3%)[152].
The incidence of neurologic complications for VA-ECMO was 13.3% (95%CI: 9.9% to 17.7%), acute kidney injury, 55.6% (95%CI: 35.5% to 74.0%) with almost half requiring renal replacement therapy [95%CI: 46.0% (36.7% to 55.5%)][152]. The risk of bleeding complications in VA-ECMO is 46.0% (95%CI: 36.7% to 55.5%) with re-exploration for bleeding or tamponade reported around 41.9% (95%CI: 24.3% to 61.8%) alongside a significant infection rate of 30.4% (95%CI: 19.5% to 44.0%) in the post-cardiotomy cohort alone[152]. The risks of the above in the post-transplant setting have not been noted via a metanalysis but one can only assume the risks of renal impairment and infections to be higher with the administration of immunosuppressive medications.
Delayed chest closure (DCC) was initially thought to be a marker for PGD with one study identifying patients with increased CXR changes as being at risk for DCC[153]. It is perhaps a surrogate marker for prolonged CPB usage, challenging intraoperative conditions, and polytransfusion. The Pittsburgh group showed no increased mortality in this group despite the increased PGD incidence, potentially indicating that DCC may be of benefit in these sub-select groups of patients as long as the skin closure method was applied[154]. They noted that acute lung oedema, oversized grafts, and coagulopathy were the most common reasons for DCC followed by haemodynamic instability. The authors noted that improved the haemodynamics and physiology of lung allografts especially when LPVV with high PEEP was utilized[154]. There is a delicate balance between the physiological and haemodynamic benefits vs the loss of the barrier function against infections, particularly in an immunosuppressed patient[154].
Aprotinin is a nonspecific serine protease inhibitor that has shown evidence of attenuating ischemia-reperfusion lung injury by inhibiting the inflammatory response and suppressing NADPH oxidase[155]. Using a rat model, the team from Tokyo noted that by using aprotinin in the flush preservation solution during organ retrieval, and 18-h lung preservation at 4 ℃ followed by normothemic reperfusion with blood, the malondialdehyde (MDA) and IL-8 levels in the lung tissue after reperfusion were reduced in the aprotinin group. The aprotinin-treated lungs also showed significantly better oxygenation throughout the reperfusion period[155]. This preservative effect of aprotinin was also confirmed by the prevention of an increase in peak airway pressure in aprotinin group. They highlighted 2 key issues which were: cold ischemic preservation followed by reperfusion results in interstitial edema and neutrophil extravasation into alveoli, secondly, aprotinin prevented these pathological changes[155]. Similar outcomes were reproduced from 2 other animal model studies[156-158]. Bittner et al and the Leipzig group then translated this into a clinical study whereby 59 patients were managed perioperatively with aprotinin infusion using a historical cohort for comparison[159]. Despite advancing donor age in the aprotinin group and longer ischaemic times, the incidence of post-transplant reperfusion injury was markedly lower than in the historical cohort[159]. There were however several limitations to their study including the use of a historical cohort, alongside the multiple interventions used by the team during the aprotinin study, namely iNO usage, and controlled reperfusion.
Prove ventilation involves the delivery of mechanical ventilation with the patient in a prone position[160]. The oxygenation is improved due to improved lung perfusion and reduced lung compression[161]. It is a technique that is used as part of the lung-protective strategy to improve survival in severe cases of ARDS with some studies showing improvements, especially in the COVID-19 pandemic[162,163]. Its role in lung transplantation however is slightly more limited due to the incision sites which tend to be via sternotomy or clamshell for bilateral lung transplants although posterolateral thoracotomies can also be performed. A team in Padua, Italy, utilized high-frequency percussive ventilation (HFPV) along with proning to good effect in 3 patients with infiltrates on CXR[164]. They noted a gradual improvement in the clearance of secretions without needing endotracheal intubation in all 3 of their patients. They also noted that HFPV accommodated volume distribution without overinflating compartments with low time constants thus potentially having a protective role against alveolar hyperinflation[164].
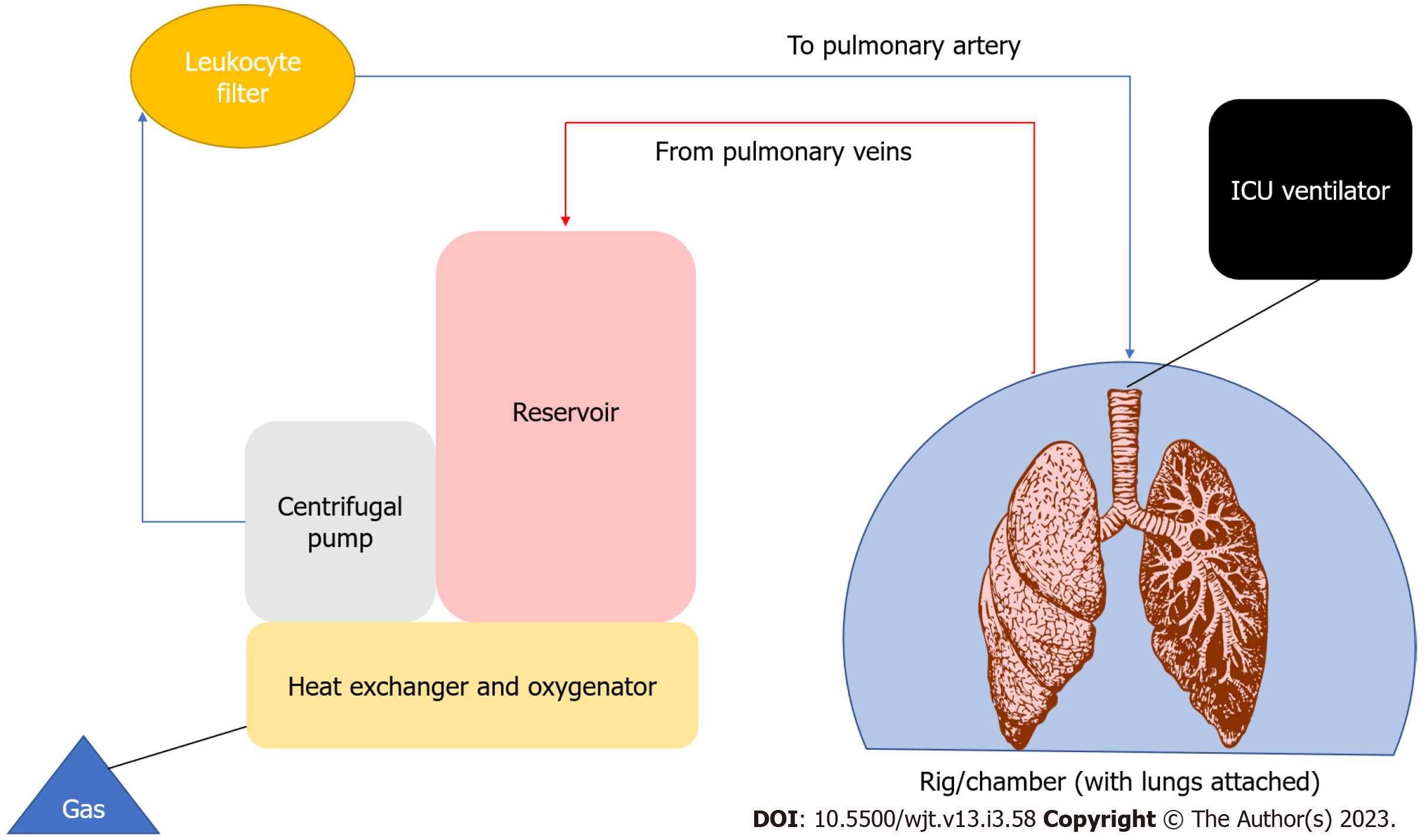
Prior to EVLP, 4/5 of donor lungs expressed some degree of potential injury therefore not considered suitable for transplantation, limiting the donor pool[165]. Another major limitation of transplantation is prolonged cold storage during transport and its perceived effects on IRI[166]. Normothermic ex vivo lung perfusion is a novel way of organ preservation, evaluation, and potential reconditioning of donor lungs. EVLP allows for perfusion of the donor lungs using an ex vivo circuit (Figure 4).
There are currently 3 EVLP protocols that have been described, the Lund protocol, the Organ Care System protocol, and the Toronto protocol. The differences between the protocols are highlighted below in Table 5.
| Description | Lund | OCS | Toronto |
| Year | 2001[176] | 2011[222] | 2008[177] |
| Perfusion parameters | |||
| Perfusate | CellularSteen solution + RBCs | CellularOCS proprietary solution + RBCs | AcellularSteen solution |
| Target haematocrit | 14% | 15%-25% | N/A |
| Target flow | 100% cardiac output | 2.0-2.5 L/min | 40% cardiac output |
| PA pressure | ≤ 20 mmHg | ≤ 20 mmHg | Flow dictated (usually < 15 mmHg) |
| Left atrium | Open | Open | Closed |
| Flow type | Continuous | Pulsatile | Continuous |
| Ventilation | |||
| Initial temperature | 32 oC | 34 oC | 32 oC |
| Tidal volume | 5-7 mL/kg | 6 mL/kg | 7 mL/kg |
| Respiratory rate | 20/min | 10/min | 7/min |
| FiO2 | 50% | 12% | 21% |
| PEEP | 5 cmH2O | 5-7 cmH2O | 5 cmH2O |
There are several EVLP kits available that differ in size, function, and portability. The specifics of these are outwith the remit of this review. EVLP was initially used by Steen et al[167] for the first human lung transplant using a DCD lung assessed by EVLP and the first successful lung transplant of an initially unacceptable donor lung reconditioned ex vivo in 2000. This proof of concept was then expanded to prolong the EVLP time up to 12 h[168] by the Toronto group. The usage of an acellular perfusate as per the Toronto Protocol is to mitigate the haemolysis of RBCs which may in itself precipitate IRI with the release of pro-inflammatory cytokines[169]. Haemolysis of RBCs also causes results in increased plasma volumes of AGEs (advanced glycation end-products), a finding noted in oxidative conditions and is associated with disease severity inflammatory conditions[170]. Since then, numerous trials have been conducted to show equivocal results to that of cold storage. This includes the NOVEL lung[171], HELP[165], and INSPIRE[172]. The HELP trial showed an increased rate of PGD at 72 h in lungs that underwent EVLP but this did not meet statistical significance (15% vs 30.1%; 95%CI: −2.6% to 32.8%; P = 0.11) although the study was not powered to calculate differences in PGD. Severe PGD rates were similar in both arms[165]. The NOVEL lung trial noted a higher number of severe PGD cases in the EVLP group [EVLP vs Control (21.4%, n = 9 vs 9.5%, n = 4), P = 0.2] although this study too was not powered for PGD outcomes[171]. Survival in both studies was similar between the groups. The UK-based DEVELOP-UK study indicated a higher rate of early severe PGD in the EVLP arm, but rates of PGD did not differ between groups after 72 h[173]. The requirement for ECMO support was higher in the EVLP arm than in the standard arm [(7/18, 38.8%) vs (6/184, 3.2%), P < 0.001][173]. The study however was concluded early and non-inferiority analysis of survival could not be performed. The EXPAND study was a single-arm trial that studied the role of EVLP using the Organ Care System Lung for procurement of extended-criteria donor lungs from brain-death donors, and donors after circulatory death, which are seldomly used for transplantation. The primary efficacy endpoint was a composite of patient survival at day 30 post-transplant and absence of primary graft dysfunction grade 3 (PGD3) within 72 h post-transplantation[174]. The authors chose a prespecified objective performance goal (OPG) of 65% based on a PGD3 within the initial 72 h post-transplant rate of 30.8% (95%CI: 28.2% to 33.3%) for standard-criteria donor lungs as noted by Diamond et al[9]. The primary effectiveness composite endpoint of patient survival at day 30 post-transplant and no PGD3 within the initial 72 h was achieved in 43/79 patients (54%) and did not meet the prespecified OPG[174]. When investigating this, they noted a perceptibly high rate of PGD3 of 44% (35/79 patients) within 72 h. At 72 h, post-transplantation, the severe PGD rate dropped to 6% (5/79) and moderate or severe PGD rate of 16% (13/79) which were comparable to the control group in INSPIRE[174]. These findings were also noted in the DEVELOP-UK study where the baseline severe PGD rate was 88.9% and dropped to 27.8% at 72 h[173].
There is a disproportionate number of severe PGD cases following DCD procurement and EVLP. This could be a result of the prolonged functional warm ischaemic time as described above. Another explanation could be the 48% rate of CPB usage in the EXPAND trial (38/79 recipients).
It should be noted that despite the rate PGD associated with EVLP at the time of writing, these were lungs that were unlikely to be used. Given the 98.7% (78/79 patients) 30-d survival rate of lungs transplanted in the EXPAND study, EVLP has a major role to play in expanding the donor pool for lung transplantation[174]. It remains a viable avenue for studies on preconditioning, attenuation, and mitigation of IRI in the near future.
Given our current understanding of PGD and the role of IRI in its pathogenesis, several experimental studies have been conducted to attenuate these. The search for a biomarker however remains elusive. Several biomarkers have been suggested including RAGE, plasminogen activator inhibitor-1 (PAI-1)[175], IL-8[176], endothelin-1 (ET-1)[177], ICAM-1[27], proadrenomedullin (proADM)[178], and several others have been mooted but none have made the transition to routine clinical practice. A list of studied biomarkers is available in Table 6.
| Biomarker | Donor/recipient | Timing | Type |
| RAGE[179] | Donor | Retrieval | Alveolar epithelium |
| Recipient | Intraoperative, post-transplant | ||
| PAI-1[175] | Recipient | Post-transplant | Vascular endothelium |
| Protein C[179] | Recipient | Post-transplant | Vascular endothelium |
| IL-8[176] | Donor | EVLP | Inflammatory marker |
| Recipient | Pre-transplant | ||
| Post-transplant | |||
| ET-1[171] | Donor | EVLP | Inflammatory marker |
| ICAM-1[27] | Recipient | Post-transplant | Vascular endothelium |
| IL-6[135] | Recipient | Pre-transplant | Inflammatory marker |
| Post-transplant | |||
| IL-10[135] | Recipient | Pre-transplant | Inflammatory marker |
| Post-transplant | |||
| proADM[178] | Recipient | Post-transplant | Vascular endothelium |
| TNF-α[139] | Recipient | Post-transplant | Inflammatory marker |
| P-selectin[26] | Recipient | Post-transplant | Vascular endothelium |
| SP-D[179] | Recipient | Post-transplant | Alveolar epithelium |
Shah et al[179] utilised a panel of biomarkers to improve the predictive value for PGD and survival by using a combination of biomarkers derived from the alveolar epithelium (sRAGE, SP-D), coagulation cascade proteins (PAI-1 and protein C), and vascular endothelium (ICAM-1). Ongoing research is needed to improve discriminatory value for PGD to allow better resource provision for these patients in the intensive care setting.
EVLP has allowed therapeutic interventions to donor lungs prior to implantation. Using IL-8 as a biomarker for inflammation, the Toronto group created an animal model using porcine lungs preserved for 18 h followed by 12 h of EVLP conditioning[180]. In the treatment arm, mesenchymal stromal cells were used in the pulmonary artery. They noted a significant reduction in IL-8 levels in the perfusate in the intervention arm compared to the control group. A similar study had also been conducted where by McAuley et al[181] whereby lungs that were rejected for transplantation were reconditioned using mesenchymal stromal cell administration into the perfusate in the intervention group increased alveolar fluid clearance in the experimental group compared to the control group. A more recent study also showed using amnion-derived mesenchymal stem cells added to Steen Solution during EVLP resulted in further attenuation of IRI effects thus improving the efficacy of EVLP[182]. Mesenchymal stromal cells also demonstrated IL-10 attenuating properties in an animal model on sepsis[183]. Whilst still at an experimental stage, stem cell therapy may provide tangible treatment options in the near future.
Ischaemic preconditioning (IPC) is an intervention whereby brief intermittent ischaemic episodes are induced interspersed with reperfusion either at the site of interest (IPC) or at a distance from the site of interest (remote ischemic preconditioning, RIPC)[184]. The evidence for IPC or RIPC has resulted in mixed outcomes with favourable results in some studies[185-188] but with little clinical benefit in others[189-191]. Many animal models however have shown a reduction in acute lung injury following IPC or RIPC[192-195]. The Dutch group performed a series of lung transplants using rabbits and a variety of IPC strategies and noted that the lungs that underwent increasing bouts of ischaemic preconditioning had less oedema[196]. The authors hinted at an additional benefit for repeated cycles of IPC[196]. Randomised studies on RIPC in lung resections have shown a decreased incidence of acute lung injury in the intervention group[185,197]. A randomised pilot study was performed by Lin et al[198] on 52 patients to study the effects of RIPC using a tourniquet on the lower limb of the recipient and noted no statistically significant differences in PaO2/FiO2 ratios or panel of inflammatory markers at different time points. They concluded that there was no significant benefit of RIPC although the incidence of IRI/PGD was lower than expected[198]. In their subsequent publication, the Australian group however noted that RIPC is feasible and despite not reaching statistical significance, they noted a trend to improvements in P/F ratio and PGD grade seen at virtually all time points during the first 72 h after lung transplantation[199]. Given the negligible cost of the intervention, a multicenter randomized study could well highlight whether RIPC has a role in lung transplantation.
Pharmacological interventions to attenuate IRI have been suggested in recent times.
Adenosine A2A receptor agonist - regadenoson is a drug that was intended for use for its coronary vasodilatory effects during stress testing in myocardial perfusion scans[200]. Animal models have shown success in using Adenosine A2A agonists with EVLP in reducing IRI[201-203]. A recently completed Phase I study was also published with no dose-limiting toxicities observed and no mortalities reported[204].
α1-antitrypsin (AAT)- is a plasma proteinase inhibitor that has a protective function for proteolytic enzymes produced by inflammatory cells[205]. In a rat model, lungs primed in AAT had decreased weight and allograft necrosis compared to the control group (primed in albumin highlighting a potential role in ameliorating IRI[205]. When used in addition to lung preservation fluid, reduced the extent of primary graft dysfunction and early neutrophil responses after extended storage for 18 h at 4 ℃ and 4-h reperfusion in another rat model study[206]. Interestingly, there were similar findings in proteinase 3/neutrophil elastase (PR3/NE) double-deficient mice, highlighting a potential pathogenetic pathway[206].
C1-esterase inhibitor (C1-INH) - In ARDS, the blood coagulation contact system and the complement system are activated, leading to capillary leakage and the pathognomic sequalae. Activation of the contact as well as the complement system is regulated by a common inhibitor, C1-INH[207]. A canine model study indicated that C1-INH administration prevented hypoxemia, activation of the complement system, and decreased expression of leukocyte adhesion molecules alongside inflammatory cell infiltrate[208].
The Hannover group initially reported 2 cases whereby C1-INH was used to good effect[207]. They reported their experience in using it in PGD3 patients whereby it was administered in the operating theatre once PGD3 was suspected[209]. When comparing the 3 groups (control vs C1-INH vs PGD3) ICU stay was longest in the PGD3 cohort and prolonged in C1-INH patients compared with the control group [29 (2-70) vs 9 (2-83) vs 3 (1-166) d, P = 0.002]. The C1-INH-treated-group had a one-year survival of 82.5% which was better than the PGD3 cohort (71.4%)[209].
The Toronto group described one of the earliest attempts at using adenovirus-mediated gene therapy in 1999[210]. They then successfully demonstrated that the adenoviral-mediated human IL-10 (hIL-10) gene to donor rat lungs 24 h before lung retrieval reduced IRI and improved post-transplant graft function[211]. Following the development of EVLP, they were able to administer the adenoviral-mediated hIL-10 gene intrabronchially with 12 h of EVLP and noted significantly better gas exchange, lower histologic inflammation score, and less interferon-gamma production when compared with non-treatment groups[212]. This will perhaps be an avenue clinical application of gene therapy to optimise donor lungs and improve outcomes post-lung transplantation.
PGD remains a life-threatening complication post-transplantation. The pathophysiology is not fully understood but ischaemic-reperfusion injury is almost certainly implicated. Diagnostic criteria make classification easier, however, it is still lacking an encompassing biomarker. Animal models and preclinical studies have played vital roles in helping us understand the pathophysiology and engineer therapeutic options which will hopefully translate to clinical benefits in the near future. The role of EVLP is still in its infancy with multiple conditioning, treatment, and assessment options available for research opportunities to guide our ongoing understanding of this condition.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Andriolo LG, Italy; Nakajima D, Japan; Thongon N, Thailand S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Machuca TN, Keshavjee S. Transplantation for lung cancer. Curr Opin Organ Transplant. 2012;17:479-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Cooper JD, Pearson FG, Patterson GA, Todd TR, Ginsberg RJ, Goldberg M, DeMajo WA. Technique of successful lung transplantation in humans. J Thorac Cardiovasc Surg. 1987;93:173-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 215] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | Chambers DC, Cherikh WS, Harhay MO, Hayes D Jr, Hsich E, Khush KK, Meiser B, Potena L, Rossano JW, Toll AE, Singh TP, Sadavarte A, Zuckermann A, Stehlik J; International Society for Heart and Lung Transplantation. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult lung and heart-lung transplantation Report-2019; Focus theme: Donor and recipient size match. J Heart Lung Transplant. 2019;38:1042-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 630] [Article Influence: 105.0] [Reference Citation Analysis (0)] |
| 4. | Christie JD, Bellamy S, Ware LB, Lederer D, Hadjiliadis D, Lee J, Robinson N, Localio AR, Wille K, Lama V, Palmer S, Orens J, Weinacker A, Crespo M, Demissie E, Kimmel SE, Kawut SM. Construct validity of the definition of primary graft dysfunction after lung transplantation. J Heart Lung Transplant. 2010;29:1231-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 5. | Christie JD, Van Raemdonck D, de Perrot M, Barr M, Keshavjee S, Arcasoy S, Orens J; ISHLT Working Group on Primary Lung Graft Dysfunction. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part I: introduction and methods. J Heart Lung Transplant. 2005;24:1451-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Chatila WM, Furukawa S, Gaughan JP, Criner GJ. Respiratory failure after lung transplantation. Chest. 2003;123:165-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Granton J. Update of early respiratory failure in the lung transplant recipient. Curr Opin Crit Care. 2006;12:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Diamond JM, Arcasoy S, Kennedy CC, Eberlein M, Singer JP, Patterson GM, Edelman JD, Dhillon G, Pena T, Kawut SM, Lee JC, Girgis R, Dark J, Thabut G. Report of the International Society for Heart and Lung Transplantation Working Group on Primary Lung Graft Dysfunction, part II: Epidemiology, risk factors, and outcomes-A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2017;36:1104-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 123] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 9. | Diamond JM, Lee JC, Kawut SM, Shah RJ, Localio AR, Bellamy SL, Lederer DJ, Cantu E, Kohl BA, Lama VN, Bhorade SM, Crespo M, Demissie E, Sonett J, Wille K, Orens J, Shah AS, Weinacker A, Arcasoy S, Shah PD, Wilkes DS, Ware LB, Palmer SM, Christie JD; Lung Transplant Outcomes Group. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2013;187:527-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 528] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 10. | Snell GI, Yusen RD, Weill D, Strueber M, Garrity E, Reed A, Pelaez A, Whelan TP, Perch M, Bag R, Budev M, Corris PA, Crespo MM, Witt C, Cantu E, Christie JD. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction, part I: Definition and grading-A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2017;36:1097-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 469] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 11. | Oto T, Griffiths AP, Levvey BJ, Pilcher DV, Williams TJ, Snell GI. Definitions of primary graft dysfunction after lung transplantation: differences between bilateral and single lung transplantation. J Thorac Cardiovasc Surg. 2006;132:140-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Van Raemdonck D, Hartwig MG, Hertz MI, Davis RD, Cypel M, Hayes D Jr, Ivulich S, Kukreja J, Lease ED, Loor G, Mercier O, Paoletti L, Parmar J, Rampolla R, Wille K, Walia R, Keshavjee S. Report of the ISHLT Working Group on primary lung graft dysfunction Part IV: Prevention and treatment: A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2017;36:1121-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 13. | Christie JD, Kotloff RM, Ahya VN, Tino G, Pochettino A, Gaughan C, DeMissie E, Kimmel SE. The effect of primary graft dysfunction on survival after lung transplantation. Am J Respir Crit Care Med. 2005;171:1312-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 264] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 14. | Verleden GM, Glanville AR, Lease ED, Fisher AJ, Calabrese F, Corris PA, Ensor CR, Gottlieb J, Hachem RR, Lama V, Martinu T, Neil DAH, Singer LG, Snell G, Vos R. Chronic lung allograft dysfunction: Definition, diagnostic criteria, and approaches to treatment-A consensus report from the Pulmonary Council of the ISHLT. J Heart Lung Transplant. 2019;38:493-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 630] [Article Influence: 105.0] [Reference Citation Analysis (0)] |
| 15. | den Hengst WA, Gielis JF, Lin JY, Van Schil PE, De Windt LJ, Moens AL. Lung ischemia-reperfusion injury: a molecular and clinical view on a complex pathophysiological process. Am J Physiol Heart Circ Physiol. 2010;299:H1283-H1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 290] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 16. | Barman SA, Ardell JL, Parker JC, Perry ML, Taylor AE. Pulmonary and systemic blood flow contributions to upper airways in canine lung. Am J Physiol. 1988;255:H1130-H1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Frye CC, Bery AI, Kreisel D, Kulkarni HS. Sterile inflammation in thoracic transplantation. Cell Mol Life Sci. 2021;78:581-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Weyker PD, Webb CA, Kiamanesh D, Flynn BC. Lung ischemia reperfusion injury: a bench-to-bedside review. Semin Cardiothorac Vasc Anesth. 2013;17:28-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Wang X, O'Brien ME, Yu J, Xu C, Zhang Q, Lu S, Liang L, An X, McDyer JF, Mallampalli RK. Prolonged Cold Ischemia Induces Necroptotic Cell Death in Ischemia-Reperfusion Injury and Contributes to Primary Graft Dysfunction after Lung Transplantation. Am J Respir Cell Mol Biol. 2019;61:244-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 20. | Kim H, Zamel R, Bai XH, Lu C, Keshavjee S, Liu M. Ischemia-reperfusion induces death receptor-independent necroptosis via calpain-STAT3 activation in a lung transplant setting. Am J Physiol Lung Cell Mol Physiol. 2018;315:L595-L608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 21. | Gielis JF, Beckers PAJ, Briedé JJ, Cos P, Van Schil PE. Oxidative and nitrosative stress during pulmonary ischemia-reperfusion injury: from the lab to the OR. Ann Transl Med. 2017;5:131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Ma J, Yang L, Ren J, Yang J. Chapter 20 - Autophagy, Oxidative Stress, and Redox Regulation. In: Ren J, Sowers JR, Zhang Y, editors. Autophagy and Cardiometabolic Diseases. Academic Press; 2018: 237-251. [DOI] [Full Text] |
| 23. | de Perrot M, Liu M, Waddell TK, Keshavjee S. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med. 2003;167:490-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 666] [Cited by in RCA: 693] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 24. | Schnickel GT, Ross DJ, Beygui R, Shefizadeh A, Laks H, Saggar R, Lynch JP 3rd, Ardehali A. Modified reperfusion in clinical lung transplantation: the results of 100 consecutive cases. J Thorac Cardiovasc Surg. 2006;131:218-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Christie JD, Shah CV, Kawut SM, Mangalmurti N, Lederer DJ, Sonett JR, Ahya VN, Palmer SM, Wille K, Lama V, Shah PD, Shah A, Weinacker A, Deutschman CS, Kohl BA, Demissie E, Bellamy S, Ware LB; Lung Transplant Outcomes Group. Plasma levels of receptor for advanced glycation end products, blood transfusion, and risk of primary graft dysfunction. Am J Respir Crit Care Med. 2009;180:1010-1015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 26. | Kawut SM, Okun J, Shimbo D, Lederer DJ, De Andrade J, Lama V, Shah A, Milstone A, Ware LB, Weinacker A, Demissie E, Christie JD; Lung Transplant Outcomes Group. Soluble p-selectin and the risk of primary graft dysfunction after lung transplantation. Chest. 2009;136:237-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Covarrubias M, Ware LB, Kawut SM, De Andrade J, Milstone A, Weinacker A, Orens J, Lama V, Wille K, Bellamy S, Shah C, Demissie E, Christie JD; Lung Transplant Outcomes Group. Plasma intercellular adhesion molecule-1 and von Willebrand factor in primary graft dysfunction after lung transplantation. Am J Transplant. 2007;7:2573-2578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Fang A, Studer S, Kawut SM, Ahya VN, Lee J, Wille K, Lama V, Ware L, Orens J, Weinacker A, Palmer SM, Crespo M, Lederer DJ, Deutschman CS, Kohl BA, Bellamy S, Demissie E, Christie JD; Lung Transplant Outcomes Group. Elevated pulmonary artery pressure is a risk factor for primary graft dysfunction following lung transplantation for idiopathic pulmonary fibrosis. Chest. 2011;139:782-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 29. | Monticelli LA, Diamond JM, Saenz SA, Tait Wojno ED, Porteous MK, Cantu E, Artis D, Christie JD. Lung Innate Lymphoid Cell Composition Is Altered in Primary Graft Dysfunction. Am J Respir Crit Care Med. 2020;201:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Porteous MK, Ky B, Kirkpatrick JN, Shinohara R, Diamond JM, Shah RJ, Lee JC, Christie JD, Kawut SM. Diastolic Dysfunction Increases the Risk of Primary Graft Dysfunction after Lung Transplant. Am J Respir Crit Care Med. 2016;193:1392-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 31. | Li D, Weinkauf J, Hirji A, Kapasi A, Lien D, Nagendran J, Kim D, Ezekowitz J, Halloran K. Elevated pre-transplant left ventricular end-diastolic pressure increases primary graft dysfunction risk in double lung transplant recipients. J Heart Lung Transplant. 2019;38:710-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Avriel A, Klement AH, Johnson SR, de Perrot M, Granton J. Impact of Left Ventricular Diastolic Dysfunction on Lung Transplantation Outcome in Patients With Pulmonary Arterial Hypertension. Am J Transplant. 2017;17:2705-2711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Whitson BA, Nath DS, Johnson AC, Walker AR, Prekker ME, Radosevich DM, Herrington CS, Dahlberg PS. Risk factors for primary graft dysfunction after lung transplantation. J Thorac Cardiovasc Surg. 2006;131:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 132] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 34. | Bonser RS, Taylor R, Collett D, Thomas HL, Dark JH, Neuberger J; Cardiothoracic Advisory Group to NHS Blood and Transplant and the Association of Lung Transplant Physicians (UK). Effect of donor smoking on survival after lung transplantation: a cohort study of a prospective registry. Lancet. 2012;380:747-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 35. | Ware LB, Lee JW, Wickersham N, Nguyen J, Matthay MA, Calfee CS; California Transplant Donor Network. Donor smoking is associated with pulmonary edema, inflammation and epithelial dysfunction in ex vivo human donor lungs. Am J Transplant. 2014;14:2295-2302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Kuschner WG, D'Alessandro A, Wong H, Blanc PD. Dose-dependent cigarette smoking-related inflammatory responses in healthy adults. Eur Respir J. 1996;9:1989-1994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 259] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 37. | Diamond JM, Porteous MK, Roberts LJ 2nd, Wickersham N, Rushefski M, Kawut SM, Shah RJ, Cantu E 3rd, Lederer DJ, Chatterjee S, Lama VN, Bhorade S, Crespo M, McDyer J, Wille K, Orens J, Weinacker A, Arcasoy S, Shah PD, Wilkes DS, Hage C, Palmer SM, Snyder L, Calfee CS, Ware LB, Christie JD; Lung Transplant Outcomes Group. The relationship between plasma lipid peroxidation products and primary graft dysfunction after lung transplantation is modified by donor smoking and reperfusion hyperoxia. J Heart Lung Transplant. 2016;35:500-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 38. | Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:360438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2367] [Cited by in RCA: 3548] [Article Influence: 322.5] [Reference Citation Analysis (0)] |
| 39. | Morrison D, Rahman I, Lannan S, MacNee W. Epithelial permeability, inflammation, and oxidant stress in the air spaces of smokers. Am J Respir Crit Care Med. 1999;159:473-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 184] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 40. | Simou E, Leonardi-Bee J, Britton J. The Effect of Alcohol Consumption on the Risk of ARDS: A Systematic Review and Meta-Analysis. Chest. 2018;154:58-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 41. | Lowery EM, Kuhlmann EA, Mahoney EL, Dilling DF, Kliethermes SA, Kovacs EJ. Heavy alcohol use in lung donors increases the risk for primary graft dysfunction. Alcohol Clin Exp Res. 2014;38:2853-2861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 42. | Pelaez A, Mitchell PO, Shah NS, Force SD, Elon L, Brown LA, Guidot DM. The role of donor chronic alcohol abuse in the development of primary graft dysfunction in lung transplant recipients. Am J Med Sci. 2015;349:117-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | Valencia-Martín JL, Galán I, Rodríguez-Artalejo F. The joint association of average volume of alcohol and binge drinking with hazardous driving behaviour and traffic crashes. Addiction. 2008;103:749-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 44. | Poulose B, Srinivasan K. High risk behaviours following alcohol use in alcohol dependent men. Indian J Med Res. 2009;129:376-381. [PubMed] |
| 45. | Pompili M, Serafini G, Innamorati M, Dominici G, Ferracuti S, Kotzalidis GD, Serra G, Girardi P, Janiri L, Tatarelli R, Sher L, Lester D. Suicidal behavior and alcohol abuse. Int J Environ Res Public Health. 2010;7:1392-1431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 323] [Cited by in RCA: 241] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 46. | Guidot DM, Brown LA. Mitochondrial glutathione replacement restores surfactant synthesis and secretion in alveolar epithelial cells of ethanol-fed rats. Alcohol Clin Exp Res. 2000;24:1070-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 47. | Moss M, Guidot DM, Wong-Lambertina M, Ten Hoor T, Perez RL, Brown LA. The effects of chronic alcohol abuse on pulmonary glutathione homeostasis. Am J Respir Crit Care Med. 2000;161:414-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 163] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 48. | Mitchell PO, Jensen JS, Ritzenthaler JD, Roman J, Pelaez A, Guidot DM. Alcohol primes the airway for increased interleukin-13 signaling. Alcohol Clin Exp Res. 2009;33:505-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | Singh SSA, Dalzell JR, Berry C, Al-Attar N. Primary graft dysfunction after heart transplantation: a thorn amongst the roses. Heart Fail Rev. 2019;24:805-820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 50. | Dayoub JC, Cortese F, Anžič A, Grum T, de Magalhães JP. The effects of donor age on organ transplants: A review and implications for aging research. Exp Gerontol. 2018;110:230-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 51. | Baldwin MR, Peterson ER, Easthausen I, Quintanilla I, Colago E, Sonett JR, D'Ovidio F, Costa J, Diamond JM, Christie JD, Arcasoy SM, Lederer DJ. Donor age and early graft failure after lung transplantation: a cohort study. Am J Transplant. 2013;13:2685-2695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 52. | De Perrot M, Waddell TK, Shargall Y, Pierre AF, Fadel E, Uy K, Chaparro C, Hutcheon M, Singer LG, Keshavjee S. Impact of donors aged 60 years or more on outcome after lung transplantation: results of an 11-year single-center experience. J Thorac Cardiovasc Surg. 2007;133:525-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 53. | Shigemura N, Horai T, Bhama JK, D'Cunha J, Zaldonis D, Toyoda Y, Pilewski JM, Luketich JD, Bermudez CA. Lung transplantation with lungs from older donors: recipient and surgical factors affect outcomes. Transplantation. 2014;98:903-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 54. | Fischer S, Gohrbandt B, Struckmeier P, Niedermeyer J, Simon A, Hagl C, Kallenbach K, Haverich A, Strüber M. Lung transplantation with lungs from donors fifty years of age and older. J Thorac Cardiovasc Surg. 2005;129:919-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 55. | Waller DA, Bennett MK, Corris PA, Dark JH. Donor-acquired fat embolism causing primary organ failure after lung transplantation. Ann Thorac Surg. 1995;59:1565-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 56. | Rosendale BE, Keenan RJ, Duncan SR, Hardesty RL, Armitage JA, Griffith BP, Yousem SA. Donor cerebral emboli as a cause of acute graft dysfunction in lung transplantation. J Heart Lung Transplant. 1992;11:72-76. [PubMed] |
| 57. | Oto T, Excell L, Griffiths AP, Levvey BJ, Snell GI. The implications of pulmonary embolism in a multiorgan donor for subsequent pulmonary, renal, and cardiac transplantation. J Heart Lung Transplant. 2008;27:78-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 58. | Oto T, Rabinov M, Griffiths AP, Whitford H, Levvey BJ, Esmore DS, Williams TJ, Snell GI. Unexpected donor pulmonary embolism affects early outcomes after lung transplantation: a major mechanism of primary graft failure? J Thorac Cardiovasc Surg. 2005;130:1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 59. | Ciccone AM, Stewart KC, Meyers BF, Guthrie TJ, Battafarano RJ, Trulock EP, Cooper JD, Patterson GA. Does donor cause of death affect the outcome of lung transplantation? J Thorac Cardiovasc Surg. 2002;123:429-434; discussion 434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 60. | Eberlein M, Reed RM, Bolukbas S, Diamond JM, Wille KM, Orens JB, Brower RG, Christie JD; Lung Transplant Outcomes Group. Lung size mismatch and primary graft dysfunction after bilateral lung transplantation. J Heart Lung Transplant. 2015;34:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 61. | Riddell P, Ma J, Dunne B, Binnie M, Cypel M, Donahoe L, de Perrot M, Pierre A, Waddell TK, Yeung J, Yasufuku K, Tomlinson G, Singer LG, Keshavjee S. A simplified strategy for donor-recipient size-matching in lung transplant for interstitial lung disease. J Heart Lung Transplant. 2021;40:1422-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 62. | Liu Y, Liu Y, Su L, Jiang SJ. Recipient-related clinical risk factors for primary graft dysfunction after lung transplantation: a systematic review and meta-analysis. PLoS One. 2014;9:e92773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 63. | Wahidi MM, Ravenel J, Palmer SM, McAdams HP. Progression of idiopathic pulmonary fibrosis in native lungs after single lung transplantation. Chest. 2002;121:2072-2076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 64. | Khalil N, O'Connor R. Idiopathic pulmonary fibrosis: current understanding of the pathogenesis and the status of treatment. CMAJ. 2004;171:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 65. | Mason DP, Brizzio ME, Alster JM, McNeill AM, Murthy SC, Budev MM, Mehta AC, Minai OA, Pettersson GB, Blackstone EH. Lung transplantation for idiopathic pulmonary fibrosis. Ann Thorac Surg. 2007;84:1121-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 66. | Kanasky WF Jr, Anton SD, Rodrigue JR, Perri MG, Szwed T, Baz MA. Impact of body weight on long-term survival after lung transplantation. Chest. 2002;121:401-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 67. | Madill J, Gutierrez C, Grossman J, Allard J, Chan C, Hutcheon M, Keshavjee SH; Toronto Lung Transplant Program. Nutritional assessment of the lung transplant patient: body mass index as a predictor of 90-day mortality following transplantation. J Heart Lung Transplant. 2001;20:288-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 111] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 68. | Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9119] [Cited by in RCA: 8851] [Article Influence: 285.5] [Reference Citation Analysis (0)] |
| 69. | Friedman JM. Modern science versus the stigma of obesity. Nat Med. 2004;10:563-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 240] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 70. | Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol. 2000;68:437-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 71. | Jain M, Budinger GR, Lo A, Urich D, Rivera SE, Ghosh AK, Gonzalez A, Chiarella SE, Marks K, Donnelly HK, Soberanes S, Varga J, Radigan KA, Chandel NS, Mutlu GM. Leptin promotes fibroproliferative acute respiratory distress syndrome by inhibiting peroxisome proliferator-activated receptor-γ. Am J Respir Crit Care Med. 2011;183:1490-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 72. | Lederer DJ, Kawut SM, Wickersham N, Winterbottom C, Bhorade S, Palmer SM, Lee J, Diamond JM, Wille KM, Weinacker A, Lama VN, Crespo M, Orens JB, Sonett JR, Arcasoy SM, Ware LB, Christie JD; Lung Transplant Outcomes Group. Obesity and primary graft dysfunction after lung transplantation: the Lung Transplant Outcomes Group Obesity Study. Am J Respir Crit Care Med. 2011;184:1055-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 73. | Medoff BD, Okamoto Y, Leyton P, Weng M, Sandall BP, Raher MJ, Kihara S, Bloch KD, Libby P, Luster AD. Adiponectin deficiency increases allergic airway inflammation and pulmonary vascular remodeling. Am J Respir Cell Mol Biol. 2009;41:397-406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 160] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 74. | Charalampidis C, Youroukou A, Lazaridis G, Baka S, Mpoukovinas I, Karavasilis V, Kioumis I, Pitsiou G, Papaiwannou A, Karavergou A, Tsakiridis K, Katsikogiannis N, Sarika E, Kapanidis K, Sakkas L, Korantzis I, Lampaki S, Zarogoulidis K, Zarogoulidis P. Pleura space anatomy. J Thorac Dis. 2015;7:S27-S32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 75. | Shigemura N, Bhama J, Gries CJ, Kawamura T, Crespo M, Johnson B, Zaldonis D, Pilewski J, Toyoda Y, Bermudez C. Lung transplantation in patients with prior cardiothoracic surgical procedures. Am J Transplant. 2012;12:1249-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 76. | Soresi S, Zeriouh M, Sabashnikov A, Sarang Z, Mohite PN, Patil NP, Mansur A, Weymann A, Wippermann J, Wahlers T, Reed A, Carby M, Simon AR, Popov AF. Extended Recipient Criteria in Lung Transplantation: Impact of Pleural Abnormalities on Primary Graft Dysfunction. Ann Thorac Surg. 2016;101:2112-2119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 77. | Toyoda Y, Bhama JK, Shigemura N, Zaldonis D, Pilewski J, Crespo M, Bermudez C. Efficacy of extracorporeal membrane oxygenation as a bridge to lung transplantation. J Thorac Cardiovasc Surg. 2013;145:1065-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 173] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 78. | Moon S, Park MS, Lee JG, Jung JY, Kang YA, Kim YS, Kim SK, Chang J, Paik HC, Kim SY. Risk factors and outcome of primary graft dysfunction after lung transplantation in Korea. J Thorac Dis. 2016;8:3275-3282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 79. | Gulack BC, Hirji SA, Hartwig MG. Bridge to lung transplantation and rescue post-transplant: the expanding role of extracorporeal membrane oxygenation. J Thorac Dis. 2014;6:1070-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 80. | George TJ, Beaty CA, Kilic A, Shah PD, Merlo CA, Shah AS. Outcomes and temporal trends among high-risk patients after lung transplantation in the United States. J Heart Lung Transplant. 2012;31:1182-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 81. | Mason DP, Thuita L, Nowicki ER, Murthy SC, Pettersson GB, Blackstone EH. Should lung transplantation be performed for patients on mechanical respiratory support? J Thorac Cardiovasc Surg. 2010;139:765-773.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 82. | Sharma NS, Hartwig MG, Hayes D Jr. Extracorporeal membrane oxygenation in the pre and post lung transplant period. Ann Transl Med. 2017;5:74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 83. | Hämmäinen P, Schersten H, Lemström K, Riise GC, Kukkonen S, Swärd K, Sipponen J, Silverborn M, Dellgren G. Usefulness of extracorporeal membrane oxygenation as a bridge to lung transplantation: a descriptive study. J Heart Lung Transplant. 2011;30:103-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 84. | Hayanga JW, Lira A, Aboagye JK, Hayanga HK, D'Cunha J. Extracorporeal membrane oxygenation as a bridge to lung transplantation: what lessons might we learn from volume and expertise? Interact Cardiovasc Thorac Surg. 2016;22:406-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 85. | Shafii AE, Mason DP, Brown CR, Vakil N, Johnston DR, McCurry KR, Pettersson GB, Murthy SC. Growing experience with extracorporeal membrane oxygenation as a bridge to lung transplantation. ASAIO J. 2012;58:526-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 86. | Magouliotis DE, Tasiopoulou VS, Svokos AA, Svokos KA, Zacharoulis D. Extracorporeal membrane oxygenation versus cardiopulmonary bypass during lung transplantation: a meta-analysis. Gen Thorac Cardiovasc Surg. 2018;66:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 87. | Bermudez CA, Shiose A, Esper SA, Shigemura N, D'Cunha J, Bhama JK, Richards TJ, Arlia P, Crespo MM, Pilewski JM. Outcomes of intraoperative venoarterial extracorporeal membrane oxygenation versus cardiopulmonary bypass during lung transplantation. Ann Thorac Surg. 2014;98:1936-42; discussion 1942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 88. | Hoetzenecker K, Donahoe L, Yeung JC, Azad S, Fan E, Ferguson ND, Del Sorbo L, de Perrot M, Pierre A, Yasufuku K, Singer L, Waddell TK, Keshavjee S, Cypel M. Extracorporeal life support as a bridge to lung transplantation-experience of a high-volume transplant center. J Thorac Cardiovasc Surg. 2018;155:1316-1328.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 89. | Avtaar Singh SS, DAS DE S, Rushton S, Berry C, Al-Attar N. PREDICTA: A Model to Predict Primary Graft Dysfunction After Adult Heart Transplantation in the United Kingdom. J Card Fail. 2019;25:971-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 90. | Khan TFT, Ahmad N, Serageldeen AS, Fourtounas K. Implantation Warm Ischemia Time in Kidney Transplant Recipients: Defining Its Limits and Impact on Early Graft Function. Ann Transplant. 2019;24:432-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 91. | Lozanovski VJ, Döhler B, Weiss KH, Mehrabi A, Süsal C. The Differential Influence of Cold Ischemia Time on Outcome After Liver Transplantation for Different Indications-Who Is at Risk? Front Immunol. 2020;11:892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 92. | Thabut G, Vinatier I, Brugière O, Lesèche G, Loirat P, Bisson A, Marty J, Fournier M, Mal H. Influence of preservation solution on early graft failure in clinical lung transplantation. Am J Respir Crit Care Med. 2001;164:1204-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 93. | Novick RJ, Bennett LE, Meyer DM, Hosenpud JD. Influence of graft ischemic time and donor age on survival after lung transplantation. J Heart Lung Transplant. 1999;18:425-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 106] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 94. | Bund M, Strüber M, Heine J, Jaeger K, Wahlers T, Haverich A, Piepenbrock S. Effect of lung allograft ischaemia duration on postreperfusion graft function and postoperative course. Thorac Cardiovasc Surg. 1998;46:93-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 95. | Grimm JC, Valero V 3rd, Kilic A, Magruder JT, Merlo CA, Shah PD, Shah AS. Association Between Prolonged Graft Ischemia and Primary Graft Failure or Survival Following Lung Transplantation. JAMA Surg. 2015;150:547-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 96. | Chambers DC, Yusen RD, Cherikh WS, Goldfarb SB, Kucheryavaya AY, Khusch K, Levvey BJ, Lund LH, Meiser B, Rossano JW, Stehlik J; International Society for Heart and Lung Transplantation. The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Lung And Heart-Lung Transplantation Report-2017; Focus Theme: Allograft ischemic time. J Heart Lung Transplant. 2017;36:1047-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 453] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 97. | Snell GI, Rabinov M, Griffiths A, Williams T, Ugoni A, Salamonsson R, Esmore D. Pulmonary allograft ischemic time: an important predictor of survival after lung transplantation. J Heart Lung Transplant. 1996;15:160-168. [PubMed] |
| 98. | Gammie JS, Stukus DR, Pham SM, Hattler BG, McGrath MF, McCurry KR, Griffith BP, Keenan RJ. Effect of ischemic time on survival in clinical lung transplantation. Ann Thorac Surg. 1999;68:2015-9; discussion 2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 99. | Ghaidan H, Fakhro M, Lindstedt S. Impact of allograft ischemic time on long-term survival in lung transplantation: a Swedish monocentric study. Scand Cardiovasc J. 2020;54:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 100. | Sandoval E, Fernández-Cisneros A, Boada M. DCD lungs: is it all the same? Ann Cardiothorac Surg. 2020;9:54-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 101. | Halazun KJ, Al-Mukhtar A, Aldouri A, Willis S, Ahmad N. Warm ischemia in transplantation: search for a consensus definition. Transplant Proc. 2007;39:1329-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 102. | Manara AR, Murphy PG, O'Callaghan G. Donation after circulatory death. Br J Anaesth. 2012;108 Suppl 1:i108-i121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 200] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 103. | Kayano K, Toda K, Naka Y, Pinsky DJ. Identification of optimal conditions for lung graft storage with Euro-Collins solution by use of a rat orthotopic lung transplant model. Circulation. 1999;100:II257-II261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 104. | DeCampos KN, Keshavjee S, Liu M, Slutsky AS. Optimal inflation volume for hypothermic preservation of rat lungs. J Heart Lung Transplant. 1998;17:599-607. [PubMed] |
| 105. | Healey A, Watanabe Y, Mills C, Stoncius M, Lavery S, Johnson K, Sanderson R, Humar A, Yeung J, Donahoe L, Pierre A, de Perrot M, Yasufuku K, Waddell TK, Keshavjee S, Cypel M. Initial lung transplantation experience with uncontrolled donation after cardiac death in North America. Am J Transplant. 2020;20:1574-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 106. | Villavicencio MA, Axtell AL, Spencer PJ, Heng EE, Kilmarx S, Dalpozzal N, Funamoto M, Roy N, Osho A, Melnitchouk S, D'Alessandro DA, Tolis G, Astor T. Lung Transplantation From Donation After Circulatory Death: United States and Single-Center Experience. Ann Thorac Surg. 2018;106:1619-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 107. | Sabashnikov A, Patil NP, Popov AF, Soresi S, Zych B, Weymann A, Mohite PN, García Sáez D, Zeriouh M, Wahlers T, Choi YH, Wippermann J, Wittwer T, De Robertis F, Bahrami T, Amrani M, Simon AR. Long-term results after lung transplantation using organs from circulatory death donors: a propensity score-matched analysis†. Eur J Cardiothorac Surg. 2016;49:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 108. | Erasmus ME, van Raemdonck D, Akhtar MZ, Neyrinck A, de Antonio DG, Varela A, Dark J. DCD lung donation: donor criteria, procedural criteria, pulmonary graft function validation, and preservation. Transpl Int. 2016;29:790-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 109. | Krutsinger D, Reed RM, Blevins A, Puri V, De Oliveira NC, Zych B, Bolukbas S, Van Raemdonck D, Snell GI, Eberlein M. Lung transplantation from donation after cardiocirculatory death: a systematic review and meta-analysis. J Heart Lung Transplant. 2015;34:675-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 110. | Copeland H, Hayanga JWA, Neyrinck A, MacDonald P, Dellgren G, Bertolotti A, Khuu T, Burrows F, Copeland JG, Gooch D, Hackmann A, Hormuth D, Kirk C, Linacre V, Lyster H, Marasco S, McGiffin D, Nair P, Rahmel A, Sasevich M, Schweiger M, Siddique A, Snyder TJ, Stansfield W, Tsui S, Orr Y, Uber P, Venkateswaran R, Kukreja J, Mulligan M. Donor heart and lung procurement: A consensus statement. J Heart Lung Transplant. 2020;39:501-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 128] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 111. | Oechslin P, Zalunardo MP, Inci I, Schlaepfer M, Grande B. Established and potential predictors of blood loss during lung transplant surgery. J Thorac Dis. 2018;10:3845-3848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 112. | Semple JW, Rebetz J, Kapur R. Transfusion-associated circulatory overload and transfusion-related acute lung injury. Blood. 2019;133:1840-1853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 183] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 113. | Weber D, Cottini SR, Locher P, Wenger U, Stehberger PA, Fasshauer M, Schuepbach RA, Béchir M. Association of intraoperative transfusion of blood products with mortality in lung transplant recipients. Perioper Med (Lond). 2013;2:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 114. | Björkbom E, Hämmäinen P, Schramko A. Effects of Perioperative Fluid Replacement Therapy in Lung Transplant Patients. Exp Clin Transplant. 2017;15:78-81. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 115. | Cunningham PS, Maidstone R, Durrington HJ, Venkateswaran RV, Cypel M, Keshavjee S, Gibbs JE, Loudon AS, Chow CW, Ray DW, Blaikley JF. Incidence of primary graft dysfunction after lung transplantation is altered by timing of allograft implantation. Thorax. 2019;74:413-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 116. | Griffin P, Dimitry JM, Sheehan PW, Lananna BV, Guo C, Robinette ML, Hayes ME, Cedeño MR, Nadarajah CJ, Ezerskiy LA, Colonna M, Zhang J, Bauer AQ, Burris TP, Musiek ES. Circadian clock protein Rev-erbα regulates neuroinflammation. Proc Natl Acad Sci USA. 2019;116:5102-5107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 190] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 117. | Montaigne D, Marechal X, Modine T, Coisne A, Mouton S, Fayad G, Ninni S, Klein C, Ortmans S, Seunes C, Potelle C, Berthier A, Gheeraert C, Piveteau C, Deprez R, Eeckhoute J, Duez H, Lacroix D, Deprez B, Jegou B, Koussa M, Edme JL, Lefebvre P, Staels B. Daytime variation of perioperative myocardial injury in cardiac surgery and its prevention by Rev-Erbα antagonism: a single-centre propensity-matched cohort study and a randomised study. Lancet. 2018;391:59-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 235] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 118. | Ikeda R, Tsuchiya Y, Koike N, Umemura Y, Inokawa H, Ono R, Inoue M, Sasawaki Y, Grieten T, Okubo N, Ikoma K, Fujiwara H, Kubo T, Yagita K. REV-ERBα and REV-ERBβ function as key factors regulating Mammalian Circadian Output. Sci Rep. 2019;9:10171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 119. | Ren D, Kaleekal TS, Graviss EA, Nguyen DT, Sinha N, Goodarzi A, Agboli I, Suarez EE, Loebe M, Scheinin SA, Bruckner BA. Retransplantation Outcomes at a Large Lung Transplantation Program. Transplant Direct. 2018;4:e404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 120. | Wallinder A, Danielsson C, Magnusson J, Riise GC, Dellgren G. Outcomes and Long-term Survival After Pulmonary Retransplantation: A Single-Center Experience. Ann Thorac Surg. 2019;108:1037-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 121. | Osaki S, Maloney JD, Meyer KC, Cornwell RD, Edwards NM, De Oliveira NC. Redo lung transplantation for acute and chronic lung allograft failure: long-term follow-up in a single center. Eur J Cardiothorac Surg. 2008;34:1191-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 122. | Aigner C, Jaksch P, Taghavi S, Lang G, Reza-Hoda MA, Wisser W, Klepetko W. Pulmonary retransplantation: is it worth the effort? J Heart Lung Transplant. 2008;27:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 123. | Schumer EM, Rice JD, Kistler AM, Trivedi JR, Black MC, Bousamra M 2nd, van Berkel V. Single Versus Double Lung Retransplantation Does Not Affect Survival Based on Previous Transplant Type. Ann Thorac Surg. 2017;103:236-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 124. | Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8487] [Cited by in RCA: 8327] [Article Influence: 333.1] [Reference Citation Analysis (3)] |
| 125. | Barnes L, Reed RM, Parekh KR, Bhama JK, Pena T, Rajagopal S, Schmidt GA, Klesney-Tait JA, Eberlein M. Mechanical ventilation for the lung transplant recipient. Curr Pulmonol Rep. 2015;4:88-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 126. | Petrucci N, De Feo C. Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database Syst Rev. 2013;2013:CD003844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 127. | Chacko B, Peter JV, Tharyan P, John G, Jeyaseelan L. Pressure-controlled versus volume-controlled ventilation for acute respiratory failure due to acute lung injury (ALI) or acute respiratory distress syndrome (ARDS). Cochrane Database Syst Rev. 2015;1:CD008807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 128. | Tague LK, Bedair B, Witt C, Byers DE, Vazquez-Guillamet R, Kulkarni H, Alexander-Brett J, Nava R, Puri V, Kreisel D, Trulock EP, Gelman A, Hachem RR. Lung protective ventilation based on donor size is associated with a lower risk of severe primary graft dysfunction after lung transplantation. J Heart Lung Transplant. 2021;40:1212-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 129. | Pierre AF, DeCampos KN, Liu M, Edwards V, Cutz E, Slutsky AS, Keshavjee SH. Rapid reperfusion causes stress failure in ischemic rat lungs. J Thorac Cardiovasc Surg. 1998;116:932-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 130. | Ardehali A, Laks H, Russell H, Levine M, Shpiner R, Lackey S, Ross D. Modified reperfusion and ischemia-reperfusion injury in human lung transplantation. J Thorac Cardiovasc Surg. 2003;126:1929-1934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 131. | Kagawa H, Morita K, Nagahori R, Shinohara G, Kinouchi K, Hashimoto K. Prevention of ischemia/reperfusion-induced pulmonary dysfunction after cardiopulmonary bypass with terminal leukocyte-depleted lung reperfusion. J Thorac Cardiovasc Surg. 2010;139:174-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 132. | Noda K, Tane S, Haam SJ, D'Cunha J, Hayanga AJ, Luketich JD, Shigemura N. Targeting Circulating Leukocytes and Pyroptosis During Ex Vivo Lung Perfusion Improves Lung Preservation. Transplantation. 2017;101:2841-2849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 133. | Date H, Triantafillou AN, Trulock EP, Pohl MS, Cooper JD, Patterson GA. Inhaled nitric oxide reduces human lung allograft dysfunction. J Thorac Cardiovasc Surg. 1996;111:913-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 150] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 134. | Gebistorf F, Karam O, Wetterslev J, Afshari A. Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) in children and adults. Cochrane Database Syst Rev. 2016;2016:CD002787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 136] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 135. | Moreno I, Vicente R, Mir A, León I, Ramos F, Vicente JL, Barbera M. Effects of inhaled nitric oxide on primary graft dysfunction in lung transplantation. Transplant Proc. 2009;41:2210-2212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 136. | Yerebakan C, Ugurlucan M, Bayraktar S, Bethea BT, Conte JV. Effects of inhaled nitric oxide following lung transplantation. J Card Surg. 2009;24:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 137. | Bhandary S, Stoicea N, Joseph N, Whitson B, Essandoh M. Pro: Inhaled Pulmonary Vasodilators Should Be Used Routinely in the Management of Patients Undergoing Lung Transplantation. J Cardiothorac Vasc Anesth. 2017;31:1123-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 138. | Shargall Y, Guenther G, Ahya VN, Ardehali A, Singhal A, Keshavjee S; ISHLT Working Group on Primary Lung Graft Dysfunction. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part VI: treatment. J Heart Lung Transplant. 2005;24:1489-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 139. | de Perrot M, Fischer S, Liu M, Jin R, Bai XH, Waddell TK, Keshavjee S. Prostaglandin E1 protects lung transplants from ischemia-reperfusion injury: a shift from pro- to anti-inflammatory cytokines. Transplantation. 2001;72:1505-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 140. | Toyoda Y, Thacker J, Santos R, Nguyen D, Bhama J, Bermudez C, Kormos R, Johnson B, Crespo M, Pilewski J, Teuteberg J, Alvarez R, Mathier M, McNamara D, McCurry K, Zenati M, Hattler B. Long-term outcome of lung and heart-lung transplantation for idiopathic pulmonary arterial hypertension. Ann Thorac Surg. 2008;86:1116-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 141. | Chiang CH, Wu K, Yu CP, Yan HC, Perng WC, Wu CP. Hypothermia and prostaglandin E(1) produce synergistic attenuation of ischemia-reperfusion lung injury. Am J Respir Crit Care Med. 1999;160:1319-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 142. | Riva CM, Morganroth ML, Ljungman AG, Schoeneich SO, Marks RM, Todd RF 3rd, Ward PA, Boxer LA. Iloprost inhibits neutrophil-induced lung injury and neutrophil adherence to endothelial monolayers. Am J Respir Cell Mol Biol. 1990;3:301-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 60] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 143. | Lee SH, Lee JG, Lee CY, Kim N, Chang MY, You YC, Kim HJ, Paik HC, Oh YJ. Effects of intraoperative inhaled iloprost on primary graft dysfunction after lung transplantation: A retrospective single center study. Medicine (Baltimore). 2016;95:e3975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 144. | Kawashima M, Nakamura T, Schneider S, Vollmar B, Lausberg HF, Bauer M, Menger MD, Schäfers HJ. Iloprost ameliorates post-ischemic lung reperfusion injury and maintains an appropriate pulmonary ET-1 balance. J Heart Lung Transplant. 2003;22:794-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 145. | Koksel O, Ozdulger A, Aytacoglu B, Tamer L, Polat A, Sucu N, Yildirim C, Degirmenci U, Kanik A. The influence of iloprost on acute lung injury induced by hind limb ischemia-reperfusion in rats. Pulm Pharmacol Ther. 2005;18:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 146. | Amital A, Shitrit D, Raviv Y, Saute M, Medalion B, Bakal L, Kramer MR. The use of surfactant in lung transplantation. Transplantation. 2008;86:1554-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 147. | Amital A, Shitrit D, Raviv Y, Saute M, Bakal I, Medalion B, Kramer MR. Surfactant as salvage therapy in life threatening primary graft dysfunction in lung transplantation. Eur J Cardiothorac Surg. 2009;35:299-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 148. | Wigfield CH, Lindsey JD, Steffens TG, Edwards NM, Love RB. Early institution of extracorporeal membrane oxygenation for primary graft dysfunction after lung transplantation improves outcome. J Heart Lung Transplant. 2007;26:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 149. | Bermudez CA, Adusumilli PS, McCurry KR, Zaldonis D, Crespo MM, Pilewski JM, Toyoda Y. Extracorporeal membrane oxygenation for primary graft dysfunction after lung transplantation: long-term survival. Ann Thorac Surg. 2009;87:854-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 150. | Hartwig MG, Appel JZ 3rd, Cantu E 3rd, Simsir S, Lin SS, Hsieh CC, Walczak R, Palmer SM, Davis RD Jr. Improved results treating lung allograft failure with venovenous extracorporeal membrane oxygenation. Ann Thorac Surg. 2005;80:1872-1879; discussion 1879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 151. | Hartwig MG, Walczak R, Lin SS, Davis RD. Improved survival but marginal allograft function in patients treated with extracorporeal membrane oxygenation after lung transplantation. Ann Thorac Surg. 2012;93:366-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 152. | Cheng R, Hachamovitch R, Kittleson M, Patel J, Arabia F, Moriguchi J, Esmailian F, Azarbal B. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1,866 adult patients. Ann Thorac Surg. 2014;97:610-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 635] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 153. | Force SD, Miller DL, Pelaez A, Ramirez AM, Vega D, Barden B, Lawrence EC. Outcomes of delayed chest closure after bilateral lung transplantation. Ann Thorac Surg. 2006;81:2020-2024; discussion 2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 154. | Shigemura N, Orhan Y, Bhama JK, D'Cunha J, Zaldonis D, Pilewski JM, Luketich JD, Bermudez CA. Delayed chest closure after lung transplantation: techniques, outcomes, and strategies. J Heart Lung Transplant. 2014;33:741-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 155. | Shimoyama T, Tabuchi N, Kojima K, Akamatsu H, Arai H, Tanaka H, Sunamori M. Aprotinin attenuated ischemia-reperfusion injury in an isolated rat lung model after 18-hours preservation. Eur J Cardiothorac Surg. 2005;28:581-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 156. | Eren S, Esme H, Balci AE, Cakir O, Buyukbayram H, Eren MN, Erdinc L, Satici O. The effect of aprotinin on ischemia-reperfusion injury in an in situ normothermic ischemic lung model. Eur J Cardiothorac Surg. 2003;23:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 157. | Roberts RF, Nishanian GP, Carey JN, Darbinian SH, Kim JD, Sakamaki Y, Chang JY, Starnes VA, Barr ML. Addition of aprotinin to organ preservation solutions decreases lung reperfusion injury. Ann Thorac Surg. 1998;66:225-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 158. | Mathias MA, Tribble CG, Dietz JF, Nguyen RP, Shockey KS, Kern JA, Kron IL. Aprotinin improves pulmonary function during reperfusion in an isolated lung model. Ann Thorac Surg. 2000;70:1671-1674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 159. | Bittner HB, Richter M, Kuntze T, Rahmel A, Dahlberg P, Hertz M, Mohr FW. Aprotinin decreases reperfusion injury and allograft dysfunction in clinical lung transplantation. Eur J Cardiothorac Surg. 2006;29:210-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 160. | Scholten EL, Beitler JR, Prisk GK, Malhotra A. Treatment of ARDS With Prone Positioning. Chest. 2017;151:215-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 248] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 161. | Lai-Fook SJ, Rodarte JR. Pleural pressure distribution and its relationship to lung volume and interstitial pressure. J Appl Physiol (1985). 1991;70:967-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 162. | Gattinoni L, Chiumello D, Rossi S. COVID-19 pneumonia: ARDS or not? Crit Care. 2020;24:154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 372] [Cited by in RCA: 433] [Article Influence: 86.6] [Reference Citation Analysis (0)] |
| 163. | Petrone P, Brathwaite CEM, Joseph DK. Prone ventilation as treatment of acute respiratory distress syndrome related to COVID-19. Eur J Trauma Emerg Surg. 2021;47:1017-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 164. | Feltracco P, Serra E, Barbieri S, Milevoj M, Michieletto E, Carollo C, Rea F, Zanus G, Boetto R, Ori C. Noninvasive high-frequency percussive ventilation in the prone position after lung transplantation. Transplant Proc. 2012;44:2016-2021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 165. | Cypel M, Yeung JC, Liu M, Anraku M, Chen F, Karolak W, Sato M, Laratta J, Azad S, Madonik M, Chow CW, Chaparro C, Hutcheon M, Singer LG, Slutsky AS, Yasufuku K, de Perrot M, Pierre AF, Waddell TK, Keshavjee S. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med. 2011;364:1431-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 743] [Cited by in RCA: 815] [Article Influence: 58.2] [Reference Citation Analysis (0)] |
| 166. | Moreno Garijo J, Roscoe A. Ex-vivo lung perfusion. Curr Opin Anaesthesiol. 2020;33:50-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 167. | Steen S, Sjöberg T, Pierre L, Liao Q, Eriksson L, Algotsson L. Transplantation of lungs from a non-heart-beating donor. Lancet. 2001;357:825-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 462] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 168. | Cypel M, Yeung JC, Hirayama S, Rubacha M, Fischer S, Anraku M, Sato M, Harwood S, Pierre A, Waddell TK, de Perrot M, Liu M, Keshavjee S. Technique for prolonged normothermic ex vivo lung perfusion. J Heart Lung Transplant. 2008;27:1319-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 401] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 169. | Andreasson AS, Dark JH, Fisher AJ. Ex vivo lung perfusion in clinical lung transplantation--state of the art. Eur J Cardiothorac Surg. 2014;46:779-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 170. | Nur E, Brandjes DP, Schnog JJ, Otten HM, Fijnvandraat K, Schalkwijk CG, Biemond BJ; CURAMA Study Group. Plasma levels of advanced glycation end products are associated with haemolysis-related organ complications in sickle cell patients. Br J Haematol. 2010;151:62-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 171. | Sanchez PG, Davis RD, D'Ovidio F, Cantu E, Weyant M, Camp P, Griffith B. The NOVEL Lung Trial One-Year Outcomes. J Heart Lung Transplant. 2014;33: S71-S72. [RCA] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 172. | Warnecke G, Van Raemdonck D, Smith MA, Massard G, Kukreja J, Rea F, Loor G, De Robertis F, Nagendran J, Dhital KK, Moradiellos Díez FJ, Knosalla C, Bermudez CA, Tsui S, McCurry K, Wang IW, Deuse T, Lesèche G, Thomas P, Tudorache I, Kühn C, Avsar M, Wiegmann B, Sommer W, Neyrinck A, Schiavon M, Calabrese F, Santelmo N, Olland A, Falcoz PE, Simon AR, Varela A, Madsen JC, Hertz M, Haverich A, Ardehali A. Normothermic ex-vivo preservation with the portable Organ Care System Lung device for bilateral lung transplantation (INSPIRE): a randomised, open-label, non-inferiority, phase 3 study. Lancet Respir Med. 2018;6:357-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 158] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 173. | Fisher A, Andreasson A, Chrysos A, Lally J, Mamasoula C, Exley C, Wilkinson J, Qian J, Watson G, Lewington O, Chadwick T, McColl E, Pearce M, Mann K, McMeekin N, Vale L, Tsui S, Yonan N, Simon A, Marczin N, Mascaro J, Dark J. An observational study of Donor Ex Vivo Lung Perfusion in UK lung transplantation: DEVELOP-UK. Health Technol Assess. 2016;20:1-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 174. | Loor G, Warnecke G, Villavicencio MA, Smith MA, Kukreja J, Ardehali A, Hartwig M, Daneshmand MA, Hertz MI, Huddleston S, Haverich A, Madsen JC, Van Raemdonck D. Portable normothermic ex-vivo lung perfusion, ventilation, and functional assessment with the Organ Care System on donor lung use for transplantation from extended-criteria donors (EXPAND): a single-arm, pivotal trial. Lancet Respir Med. 2019;7:975-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 175. | Hamilton BCS, Dincheva GR, Zhuo H, Golden JA, Brzezinski M, Singer JP, Matthay MA, Kukreja J. Elevated donor plasminogen activator inhibitor-1 levels and the risk of primary graft dysfunction. Clin Transplant. 2018;32:e13210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 176. | Fisher AJ, Donnelly SC, Hirani N, Haslett C, Strieter RM, Dark JH, Corris PA. Elevated levels of interleukin-8 in donor lungs is associated with early graft failure after lung transplantation. Am J Respir Crit Care Med. 2001;163:259-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 192] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 177. | Salama M, Andrukhova O, Hoda MA, Taghavi S, Jaksch P, Heinze G, Klepetko W, Aharinejad S. Concomitant endothelin-1 overexpression in lung transplant donors and recipients predicts primary graft dysfunction. Am J Transplant. 2010;10:628-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 178. | Riera J, Senna A, Cubero M, Roman A, Rello J; Vall d'Hebron Lung Transplant Study Group Investigators. Primary Graft Dysfunction and Mortality Following Lung Transplantation: A Role for Proadrenomedullin Plasma Levels. Am J Transplant. 2016;16:634-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 179. | Shah RJ, Bellamy SL, Localio AR, Wickersham N, Diamond JM, Weinacker A, Lama VN, Bhorade S, Belperio JA, Crespo M, Demissie E, Kawut SM, Wille KM, Lederer DJ, Lee JC, Palmer SM, Orens J, Reynolds J, Shah A, Wilkes DS, Ware LB, Christie JD. A panel of lung injury biomarkers enhances the definition of primary graft dysfunction (PGD) after lung transplantation. J Heart Lung Transplant. 2012;31:942-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 180. | Mordant P, Nakajima D, Kalaf R, Iskender I, Maahs L, Behrens P, Coutinho R, Iyer RK, Davies JE, Cypel M, Liu M, Waddell TK, Keshavjee S. Mesenchymal stem cell treatment is associated with decreased perfusate concentration of interleukin-8 during ex vivo perfusion of donor lungs after 18-hour preservation. J Heart Lung Transplant. 2016;35:1245-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 181. | McAuley DF, Curley GF, Hamid UI, Laffey JG, Abbott J, McKenna DH, Fang X, Matthay MA, Lee JW. Clinical grade allogeneic human mesenchymal stem cells restore alveolar fluid clearance in human lungs rejected for transplantation. Am J Physiol Lung Cell Mol Physiol. 2014;306:L809-L815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 182. | Miceli V, Bertani A, Chinnici CM, Bulati M, Pampalone M, Amico G, Carcione C, Schmelzer E, Gerlach JC, Conaldi PG. Conditioned Medium from Human Amnion-Derived Mesenchymal Stromal/Stem Cells Attenuating the Effects of Cold Ischemia-Reperfusion Injury in an In Vitro Model Using Human Alveolar Epithelial Cells. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 183. | Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1919] [Cited by in RCA: 1818] [Article Influence: 113.6] [Reference Citation Analysis (1)] |
| 184. | Cocking S, Jones H, Cable NT, Thijssen DH. Chapter 19 - Enhancing Sports Performance Through Ischemic Preconditioning: Moderating Factors and Potential Mechanisms. In: Rattan SIS, Kyriazis M, editors. The Science of Hormesis in Health and Longevity. Academic Press; 2019: 213-222. [DOI] [Full Text] |
| 185. | Li C, Xu M, Wu Y, Li YS, Huang WQ, Liu KX. Limb remote ischemic preconditioning attenuates lung injury after pulmonary resection under propofol-remifentanil anesthesia: a randomized controlled study. Anesthesiology. 2014;121:249-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 186. | Li C, Li YS, Xu M, Wen SH, Yao X, Wu Y, Huang CY, Huang WQ, Liu KX. Limb remote ischemic preconditioning for intestinal and pulmonary protection during elective open infrarenal abdominal aortic aneurysm repair: a randomized controlled trial. Anesthesiology. 2013;118:842-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 187. | Jensen HA, Loukogeorgakis S, Yannopoulos F, Rimpiläinen E, Petzold A, Tuominen H, Lepola P, Macallister RJ, Deanfield JE, Mäkelä T, Alestalo K, Kiviluoma K, Anttila V, Tsang V, Juvonen T. Remote ischemic preconditioning protects the brain against injury after hypothermic circulatory arrest. Circulation. 2011;123:714-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 188. | Hausenloy DJ, Mwamure PK, Venugopal V, Harris J, Barnard M, Grundy E, Ashley E, Vichare S, Di Salvo C, Kolvekar S, Hayward M, Keogh B, MacAllister RJ, Yellon DM. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet. 2007;370:575-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 523] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 189. | Kim JC, Shim JK, Lee S, Yoo YC, Yang SY, Kwak YL. Effect of combined remote ischemic preconditioning and postconditioning on pulmonary function in valvular heart surgery. Chest. 2012;142:467-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 190. | Hausenloy DJ, Candilio L, Evans R, Ariti C, Jenkins DP, Kolvekar S, Knight R, Kunst G, Laing C, Nicholas J, Pepper J, Robertson S, Xenou M, Clayton T, Yellon DM; ERICCA Trial Investigators. Remote Ischemic Preconditioning and Outcomes of Cardiac Surgery. N Engl J Med. 2015;373:1408-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 559] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 191. | Meybohm P, Bein B, Brosteanu O, Cremer J, Gruenewald M, Stoppe C, Coburn M, Schaelte G, Böning A, Niemann B, Roesner J, Kletzin F, Strouhal U, Reyher C, Laufenberg-Feldmann R, Ferner M, Brandes IF, Bauer M, Stehr SN, Kortgen A, Wittmann M, Baumgarten G, Meyer-Treschan T, Kienbaum P, Heringlake M, Schön J, Sander M, Treskatsch S, Smul T, Wolwender E, Schilling T, Fuernau G, Hasenclever D, Zacharowski K; RIPHeart Study Collaborators. A Multicenter Trial of Remote Ischemic Preconditioning for Heart Surgery. N Engl J Med. 2015;373:1397-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 496] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 192. | Liu Q, Rehman H, Krishnasamy Y, Lemasters JJ, Zhong Z. Ischemic preconditioning attenuates acute lung injury after partial liver transplantation. Int J Physiol Pathophysiol Pharmacol. 2018;10:83-94. [PubMed] |
| 193. | Olguner C, Koca U, Kar A, Karci A, Işlekel H, Canyilmaz M, Mavioĝlu O, Kizildaĝ S, Unlü G, Elar Z. Ischemic preconditioning attenuates the lipid peroxidation and remote lung injury in the rat model of unilateral lower limb ischemia reperfusion. Acta Anaesthesiol Scand. 2006;50:150-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 194. | Jan WC, Chen CH, Tsai PS, Huang CJ. Limb ischemic preconditioning mitigates lung injury induced by haemorrhagic shock/resuscitation in rats. Resuscitation. 2011;82:760-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 195. | Luo N, Liu J, Chen Y, Li H, Hu Z, Abbott GW. Remote ischemic preconditioning STAT3-dependently ameliorates pulmonary ischemia/reperfusion injury. PLoS One. 2018;13:e0196186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 196. | Gasparri RI, Jannis NC, Flameng WJ, Lerut TE, Van Raemdonck DE. Ischemic preconditioning enhances donor lung preservation in the rabbit. Eur J Cardiothorac Surg. 1999;16:639-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 197. | García-de-la-Asunción J, Bruno L, Perez-Griera J, Galan G, Morcillo A, Wins R, García-Del-Olmo E, Guijarro R, Sarriá B, Martí F, Soro M, Belda FJ. Remote Ischemic Preconditioning Decreases Oxidative Lung Damage After Pulmonary Lobectomy: A Single-Center Randomized, Double-Blind, Controlled Trial. Anesth Analg. 2017;125:499-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 198. | Lin E, Snell GI, Levvey BJ, Mitchell LE, Marasco S, Kotsimbos T, Mifsud NA, Sharland AF, Myles P. 532 Remote Ischemic Pre-Conditioning (RIPC) To Improve Lung Transplant (LTx) Outcomes: A Randomized, Placebo-Controlled, Clinical and Mechanistic Trial. J Heart Lung Transplant. 2012;31:S184-S185. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 199. | Lin E, Snell GI, Levvey BJ, Mifsud N, Paul M, Buckland MR, Gooi J, Marasco S, Sharland AF, Myles PS. Safety, feasibility, and effect of remote ischemic conditioning in patients undergoing lung transplantation. J Heart Lung Transplant. 2014;33:1139-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 200. | Seto AH. 3 - Interventional Pharmacology. In: Kern MJ, Sorajja P, Lim MJ, editors. The Interventional Cardiac Catheterization Handbook (Fourth Edition): Elsevier. 2018: 88-106. [DOI] [Full Text] |
| 201. | Emaminia A, Lapar DJ, Zhao Y, Steidle JF, Harris DA, Laubach VE, Linden J, Kron IL, Lau CL. Adenosine A₂A agonist improves lung function during ex vivo lung perfusion. Ann Thorac Surg. 2011;92:1840-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 202. | Wagner CE, Pope NH, Charles EJ, Huerter ME, Sharma AK, Salmon MD, Carter BT, Stoler MH, Lau CL, Laubach VE, Kron IL. Ex vivo lung perfusion with adenosine A2A receptor agonist allows prolonged cold preservation of lungs donated after cardiac death. J Thorac Cardiovasc Surg. 2016;151:538-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 203. | Gazoni LM, Laubach VE, Mulloy DP, Bellizzi A, Unger EB, Linden J, Ellman PI, Lisle TC, Kron IL. Additive protection against lung ischemia-reperfusion injury by adenosine A2A receptor activation before procurement and during reperfusion. J Thorac Cardiovasc Surg. 2008;135:156-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 204. | Lau CL, Beller JP, Boys JA, Zhao Y, Phillips J, Cosner M, Conaway MR, Petroni G, Charles EJ, Mehaffey JH, Mannem HC, Kron IL, Krupnick AS, Linden J. Adenosine A2A receptor agonist (regadenoson) in human lung transplantation. J Heart Lung Transplant. 2020;39:563-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 205. | Emtiazjoo AM, Hu H, Lu L, Brantly ML. Alpha-1 Antitrypsin Attenuates Acute Lung Allograft Injury in a Rat Lung Transplant Model. Transplant Direct. 2019;5:e458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 206. | Götzfried J, Smirnova NF, Morrone C, Korkmaz B, Yildirim AÖ, Eickelberg O, Jenne DE. Preservation with α(1)-antitrypsin improves primary graft function of murine lung transplants. J Heart Lung Transplant. 2018;37:1021-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 207. | Strüber M, Hagl C, Hirt SW, Cremer J, Harringer W, Haverich A. C1-esterase inhibitor in graft failure after lung transplantation. Intensive Care Med. 1999;25:1315-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 208. | Salvatierra A, Velasco F, Rodriguez M, Alvarez A, Lopez-Pedrera R, Ramirez R, Carracedo J, Lopez-Rubio F, Lopez-Pujol J, Guerrero R. C1-esterase inhibitor prevents early pulmonary dysfunction after lung transplantation in the dog. Am J Respir Crit Care Med. 1997;155:1147-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 209. | Sommer W, Tudorache I, Kühn C, Avsar M, Salman J, Ius F, Gras C, Weber P, Welte T, Gottlieb J, Haverich A, Warnecke G. C1-esterase-inhibitor for primary graft dysfunction in lung transplantation. Transplantation. 2014;97:1185-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 210. | Cassivi SD, Liu M, Boehler A, Tanswell AK, Slutsky AS, Keshavjee S, Todd STRJ. Transgene expression after adenovirus-mediated retransfection of rat lungs is increased and prolonged by transplant immunosuppression. J Thorac Cardiovasc Surg. 1999;117:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 211. | Fischer S, Liu M, MacLean AA, de Perrot M, Ho M, Cardella JA, Zhang XM, Bai XH, Suga M, Imai Y, Keshavjee S. In vivo transtracheal adenovirus-mediated transfer of human interleukin-10 gene to donor lungs ameliorates ischemia-reperfusion injury and improves early posttransplant graft function in the rat. Hum Gene Ther. 2001;12:1513-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 212. | Machuca TN, Cypel M, Bonato R, Yeung JC, Chun YM, Juvet S, Guan Z, Hwang DM, Chen M, Saito T, Harmantas C, Davidson BL, Waddell TK, Liu M, Keshavjee S. Safety and Efficacy of Ex Vivo Donor Lung Adenoviral IL-10 Gene Therapy in a Large Animal Lung Transplant Survival Model. Hum Gene Ther. 2017;28:757-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |