Published online Sep 19, 2025. doi: 10.5498/wjp.v15.i9.108525
Revised: May 14, 2025
Accepted: July 15, 2025
Published online: September 19, 2025
Processing time: 131 Days and 13.3 Hours
Clozapine, the gold standard for resistant schizophrenia, is underused due to risks like clozapine-induced myocarditis (CIM). Non-specific biomarkers and inconsistent imaging, and the significant overlap with clozapine-induced pneumonia (CIP) lead to misdiagnosis and premature discontinuation.
To develop a diagnostic algorithm for CIM to enhance accuracy, differentiate from CIP, and guide safe clozapine rechallenge.
A systematic review of 119 PubMed studies (published between 1990 and April 2025) was conducted in accordance with PRISMA guidelines. The review analyzed CIM diagnosis and rechallenge outcomes, with a focus on biomarkers, imaging, and collaboration with cardiology.
CIM diagnosis relies on troponin and C-reactive protein; electrocardiography and echocardiography are inconsistently applied, and cardiac magnetic resonance imaging (CMR) is underused. Rechallenge was successful in 64.7% to 68.9% of 136 cases, with 2.9% resulting in fatal outcomes. Up to 65% of presumed CIM cases lack confirmation. A proposed protocol integrates chest computed tomography to exclude pneumonia and CMR for CIM confirmation, with echocardiography as an alternative.
A protocol involving multidisciplinary collaboration among computed tomography, CMR, and cardiology improves CIM diagnosis. Slow titration prevents CIM; adjust the dose for CIP and discontinue for confirmed CIM.
Core Tip: This systematic review proposes a multidisciplinary diagnostic algorithm for clozapine-induced myocarditis to reduce misdiagnosis with clozapine-induced pneumonia. Integrating chest computed tomography to exclude clozapine-induced pneumonia, cardiac magnetic resonance imaging for clozapine-induced myocarditis confirmation, and echocardiography in resource-limited settings, the protocol emphasizes slower clozapine titration and collaboration with cardiology to enhance diagnostic accuracy and ensure the safe use of clozapine in the treatment of treatment-resistant schizophrenia.
- Citation: Mahgoub Y, Alhau R, Magzoub Y, Ali A, Nour E, Saeed MEE, Mohamed SGM, Hassan AOS, Ali O. Diagnostic algorithm for clozapine-induced myocarditis: A systematic review. World J Psychiatry 2025; 15(9): 108525
- URL: https://www.wjgnet.com/2220-3206/full/v15/i9/108525.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i9.108525
Clozapine is unparalleled in reducing hospitalization and suicidality in treatment-resistant schizophrenia[1]. However, its use is limited by rare but potentially fatal adverse effects, including clozapine-induced myocarditis (CIM) and clozapine-induced pneumonia (CIP)[2,3]. These conditions pose diagnostic challenges due to overlapping symptoms (e.g., fever, dyspnea) and elevated biomarkers [e.g., troponin, C-reactive protein (CRP)][4]. Current CIM screening protocols primarily rely on clinical suspicion and nonspecific biomarkers, with inconsistent application of electrocardiography (EKG), echocardiography, or advanced diagnostics, such as cardiac magnetic resonance imaging (CMR) or biopsy. Exclusion of overlapping conditions like CIP and cardiologist consultation are rarely standardized, and guidelines often recommend discontinuing clozapine at the first suspicion of CIM, risking premature abandonment of an effective therapy. The absence of uniform diagnostic criteria for CIM and its overlap with CIP heighten the risk of misdiagnosis. Given CIM’s life-threatening potential, refining diagnostic approaches is critical to balance patient safety with clozapine’s therapeutic benefits.
This systematic review evaluates existing clinical information management screening and diagnostic guidelines, assesses clozapine rechallenge outcomes following presumed myocarditis, examines gaps in differentiating CIM from clozapine-induced psychosis (CIP), and proposes a structured protocol integrating advanced imaging and multidisciplinary care to improve diagnostic accuracy and reduce unnecessary clozapine discontinuation. This review aims to develop a diagnostic algorithm for CIM that minimizes misdiagnosis with CIP and informs safe clozapine rechallenge.
This work is a systematic review, not a clinical trial. It synthesizes existing literature on CIM diagnosis and management rather than prospectively assigning human subjects to intervention or comparison groups to explore cause-and-effect relationships.
We conducted a systematic review of literature indexed in PubMed from 1990 to April 2025, using the search string: “clozapine AND (myocarditis OR cardio* OR pericarditis) AND (evaluation OR algorithm OR guidelines OR monitoring)”. This timeframe encompasses early CIM reports, which reflect the evolving diagnostic and management approaches. Studies were eligible if they were clinical investigations, diagnostic guidelines, or high-quality case series addressing the diagnosis of CIM or the outcomes of clozapine rechallenge. Single or low-quality case reports, animal studies, and non-English publications were excluded to prioritize robust, generalizable evidence. Two independent reviewers screened titles and abstracts, followed by full-text evaluation, resolving discrepancies through consensus.
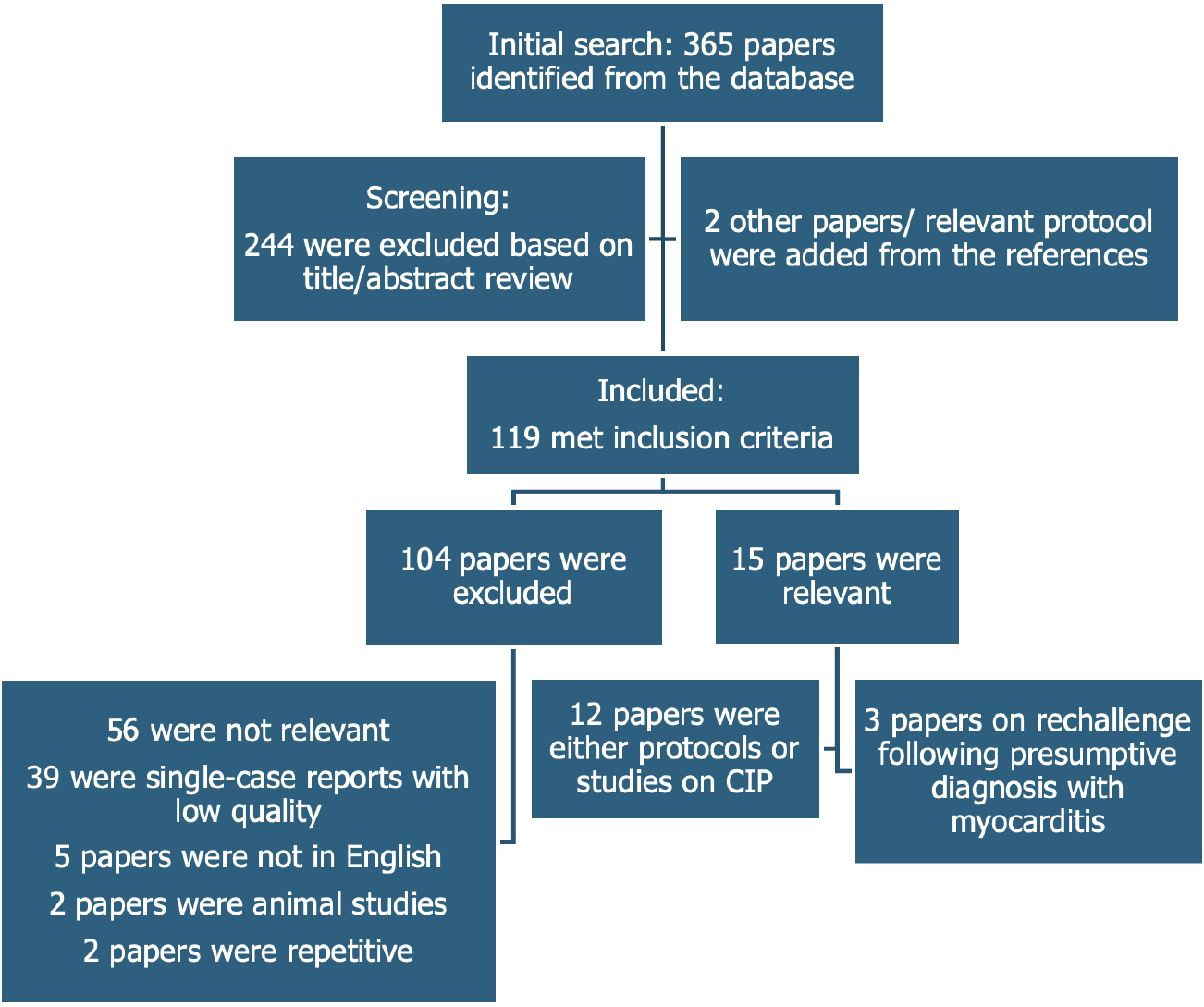
The review adhered to the PRISMA framework to ensure a structured and transparent process. The PRISMA checklist guided the formulation of the research question (“How can CIM be accurately diagnosed and differentiated from CIP in clozapine-treated patients?”), the development of the search strategy, study selection, data extraction, and synthesis. PRISMA principles also informed workflow development, including the use of structured questions (e.g., Population: Clozapine-treated patients; intervention: Diagnostic protocols; comparison: Standard vs advanced imaging; outcome: Diagnostic accuracy, rechallenge success) and process validation across reviewers. Figure 1 presents the PRISMA flowchart detailing the study selection process.
Data extraction focused on diagnostic protocols and rechallenge outcomes, which were synthesized and compared across studies (Tables 1 and 2), allowing for a critical assessment of current practices and identifying evidence gaps. To address the specificity of biomarker thresholds for CIM, we extracted troponin and CRP cut-offs from CIM-focused studies (e.g., Ronaldson et al[5]; Tirupati et al[6]) where available. Where CIM-specific thresholds were not reported, we adopted conservative thresholds (troponin > 0.04 ng/mL, CRP > 10 mg/L) from the literature on viral myocarditis, acknowledging potential differences in clozapine-related inflammation. These thresholds were selected to prioritize sensitivity for early detection, with the understanding that imaging [chest computed tomography (CT), CMR, or echocardiography] is required for confirmation and verification. We also assessed studies for dynamic biomarker trends (e.g., rising troponin over 24-48 hours) to enhance diagnostic precision.
| Ref. | Study type | Sample size | Monitoring protocol | Biomarkers used | Imaging techniques | Outcomes reported | NOS scores | Certainty of evidence | Strengths | Limitations |
| Berk et al[45], 2007 | Guidelines | N/A | Baseline: Clinical evaluation, EKG, plasma troponin, CK-MB, TEE-days 7 and 14: Clinical evaluation, EKG, plasma troponin, CK-MB-6 months and annually: TEE | Troponin, CK-MB (routine); WBC, eosinophil count, ESR, CRP (suspected cases) | Echo (routine) | No outcome reported for guidelines | 6/9 | Low: Expert-driven with no supporting data | First suggested protocol | Based on expert opinion and clinical consensus, with no data supporting the guidelines |
| Ronaldson et al[5], 2010 | Study | 75 | Baseline: Echo, troponin I or T, CRP-weekly for 4 weeks: Troponin I or T, CRP | Troponin and CRP | Echo (baseline), EKG (suspected cases) | Reduced mortality and early detection | 7/9 | Moderate: Strong evidence but a moderate sample | Strong evidence, comprehensive | Moderate sample size, limited follow-up, limited imaging role, and lack of guidelines for overlapping conditions |
| Murch et al[52], 2013 | Study | 122 | Baseline Echo and repeated Echo at 3 and 6 months | CBC and CRP | Echo | 73% had the Echo before starting clozapine 65% had one follow-up echo | 4/9 | Low: Small study with high bias risk | Examine the role and benefit of echo in patients with myocarditis | Limited utility, high cost of using echo for screening, and lack of guidelines for overlapping conditions |
| Symptomatic patients had CBC, CRP | 3 patients screened positive, 2/3 had findings in the echo suggesting myocarditis | |||||||||
| Youssef et al[55], 2016 | Retrospective study | 129 | No protocol. They had criteria for myocarditis based on symptoms and one of the following (elevated troponin, EKG changes, or echo changes) | Troponin | Echo and EKG were inconsistently assessed | 3.88% met the diagnosis of myocarditis | 6/9 | Moderate: Large sample but retrospective | Large sample | Inconsistent use of imaging and lack of exclusion of overlapping conditions |
| McNutt et al[53], 2021 | Study | 38 | Baseline and weekly for 4 weeks: Troponin I or T, CRP | Troponin, CRP | Cardiology involved 2 cases had echo/CMR 2 other patients only had elevated CRP | 50% of patients who screened positive were confirmed to have myocarditis | 4/9 | Low: Small study, inconsistent imaging | Cardiologist for suspected cases | Small study, inconsistent use of images, and lack of guidelines for overlapping conditions |
| Anıl Yağcıoğlu et al[46], 2019 | Study | 38 | Baseline and weekly for 4 weeks: Clinical evaluation, troponin, CRP, ESR, eosinophil count | Troponin, CRP, ESR, and eosinophil count | Echo (suspected cases), CMR (1 patient) | 11.3% suspected myocarditis, 1.4% confirmed | 6/9 | Low: Small study | Echo for suspected cases | Small study, no clear management consensus |
| Nachmani Major et al[48], 2020 | Retrospective study | 24 | Routine: Clinical symptoms, WBC, troponin, CRP, BNP- Suspected cases: Clinical assessment, EKG, echo, MRI | Troponin, CRP, WBC, BNP | EKG, echo, MRI (suspected cases) | 8.6% suspected myocarditis, 1.4% confirmed with imaging | 5/9 | Low: Small retrospective study | Moderate sample size | Retrospective study and lacks guidelines for overlapping conditions |
| Kanniah et al[51], 2020 | Guidelines | N/A | Baseline and weekly for 4 weeks: Vital signs, CRP, ESR, CPK, troponin-after 4 weeks: Eosinophil count monitoring | CRP, ESR, CPK, troponin, eosinophil count | EKG (symptom-driven) | No outcome reported for guidelines | 5/9 | Low: Screening-focused, expert-driven | Focused on screening | There are no work-up recommendations for suspected cases or guidelines for overlapping conditions |
| Sandarsh et al[47], 2021 | Study | 100 | Baseline and weekly for 4 weeks: Clinical symptoms, CRP, eosinophil count, CPK | CRP, eosinophil count, CPK, troponin I | EKG, echo (2/6 positive cases) | 5.3% suspected myocarditis after initial exposure, 3.5% after restarting | 6/9 | Moderate: Larger study | Larger study | Screening-focused, no further workup guidance |
| Segev et al[54], 2021 | Retrospective study | 228 | Undetermined | CRP and troponin | Echo/MRI for suspected cases | Myocarditis was ruled out in 78.7%, confirmed in 9.8%, and undetermined in 11.5% | 6/9 | Moderate: Large study with cardiology input | A large study involved cardiology for further workup | Retrospective study, studied other antipsychotics other than clozapine, no workup for overlapping conditions |
| de Leon et al[56], 2022 | Guidelines | N/A | Baseline and weekly for 4 weeks: CBC, CRP; troponin optional in resource-rich settings | WBC, CRP, troponin (optional) | None specified | No outcome reported for guidelines | NA | Low: International guidelines, no outcomes | International guidelines | Focused on screening with no workup for presumptive cases or workup to exclude overlapping conditions |
| NSW Health[50], 2022 | Guidelines | N/A | Baseline: Clinical symptoms, CRP, CPK, troponin, EKG, echo-weekly for 6 weeks: Troponin, CRP - every 6 months: EKG - every 12 months: Echo | CRP, CPK, troponin | EKG (routine), echo(routine), MRI (suspected cases) | No outcome reported for guidelines | NA | Low: Expert-driven, no outcomes | Clear workup for suspected cases | No outcome data |
| Tirupati et al[6], 2024 | Retrospective study | 327 | Baseline and weekly for 6 weeks: Troponin I or T, CRP | Troponin, CRP | EKG, echo (most positive cases) | 9.8% incidence of clozapine-induced myocarditis | 7/9 | Moderate: Large study, comprehensive | Large study, comprehensive follow-up | Retrospective, no exclusion of overlapping conditions |
| Griffin et al[49], 2021 | Expert opinion and case series | NA | Baseline and weekly for 8 weeks: Troponin, CRP, BNP/NP-pro BNP and EKG | CRP, BNP/pro-BNP | Consult cardiology for suspected cases. TEE, CMR, and LVEF should be assessed in 6 months, and based on the results, clozapine can be reinstated | No outcome reported for guidelines | 7/9 | Low: Expert opinion, no outcomes | Expert opinion. Includes more workup for presumptive cases | The suggested protocol does not have data to support it and does not include a workup for overlapping presentations |
| Ref. | Study type | Sample size | Rechallenge success rate | Fatal failure | Diagnostic certainty | Confirmatory test used | Key findings | Limitations |
| Richardson et al[57], 2021 | Systematic review | 88 cases from 88 case reports/case series | 64.7% | 2.9% | Low (4.5% biopsy-confirmed) | Biopsy (4.5%) No CMR | High failure rate, rare fatalities | Based on the case report, we rely on biomarkers for diagnosis, with no exclusion of overlapping conditions |
| McMahon et al[58], 2024 | Systematic review | 45 cases | 68.9% | 0% | Moderate (EKG/TEE in < 33%) | EKG, TEE (< 33%) | No fatalities, 1/3 failed | Based on case reports |
| No CMR | Most of the studies diagnosed myocarditis based on biomarkers alone | |||||||
| With no workup overlapping conditions | ||||||||
| Noël et al[59], 2019 | Case series | 3 cases | 33.3% | 0% | High (EKG/TEE evidence) | EKG, TEE | Success is only in mild cases | Case series |
Evidence certainty for CIM incidence, diagnostic accuracy, and rechallenge outcomes was evaluated narratively, considering study design (retrospective vs. prospective), sample size, diagnostic method consistency (e.g., CMR use), and risk of bias. Evidence was rated as high, moderate, low, or very low, reflecting the level of confidence in clinical reliability.
Risk of bias in included studies was assessed using a modified Newcastle-Ottawa Scale for observational studies and a checklist for guidelines. Newcastle-Ottawa Scale scores for each included study are now included in Table 1 for transparency. To assess reporting bias, we searched conference abstracts from the American Psychiatric Association Annual Meetings and European Society of Cardiology (ESC) Congresses (1990 to April 2025) via available online archives (e.g., American Journal of Psychiatry supplements, European Society of Cardiology 366 ESC 365) and manually reviewed reference lists for unpublished reports.
Of the 365 initial articles, 119 met the inclusion criteria (Figure 1), excluding single case reports. Fourteen key protocols and studies on CIM screening and diagnosis were identified (12 via PubMed and two from reference lists), and three studies met the criteria for clozapine rechallenge.
Retrospective studies carried a moderate risk, case series had a high risk, and guidelines had a low risk, although they were expert-driven (e.g., New South Wales Health guidelines). Rechallenge success (64.7%-68.9%) evidence had low certainty due to small sample sizes, inconsistent diagnostics, and rare fatal failures (2.9%). CIM incidence evidence (0.06%-3.88%) had moderate certainty, supported by large cohorts but weakened by diagnostic variability.
Troponin and CRP were the most commonly used biomarkers across 14 protocols or studies that utilized various imaging techniques. Suspected CIM ranged from 5.3 to 11.3% (median 8.6%), and confirmed cases ranged from 1.4% to 9.8% (median 3.5%). Moderate certainty is reflected in large cohorts (e.g., n = 327, 228) but is undermined by variable diagnostics and the lack of CIP exclusion (Table 1).
Three studies reported rechallenge success rates of 64.7%-68.9%, with rare cases of fatal failure. Inconsistent use of cardiologists, imaging, and confirmatory tests raises safety concerns (Table 2).
CIM was first reported in 1980 during post-marketing surveillance, with a suspected overdose case[7]. Subsequent reports linked myocarditis to overdose[8] and rapid titration[9], while an Australian case series (n = 23) solidified the association[2]. Several hypotheses have been proposed to explain the mechanism of CIM. One suggested that a type 1 immunoglobulin E-mediated hypersensitivity reaction is involved, supported by eosinophilia in two-thirds of patients[5,10]. Other reports indicate that clozapine causes an increase in both epinephrine and norepinephrine[11]. This increase in catecholamines has been found to worsen myocarditis in animal studies[12]. Another possible mechanism is associated with oxidative stress, downregulation of antioxidants, and apoptosis[13].
Several risk factors have been identified, including genetic susceptibility, advanced age, and rapid titration of clozapine doses[14]. Additionally, the concurrent use of sodium valproate, a common augmenting strategy for augmentation with clozapine, is suggested to increase the risk of CIM[15]. Myocarditis typically presents within the first two to eight weeks of clozapine initiation, with the highest risk occurring in the first 30 days. However, delayed myocarditis can occur years after initiating clozapine[14,16].
The reported rates of CIM varied significantly, ranging from as low as 0.06% to as high as 3.88%[14]. This variability may stem from challenges in diagnosing CIM, as the clinical symptoms often overlap with those of other conditions, such as pneumonia, pulmonary embolism, and other cardiopulmonary diseases. Additionally, the diagnostic methods for myocarditis lack specificity, which can result in mistakenly diagnosing other conditions as myocarditis or failing to recognize this potentially life-threatening condition[14].
CIM varies from mild to severe, with symptoms overlapping those of CIP and clozapine side effects, including fever, fatigue, dizziness, chest pain, tachycardia, shortness of breath, abnormal heart rhythm, and peripheral edema[14,17,18], necessitating advanced diagnostics beyond clinical suspicion.
The current diagnostic criteria for CIM are not well-defined, leading to treatment termination based solely on clinical suspicion[19]. Up to 65% of patients presumed to have CIM do not meet ESC criteria for myocarditis[1]. The ESC working group on myocardial and pericardial disease has proposed diagnostic criteria based on an integrative approach for myocarditis. This approach was developed in response to the challenges of confirming or excluding myocarditis based on a single diagnostic test. Their recommendations were primarily focused on viral myocarditis, the most common cause, but have also been extended to consider other underlying causes, such as hypersensitivity or toxicity from medications, including clozapine[20]. These criteria and the workup are designed to assist in making a diagnosis and ruling out other conditions with similar clinical presentations.
These criteria include the presence of suggestive clinical presentations such as acute chest pain, new onset or worsening of dyspnea, fatigue, subacute/chronic worsening of dyspnea and fatigue, palpitations, unexplained arrhythmia, or unexplained cardiogenic shock. These clinical features should be followed by investigative findings of the following: (1) Biomarkers of myocardial injury; (2) EKG/Holter/Stress test features; (3) Cardiac imaging or CMR changes[21].
The utility of standard tests in diagnosing myocarditis can be significantly limited. EKG changes were seen in 78% of patients with CMR-proven myocarditis; however, these features are primarily nonspecific, and up to 22% of patients with CMR-diagnosed myocarditis showed normal EKG[21]. Cardiac markers such as high-sensitivity cardiac troponin can be a valuable indicator of myocardial tissue damage. Cardiac troponin was reported to have a sensitivity of 34%, specificity of 89%, and positive predictive value of 82%. CRP is another marker used for myocarditis, with a sensitivity of 52% and a specificity of 81%[4,22]. However, these data were recovered from studies for all causes of myocarditis, where viral myocarditis is the most common etiology[4].
Echocardiographic changes can be nonspecific and reported in about 30%-60% of patients with myocarditis[23,24]. Hence, the absence of abnormalities in cardiac echocardiograms does not exclude myocarditis. Endomyocardial biopsy (EMB) is the gold standard for diagnosing myocarditis. It involves using the updated Dallas criteria and polymerase chain reaction, as well as conducting immunohistochemical analysis of inflammatory cells. EMB provides detailed information about the histological composition of inflammatory cell infiltrations, cardiomyocyte damage, necrosis, fibrosis, atrophy, and hypertrophy. These findings can help determine the underlying cause of myocarditis, particularly when combined with polymerase chain reaction or immunohistochemical analysis of inflammatory cells[25]. However, it is essential to note that EMB is an invasive procedure, and the results can vary depending on the location of the biopsy, particularly in cases of patchy myocardial involvement. Sensitivity can increase from 50% for one biopsy to 90% for seven biopsies[26,27]. The updated Dallas criteria, used for interpreting EMB results, have a sensitivity of 60% and a specificity of 80%[28]. EMB is generally recommended for patients with severe or rapidly progressing symptoms, especially when less invasive tests are insufficient for a definitive diagnosis[29]. CMR has become a valuable and non-invasive option for diagnosing myocarditis in recent years due to its ability to characterize inflamed tissues in multiple ways[30]. The CMR findings of myocardial inflammation were outlined in the Lake Louise Criteria 2009. However, the initial criteria were criticized for their moderate diagnostic sensitivity and high subjectivity in qualitative assessment[31]. The Lake Louise Criteria was revised in 2018 to include parametric mapping and several quantitative evaluations, which increased sensitivity to approximately 88% while maintaining high specificity for patients with a strong clinical suspicion of myocarditis[32,33]. The results of CMR can help identify the cause of myocarditis, especially when combined with the patient's clinical symptoms[31]. This capability was used during the coronavirus disease 2019 pandemic to distinguish between myocarditis related to the coronavirus disease 2019 virus and that caused by vaccination[31]. Despite the clear benefits of CMR, challenges such as cost, availability, and accessibility hinder its widespread use for diagnosing myocarditis. In many centers, the approximate wait time for patients is between 2 weeks and 4 weeks[33]. Nonetheless, CMR has emerged as a non-invasive, highly sensitive tool for diagnosing myocarditis. Table 2 provides a summary of the significance of various tests used for assessing myocarditis.
The Food and Drug Administration has issued a warning about second-generation antipsychotics due to the increased mortality among older adults with behavioral disturbances. Pneumonia was reported to be the most common cause of death in this population[34]. Patients taking clozapine are significantly more susceptible to pneumonia, with an odds ratio of 4.07 compared to the general population[3]. The risk of pneumonia depends on the dosage, with the frequency among clozapine patients ranging from 19% to 34%[35].
Several factors are believed to increase the risk of pneumonia in patients taking clozapine. These factors include sedation, excessive salivation, difficulty swallowing, and alterations to the immune system[35]. Managing excessive salivation and monitoring sedation levels is recommended to lower the risk of inhaling saliva or food particles and developing pneumonia[36]. Patients with pneumonia may experience increased clozapine levels due to the release of cytokines that can inhibit the activity of the cytochrome P450 enzyme, the primary enzyme responsible for clozapine metabolism[37]. Therefore, many medical professionals recommend reducing the clozapine dose by half during an upper respiratory infection accompanied by fever. The microbiology of clozapine-associated pneumonia has not been extensively studied; however, aspiration is a suspected mechanism of infection. Several bacteria are common during aspiration, including Streptococcus pneumoniae, Staphylococcus aureus, Haemophilus influenzae, and Enterobacteriaceae[38].
The symptoms of CIP can overlap with the symptoms of myocarditis. They include fever, cough, chest pain, shortness of breath, fatigue, rapid heartbeat, and dizziness[39-41]. While chest radiographs are considered the gold standard for diagnosing pneumonia, they have a lower positive finding among culture-positive patients than chest CT scans (51.5% vs 90.7%)[42], all of which should mandate the need for careful interpretation of a normal chest X-ray (CXR) and a follow-up with a chest CT scan might occasionally be required. Table 3 highlights the overlap between symptoms of clozapine-associated pneumonia and myocarditis.
The current approaches to diagnosing CIM are hindered by an overreliance on nonspecific biomarkers and inconsistent imaging practices, which are further complicated by inadequate differentiation from CIP. Troponin and CRP are cornerstone screening tools, reflecting myocardial inflammation with sensitivities of 34% and 52%, and specificities of 89% and 81%, respectively[4,22]. However, these markers lack specificity for CIM, as they are elevated in up to 52% and 75% of pneumonia cases, respectively[43,44], a condition that is frequently observed among clozapine users (odds ratio = 4.07)[3]. The proposed troponin (> 0.04 ng/mL) and CRP (> 10 mg/L) thresholds are based on viral myocarditis studies, as there is limited CIM-specific data available. However, CIM may present with higher troponin elevations, as seen in cohorts by Ronaldson et al[5] and Tirupati et al[6], suggesting a need for recalibration. For instance, Ronaldson et al[5] reported troponin levels greater than 0.1 ng/mL in confirmed cases of CIM, indicating a potential for higher specificity with adjusted cut-offs. Dynamic monitoring of biomarkers over 24-48 hours, rather than single-point measurements, may further enhance diagnostic accuracy, particularly when distinguishing between CIM and CIP, as CRP elevations are common, occurring in up to 75% of cases. Additional biomarkers, such as erythrocyte sedimentation rate, eosinophil count, and creatine phosphokinase (CPK), are variably employed across protocols without a consensus on their optimal use (Table 1), further clouding diagnostic precision. The overlap in clinical presentation - fever, dyspnea, chest pain, and tachycardia - which is common to both CIM and CIP (Table 3) - exacerbates this uncertainty, risking misdiagnosis in up to 65% of presumed CIM cases that fail to meet the ESC criteria.
Imaging modalities provide limited support in resolving these challenges. EKG reveals non-specific changes in 78% of CMR-confirmed myocarditis cases, yet 22% of affected patients show normal results[21]. Echocardiography, used routinely in some protocols (e.g., Berk et al[45] in 2007) or reserved for suspected cases (e.g., Anıl Yağcıoğlu et al[46] in 2019), detects abnormalities in 30%-60% of CIM patients but lacks specificity and often appears normal in CIP[23]. CXRs, the standard for CIP diagnosis, miss the condition in nearly half of the culture-positive cases (51.5% sensitivity vs 90.7% for chest CT)[42], undermining their utility in ruling out this confounder.
Endomyocardial biopsy, the gold standard for CIM, has a sensitivity of 60% and specificity of 80%[28], but is invasive and impractical for routine use. In comparison, CMR offers high sensitivity (88%) and specificity (91%) non-invasively[32] but remains underutilized due to cost and access barriers[33]. Current protocols rarely standardize advanced imaging or CIP exclusion, thereby perpetuating diagnostic variability. Suspected CIM rates range from 5.3% to 11.3%, with confirmed cases spanning 1.4% to 9.8% (Table 1), highlighting the need for a more robust and integrated approach. Table 4 summarizes the significance of various tests for both CIM and CIP.
| Test | CIM sensitivity/specificity | CIP sensitivity/specificity | Dynamic monitoring | Comments |
| EKG changes | Non-specific and seen in 78% of patients[21] | Non-specific[40] | NA | Feasible in all settings |
| Non-specific changes (e.g., ST changes, T-wave inversion) in CIM; normal in 22% of cases | ||||
| Limited use in CIP | ||||
| cTnl | 34%/89% | Elevated by 52%[43] | Cornerstone for CIM screening; elevated in CIP | Feasible in all settings |
| Repeating testing is critical for trends (> 20% rise suggests escalation) | ||||
| CRP | 52%/81%[22] | Elevated in 75%[44] | Yes (24-48 hours) in CIM Repeat testing (e.g., > 50 mg/L) informs interim management | Feasible in all settings |
| Echo changes | Nonspecific and seen in 30-60%[23] | Likely normal | Yes (48 hours-5 days) | Feasible in most settings |
| Detects wall motion defects in CIM, normal in CIP. Serial testing is key in resource-limited settings (echo-only pathway) | ||||
| Chest X-ray | NA | 51% sensitivity | NA | Feasible in most settings |
| Misses CIP in approximately 50% of cases; used in resource-limited settings. Normal X-ray requires clinical correlation | ||||
| Chest CT | NA | 90.7% | NA | Gold standard for CIP; critical for excluding CIP in CIM workup |
| Limited to well-resourced settings due to cost (300 dollars-1000 dollars) | ||||
| Endomyocardial biopsy | 60%/80%[28] | NA | NA | Gold standard for CIM; invasive, rarely used. Feasible only in specialized centers with severe cases |
| Cardiac MRI | 88%/91%[32] | NA | NA | Non-invasive, highly sensitive for CIM |
| Limited by cost (1000 dollars-3000 dollars) and access (2-4-week wait) | ||||
| Preferred in well-resourced settings |
The serious risk of inducing myocarditis necessitates effective monitoring protocols to ensure early detection and management. Hence, several studies and protocols have been developed for screening and diagnosing CIM (Table 1). Variability in diagnostic thresholds across protocols has led to significant disparities in reported CIM rates. Suspected cases range from 5.3%[47] to 11.3%[46], while confirmed cases span 1.4%[48,46] to 9.8%[49]. These differences likely arise from variations in monitoring intensity, patient demographics, and diagnostic criteria, as detailed in Table 1. For instance, protocols like Ronaldson et al[5] emphasize weekly troponin and CRP monitoring for four weeks, while others extend this to eight weeks[49] or incorporate annual echocardiography[50]. Such inconsistencies reflect a lack of consensus on optimal timing and tools.
A key observation of most protocols is their reliance on troponin and CRP as cornerstone biomarkers, widely recognized for their ability to detect myocardial inflammation early[5,6]. Additional markers - ESR, eosinophil count, creatinine phosphokinase, B-type natriuretic peptide, and white blood count - are variably employed, offering flexibility but no standardized panel (e.g., Kanniah et al[51]; Nachmani Major et al[48]. Some protocols integrate routine imaging, like echocardiography[45,50], or reserve it for suspected cases[46,48], while advanced tools like CMR are occasionally used for confirmation[50,52]. Large-scale studies (e.g., Tirupati et al[6], n = 327) and clear workup pathways outlined in the New South Wales Health guidelines[50] further bolster the evidence base, with progression from expert-driven guidelines, such as those by Berk et al[45], to data-driven approaches marking a significant advancement.
However, limitations persist. Small sample sizes in early studies (e.g., Murch et al[52], n = 122; McNutt et al[53], n = 38) and retrospective designs (e.g., Nachmani Major et al[48]; Segev et al[54]) limit the generalizability of the findings[48]. Inconsistent imaging use - ranging from routine to symptom-driven - undermines diagnostic reliability, as does the rare application of CMR, despite its high sensitivity[32,55]. Many protocols fail to address overlapping conditions, such as CIP, a frequent confounder that shares symptoms and biomarkers with other conditions (Table 3). For example, Kanniah et al[51] and de Leon et al[56] focus narrowly on screening without guidance for suspected cases or CIP exclusion, which can lead to misdiagnosis in up to 65% of presumed CIM cases[1].
Recommendations for clozapine rechallenge after presumed CIM rest on limited evidence from small case series and reviews of isolated case reports. Diagnoses in these studies often rely on non-specific biomarkers (e.g., troponin, CRP) or suggestive clinical features, lacking rigorous confirmation. Richardson et al[57] analyzed 88 cases, with only 4.5% confirmed by biopsy and no use of transesophageal echocardiography (TEE) or CMR. Despite this, 35.3% of rechallenges failed, with 2.9% resulting in death. McMahon et al[58] studied 45 patients, employing EKG and TEE in fewer than one-third of the cases and involving cardiologists in nearly half; however, they did not include CMR or biopsy. The rechallenge failed in approximately one-third of cases, although no fatalities occurred. In a series of three patients, Noël et al[59] reported success only in one, who had asymptomatic, mild elevations of CRP and troponin; the other two, with flu-like symptoms and EKG/TEE evidence of myocarditis, experienced recurrence.
Current screening protocols further complicate the decision-making process for rechallenge. Anıl Yağcıoğlu et al[46] found that up to 10% of patients screen positive for CIM, yet existing methods rarely confirm the diagnosis. This diagnostic uncertainty, combined with the risk of fatal outcomes[57,58], argues against rechallenging patients with presumed CIM unless definitive confirmation excludes alternative causes.
This review highlights critical gaps in current diagnostic approaches, including an overreliance on non-specific biomarkers, inconsistent imaging practices, and insufficient differentiation between CIP and CIM. Given the high incidence of CIP, its significant overlap with CIM in clinical presentation and biomarker profiles, and the potential for misdiagnosis, incorporating a workup for CIP is essential when evaluating patients for suspected CIM. CXRs, commonly used to diagnose CIP, frequently fail to detect it, rendering chest CT scans a more reliable tool for this population. Similarly, while EKG and echocardiography are routinely employed for suspected myocarditis, their findings often lack specificity. The diagnostic gold standard - endomyocardial biopsy—is invasive and carries significant risks. In contrast, CMR offers a non-invasive, highly sensitive alternative that confirms CIM, elucidates etiology, and informs treatment strategies. Expanding access to and utilization of CMR is therefore strongly recommended for evaluating CIM[33].
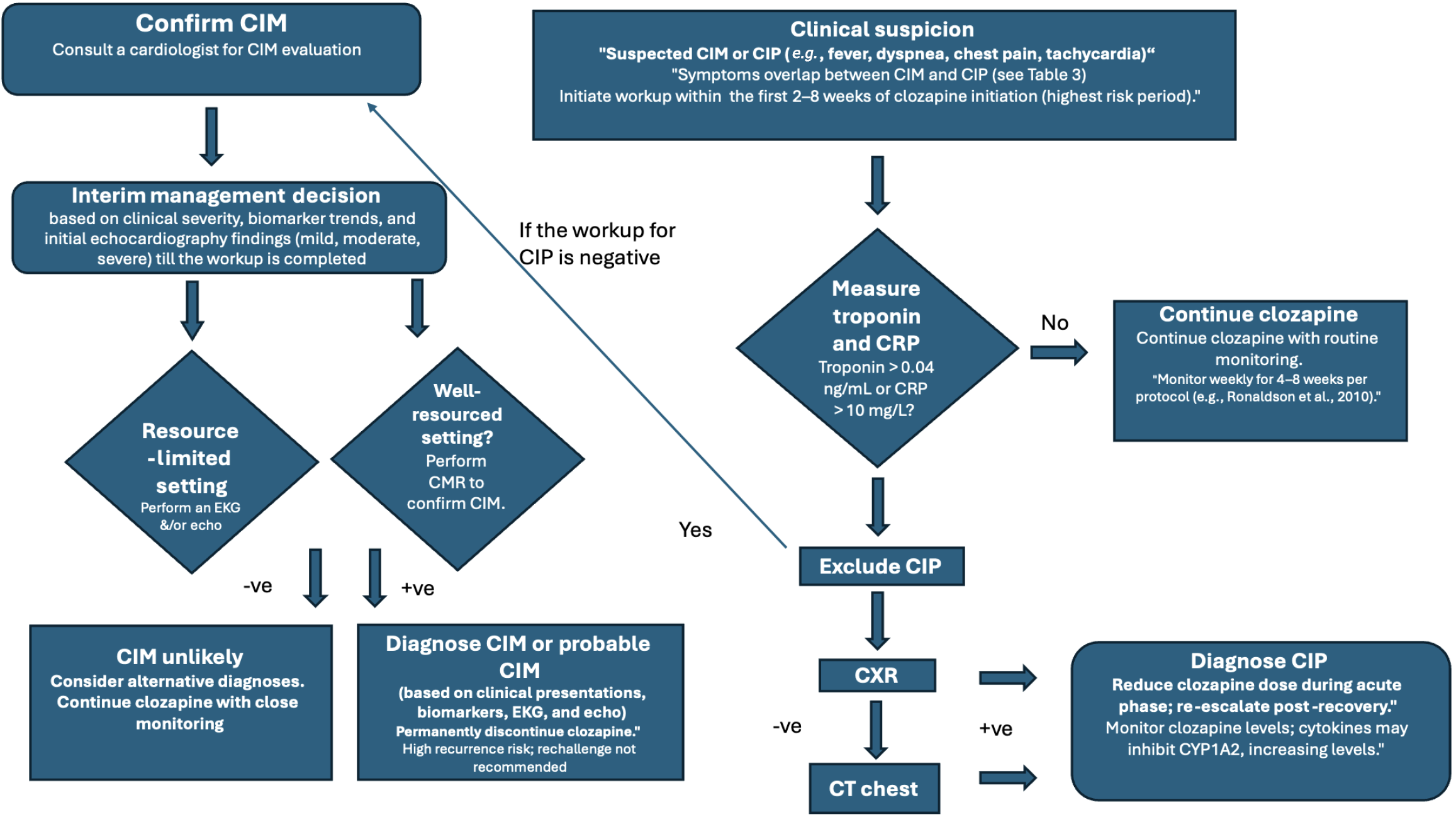
To address these challenges, we propose an algorithm that integrates chest CT to exclude CIP, leverages cardiology collaboration with CMR to confirm CIM, or utilizes CXR and echocardiography when resources are limited. If troponin > 0.04 ng/mL or CRP > 10 mg/L, proceed to chest CT; if negative, perform CMR or perform CXR followed by echocardiography if CMR is inaccessible.
Monitoring protocols: While current protocols recommend monitoring for myocarditis during the first 4-8 weeks, their utility remains questionable. Elevated troponin and CRP levels alone should not prompt discontinuation of clozapine, as these markers are nonspecific and more likely to indicate pneumonia than myocarditis.
Diagnostic approaches: Prioritize CT scans for diagnosing pneumonia and advocate for the use of CMR in suspected myocarditis cases in well-resourced settings. In limited-resource settings, utilize CXR and serial echocardiography due to their non-invasive nature and reasonable diagnostic value.
Interim management during diagnostic workup: Given CMR wait times of 2-4 weeks in many centers, interim management of clozapine is crucial to balance the risk of CIM with therapeutic continuity. For patients with suspected cardiac injury syndrome (troponin > 0.04 ng/mL or CRP > 10 mg/L), perform immediate echocardiography and repeat biomarker testing within 24-48 hours. The following decision tree is proposed: (1) Mild presentation: (e.g., isolated biomarker elevation, stable vitals, no dyspnea or chest pain): Continue clozapine at current dose, monitor biomarkers daily, and repeat echocardiography in 3-5 days if biomarkers rise (e.g., troponin increase > 20% or CRP > 50 mg/L). Expedite chest CT to exclude CIP; (2) Moderate presentation: (e.g., fever, tachycardia, mild dyspnea, biomarker elevation): Reduce clozapine dose by 25%-50%, monitor biomarkers and vitals twice daily, and repeat echocardiography in 48 hours. If a chest CT confirms CIP, manage the patient according to infectious disease protocols and consider a temporary dose reduction rather than discontinuation; and (3) Severe presentation: (e.g., chest pain, significant dyspnea, arrhythmias, or troponin > 0.1 ng/mL): Hold clozapine, admit for cardiology consultation, and pursue urgent CMR or endomyocardial biopsy if echocardiography is inconclusive. Restart clozapine only after CIM is excluded.
This approach ensures timely CIP exclusion via chest CT, leverages echocardiography for interim monitoring, and minimizes the need for unnecessary discontinuation of clozapine. This approach strikes a balance between diagnostic precision and clinical feasibility. For CIP, the protocol supports dose reduction during acute illness and gradual re-escalation post-recovery to preserve clozapine’s therapeutic benefits. In contrast, permanent discontinuation is advised for confirmed or strongly suspected CIM due to the risk of recurrence. By harnessing the precision of advanced imaging, this framework addresses overlapping conditions, optimizes the role of clozapine in treatment-resistant schizophrenia, and provides a practical strategy to refine clinical practice. Ultimately, this protocol has the potential to enhance patient outcomes, minimize diagnostic errors, and improve the safe use of clozapine in psychiatric care.
Rechallenge: The current evidence supporting the rechallenge of clozapine following a diagnosis of myocarditis is limited. The limited number of confirmed cases and a high failure rate make it unsafe. Based on this evidence, the authors strongly discourage rechallenge with clozapine following the diagnosis of CIM.
Multidisciplinary care: Manage suspected myocarditis cases with a multidisciplinary team, including a cardiologist familiar with the risks and benefits of clozapine.
Clinical awareness: Educate clinicians about the challenges of diagnosing myocarditis in patients taking clozapine and the importance of considering alternative diagnoses, such as pneumonia, to ensure accurate diagnosis and treatment.
Figure 2 presents our recommendations for improving the recognition of CIM. Our proposed protocol addresses critical gaps in diagnosing CIM and pneumonia by integrating advanced imaging techniques, such as CT and CMR, with multidisciplinary collaboration. This approach not only enhances diagnostic accuracy but also ensures that patients continue to benefit from clozapine's unparalleled efficacy in treatment-resistant schizophrenia.
Strengths and weaknesses: Implementing the proposed CIM diagnostic algorithm requires addressing cost, access, and workflow integration. Chest CT costs range from 300 dollars to 1000 United States dollars, and CMR costs 1000 dollars to 3000 dollars, varying by region and facility[33]. These expenses may deter adoption in resource-limited settings, though echocardiography (100-500 dollars) offers a cost-effective alternative. In the United States, Medicare and private insurers often reimburse CT and CMR for myocarditis evaluation (current procedural terminology codes 75561, 71260). Still, coverage for clozapine-specific indications may require justification of medical necessity. Workflow integration involves coordinating services across psychiatry, cardiology, and radiology, potentially through multidisciplinary clinics or teleconsultation for rural facilities. Training psychiatrists to interpret biomarker trends and recognize the overlap between CIP and CIM can streamline referrals and reduce diagnostic delays.
A preliminary cost–benefit analysis suggests that accurate CIM diagnosis could reduce unnecessary clozapine discontinuations, which incur significant costs from schizophrenia relapses (e.g., hospitalizations averaging 15000 dollars-20000 dollars per episode). By preventing misdiagnosis in up to 65% of presumed CIM cases, the algorithm may yield net savings, though formal economic modeling is needed. Barriers include CMR wait times (2-4 weeks) and limited radiology capacity in low-resource settings, necessitating reliance on echocardiography and clinical assessment.
Our findings are limited by the heterogeneity of the included studies and the lack of standardized diagnostic criteria across protocols. We propose a prospective validation study to transition from narrative recommendations to evidence-based practice. A multicenter cohort of 200-300 clozapine-treated patients with suspected CIM would assess the algorithm’s sensitivity, specificity, positive predictive value, and time to diagnosis. Patients would undergo chest CT, CMR (or echocardiography), and biomarker monitoring per the protocol, with outcomes compared to endomyocardial biopsy or clinical follow-up as reference standards. Secondary outcomes include clozapine continuation rates and the cost-effectiveness of the treatment. This study, ideally conducted across high- and low-resource settings, would refine biomarker thresholds, suggest and validate the clinical CIM Risk assessment, and inform global implementation strategies.
Future studies should focus on validating this protocol in diverse clinical settings and exploring cost-effective strategies to improve access to advanced imaging in resource-limited environments. In addition, studies should prioritize establishing specific biomarker thresholds and validating their predictive value in prospective cohorts of patients treated with clozapine.
This systematic review underscores the diagnostic challenges of CIM and its frequent misdiagnosis with CIP, driven by non-specific biomarkers and inconsistent imaging practices. Our analysis of 119 studies reveals that up to 65% of presumed CIM cases lack confirmatory evidence, risking premature clozapine discontinuation, while rechallenge succeeds in 64.7%-68.9% of cases, albeit with a 2.9% fatality risk. To address these gaps, we propose a multidisciplinary diagnostic algorithm that integrates chest CT to exclude CIP, CMR for CIM confirmation in well-resourced settings, and echocardiography as a practical alternative in other settings, such as CXR and serial echocardiography. Emphasizing slower titration and cardiology collaboration, this protocol balances diagnostic precision with clozapine’s unparalleled benefits for treatment-resistant schizophrenia. Our approach aims to enhance patient safety and therapeutic outcomes by reducing misdiagnosis and optimizing management. Future research should validate this algorithm across diverse clinical settings, explore cost-effective imaging solutions for resource-limited environments, and refine the criteria for safe clozapine rechallenge to further support its role in psychiatric care.
| 1. | Kane JM, Honigfeld G, Singer J, Meltzer H; the Clozaril Collaborative Study Group. Clozapine for the treatment-resistant schizophrenic: results of a US multicenter trial. Psychopharmacology. 1989;99:S60-S63. [DOI] [Full Text] |
| 2. | Kilian JG, Kerr K, Lawrence C, Celermajer DS. Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999;354:1841-1845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 343] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 3. | Stoecker ZR, George WT, O'Brien JB, Jancik J, Colon E, Rasimas JJ. Clozapine usage increases the incidence of pneumonia compared with risperidone and the general population: a retrospective comparison of clozapine, risperidone, and the general population in a single hospital over 25 months. Int Clin Psychopharmacol. 2017;32:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Smith SC, Ladenson JH, Mason JW, Jaffe AS. Elevations of cardiac troponin I associated with myocarditis. Experimental and clinical correlates. Circulation. 1997;95:163-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 310] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 5. | Ronaldson KJ, Taylor AJ, Fitzgerald PB, Topliss DJ, Elsik M, McNeil JJ. Diagnostic characteristics of clozapine-induced myocarditis identified by an analysis of 38 cases and 47 controls. J Clin Psychiatry. 2010;71:976-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Tirupati S, Arachchi MK. High rates of myocarditis with clozapine in the Hunter region of Australia. Schizophr Res. 2024;264:543-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Vesterby A, Pedersen JH, Kaempe B, Thomsen NJ. [Sudden death during treatment with clozapine (Leponex)]. Ugeskr Laeger. 1980;142:170-171. [PubMed] |
| 8. | Meeker JE, Herrmann PW, Som CW, Reynolds PC. Clozapine tissue concentrations following an apparent suicidal overdose of Clozaril. J Anal Toxicol. 1992;16:54-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Bandelow B, Degner D, Kreusch U, Rüther E. Myocarditis under therapy with clozapine. Schizophr Res. 1995;17:293-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Haas SJ, Hill R, Krum H, Liew D, Tonkin A, Demos L, Stephan K, McNeil J. Clozapine-associated myocarditis: a review of 116 cases of suspected myocarditis associated with the use of clozapine in Australia during 1993-2003. Drug Saf. 2007;30:47-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 139] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 11. | Li JK, Yeung VT, Leung CM, Chow CC, Ko GT, So WY, Cockram CS. Clozapine: a mimicry of phaeochromocytoma. Aust N Z J Psychiatry. 1997;31:889-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Wang JF, Min JY, Hampton TG, Amende I, Yan X, Malek S, Abelmann WH, Green AI, Zeind J, Morgan JP. Clozapine-induced myocarditis: role of catecholamines in a murine model. Eur J Pharmacol. 2008;592:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Abdel-Wahab BA, Metwally ME. Clozapine-Induced Cardiotoxicity: Role of Oxidative Stress, Tumour Necrosis Factor Alpha and NF-κβ. Cardiovasc Toxicol. 2015;15:355-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Higgins JM, San C, Lagnado G, Chua D, Mihic T. Incidence and Management of Clozapine-Induced Myocarditis in a Large Tertiary Hospital. Can J Psychiatry. 2019;64:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Vickers M, Ramineni V, Malacova E, Eriksson L, McMahon K, Moudgil V, Scott J, Siskind D. Risk factors for clozapine-induced myocarditis and cardiomyopathy: A systematic review and meta-analysis. Acta Psychiatr Scand. 2022;145:442-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 16. | Datta T, Solomon AJ. Clozapine-induced myocarditis. Oxf Med Case Reports. 2018;2018:omx080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Layland JJ, Liew D, Prior DL. Clozapine-induced cardiotoxicity: a clinical update. Med J Aust. 2009;190:190-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Iqbal E, Govind R, Romero A, Dzahini O, Broadbent M, Stewart R, Smith T, Kim CH, Werbeloff N, MacCabe JH, Dobson RJB, Ibrahim ZM. The side effect profile of Clozapine in real world data of three large mental health hospitals. PLoS One. 2020;15:e0243437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 19. | Patel RK, Moore AM, Piper S, Sweeney M, Whiskey E, Cole G, Shergill SS, Plymen CM. Clozapine and cardiotoxicity - A guide for psychiatrists written by cardiologists. Psychiatry Res. 2019;282:112491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, Fu M, Heliö T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss HP, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM; European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636-2648, 2648a. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 2273] [Article Influence: 189.4] [Reference Citation Analysis (0)] |
| 21. | Fischer K, Marggraf M, Stark AW, Kaneko K, Aghayev A, Guensch DP, Huber AT, Steigner M, Blankstein R, Reichlin T, Windecker S, Kwong RY, Gräni C. Association of ECG parameters with late gadolinium enhancement and outcome in patients with clinical suspicion of acute or subacute myocarditis referred for CMR imaging. PLoS One. 2020;15:e0227134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Liu Y, Huang X, Liu Y, Li D, Zhang J, Yang L. Application value of hypersensitive C-reactive protein, lactic acid and myoglobin in the combined detection of myocarditis. Exp Ther Med. 2019;17:4471-4476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 23. | Goitein O, Matetzky S, Beinart R, Di Segni E, Hod H, Bentancur A, Konen E. Acute myocarditis: noninvasive evaluation with cardiac MRI and transthoracic echocardiography. AJR Am J Roentgenol. 2009;192:254-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Mavrogeni S, Sfikakis PP, Dimitroulas T, Kolovou G, Kitas GD. Cardiac and muscular involvement in idiopathic inflammatory myopathies: noninvasive diagnostic assessment and the role of cardiovascular and skeletal magnetic resonance imaging. Inflamm Allergy Drug Targets. 2014;13:206-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Vidusa L, Kalejs O, Maca-Kaleja A, Strumfa I. Role of Endomyocardial Biopsy in Diagnostics of Myocarditis. Diagnostics (Basel). 2022;12:2104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 26. | Rizkallah J, Desautels A, Malik A, Zieroth S, Jassal D, Hussain F, Cordova F. Eosinophilic myocarditis: two case reports and review of the literature. BMC Res Notes. 2013;6:538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Tang WHW. Myocarditis Workup. [cited 16 April 2025]. Available from: https://emedicine.medscape.com/article/156330-workup. |
| 28. | Akcha MA, Shams P, Kiel J. Acute Myocarditis. 2023 [cited 16 April 2025]. Available from: https://ecommons.aku.edu/pakistan_fhs_mc_med_cardiol/264. |
| 29. | McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599-3726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8225] [Cited by in RCA: 7283] [Article Influence: 1820.8] [Reference Citation Analysis (0)] |
| 30. | Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, Kindermann I, Gutberlet M, Cooper LT, Liu P, Friedrich MG. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J Am Coll Cardiol. 2018;72:3158-3176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 762] [Cited by in RCA: 1340] [Article Influence: 223.3] [Reference Citation Analysis (0)] |
| 31. | Sanchez Tijmes F, Thavendiranathan P, Udell JA, Seidman MA, Hanneman K. Cardiac MRI Assessment of Nonischemic Myocardial Inflammation: State of the Art Review and Update on Myocarditis Associated with COVID-19 Vaccination. Radiol Cardiothorac Imaging. 2021;3:e210252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 32. | Luetkens JA, Faron A, Isaak A, Dabir D, Kuetting D, Feisst A, Schmeel FC, Sprinkart AM, Thomas D. Comparison of Original and 2018 Lake Louise Criteria for Diagnosis of Acute Myocarditis: Results of a Validation Cohort. Radiol Cardiothorac Imaging. 2019;1:e190010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 33. | Li JM, Ho DR, Husain N, Biederman RW, Finn JP, Fuisz AR, Saeed IM, Nguyen KL. Regional variability of cardiovascular magnetic resonance access and utilization in the United States. J Cardiovasc Magn Reson. 2024;26:101061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 34. | Cicala G, Barbieri MA, Spina E, de Leon J. A comprehensive review of swallowing difficulties and dysphagia associated with antipsychotics in adults. Expert Rev Clin Pharmacol. 2019;12:219-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 35. | Leung JG, Hasassri ME, Barreto JN, Nelson S, Morgan RJ 3rd. Characterization of Admission Types in Medically Hospitalized Patients Prescribed Clozapine. Psychosomatics. 2017;58:164-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | de Leon J, Ruan CJ, Verdoux H, Wang C. Clozapine is strongly associated with the risk of pneumonia and inflammation. Gen Psychiatr. 2020;33:e100183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 37. | de Leon J. Respiratory infections rather than antibiotics may increase clozapine levels: a critical review of the literature. J Clin Psychiatry. 2004;65:1144-1145. [PubMed] [DOI] [Full Text] |
| 38. | Suzuki J, Ikeda R, Kato K, Kakuta R, Kobayashi Y, Ohkoshi A, Ishii R, Hirano-Kawamoto A, Ohta J, Kawata R, Kanbayashi T, Hatano M, Shishido T, Miyakura Y, Ishigaki K, Yamauchi Y, Nakazumi M, Endo T, Tozuka H, Kitaya S, Numano Y, Koizumi S, Saito Y, Unuma M, Hashimoto K, Ishida E, Kikuchi T, Kudo T, Watanabe K, Ogura M, Tateda M, Sasaki T, Ohta N, Okazaki T, Katori Y. Characteristics of aspiration pneumonia patients in acute care hospitals: A multicenter, retrospective survey in Northern Japan. PLoS One. 2021;16:e0254261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 39. | Kikuchi Y, Komatsu H, Sakuma A, Tanifuji H, Tomita H. Successful rechallenge with clozapine after discontinuation due to drug-induced pneumonia: A case report. PCN Rep. 2022;1:e38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Stein PD, Matta F, Ekkah M, Saleh T, Janjua M, Patel YR, Khadra H. Electrocardiogram in pneumonia. Am J Cardiol. 2012;110:1836-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | Tintinalli JE, Gabor DK, Stephan Stapczynski J. Emergency Medicine: A Comprehensive Study Guide. 6th ed. NY: McGraw-Hill, 2010: 480. |
| 42. | Ibrahim D, Bizri AR, El Amine MA, Halabi Z. Chest computed tomography and chest X-ray in the diagnosis of community-acquired pneumonia: a retrospective observational study. J Int Med Res. 2021;49:3000605211039791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 43. | Ramirez J, Aliberti S, Mirsaeidi M, Peyrani P, Filardo G, Amir A, Moffett B, Gordon J, Blasi F, Bordon J. Acute myocardial infarction in hospitalized patients with community-acquired pneumonia. Clin Infect Dis. 2008;47:182-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 152] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 44. | Smith RP, Lipworth BJ, Cree IA, Spiers EM, Winter JH. C-reactive protein. A clinical marker in community-acquired pneumonia. Chest. 1995;108:1288-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 109] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 45. | Berk M, Fitzsimons J, Lambert T, Pantelis C, Kulkarni J, Castle D, Ryan EW, Jespersen S, McGorry P, Berger G, Kuluris B, Callaly T, Dodd S. Monitoring the safe use of clozapine: a consensus view from Victoria, Australia. CNS Drugs. 2007;21:117-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 46. | Anıl Yağcıoğlu AE, Ertuğrul A, Karakaşlı AA, Ağaoğlu E, Ak S, Karahan S, Yazıcı MK. A comparative study of detection of myocarditis induced by clozapine: With and without cardiac monitoring. Psychiatry Res. 2019;279:90-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 47. | Sandarsh S, Bishnoi RJ, Shashank RB, Miller BJ, Freudenreich O, McEvoy JP. Monitoring for myocarditis during treatment initiation with clozapine. Acta Psychiatr Scand. 2021;144:194-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 48. | Nachmani Major N, Dawson BPharm Hons JL, Clark SR. Implementation and Outcomes of a Clozapine-Associated Myocarditis Screening Program in a Region of South Australia-Lessons Learned. J Clin Psychopharmacol. 2020;40:250-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 49. | Griffin JM, Woznica E, Gilotra NA, Nucifora FC Jr. Clozapine-Associated Myocarditis: A Protocol for Monitoring Upon Clozapine Initiation and Recommendations for How to Conduct a Clozapine Rechallenge. J Clin Psychopharmacol. 2021;41:180-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 50. | NSW Health. Monitoring clozapine-induced myocarditis. Sydney: NSW Government; 2022 [cited 2025 Jun 30]. Available from: https://www1.health.nsw.gov.au/pds/ActivePDSDocuments/GL2022_011.pdf. |
| 51. | Kanniah G, Kumar S. Clozapine associated cardiotoxicity: Issues, challenges and way forward. Asian J Psychiatr. 2020;50:101950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 52. | Murch S, Tran N, Liew D, Petrakis M, Prior D, Castle D. Echocardiographic monitoring for clozapine cardiac toxicity: lessons from real-world experience. Australas Psychiatry. 2013;21:258-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 53. | McNutt SE, Morrow G, Waters K, Cooper C, Crish E. Health System Pharmacist Protocol for Myocarditis Monitoring in Clozapine-Naïve Inpatients-Two Years' Experience. J Pharm Pract. 2021;34:542-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 54. | Segev A, Iqbal E, McDonagh TA, Casetta C, Oloyede E, Piper S, Plymen CM, MacCabe JH. Clozapine-induced myocarditis: electronic health register analysis of incidence, timing, clinical markers and diagnostic accuracy. Br J Psychiatry. 2021;219:644-651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 55. | Youssef DL, Narayanan P, Gill N. Incidence and risk factors for clozapine-induced myocarditis and cardiomyopathy at a regional mental health service in Australia. Australas Psychiatry. 2016;24:176-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 56. | de Leon J, Schoretsanitis G, Smith RL, Molden E, Solismaa A, Seppälä N, Kopeček M, Švancer P, Olmos I, Ricciardi C, Iglesias-Garcia C, Iglesias-Alonso A, Spina E, Ruan CJ, Wang CY, Wang G, Tang YL, Lin SK, Lane HY, Kim YS, Kim SH, Rajkumar AP, González-Esquivel DF, Jung-Cook H, Baptista T, Rohde C, Nielsen J, Verdoux H, Quiles C, Sanz EJ, De Las Cuevas C, Cohen D, Schulte PFJ, Ertuğrul A, Anıl Yağcıoğlu AE, Chopra N, McCollum B, Shelton C, Cotes RO, Kaithi AR, Kane JM, Farooq S, Ng CH, Bilbily J, Hiemke C, López-Jaramillo C, McGrane I, Lana F, Eap CB, Arrojo-Romero M, Rădulescu FŞ, Seifritz E, Every-Palmer S, Bousman CA, Bebawi E, Bhattacharya R, Kelly DL, Otsuka Y, Lazary J, Torres R, Yecora A, Motuca M, Chan SKW, Zolezzi M, Ouanes S, De Berardis D, Grover S, Procyshyn RM, Adebayo RA, Kirilochev OO, Soloviev A, Fountoulakis KN, Wilkowska A, Cubała WJ, Ayub M, Silva A, Bonelli RM, Villagrán-Moreno JM, Crespo-Facorro B, Temmingh H, Decloedt E, Pedro MR, Takeuchi H, Tsukahara M, Gründer G, Sagud M, Celofiga A, Ignjatovic Ristic D, Ortiz BB, Elkis H, Pacheco Palha AJ, LLerena A, Fernandez-Egea E, Siskind D, Weizman A, Masmoudi R, Mohd Saffian S, Leung JG, Buckley PF, Marder SR, Citrome L, Freudenreich O, Correll CU, Müller DJ. Correction: An International Adult Guideline for Making Clozapine Titration Safer by Using Six Ancestry-Based Personalized Dosing Titrations, CRP, and Clozapine Levels. Pharmacopsychiatry. 2022;55:e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 57. | Richardson N, Greenway SC, Bousman CA. Clozapine-induced myocarditis and patient outcomes after drug rechallenge following myocarditis: A systematic case review. Psychiatry Res. 2021;305:114247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 58. | McMahon L, Giudice M, Wagner E, Hasan A, Burrage MK, Amerena J, Fox C, Winckel K, Tanzer T, Smith L, Warren N, Siskind D, Korman N. Clozapine rechallenge following myocarditis: a systematic review of rechallenge cases. CNS Spectr. 2024;29:585-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 59. | Noël MC, Powell V, Burton L, Panda R, Remington G. Clozapine-Related Myocarditis and Rechallenge: A Case Series and Clinical Review. J Clin Psychopharmacol. 2019;39:380-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |