Published online Mar 31, 2020. doi: 10.5500/wjt.v10.i3.64
Peer-review started: December 4, 2019
First decision: December 12, 2019
Revised: February 10, 2020
Accepted: March 23, 2020
Article in press: March 23, 2020
Published online: March 31, 2020
Processing time: 117 Days and 20.2 Hours
Orthotopic liver transplantation (OLT) is the only treatment for end-stage liver failure; however, graft shortage impedes its applicability. Therefore, studies investigating alternative therapies are plenty. Nevertheless, no study has comprehensively analyzed these therapies from different perspectives.
To summarize the current status of alternative transplantation therapies for OLT and to support future research.
A systematic literature search was performed using PubMed, Cochrane Library and EMBASE for articles published between January 2010 and 2018, using the following MeSH terms: [(liver transplantation) AND cell] OR [(liver transplantation) AND differentiation] OR [(liver transplantation) AND organoid] OR [(liver transplantation) AND xenotransplantation]. Various types of studies describing therapies to replace OLT were retrieved for full-text evaluation. Among them, we selected articles including in vivo transplantation.
A total of 89 studies were selected. There are three principle forms of treatment for liver failure: Xeno-organ transplantation, scaffold-based transplantation, and cell transplantation. Xeno-organ transplantation was covered in 14 articles, scaffold-based transplantation was discussed in 22 articles, and cell transplantation was discussed in 53 articles. Various types of alternative therapies were discussed: Organ liver, 25 articles; adult hepatocytes, 31 articles; fetal hepatocytes, three articles; mesenchymal stem cells (MSCs), 25 articles; embryonic stem cells, one article; and induced pluripotent stem cells, three articles and other sources. Clinical applications were discussed in 12 studies: Cell transplantation using hepatocytes in four studies, five studies using umbilical cord-derived MSCs, three studies using bone marrow-derived MSCs, and two studies using hematopoietic stem cells.
The clinical applications are present only for cell transplantation. Scaffold-based transplantation is a comprehensive treatment combining organ and cell transplantations, which warrants future research to find relevant clinical applications.
Core tip: This systematic review analyzes the current status of transplantation treatments in place of liver organ transplantation from multiple viewpoints. We classified reports into three types: Xeno-organ transplantation, scaffold-based transplantation, and cell transplantation. Clinical application occurred for cell transplantation with hepatocytes and mesenchymal stem cells; however, the effect was limited. On the other hand, scaffold-based transplantation is a comprehensive treatment that combines organ transplantation and cell transplantation. Future research for clinical application is expected. The present article provides researchers with a summary and updated information on recent trends in alternatives to liver transplantation and support for future research.
- Citation: Furuta T, Furuya K, Zheng YW, Oda T. Novel alternative transplantation therapy for orthotopic liver transplantation in liver failure: A systematic review. World J Transplant 2020; 10(3): 64-78
- URL: https://www.wjgnet.com/2220-3230/full/v10/i3/64.htm
- DOI: https://dx.doi.org/10.5500/wjt.v10.i3.64
Liver diseases lead the causes of mortality worldwide, accounting for approximately 1-2 million deaths per annum according to the World Health Organization[1]. Orthotopic liver transplantation (OLT) remains as the only curative therapy for end-stage liver diseases. However, the shortage of donor organs limits its application.
Alternatives to OLT such as liver support systems, including bioartificial livers, and hepatocyte transplantation have been extensively explored; however, none could be adopted in clinical practice[2]. Thus, to overcome the organ shortage, many researchers attempted to find alternatives to the traditional solid-organ transplantation method[3].
Various alternative treatments are available, including organ transplantations from other human beings, transplanting cells from other species, or transplanting processed cells from humans or transplanting processed cells from other species.
Alternative therapies investigated in the past include xenotransplantation, scaffold-based transplantation, and cell transplantation therapies. In particular, the use of animal livers for human patients, i.e., xenotransplantation, has been deemed as a solution for donor shortage. If the organ of other species could be transplanted, there are many advantages about the supply of organ[4]. Although this approach has still several problems, such as immune rejection and coagulopathy, α-1,3-galactosyltransferase gene-knockout (GT-KO) pigs that do not express the α1,3Gal (Gal) antigens have improved the potential of this therapy[5,6]. In fact, it underwent many advancements through genome editing technologies[7].
Scaffold-based transplantation is a novel method, which aims to generate tissues and organs ex vivo or in vivo with biological materials that can be used to repair, regenerate, or even replace malfunctioning tissues and organs. Essentially, to create scaffolds, all the cells from animal organs are removed while retaining the structural, mechanical, and chemical attributes of the native tissue[8]. Then, the human-derived cells are embedded in the scaffold that serves as an ideal container to generate humanized organs.
In parallel, cell transplantation research has undergone vast advancements with the establishment of induced pluripotent stem cells (iPSCs). Clinical human-to-human hepatocyte transplantation following host conditioning has been reported[9]. However, hepatocytes have limitations with respect to proliferation, function, and immunity. Recently, pluripotent or somatic stem cells were used as new sources in place of hepatocytes[10]. Further, researchers tried to direct pluripotent or somatic stem cells toward differentiation into hepatocytes in various studies[11].
Thus, alternative therapies manifest various combinations depending on different resources. Still, no study has comprehensively analyzed these different viewpoints yet, although such studies are instrumental while considering novel alternatives for the future regarding the utility of these kinds of treatments.
Therefore, we aimed to discuss the current status of alternative transplantation therapies to replace liver organ transplantation and to support their research and development.
The methodological approach included the development of selection criteria, defining the search strategies, assessing the study quality, and abstracting the relevant data. The PRISMA statements checklist for reporting a systematic review was followed[12].
This systematic literature review was performed to select articles discussing alternatives to liver organ transplantation. The PubMed, Cochrane Library, and EMBASE were electronically searched for articles published between January 2010 and December 2018, using the following MeSH terms: [(liver transplantation) AND cell] OR [(liver transplantation) AND differentiation] OR [(liver transplantation) AND organoid] OR [(liver transplantation) AND xenotransplantation].
The study selection criteria were defined before initiating data collection to identify eligible studies for the analysis. Only studies written in English were selected. We retrieved all studies in which the primary objective was to evaluate new transplantation therapies in place of OLT for our analysis.
Exclusion criteria were as follows: (1) Studies not including in vivo transplantation; (2) Studies lacking sufficient details; (3) Review articles; (4) Expert opinions; (5) Letters; and (6) Conference summaries.
The titles and abstracts of the retrieved studies were independently and blindly screened for relevance by two reviewers (Furuta T and Furuya K), who assessed the study quality and extracted data. To enhance sensitivity, records were removed only in case both reviewers judged them to be inappropriate. All disagreements were resolved by discussion and consensus. The study design, quality, level of evidence, and the relevance of the studies were analyzed according to the objective of this study.
We classified the reports into three types: Xeno-organ transplantation, scaffold-based transplantation, and cell transplantation. Further, we categorized the source of donor or donor species, recipients, and the clinical applications.
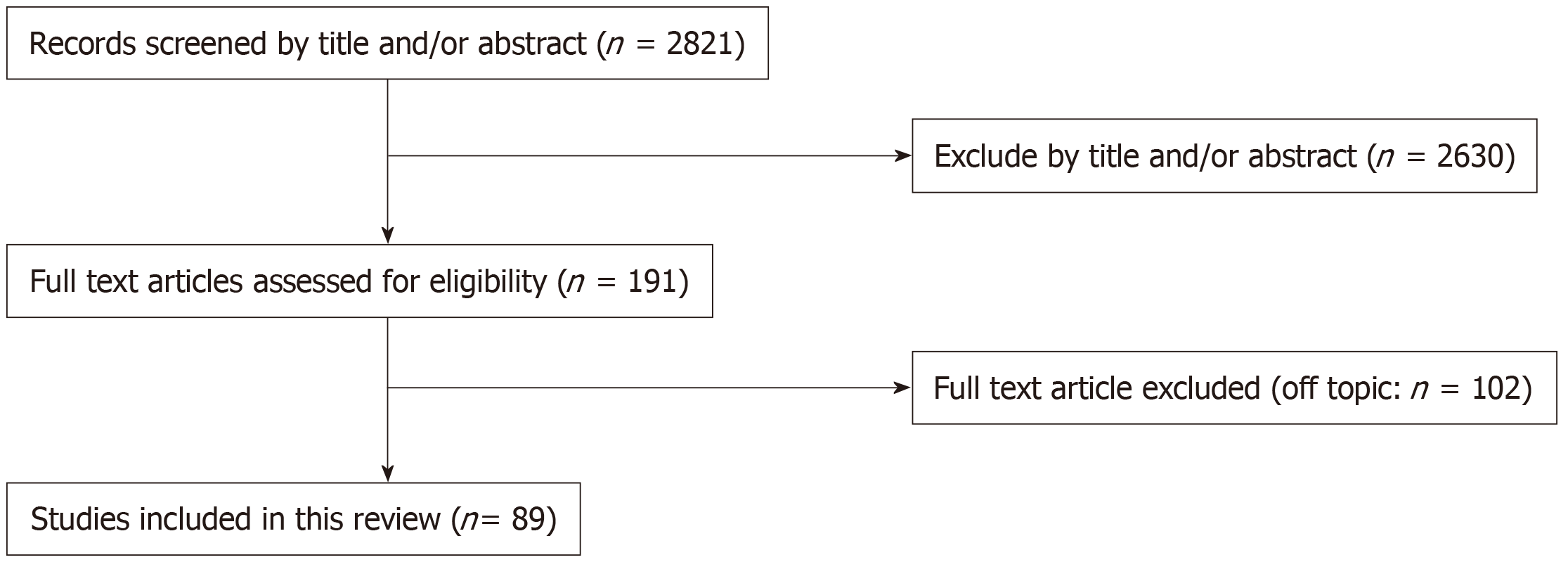
The combined search identified 2821 articles. Of these, 2630 were removed after evaluating the title and abstract. By checking the full text, 89 articles were considered eligible for the systematic review and were analyzed qualitatively and quantitatively. The entire study selection process is summarized in Figure 1.
From our qualitative analysis on the selected articles, there were 14 xeno-organ transplantation studies, 22 scaffold-based transplantation studies, and 53 cell transplantation studies. The study selection is displayed in Tables 1-3[2,5,13-99]. There were various sources of alternative therapy, including organ liver (25 studies), adult hepatocytes (31 studies), fetal hepatocytes (three studies), mesenchymal stem cells (MSCs; 25 studies), embryonic stem cells (ESCs; one study), and iPSCs (three studies) and others (Table 4)[2,5,13-45,48-70,72-99]. Clinical application was discussed in 12 studies. In particular, hepatocyte transplantation was discussed in four studies, umbilical cord derived MSCs (UC-MSCs) transplantation was described in five studies, bone marrow derived MSCs (BM- MSCs) was described three studies and hematopoietic stem cells was described two studies.
| Donor | Recipients (disease, strain etc.) | Outcomes | Year | ||
| Cells | Species | Treatments [co-culture (Co), organoid generated] | |||
| Hepatocytes | Human | - | Human (ALF) | Hepatic function | 2018[13] |
| - | Human (ACLF) | Hepatic function | 2014[22] | ||
| - | Human (metabolic disease) | Engraftment, hepatic function | 2012[23] | ||
| - | Human (oxalosis) | Hepatic function | 2012[24] | ||
| - | Rat (SD) | Hepatic function, survival extension | 2017[25] | ||
| - | Mouse (NOD/SCID) | Alb secretion, engraftment | 2017[26] | ||
| - | Mouse (FRG) | Engraftment, hepatic function | 2013[27] | ||
| - | Mouse (SCID/Alb-uPA) | Analysis of NK cell | 2010[28] | ||
| UC-MSC (human) | Mouse (BALB/c) | Engraftment, hepatic function | 2018[29] | ||
| Rat | - | Mouse (C57BL/6 FRG) | 2018[30] | ||
| - | Rat (Wistar) | Engraftment | 2015[31] | ||
| - | Rat (SD) | Engraftment, hepatic function | 2015[32] | ||
| - | Rat | Engraftment | 2014[33] | ||
| - | Rat (DPP4-) | Engraftment, repopulation | 2014[34] | ||
| - | Rat (An alb) | Engraftment, hepatic function | 2014[35] | ||
| HSCs (Rat), SECs (Rat)/Co | Mouse (C57BL/6) | Engraftment, survival extension | 2014[36] | ||
| - | Rat (SD) | Hepatic function | 2010[37] | ||
| Mouse | Organoid | Mouse (C57BL/6) | Engraftment | 2017[38] | |
| - | Mouse (emdr2−/−) | Engraftment, Repopulation | 2015[39] | ||
| - | Mouse (Fah-/-) | Hepatic function | 2010[40] | ||
| - | Mouse (FVB/N) | Engraftment, analysis of metabolite | 2010[41] | ||
| - | Mouse (C57BL/6) | Engraftment | 2010[42] | ||
| Hepatocytes (fetal) | Rat | - | Rat (DPPIV-) | Engraftment, repopulation | 2018[43] |
| Mouse | - | Mouse (C57BL/6) | Engraftment, hepatic function | 2012[44] | |
| Liver cells | Rabbit | - | Rabbit (New Zealand) | Hepatic function | 2012[45] |
| Hepatic oval cells | Rat | - | Rat (Lewis) | Hepatic function, survival extension | 2013[46] |
| Hepatoma cell line | - | Rat (SD) | Hepatic function, survival extension | 2013[47] | |
| UC-MSCs | Human | - | Human after OLT | Hepatic function, intervention rate | 2017[48] |
| - | Human after OLT | Hepatic function | 2017[49] | ||
| BM-MSCs/BM-MNCs | Human | - | Human (LC) | Hepatic function | 2017[50] |
| - | 2016[51] | ||||
| - | Human (Liver failure) | Hepatic function | 2013[52] | ||
| Rabbit | - | Rabbit | Remodeling | 2011[53] | |
| BM-MSCs/HSCs | Human | - | Human (EPP) | Engraftment | 2010[54] |
| BM-MSC | Human | - | Human (LC) | Engraftment, hepatic function | 2011[55] |
| - | Rat (Wistar) | Hepatic function | 2014[56] | ||
| - | Mouse (SCID) | Engraftment, analysis of glucose | 2017[57] | ||
| - | Mouse (Pfp/Rag2−/−) | Engraftment | 2010[58] | ||
| Rhesus macaque | - | Mouse | Hepatic function | 2018[59] | |
| Rat | - | Rat (SD) | Hepatic function | 2014[60] | |
| BM-MNC-EPC | Rat | - | Rat (SD) | Remodeling | 2012[61] |
| Liver-MSCs | Human | - | Mouse (NOD/SCID) | Engraftment, repopulation | 2011[62] |
| AD-MSCs | Human | - | Mouse (c57/B6) | Analysis of IRI | 2014[63] |
| Mouse | - | Mouse (Swiss CD1) | Repopulation | 2012[64] | |
| AD-MSC-Hep | Mouse | - | Mouse (C57BL/6) | Engraftment | 2015[65] |
| CD34+ cells | Human | - | Human (LC) | Hepatic function | 2015[66] |
| ESCs-Hep | Mouse | - | Mouse (BALB/c) | Engraftment, hepatic function | 2012[67] |
| iPSC-Hep | Human | Organoid | Mouse (Alb-Tk-NOG) | Survival extension, hepatic function | 2017[68] |
| Organoid | Mouse (NOD/SCID) | Engraftment | 2013[69] | ||
| Mouse | - | Mouse (Fah-/- C57Bl/6) | Engraftment | 2010[70] | |
| iMPC-Hep | Human | - | Mouse (FRG) | Engraftment | 2014[71] |
| GPSCs-Hep | Mouse | - | Mouse (Hfe-null) | Engraftment | 2015[72] |
| Liver stem cells | Rat | Organoid | Rat (Fah−/−Il2rg−/−) | Engraftment, hepatic function | 2016[73] |
| Donor organ | Recipients | Outcomes | Year |
| GTKO pig | Tibetan macaques | Cytokine profile | 2017[74] |
| Baboon | Survival extension | 2018[5]; 2017[14]; 2014[75]; 2012[76]; 2010[77] | |
| Analysis of thrombotic microangiopathy | 2016[78] | ||
| Analysis of platelet | 2014[79] | ||
| Analysis of rejection | 2012[80] | ||
| Platelet aggregation | 2012[81] | ||
| Analysis of coagulopathy | 2012 [82] | ||
| Hepatic function | 2010[83] | ||
| Pig | Baboon | Analysis of immunoglobulin | 2018 [84] |
| Rabbit | Porcine, rabbit | Analysis of IgG | 2012[85] |
| Donor | Recipients (strain) | Outcomes | Year | ||
| Scaffold | Species | Seeding cell | |||
| Decellularized organ liver | Human | Mouse (C57BL/6J) | Immunogenicity | 2015[2] | |
| Porcine | Rat (F344) | Immunogenicity | 2013[86] | ||
| Porcine | Immunogenicity | 2013[87] | |||
| Porcine | Engraftment | 2012[88] | |||
| Sheep, rat | Sheep, rat | Engraftment | 2015[89] | ||
| Rat | Hepatocytes (rat), BM- MSCs (Rat) | Rat (Lewis) | Engraftment | 2014[90] | |
| Hepatocytes (rat) | Rat (Lewis) | Engraftment, Hepatic function | 2010[91], 2011[92] | ||
| Mouse | Hematopoietic progenitor cells (mouse) | Mouse (C57Bl/6) | Hepatic function, metabolic function | 2018 [93] | |
| BM-MSCs (mouse) | Mouse (NOD-SCID) | Survival extension, hepatic function | 2014[94] | ||
| Placenta | Human | Liver cells (sheep) | Sheep | Survival extension, hepatic function | 2018[15] |
| Amniotic membrane | Human | AD-MSCs (human) | Mouse | Survival extension, hepatic function | 2015[95] |
| Nonwoven polyglycolic acid scaffolds | Liver cells (human, mouse) | Mouse (NOD/SCID) | Analysis of human metabolite | 2017[19] | |
| 3D hydrogel | Hepatocytes (human) | Mouse (nude) | Engraftment, hepatic function | 2016[16] | |
| Hyaluronan tube | Hepatocytes (rat), adipose-MSCs (human) | Rat (nude) | Engraftment, hepatic function | 2016[17] | |
| Polyethylene glycol hydrogels | Hepatocytes (rat) | Mouse (Nude) | Engraftment | 2015[20] | |
| Microbeads | Hepatocytes (rat) | Rat (SD) | Hepatic function | 2014 [96] | |
| Poly-L-glycolic acid | Hepatocytes (mouse) | Mouse (NOD/SCID) | Engraftment | 2014[21] | |
| Hyaluronan hydrogels | Hepatic stem cells (human) | Mouse (Athymic nude) | Engraftment | 2013[97] | |
| Apatite-fiber scaffold | Hepatocytes (mouse) + HSC + SECs | Mouse (BALB/CA nu) | Hepatic function | 2011[98] | |
| Chitosan-alginate fibrous scaffolds | BM-MSCs (human) | Rat (Wistar) | Hepatic function | 2010[99] | |
| Hyaluronic acid sponge | Fetal hepatocyte (rat) | Rat (LEC) | Engraftment, hepatic function | 2010[18] | |
| Donors | Species | Numbers |
| Organ liver | Total | 25 |
| Human | 1[2] | |
| Porcine | 16[5,14,74-84,86-88] | |
| Sheep | 1[89] | |
| Rabbit | 1[85] | |
| Rat | 4[89-92] | |
| Mouse | 2[93,94] | |
| Hepatocytes (adult) | Total | 31 |
| Human | 10[13,16,22-29] | |
| Rat | 14[17,20,30-37,90-92,96] | |
| Mouse | 7[21,38-42,98] | |
| Hepatocytes (fetal) | Total | 3 |
| Rat | 2[18,43] | |
| Mouse | 1[44] | |
| Liver cells | Total | 3 |
| Human | 1[19] | |
| Sheep | 1[15] | |
| Rabbit | 1[45] | |
| MSCs (umbilical cord) | Human | 3[29,48,49] |
| MSCs (bone marrow) | Total | 15 |
| Human | 9[50-52,54-58,99] | |
| Macaques | 1[59] | |
| Rabbit | 1[53] | |
| Rat | 3[60,61,90] | |
| Mouse | 1[94] | |
| MSCs (Adipose) | Total | 4 |
| Human | 2[17,63] | |
| Mouse | 2[64,65] | |
| MSCs (liver) | Human | 1[62] |
| Hematopoietic stem cells | Human | 2[54,66] |
| ESCs | Mouse | 1[67] |
| iPSCs | Total | 3 |
| Human | 2[68,69] | |
| Mouse | 1[70] | |
| GPSCs | Mouse | 1[72] |
| Liver stem cells | Total | 2 |
| Human | 1[97] | |
| Rat | 1[73] |
Among various alternative OLT therapies, only cell transplantation has been adopted in clinical practice. However, its long-term improvement effects are yet to be proven. In particular, few studies report that it can become a bridge for OLT. Considering the viewpoint of cell transplantation, cell processing strategies such as proliferation or hepatic differentiation might assume paramount significance. On the other hand, although scaffold-based transplantation is far from being applied clinically, it is deemed as attractive and promising. This approach has been devised as a treatment method that combines the efficiency of solid organ transplantation with the control of rejection. It is also a comprehensive treatment incorporating cell processing technologies.
Although many patients die from liver failure, there is no other curative treatment other than OLT. However, organ shortage remains as the major shortcoming for transplantation globally. Because of graft shortages, alternative treatments for OLT have received significant research attention.
The concept of scaffold-based transplantation was developed to substitute for the damaged human liver requiring immediate transplantation. In particular, many studies discussed xeno-organ transplantation using decellularized liver scaffolds from other species embedded with human derived hepatic cells.
Our search revealed articles on xeno-organ transplantation (n = 14), scaffold-based transplantation (n = 22), and cell transplantation (n = 53), with the majority being related to “cell therapy”.
Cell transplantation is an attractive alternative to conventional organ transplantation. Hepatocyte transplantation has also been applied clinically, however, with limited effect. To obtain better transplantation efficiency, studies were conducted to evaluate the differentiation quality and administration methods.
In this study, regarding transplantation cell sources, we found that adult hepatocytes, fetal hepatocytes, stem cells such as iPSCs, ESCs, MSCs, and differentiated hepatocytes-like cells (HLCs) have been used and most report used hepatocytes as the cell source. In addition, our article showed that only cell transplantation was clinically applied.
Lee et al[13] reported the application of neonatal hepatocytes encapsulated in alginate microbeads transplanted in three patients with acute liver failure from error of sulfite metabolism. Hansel et al[100] reported hepatocyte transplantation applied in 100 patients with errors of metabolism and acute-on-chronic liver failure (ACLF). Nevertheless, the use of human hepatocytes has limitations including limited organ availability, limited cell proliferation, loss of function, and risk for immune rejection[101,102]. Previous studies have explored the application of not only hepatocytes but other cell sources as well. Xue et al[103] performed a meta-analysis of cell transplantation for ACLF including nine RCTs. In this report, UC-MSCs and bone marrow-derived MSCs (BM-MSCs) were used as the cell source, which improved the survival period and liver function.
MSCs, especially BM-MSCs, have shown immunomodulatory and antifibrotic effects in other organ systems, and MSC transplantation has shown positive results in the treatment of liver fibrosis[104,105]. We also found 2 reports of hematopoietic stem cell transplantation, but they were relatively less applied than UC-MSCs and BM-MSCs.
Most importantly, MSCs can secure more sources than hepatocytes, but the problem of cell quality still remains. As a stem cell therapy, iPSCs attract considerable attention in the field of transplantation. iPSCs were established from adult fibroblasts by introducing different transcription factors[106]. They overcame the ethical aspects of ESCs and have the self-renewal properties and pluripotency, the ability to differentiate into various somatic cells, including hepatocytes[107].
HLCs derived from human iPSCs have been researched as a potential alternative to hepatocytes for cell therapy, disease models, and evaluating drugs[108,109]
Takebe et al[3] succeeded in creating a liver bud with iPSCs derived HLCs. This study demonstrated a three-dimensional liver bud produced by co-culturing with Human Umbilical Vein Endothelial Cells and MSCs was able to improve the liver function of recipient following transplantation.
A 3 dimensional (3D) culture is effective for hepatocyte functionality[110], and using a method combining iPSCs and 3D culture may eventually assure high cell quality and quantity.
Nevertheless, because of potential tumorigenicity, the risks of developing teratomas, and the lack of long-term safety and efficacy, 3D cultures and iPSCs have not been clinically applied yet[111,112]. In our search, we did not find many studies elucidating the in vivo application of iPSCs.
Cell transplantation also suffers from these above-mentioned challenges. Moreover, in the recent years, in vitro expansion of human hepatocytes has been explored[113] to overcome the challenges with iPSCs. The improvements in these approaches may lead to the development of alternative therapies.
The first successful animal-to-animal liver xenotransplantation was reported in 1968[114]. Because of the development of immunosuppressive drugs, various studies were conducted that targeted the applicability of harvested organs from other species. Among animals, pigs were proved as useful in terms of size and rejection strength; therefore, genetically modified porcine organs hold enormous potential for this purpose. Although the cornea and skin of pig have been clinically applied, for OLT, the survival period is so short that liver xenotransplantation could not been applied clinically. To solve the problem of severe rejection, GT-KO pig was developed, intending to reduce the risk of GVHD[115]. The recent development of CRISPR/Cas9 has made this animal model more suitable[116].
Regarding xenotransplantation, 12 of 14 articles in our search used GT-KO pigs. Shah et al[14] reported that a human prothrombin-concentrate complex and immunosuppression was used on GT-KO pigs and that the survival was improved. Even then, it is necessary to improve physiological problems such as rejection, coagulation factors, and complementary species specific for application in humans.
Regarding rejection and infection, decellularization of tissue is an attractive method. Decellularization of tissues and even whole organs represents a novel approach for developing perfusable extracellular matrix (ECM)-derived scaffolds with preserved vascular integrity. Decellularized tissue is rarely rejected and is used for tissue reconstruction as scaffold material[117]. This decellularized scaffold is transplanted orthotopically or ectopically. The decellularization of whole organ was first introduced by Ott et al[118] in 2008 with the aim of developing acellular hearts from mice. Bovine heart valves and corneas or those from pigs have already been commercialized and clinically applied[119]. In recent years, research has been conducted on human liver and hepatocytes. Mazza et al[2] reported in 2015 that human liver was decellularized and re-cellularized with a liver cell line to create engineered livers.
KaKabadze et al[15] engrafted sheep liver cells on decellularized human placenta and transplanted them into sheep that underwent partial hepatectomy. Human placenta was considered as an attractive source because it has a well-developed vascular network and ECM for tissue engineering. Moreover, it is usually discarded and widely available.
In addition, many articles exhibited the application of decellularized tissues and biomaterial-based scaffold.
As biomaterials, natural biomaterials are applied such as collagen and hyaluronic acid, and synthetic materials such as polymers based on polylactic acid and polyglycolic acid, among others[16-18]. Previous reports show that after transplanting these scaffolds, the liver function in recipients improved[19-21].
More recently, bio-printed scaffolds have been developed that mimic the tissue using these biomaterials[120]. However, they have problems of vascularization for tissue engraftment and repopulation, which warrant further research.
Meanwhile, scaffold-based transplantation with an ECM was proven effective, and further research is underway with an aim to select ideal cells for humans[119].
iPSCs and few other cell sources are seeded and cultured in decellularized tissue and other scaffolds such that tissue regeneration in vitro can be performed. Therefore, further research should aim to solve this problem for actualizing its application clinically.
Our study summarized alternative therapies for OLT. Alternative therapies have been deeply researched, particularly xeno-organ, scaffold-based, and cell transplantations. Clinically, only cell transplantation with hepatocytes or MSCs has been applied.
Scaffold-based transplantation is a comprehensive treatment that combines xeno-organ and cell transplantations. Future research on the clinical application of scaffold-based transplantation is expected.
Orthotopic liver transplantation (OLT) is the only treatment for end-stage liver failure; however, the shortage of donor organs limits its application. To overcome this problem, many researchers have attempted to develop alternatives to OLT.
There are several reports of alternative therapies. Nevertheless, no study has comprehensively analyzed these therapies from varying perspectives.
This systematic review aims to summarize the current status of alternative transplantation therapies for OLT and to support future research.
A systematic review was performed by searching the PubMed, Cochrane Library and EMBASE databases for studies concerning alternative transplantation therapy for OLT. We used the following MeSH terms: “liver transplantation”, “cell”, “differentiation”, “organoid”, and “xenotransplantation”. Various types of studies were retrieved for full-text evaluation. Of these, we selected articles involving in vivo transplantation.
A total of 89 studies were selected. There are three principle forms of treatment: Xeno-organ transplantation (14 articles), scaffold-based transplantation (22 articles), and cell transplantation (53 articles). Various types of sources for transplantation were discussed: Organ liver, 25 articles; adult hepatocytes, 31 articles; mesenchymal stem cells (MSCs), 25 articles; induced pluripotent stem cells, three articles and other sources. Clinical applications were discussed only for cell transplantation (12 studies; four studies using hepatocytes, five studies using umbilical cord-derived MSCs, three studies using bone marrow-derived MSCs, and two studies using hematopoietic stem cells).
This systematic review summarized alternative therapies for OLT from varying perspectives. Alternative therapies have been deeply researched, particularly xeno-organ, scaffold-based, and cell transplantation. Clinically, only cell transplantation with hepatocytes and MSCs have been applied. Scaffold-based transplantation is a comprehensive treatment that combines xeno-organ and cell transplantations. Future research on the clinical application of scaffold-based transplantation is expected.
This systematic review describes the current status of alternative therapy for OLT in end-stage liver failure. Further studies are needed for clinical applications in the future.
We would like to thank Vikas Narang for English language editing.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gavriilidis P, Qin JM, Tao R S-Editor: Tang JZ L-Editor: A E-Editor: Qi LL
| 1. | Brown RS. Live donors in liver transplantation. Gastroenterology. 2008;134:1802-1813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 2. | Mazza G, Rombouts K, Rennie Hall A, Urbani L, Vinh Luong T, Al-Akkad W, Longato L, Brown D, Maghsoudlou P, Dhillon AP, Fuller B, Davidson B, Moore K, Dhar D, De Coppi P, Malago M, Pinzani M. Decellularized human liver as a natural 3D-scaffold for liver bioengineering and transplantation. Sci Rep. 2015;5:13079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 296] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 3. | Takebe T, Zhang RR, Koike H, Kimura M, Yoshizawa E, Enomura M, Koike N, Sekine K, Taniguchi H. Generation of a vascularized and functional human liver from an iPSC-derived organ bud transplant. Nat Protoc. 2014;9:396-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 273] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 4. | Ekser B, Gridelli B, Tector AJ, Cooper DK. Pig liver xenotransplantation as a bridge to allotransplantation: which patients might benefit? Transplantation. 2009;88:1041-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Navarro-Alvarez N, Machaidze Z, Schuetz C, Zhu A, Liu WH, Shah JA, Vagefi PA, Elias N, Buhler L, Sachs DH, Markmann JF, Yeh H. Xenogeneic Heterotopic Auxiliary Liver transplantation (XHALT) promotes native liver regeneration in a Post-Hepatectomy Liver failure model. PLoS One. 2018;13:e0207272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Nicolas CT, Hickey RD, Chen HS, Mao SA, Lopera Higuita M, Wang Y, Nyberg SL. Concise Review: Liver Regenerative Medicine: From Hepatocyte Transplantation to Bioartificial Livers and Bioengineered Grafts. Stem Cells. 2017;35:42-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 7. | Butler JR, Ladowski JM, Martens GR, Tector M, Tector AJ. Recent advances in genome editing and creation of genetically modified pigs. Int J Surg. 2015;23:217-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Chen Y, Geerts S, Jaramillo M, Uygun BE. Preparation of Decellularized Liver Scaffolds and Recellularized Liver Grafts. Methods Mol Biol. 2018;1577:255-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Soltys KA, Setoyama K, Tafaleng EN, Soto Gutiérrez A, Fong J, Fukumitsu K, Nishikawa T, Nagaya M, Sada R, Haberman K, Gramignoli R, Dorko K, Tahan V, Dreyzin A, Baskin K, Crowley JJ, Quader MA, Deutsch M, Ashokkumar C, Shneider BL, Squires RH, Ranganathan S, Reyes-Mugica M, Dobrowolski SF, Mazariegos G, Elango R, Stolz DB, Strom SC, Vockley G, Roy-Chowdhury J, Cascalho M, Guha C, Sindhi R, Platt JL, Fox IJ. Host conditioning and rejection monitoring in hepatocyte transplantation in humans. J Hepatol. 2017;66:987-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 10. | Alwahsh SM, Rashidi H, Hay DC. Liver cell therapy: is this the end of the beginning? Cell Mol Life Sci. 2018;75:1307-1324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Gerbal-Chaloin S, Funakoshi N, Caillaud A, Gondeau C, Champon B, Si-Tayeb K. Human induced pluripotent stem cells in hepatology: beyond the proof of concept. Am J Pathol. 2014;184:332-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47082] [Article Influence: 2942.6] [Reference Citation Analysis (0)] |
| 13. | Lee CA, Dhawan A, Iansante V, Lehec S, Khorsandi SE, Filippi C, Walker S, Fernandez-Dacosta R, Heaton N, Bansal S, Mitry RR, Fitzpatrick E. Cryopreserved neonatal hepatocytes may be a source for transplantation: Evaluation of functionality toward clinical use. Liver Transpl. 2018;24:394-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Shah JA, Patel MS, Elias N, Navarro-Alvarez N, Rosales I, Wilkinson RA, Louras NJ, Hertl M, Fishman JA, Colvin RB, Cosimi AB, Markmann JF, Sachs DH, Vagefi PA. Prolonged Survival Following Pig-to-Primate Liver Xenotransplantation Utilizing Exogenous Coagulation Factors and Costimulation Blockade. Am J Transplant. 2017;17:2178-2185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 15. | Kakabadze Z, Kakabadze A, Chakhunashvili D, Karalashvili L, Berishvili E, Sharma Y, Gupta S. Decellularized human placenta supports hepatic tissue and allows rescue in acute liver failure. Hepatology. 2018;67:1956-1969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Zhong C, Xie HY, Zhou L, Xu X, Zheng SS. Human hepatocytes loaded in 3D bioprinting generate mini-liver. Hepatobiliary Pancreat Dis Int. 2016;15:512-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Carraro A, Buggio M, Gardin C, Tedeschi U, Ferroni L, Zavan PB. Mesenchymal Stem Cells Increase Neo-Angiogenesis and Albumin Production in a Liver Tissue-Engineered Engraftment. Int J Mol Sci. 2016;17:374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Katsuda T, Teratani T, Ochiya T, Sakai Y. Transplantation of a fetal liver cell-loaded hyaluronic acid sponge onto the mesentery recovers a Wilson's disease model rat. J Biochem. 2010;148:281-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Mavila N, Trecartin A, Spurrier R, Xiao Y, Hou X, James D, Fu X, Truong B, Wang C, Lipshutz GS, Wang KS, Grikscheit TC. Functional Human and Murine Tissue-Engineered Liver Is Generated from Adult Stem/Progenitor Cells. Stem Cells Transl Med. 2017;6:238-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Stevens KR, Miller JS, Blakely BL, Chen CS, Bhatia SN. Degradable hydrogels derived from PEG-diacrylamide for hepatic tissue engineering. J Biomed Mater Res A. 2015;103:3331-3338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Zhang S, Zhang B, Chen X, Chen L, Wang Z, Wang Y. Three-dimensional culture in a microgravity bioreactor improves the engraftment efficiency of hepatic tissue constructs in mice. J Mater Sci Mater Med. 2014;25:2699-2709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Wang F, Zhou L, Ma X, Ma W, Wang C, Lu Y, Chen Y, An L, An W, Yang Y. Monitoring of intrasplenic hepatocyte transplantation for acute-on-chronic liver failure: a prospective five-year follow-up study. Transplant Proc. 2014;46:192-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Ribes-Koninckx C, Ibars EP, Calzado Agrasot MÁ, Bonora-Centelles A, Miquel BP, Vila Carbó JJ, Aliaga ED, Pallardó JM, Gómez-Lechón MJ, Castell JV. Clinical outcome of hepatocyte transplantation in four pediatric patients with inherited metabolic diseases. Cell Transplant. 2012;21:2267-2282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Beck BB, Habbig S, Dittrich K, Stippel D, Kaul I, Koerber F, Goebel H, Salido EC, Kemper M, Meyburg J, Hoppe B. Liver cell transplantation in severe infantile oxalosis--a potential bridging procedure to orthotopic liver transplantation? Nephrol Dial Transplant. 2012;27:2984-2989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Hang HL, Liu XY, Wang HT, Xu N, Bian JM, Zhang JJ, Xia L, Xia Q. Hepatocyte nuclear factor 4A improves hepatic differentiation of immortalized adult human hepatocytes and improves liver function and survival. Exp Cell Res. 2017;360:81-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 26. | Sasaki K, Akagi T, Asaoka T, Eguchi H, Fukuda Y, Iwagami Y, Yamada D, Noda T, Wada H, Gotoh K, Kawamoto K, Doki Y, Mori M, Akashi M. Construction of three-dimensional vascularized functional human liver tissue using a layer-by-layer cell coating technique. Biomaterials. 2017;133:263-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 27. | Gramignoli R, Tahan V, Dorko K, Skvorak KJ, Hansel MC, Zhao W, Venkataramanan R, Ellis EC, Jorns C, Ericzon BG, Rosenborg S, Kuiper R, Soltys KA, Mazariegos GV, Fox IJ, Wilson EM, Grompe M, Strom SC. New potential cell source for hepatocyte transplantation: discarded livers from metabolic disease liver transplants. Stem Cell Res. 2013;11:563-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Kawahara T, Douglas DN, Lewis J, Lund G, Addison W, Tyrrell DL, Churchill TA, Kneteman NM. Critical role of natural killer cells in the rejection of human hepatocytes after xenotransplantation into immunodeficient mice. Transpl Int. 2010;23:934-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | El Baz H, Demerdash Z, Kamel M, Atta S, Salah F, Hassan S, Hammam O, Khalil H, Meshaal S, Raafat I. Transplant of Hepatocytes, Undifferentiated Mesenchymal Stem Cells, and In Vitro Hepatocyte-Differentiated Mesenchymal Stem Cells in a Chronic Liver Failure Experimental Model: A Comparative Study. Exp Clin Transplant. 2018;16:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Oldani G, Peloso A, Vijgen S, Wilson EM, Slits F, Gex Q, Morel P, Delaune V, Orci LA, Yamaguchi T, Kobayashi T, Rubbia-Brandt L, Nakauchi H, Lacotte S, Toso C. Chimeric liver transplantation reveals interspecific graft remodelling. J Hepatol. 2018;69:1025-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Ye J, Shirakigawa N, Ijima H. Hybrid organoids consisting of extracellular matrix gel particles and hepatocytes for transplantation. J Biosci Bioeng. 2015;120:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Ho CM, Chen YH, Chien CS, Ho YT, Ho SL, Hu RH, Chen HL, Lee PH. Transplantation speed offers early hepatocyte engraftment in acute liver injured rats: A translational study with clinical implications. Liver Transpl. 2015;21:652-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Olszewski WL, Charysz A, Gewartowska M, Nagui ME. Intrasplenic transplanted adult rat isolated hepatocyte fraction but not cholangiocytes forms bile canaliculi. Transplant Proc. 2014;46:2894-2896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 34. | Bahde R, Kapoor S, Viswanathan P, Spiegel HU, Gupta S. Endothelin-1 receptor A blocker darusentan decreases hepatic changes and improves liver repopulation after cell transplantation in rats. Hepatology. 2014;59:1107-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Hayashi C, Ito M, Ito R, Murakumo A, Yamamoto N, Hiramatsu N, Fox IJ, Horiguchi A. Effects of edaravone, a radical scavenger, on hepatocyte transplantation. J Hepatobiliary Pancreat Sci. 2014;21:919-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | No da Y, Jeong GS, Lee SH. Immune-protected xenogeneic bioartificial livers with liver-specific microarchitecture and hydrogel-encapsulated cells. Biomaterials. 2014;35:8983-8991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Yu M, Zhang W, Qin L, Tian L, Zhou C. Enhancement of P-glycoprotein expression by hepatocyte transplantation in carbon tetrachloride-induced rat liver. Anat Rec (Hoboken). 2010;293:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Zhou VX, Lolas M, Chang TT. Direct orthotopic implantation of hepatic organoids. J Surg Res. 2017;211:251-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Boudechiche L, Tranchart H, Branchereau S, Davit-Spraul A, Laïnas P, Groyer-Picard MT, Weber A, Hadchouel M, Dagher I. Improvement of hepatocyte transplantation efficiency in the mdr2-/- mouse model by glyceryl trinitrate. Transplantation. 2015;99:36-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Hoppo T, Komori J, Manohar R, Stolz DB, Lagasse E. Rescue of lethal hepatic failure by hepatized lymph nodes in mice. Gastroenterology. 2011;140:656-666.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 41. | Ohashi K, Koyama F, Tatsumi K, Shima M, Park F, Nakajima Y, Okano T. Functional life-long maintenance of engineered liver tissue in mice following transplantation under the kidney capsule. J Tissue Eng Regen Med. 2010;4:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 42. | Leconte I, Pallu S, Abarca-Quinones J, Michoux N, Peeters F, Radermacher K, Sempoux C, Najimi M, Sokal E, Van Beers BE. MRI of iron-oxide labelled transplanted hepatocytes in mice: effect of treatment with cyclophosphamide. J Magn Reson Imaging. 2010;32:367-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 43. | Boylan JM, Francois-Vaughan H, Gruppuso PA, Sanders JA. Engraftment and Repopulation Potential of Late Gestation Fetal Rat Hepatocytes. Transplantation. 2017;101:2349-2359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Buck NE, Pennell SD, Wood LR, Pitt JJ, Allen KJ, Peters HL. Fetal progenitor cell transplantation treats methylmalonic aciduria in a mouse model. Biochem Biophys Res Commun. 2012;427:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 45. | Kafert-Kasting S, Schneider A, Attaran M, Priesner C, Barthold M, Perrier AL, Kriegbaum H, Ott M, Meyburg J. Safety assessment of intraportal liver cell application in New Zealand white rabbits under GLP conditions. Arch Toxicol. 2012;86:1413-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 46. | Li Z, Chen J, Li L, Ran JH, Li XH, Liu ZH, Liu GJ, Gao YC, Zhang XL, Sun HD. In vitro and in vivo characteristics of hepatic oval cells modified with human hepatocyte growth factor. Cell Mol Biol Lett. 2013;18:507-521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | Rashid ST, Alexander GJ. Induced pluripotent stem cells: from Nobel Prizes to clinical applications. J Hepatol. 2013;58:625-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Zhang YC, Liu W, Fu BS, Wang GY, Li HB, Yi HM, Jiang N, Wang G, Zhang J, Yi SH, Li H, Zhang Q, Yang Y, Chen GH. Therapeutic potentials of umbilical cord-derived mesenchymal stromal cells for ischemic-type biliary lesions following liver transplantation. Cytotherapy. 2017;19:194-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 49. | Shi M, Liu Z, Wang Y, Xu R, Sun Y, Zhang M, Yu X, Wang H, Meng L, Su H, Jin L, Wang FS. A Pilot Study of Mesenchymal Stem Cell Therapy for Acute Liver Allograft Rejection. Stem Cells Transl Med. 2017;6:2053-2061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 50. | Kim JK, Kim SJ, Kim Y, Chung YE, Park YN, Kim HO, Kim JS, Park MS, Sakaida I, Kim DY, Lee JI, Ahn SH, Lee KS, Han KH. Long-Term Follow-Up of Patients After Autologous Bone Marrow Cell Infusion for Decompensated Liver Cirrhosis. Cell Transplant. 2017;26:1059-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 51. | Shevela EY, Starostina NM, Pal'tsev AI, Shipunov MV, Zheltova OI, Meledina IV, Khvan LA, Leplina OY, Ostanin AA, Chernykh ER, Kozlov VA. Efficiency of Cell Therapy in Liver Cirrhosis. Bull Exp Biol Med. 2016;160:542-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 52. | Park CH, Bae SH, Kim HY, Kim JK, Jung ES, Chun HJ, Song MJ, Lee SE, Cho SG, Lee JW, Choi JY, Yoon SK, Han NI, Lee YS. A pilot study of autologous CD34-depleted bone marrow mononuclear cell transplantation via the hepatic artery in five patients with liver failure. Cytotherapy. 2013;15:1571-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 53. | Shang QL, Xiao EH, Zhou QC, Luo JG, Wu HJ. Pathological and MR-DWI study of the acute hepatic injury model after stem cell transplantation. World J Gastroenterol. 2011;17:2821-2828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 54. | Smiers FJ, Van de Vijver E, Delsing BJ, Lankester AC, Ball LM, Rings EH, van Rheenen PF, Bredius RG. Delayed immune recovery following sequential orthotopic liver transplantation and haploidentical stem cell transplantation in erythropoietic protoporphyria. Pediatr Transplant. 2010;14:471-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 55. | Gholamrezanezhad A, Mirpour S, Bagheri M, Mohamadnejad M, Alimoghaddam K, Abdolahzadeh L, Saghari M, Malekzadeh R. In vivo tracking of 111In-oxine labeled mesenchymal stem cells following infusion in patients with advanced cirrhosis. Nucl Med Biol. 2011;38:961-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 198] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 56. | Ayatollahi M, Hesami Z, Jamshidzadeh A, Gramizadeh B. Antioxidant Effects of Bone Marrow Mesenchymal Stem Cell against Carbon Tetrachloride-Induced Oxidative Damage in Rat Livers. Int J Organ Transplant Med. 2014;5:166-173. [PubMed] |
| 57. | Baligar P, Kochat V, Arindkar SK, Equbal Z, Mukherjee S, Patel S, Nagarajan P, Mohanty S, Teckman JH, Mukhopadhyay A. Bone marrow stem cell therapy partially ameliorates pathological consequences in livers of mice expressing mutant human α1-antitrypsin. Hepatology. 2017;65:1319-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 58. | Stock P, Brückner S, Ebensing S, Hempel M, Dollinger MM, Christ B. The generation of hepatocytes from mesenchymal stem cells and engraftment into murine liver. Nat Protoc. 2010;5:617-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 59. | Fu X, Jiang B, Zheng B, Yan Y, Wang J, Duan Y, Li S, Yan L, Wang H, Chen B, Sang X, Ji W, Xu RH, Si W. Heterogenic transplantation of bone marrow-derived rhesus macaque mesenchymal stem cells ameliorates liver fibrosis induced by carbon tetrachloride in mouse. PeerJ. 2018;6:e4336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 60. | Yuan S, Jiang T, Zheng R, Sun L, Cao G, Zhang Y. Effect of bone marrow mesenchymal stem cell transplantation on acute hepatic failure in rats. Exp Ther Med. 2014;8:1150-1158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 61. | Nakamura T, Torimura T, Iwamoto H, Masuda H, Naitou M, Koga H, Abe M, Hashimoto O, Tsutsumi V, Ueno T, Sata M. Prevention of liver fibrosis and liver reconstitution of DMN-treated rat liver by transplanted EPCs. Eur J Clin Invest. 2012;42:717-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 62. | Pan Q, Fouraschen SM, Kaya FS, Verstegen MM, Pescatori M, Stubbs AP, van Ijcken W, van der Sloot A, Smits R, Kwekkeboom J, Metselaar HJ, Kazemier G, de Jonge J, Tilanus HW, Wagemaker G, Janssen HL, van der Laan LJ. Mobilization of hepatic mesenchymal stem cells from human liver grafts. Liver Transpl. 2011;17:596-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 63. | Saidi RF, Rajeshkumar B, Shariftabrizi A, Bogdanov AA, Zheng S, Dresser K, Walter O. Human adipose-derived mesenchymal stem cells attenuate liver ischemia-reperfusion injury and promote liver regeneration. Surgery. 2014;156:1225-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 64. | Di Rocco G, Gentile A, Antonini A, Truffa S, Piaggio G, Capogrossi MC, Toietta G. Analysis of biodistribution and engraftment into the liver of genetically modified mesenchymal stromal cells derived from adipose tissue. Cell Transplant. 2012;21:1997-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 65. | Winkler S, Hempel M, Brückner S, Mallek F, Weise A, Liehr T, Tautenhahn HM, Bartels M, Christ B. Mouse white adipose tissue-derived mesenchymal stem cells gain pericentral and periportal hepatocyte features after differentiation in vitro, which are preserved in vivo after hepatic transplantation. Acta Physiol (Oxf). 2015;215:89-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 66. | Sharma M, Rao PN, Sasikala M, Kuncharam MR, Reddy C, Gokak V, Raju B, Singh JR, Nag P, Nageshwar Reddy D. Autologous mobilized peripheral blood CD34(+) cell infusion in non-viral decompensated liver cirrhosis. World J Gastroenterol. 2015;21:7264-7271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 67. | Deng XG, Qiu RL, Li ZX, Zhang J, Zhou JJ, Wu YH, Zeng LX, Tang J. Selection of hepatocyte-like cells from mouse differentiated embryonic stem cells and application in therapeutic liver repopulation. Cell Physiol Biochem. 2012;30:1271-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 68. | Takebe T, Sekine K, Kimura M, Yoshizawa E, Ayano S, Koido M, Funayama S, Nakanishi N, Hisai T, Kobayashi T, Kasai T, Kitada R, Mori A, Ayabe H, Ejiri Y, Amimoto N, Yamazaki Y, Ogawa S, Ishikawa M, Kiyota Y, Sato Y, Nozawa K, Okamoto S, Ueno Y, Taniguchi H. Massive and Reproducible Production of Liver Buds Entirely from Human Pluripotent Stem Cells. Cell Rep. 2017;21:2661-2670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 275] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 69. | Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, Zhang RR, Ueno Y, Zheng YW, Koike N, Aoyama S, Adachi Y, Taniguchi H. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1763] [Cited by in RCA: 1511] [Article Influence: 125.9] [Reference Citation Analysis (0)] |
| 70. | Espejel S, Roll GR, McLaughlin KJ, Lee AY, Zhang JY, Laird DJ, Okita K, Yamanaka S, Willenbring H. Induced pluripotent stem cell-derived hepatocytes have the functional and proliferative capabilities needed for liver regeneration in mice. J Clin Invest. 2010;120:3120-3126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 71. | Zhu S, Rezvani M, Harbell J, Mattis AN, Wolfe AR, Benet LZ, Willenbring H, Ding S. Mouse liver repopulation with hepatocytes generated from human fibroblasts. Nature. 2014;508:93-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 203] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 72. | Fagoonee S, Famulari ES, Silengo L, Tolosano E, Altruda F. Long Term Liver Engraftment of Functional Hepatocytes Obtained from Germline Cell-Derived Pluripotent Stem Cells. PLoS One. 2015;10:e0136762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 73. | Kuijk EW, Rasmussen S, Blokzijl F, Huch M, Gehart H, Toonen P, Begthel H, Clevers H, Geurts AM, Cuppen E. Generation and characterization of rat liver stem cell lines and their engraftment in a rat model of liver failure. Sci Rep. 2016;6:22154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 74. | Zhang Z, Li X, Zhang H, Zhang X, Chen H, Pan D, Ji H, Zhou L, Ling J, Zhou J, Yue S, Wang D, Yang Z, Tao K, Dou K. Cytokine profiles in Tibetan macaques following α-1,3-galactosyltransferase-knockout pig liver xenotransplantation. Xenotransplantation. 2017;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 75. | Yeh H, Machaidze Z, Wamala I, Fraser JW, Navarro-Alvarez N, Kim K, Schuetz C, Shi S, Zhu A, Hertl M, Elias N, Farkash EA, Vagefi PA, Varma M, Smith RN, Robson SC, Van Cott EM, Sachs DH, Markmann JF. Increased transfusion-free survival following auxiliary pig liver xenotransplantation. Xenotransplantation. 2014;21:454-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 76. | Kim K, Schuetz C, Elias N, Veillette GR, Wamala I, Varma M, Smith RN, Robson SC, Cosimi AB, Sachs DH, Hertl M. Up to 9-day survival and control of thrombocytopenia following alpha1,3-galactosyl transferase knockout swine liver xenotransplantation in baboons. Xenotransplantation. 2012;19:256-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 77. | Ekser B, Long C, Echeverri GJ, Hara H, Ezzelarab M, Lin CC, de Vera ME, Wagner R, Klein E, Wolf RF, Ayares D, Cooper DK, Gridelli B. Impact of thrombocytopenia on survival of baboons with genetically modified pig liver transplants: clinical relevance. Am J Transplant. 2010;10:273-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 78. | Navarro-Alvarez N, Shah JA, Zhu A, Ligocka J, Yeh H, Elias N, Rosales I, Colvin R, Cosimi AB, Markmann JF, Hertl M, Sachs DH, Vagefi PA. The Effects of Exogenous Administration of Human Coagulation Factors Following Pig-to-Baboon Liver Xenotransplantation. Am J Transplant. 2016;16:1715-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 79. | LaMattina JC, Burdorf L, Zhang T, Rybak E, Cheng X, Munivenkatappa R, Salles II, Broos K, Sievert E, McCormick B, Decarlo M, Ayares D, Deckmyn H, Azimzadeh AM, Pierson RN, Barth RN. Pig-to-baboon liver xenoperfusion utilizing GalTKO.hCD46 pigs and glycoprotein Ib blockade. Xenotransplantation. 2014;21:274-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 80. | Ekser B, Klein E, He J, Stolz DB, Echeverri GJ, Long C, Lin CC, Ezzelarab M, Hara H, Veroux M, Ayares D, Cooper DK, Gridelli B. Genetically-engineered pig-to-baboon liver xenotransplantation: histopathology of xenografts and native organs. PLoS One. 2012;7:e29720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 81. | Ezzelarab M, Ekser B, Gridelli B, Iwase H, Ayares D, Cooper DK. Thrombocytopenia after pig-to-baboon liver xenotransplantation: where do platelets go? Xenotransplantation. 2011;18:320-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 82. | Ekser B, Lin CC, Long C, Echeverri GJ, Hara H, Ezzelarab M, Bogdanov VY, Stolz DB, Enjyoji K, Robson SC, Ayares D, Dorling A, Cooper DK, Gridelli B. Potential factors influencing the development of thrombocytopenia and consumptive coagulopathy after genetically modified pig liver xenotransplantation. Transpl Int. 2012;25:882-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 83. | Ekser B, Echeverri GJ, Hassett AC, Yazer MH, Long C, Meyer M, Ezzelarab M, Lin CC, Hara H, van der Windt DJ, Dons EM, Phelps C, Ayares D, Cooper DK, Gridelli B. Hepatic function after genetically engineered pig liver transplantation in baboons. Transplantation. 2010;90:483-493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 84. | Ramis G, Martínez-Alarcón L, Medina-Moreno E, Abellaneda JM, Quereda JJ, Febrero B, Sáez-Acosta A, Ríos A, Muñoz A, Ramírez P, Majado MJ. Presence of Pig IgG and IgM in Sera Samples From Baboons After an Orthotopic Liver Xenotransplantation. Transplant Proc. 2018;50:2842-2846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 85. | Galvao FH, Soler W, Pompeu E, Waisberg DR, Mello ES, Costa AC, Teodoro W, Velosa AP, Capelozzi VL, Antonangelo L, Catanozi S, Martins A, Malbouisson LM, Cruz RJ, Figueira ER, Filho JA, Chaib E, D'Albuquerque LA. Immunoglobulin G profile in hyperacute rejection after multivisceral xenotransplantation. Xenotransplantation. 2012;19:298-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 86. | Mirmalek-Sani SH, Sullivan DC, Zimmerman C, Shupe TD, Petersen BE. Immunogenicity of decellularized porcine liver for bioengineered hepatic tissue. Am J Pathol. 2013;183:558-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 87. | Park KM, Park SM, Yang SR, Hong SH, Woo HM. Preparation of immunogen-reduced and biocompatible extracellular matrices from porcine liver. J Biosci Bioeng. 2013;115:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 88. | Barakat O, Abbasi S, Rodriguez G, Rios J, Wood RP, Ozaki C, Holley LS, Gauthier PK. Use of decellularized porcine liver for engineering humanized liver organ. J Surg Res. 2012;173:e11-e25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 155] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 89. | Sabetkish S, Kajbafzadeh AM, Sabetkish N, Khorramirouz R, Akbarzadeh A, Seyedian SL, Pasalar P, Orangian S, Beigi RS, Aryan Z, Akbari H, Tavangar SM. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix liver scaffolds. J Biomed Mater Res A. 2015;103:1498-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 90. | Kadota Y, Yagi H, Inomata K, Matsubara K, Hibi T, Abe Y, Kitago M, Shinoda M, Obara H, Itano O, Kitagawa Y. Mesenchymal stem cells support hepatocyte function in engineered liver grafts. Organogenesis. 2014;10:268-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 91. | Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, Milwid J, Kobayashi N, Tilles A, Berthiaume F, Hertl M, Nahmias Y, Yarmush ML, Uygun K. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16:814-820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1162] [Cited by in RCA: 999] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 92. | Bao J, Shi Y, Sun H, Yin X, Yang R, Li L, Chen X, Bu H. Construction of a portal implantable functional tissue-engineered liver using perfusion-decellularized matrix and hepatocytes in rats. Cell Transplant. 2011;20:753-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 93. | Zhang H, Siegel CT, Li J, Lai J, Shuai L, Lai X, Zhang Y, Jiang Y, Bie P, Bai L. Functional liver tissue engineering by an adult mouse liver-derived neuro-glia antigen 2-expressing stem/progenitor population. J Tissue Eng Regen Med. 2018;12:e190-e202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 94. | Jiang WC, Cheng YH, Yen MH, Chang Y, Yang VW, Lee OK. Cryo-chemical decellularization of the whole liver for mesenchymal stem cells-based functional hepatic tissue engineering. Biomaterials. 2014;35:3607-3617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 95. | Yuan J, Li W, Huang J, Guo X, Li X, Lu X, Huang X, Zhang H. Transplantation of human adipose stem cell-derived hepatocyte-like cells with restricted localization to liver using acellular amniotic membrane. Stem Cell Res Ther. 2015;6:217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 96. | Jitraruch S, Dhawan A, Hughes RD, Filippi C, Soong D, Philippeos C, Lehec SC, Heaton ND, Longhi MS, Mitry RR. Alginate microencapsulated hepatocytes optimised for transplantation in acute liver failure. PLoS One. 2014;9:e113609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 97. | Turner RA, Wauthier E, Lozoya O, McClelland R, Bowsher JE, Barbier C, Prestwich G, Hsu E, Gerber DA, Reid LM. Successful transplantation of human hepatic stem cells with restricted localization to liver using hyaluronan grafts. Hepatology. 2013;57:775-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 98. | Saito R, Ishii Y, Ito R, Nagatsuma K, Tanaka K, Saito M, Maehashi H, Nomoto H, Ohkawa K, Mano H, Aizawa M, Hano H, Yanaga K, Matsuura T. Transplantation of liver organoids in the omentum and kidney. Artif Organs. 2011;35:80-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 99. | Tai BC, Du C, Gao S, Wan AC, Ying JY. The use of a polyelectrolyte fibrous scaffold to deliver differentiated hMSCs to the liver. Biomaterials. 2010;31:48-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 100. | Hansel MC, Gramignoli R, Skvorak KJ, Dorko K, Marongiu F, Blake W, Davila J, Strom SC. The history and use of human hepatocytes for the treatment of liver diseases: the first 100 patients. Curr Protoc Toxicol. 2014;62:14.12.1-14.1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 101. | Iansante V, Mitry RR, Filippi C, Fitzpatrick E, Dhawan A. Human hepatocyte transplantation for liver disease: current status and future perspectives. Pediatr Res. 2018;83:232-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 151] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 102. | Nagamoto Y, Takayama K, Ohashi K, Okamoto R, Sakurai F, Tachibana M, Kawabata K, Mizuguchi H. Transplantation of a human iPSC-derived hepatocyte sheet increases survival in mice with acute liver failure. J Hepatol. 2016;64:1068-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 110] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 103. | Xue R, Meng Q, Li J, Wu J, Yao Q, Yu H, Zhu Y. The assessment of multipotent cell transplantation in acute-on-chronic liver failure: a systematic review and meta-analysis. Transl Res. 2018;200:65-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 104. | Hardjo M, Miyazaki M, Sakaguchi M, Masaka T, Ibrahim S, Kataoka K, Huh NH. Suppression of carbon tetrachloride-induced liver fibrosis by transplantation of a clonal mesenchymal stem cell line derived from rat bone marrow. Cell Transplant. 2009;18:89-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 105. | Rabani V, Shahsavani M, Gharavi M, Piryaei A, Azhdari Z, Baharvand H. Mesenchymal stem cell infusion therapy in a carbon tetrachloride-induced liver fibrosis model affects matrix metalloproteinase expression. Cell Biol Int. 2010;34:601-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 106. | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17989] [Cited by in RCA: 18159] [Article Influence: 955.7] [Reference Citation Analysis (0)] |
| 107. | Bizzaro D, Russo FP, Burra P. New Perspectives in Liver Transplantation: From Regeneration to Bioengineering. Bioengineering (Basel). 2019;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 108. | Kia R, Sison RL, Heslop J, Kitteringham NR, Hanley N, Mills JS, Park BK, Goldring CE. Stem cell-derived hepatocytes as a predictive model for drug-induced liver injury: are we there yet? Br J Clin Pharmacol. 2013;75:885-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 109. | Gómez-Lechón MJ, Tolosa L. Human hepatocytes derived from pluripotent stem cells: a promising cell model for drug hepatotoxicity screening. Arch Toxicol. 2016;90:2049-2061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 110. | Rebelo SP, Costa R, Silva MM, Marcelino P, Brito C, Alves PM. Three-dimensional co-culture of human hepatocytes and mesenchymal stem cells: improved functionality in long-term bioreactor cultures. J Tissue Eng Regen Med. 2017;11:2034-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 111. | Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature 2011; 474: 212-215 [PMID: 21572395 DOI: 10.1038/nature10135] 112 Tolosa L, Pareja E, Gómez-Lechón MJ. Clinical Application of Pluripotent Stem Cells: An Alternative Cell-Based Therapy for Treating Liver Diseases? Transplantation. 2016;100:2548-2557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 112. | Tolosa L, Pareja E, Gómez-Lechón MJ. Clinical Application of Pluripotent Stem Cells: An Alternative Cell-Based Therapy for Treating Liver Diseases? Transplantation. 2016;100:2548-2557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 113. | Zhang K, Zhang L, Liu W, Ma X, Cen J, Sun Z, Wang C, Feng S, Zhang Z, Yue L, Sun L, Zhu Z, Chen X, Feng A, Wu J, Jiang Z, Li P, Cheng X, Gao D, Peng L, Hui L. In Vitro Expansion of Primary Human Hepatocytes with Efficient Liver Repopulation Capacity. Cell Stem Cell. 2018;23:806-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 173] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 114. | Cooper DKC. Early clinical xenotransplantation experiences-An interview with Thomas E. Starzl, MD, PhD. Xenotransplantation. 2017;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 115. | Ekser B, Ezzelarab M, Hara H, van der Windt DJ, Wijkstrom M, Bottino R, Trucco M, Cooper DK. Clinical xenotransplantation: the next medical revolution? Lancet. 2012;379:672-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 258] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 116. | Black CK, Termanini KM, Aguirre O, Hawksworth JS, Sosin M. Solid organ transplantation in the 21st century. Ann Transl Med. 2018;6:409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 201] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 117. | Mazza G, Al-Akkad W, Rombouts K, Pinzani M. Liver tissue engineering: From implantable tissue to whole organ engineering. Hepatol Commun. 2018;2:131-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 118. | Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1993] [Cited by in RCA: 1823] [Article Influence: 107.2] [Reference Citation Analysis (0)] |
| 119. | Rana D, Zreiqat H, Benkirane-Jessel N, Ramakrishna S, Ramalingam M. Development of decellularized scaffolds for stem cell-driven tissue engineering. J Tissue Eng Regen Med. 2017;11:942-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 157] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 120. | Hospodiuk M, Dey M, Sosnoski D, Ozbolat IT. The bioink: A comprehensive review on bioprintable materials. Biotechnol Adv. 2017;35:217-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 618] [Article Influence: 77.3] [Reference Citation Analysis (0)] |