Published online Aug 19, 2025. doi: 10.5498/wjp.v15.i8.109280
Revised: June 3, 2025
Accepted: July 2, 2025
Published online: August 19, 2025
Processing time: 93 Days and 8.6 Hours
The neural mechanisms underlying aggressive behavior in schizophrenia (SCZ) remain poorly understood. To date, no studies have reported on the event-related potential (ERP) characteristics of aggression in SCZ using the competitive reaction time task (CRTT). Further investigation into the ERP correlates of aggression in SCZ would provide valuable insights into the neural processes involved.
To explore the neural mechanism of aggressive behavior in SCZ.
Participants of this study included 40 SCZ patients and 42 healthy controls (HCs). The Reactive Proactive Aggression Questionnaire was used to assess trait of aggression. The Barratt Impulsiveness Scale 11 was used to measure impulsiveness. The Positive and Negative Symptom Scale (PANSS) was used to evaluate psychopathological features and disease severity. All participants were measured with ERP while performing the CRTT. Data of behavior, ERP components (P2, N2, and P3), and feedback-related negativity (FRN) were analyzed.
Analysis of the behavioral data revealed that compared with HCs, SCZ patients exhibited higher punishment choices. Analysis of ERP components showed that compared with HCs, SCZ patients exhibited higher N2 amplitudes and P2 amplitudes during the decision phase of the CRTT; however, SCZ patients exhibited lower FRN amplitudes and lower P3 amplitudes during the outcome phase of the CRTT. The N2 amplitudes evoked by high-intensity provocation were positively related to PANSS-P scores. And the P3 amplitudes evoked in the winning trials were negatively correlated with the PANSS-G scores.
SCZ patients exhibit abnormal ERP characteristics evoked by the CRTT, which suggests the neural correlates of aggressive behavior in SCZ.
Core Tip: This study represents the first investigation employing the competitive reaction time task paradigm to examine the neurocognitive mechanisms underlying aggressive behavior in schizophrenia (SCZ) patients. Our findings demonstrate a complex interplay among positive psychotic symptoms, theory of mind deficits, and negative affect dysregulation in mediating aggression in SCZ. These findings provide valuable insights into the understanding of the neural mechanisms of aggression and may guide targeted interventions in SCZ.
- Citation: Zhang L, Mei Q, Zhang JZ, Chen LM, Liu XH, Zhou ZH, Zhou HL. Neural correlates of aggression in schizophrenia: An event-related potential study using the competitive reaction time task. World J Psychiatry 2025; 15(8): 109280
- URL: https://www.wjgnet.com/2220-3206/full/v15/i8/109280.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i8.109280
Schizophrenia (SCZ) is defined as a group of severe mental disorders of unknown etiology with an onset typically occurring during adolescence or early adulthood. Moreover, the condition is characterized by abnormalities in perception, thinking, emotions, and behaviors[1]. Although the symptoms of SCZ are varied, aggression is one of the most prominent and has significant social impact[2]. Aggression is defined as any behavior directed toward another individual that is performed with the proximate intent to cause harm[3]. Research has indicated a global prevalence rate of aggression among SCZ patients of 33.3%[4], and the incidence of aggressive behaviors in SCZ patients is higher than that in the general population[5]. Aggression in SCZ patients can lead to severe negative outcomes, including serious harm to other individuals, excessive treatment burdens[6], extended hospitalizations[7], increased antipsychotic dosages, and greater stigmatization[8].
Aggression in clinical populations can be operationally divided into two neurobehaviorally distinct subtypes: Proactive aggression and reactive aggression[9]. Proactive aggression refers to planned, goal-oriented behaviors without provocation, whereas reactive aggression is associated with increased levels of autonomic arousal, and is triggered by negative emotions such as anger, frustration, or perceived threat[10]. Aggression in SCZ typically presents as a behavior that is marked by a lack of clear intent or planning[11].
Studies have demonstrated that aggression may be a precursor to SCZ and may be closely related to positive symptoms, which is a potential new dimension of SCZ[12]. Many studies have demonstrated that cognitive deficits are a major driver of aggression in patients with SCZ[13-16]. The brain regions implicated in aggression include the orbitofrontal cortex, dorsolateral prefrontal cortex[17], anterior cingulate cortex, posterior cingulate cortex, insula, hippocampus, amygdala[18], and striatum[19]. In addition, recent studies have emphasized the potential importance of the precuneus and inferior parietal lobule in various cognitive processes[20,21]. Impaired microstructural integrity in these regions or their connected white matter tracts may contribute to aggression by disrupting emotion recognition and regulation, reward and avoidance learning, and decision-making processes[22].
Event-related potential (ERP) technology provides high temporal resolution electrophysiological data by recording brain electrical activity that occurs during specific events, thus capturing millisecond-level dynamic changes. Compared with other neuroimaging techniques [such as functional magnetic resonance imaging (fMRI) and positron emission tomography], ERP offers noninvasive advantages without long-term psychophysiological impacts, and is suitable for widespread research and clinical applications[23].
Previous ERP studies focusing on aggression in patients with SCZ have utilized the Go/No-Go task or the emotional facial recognition paradigm[24]. These passive response tasks poorly simulate real-world dynamic interactions between motivational conflicts and behavioral decisions. In fact, SCZ patients exhibit significant deficits in their ability to suppress the dominant response, and response inhibition has been shown to be only weakly associated with aggression[25,26].
The Taylor Aggression Paradigm (TAP) is one of the most frequently used measures of reactive aggression[27]. In the TAP, participants believe that they are competing with another participant to achieve the fastest completion on a competitive reaction time (RT) task (CRTT), with the winner choosing the level of an aversive stimulus to be administered to the loser. This stimulus level represents the measure of aggression. Previous studies have shown that ERP components (N2, P2, and P3) and feedback-related negativity (FRN) evoked by the TAP are associated with aggression in healthy individuals. For example, a previous study demonstrated that at high provocation, adult participants with trait aggression had increased frontal N2 amplitudes during the decision phase; another study indicated that N2 amplitudes were increased in nonviolent men and individuals with high psychopathic traits in the decision phase[28]. Many studies have reported increased P2 amplitudes in violent participants during the decision phase[29], as well as increased P2 amplitudes in response to emotional images in provoked groups compared with unprovoked groups[30]. In addition, during the outcome phase, a study reported that the FRN in the violent adolescent group was significantly lower overall than that in the normal adolescent group, regardless of whether the participants were winning or losing[31]. Fur
The CRTT is a widely implemented variant of the TAP[33]. In this competitive paradigm, participants engage in sequential trials against opponents. Upon winning a trial, the losing opponent receives noise punishment; conversely, if they lose, the participants will be punished. Evidence indicates that the CRTT exhibits high internal, convergent, discriminant, and external validity[34].
To date, no studies on the ERP characteristics of aggression with the use of the CRTT in SCZ patients have been reported. Further investigations of the ERP characteristics of aggression in SCZ would be helpful in understanding the neural process of aggression. Moreover, insights into the neural mechanisms underlying these deficits could provide novel targets for pharmacological and neuromodulatory treatments in SCZ.
In the present study, the participants included SCZ patients and healthy controls (HCs), and the measurements of ERPs during the CRTT were used to investigate the neural process of aggression. The purpose of this study was to investigate the ERP characteristics of aggression and to further explore the neural mechanism of the cognitive processing of aggression in patients with SCZ.
This study was conducted in the Department of Clinical Psychiatry, the Affiliated Mental Health Center of Jiangnan Medical University from January 2025 to April 2025. The study protocol was approved by the Ethics Committee of the Affiliated Mental Health Center of Jiangnan Medical University and was conducted according to the Declaration of Helsinki (Reference No. WXMHCIRB2025 LLky020).
The inclusion criteria for the SCZ group were as follows: (1) Met the criteria for SCZ according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5); (2) Were aged 18-65 years; and (3) Had no current or history of neurological illness or any other kind of severe physical illness that would affect his or her cognitive function. The exclusion criteria were as follows: (1) Met the criteria for any other mental disorder according to DSM-5; (2) Was treated by electroconvulsive therapy (ECT) or modified ECT within 6 months before recruitment; (3) Had nicotine/other substance misuse or dependence within 6 months before recruitment; and (4) Had hearing and visual impairments that cannot be corrected to satisfy the demands of the current study. The inclusion criteria for the HCs were as follows: (1) No diagnosis of psychiatric disorders according to the DSM-5 criteria; (2) No family history of any mental disorders; and (3) Were aged 18-65 years. The exclusion criteria were as follows: (1) Had hearing and visual impairments that could not be corrected to satisfy the demands of the current study; and (2) Had nicotine/other substance misuse or dependence within 6 months before recruitment. HCs were selected from residents of Wuxi City, Jiangsu Province, China via local media advertising.
The G*Power software (version. 3.1.9.7; Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany) was used to estimate the sample size in this study. In the F tests, α = 0.05 was considered to be statistically significant, and a minimum requirement of 32 subjects per group met the sample size requirement. A total of 40 SCZ patients and 42 HCs were included in this study. For SCZ patients, the dose of antipsychotic medication was calculated based on chlorpromazine equivalence[35]. Prior to the study, participants or their guardians were informed about the study's purpose and procedures, and then provided written informed consent.
The Reactive Proactive Aggression Questionnaire was used to assess the trait of aggression[36]. The Barratt Impulsiveness Scale Version 11 was used to measure impulsiveness[37]. Additionally, the Modified Overt Aggression Scale and Buss-Perry Questionnaire were used to evaluate aggression[38]. The Positive and Negative Symptom Scale (PANSS) was used to assess psychopathological features and disease severity[39]. Furthermore, the Annett handedness scale was used to rate the participant’s handedness[40].
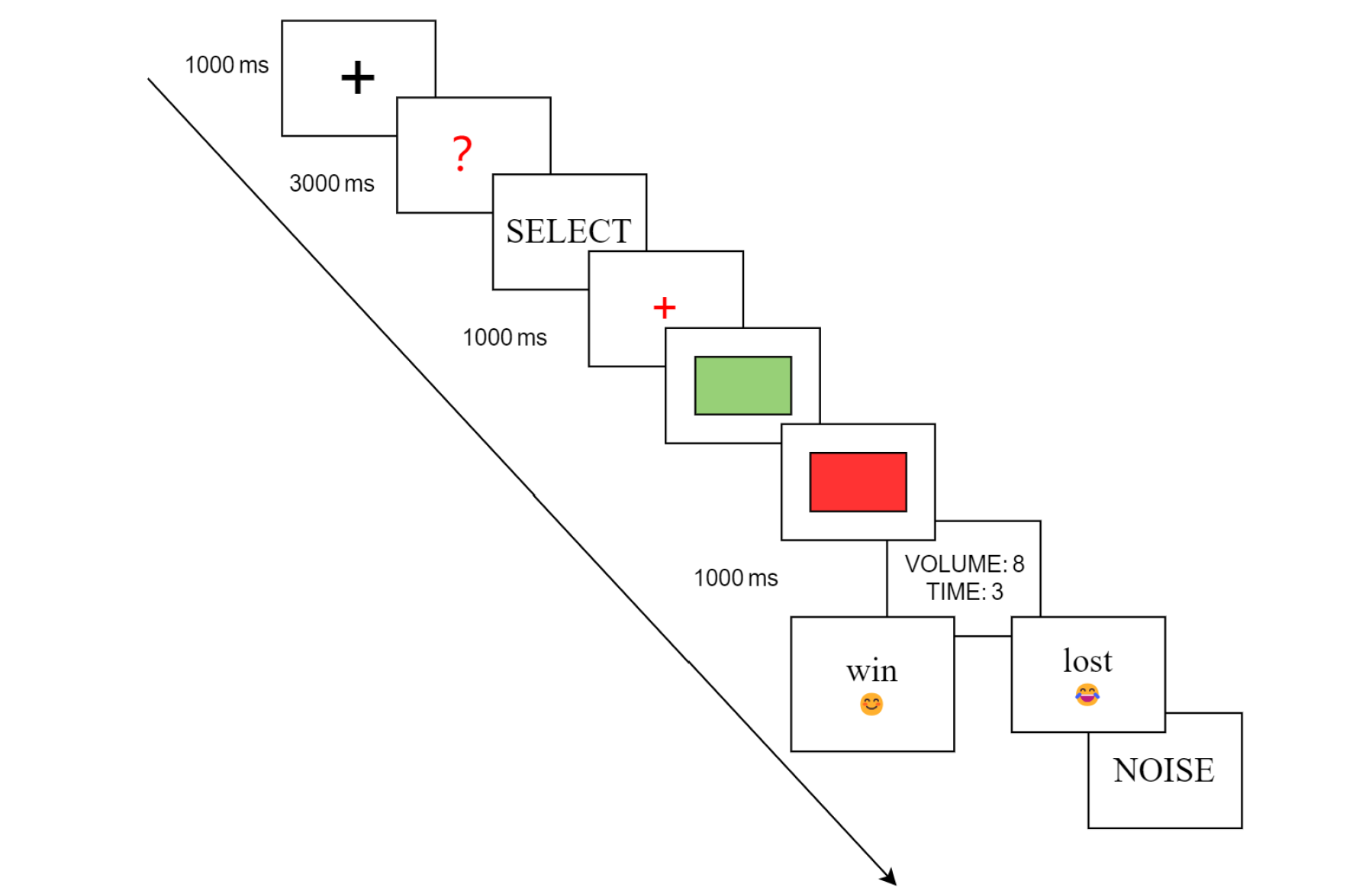
The CRTT was conferred from the TAP[27-29,31,32,41-43], and was programmed on computer using E-Prime 3.0 (Psychology Software Tools Incorporated, Pittsburgh, United States). Participants were instructed to believe that they were engaging in a competitive task against an opponent. Each trial commenced with a focus point shown in the center of the screen (“+”, 1000 milliseconds), followed by a red question mark “?” shown for 3 seconds, then the participants were asked to choose the duration and volume of the noise blast on a 10-point scale (volume: 0-85 decibels, duration: 0-5 seconds). The first provocation (first noise feedback not being zero) was given in the seventh trial. After selection, they had to try to outperform the opponent by pressing the space key when the rectangle turned from a green color into a red color on the screen. The duration was randomized, ranging from 1000 to 2000 milliseconds. Subsequently, the intensity of the punishment chosen by the opponent was presented on the screen (1000 milliseconds). The visual feedback indicated whether the participants had won or lost the trial by showing a smiling face with “win” or a sad face with “lose” for 1500 milliseconds. Finally, the participants received the noise stimulus chosen by the opponent. The participants avoided the punishment if they had won the competition. Furthermore, at the end of the study, participants were carefully probed for suspicion of the real existence of the opponent. We used the phrase “What was your impression of your opponent?” to avoid participants becoming suspicious of the questions themselves (Figure 1).
In reality, we predetermined the frequencies of the wins and losses, and the effective RTs were 0-2000 milliseconds. When participants reacted more slowly, they automatically lost the trial to ensure credibility. Furthermore, the volume and duration of the noise feedback that the participants experienced were preprogrammed and standardized for each participant. We considered volumes 1-5 as indicating low noise provocation levels and volumes 6-10 as indicating high noise provocation levels.
The task included a total of 60 trials that were divided into two blocks, with a 10-minute rest occurring between the blocks. Before the formal task, a practice session was conducted to ensure that all of the participants were familiar with the test. After completing the entire experiment, each participant was compensated with a payment of 150.0 RMB. The noise level utilized in the trial meets China’s occupational safety and health standards for full-time workers exposed to 85 decibels for a duration of 8 hours, which is within the pain threshold of 125 decibels. The software “GOLDWAVE” (v7.2, Goldwave Inc., St. John’s, Newfoundland, Canada, 2010) was used to assess the noise levels.
Continuous electroencephalograph (EEG) recordings (bandpass filtered between 0.05 to 100 Hz, digitized at 500 Hz) were conducted using a 64-channel Ag-AgCl elastic cap, adhering to the International 10/20 System, via the BioSemi Active Two system (https://www.biosemi.com/Products_ActiveTwo.htm). The impedance across all electrodes was kept under 5 kΩ during the sessions. The EEG was epoched from -200 milliseconds to 1000 milliseconds before and after the stimulus marker and the baseline was corrected by using the mean (ranging from -200 milliseconds to 0 milliseconds). Data processing was performed offline using MATLAB version 2020b (MathWorks, Inc., Natick, MA, United States) in conjunction with the EEGLAB toolbox. All of the EEG data were re-referenced to the average of the bilateral mastoids and were bandpass filtered from 0.1 to 30 Hz (via a low-pass cutoff at 12 Hz for N2 and P2) utilizing a notch filter at 50 Hz[42]. Both electric eye artifact elimination and data correction were performed by the independent component analysis statistical procedure. For the subsequent analysis, trials that demonstrated EEG voltages exceeding the ± 80 μV threshold were excluded.
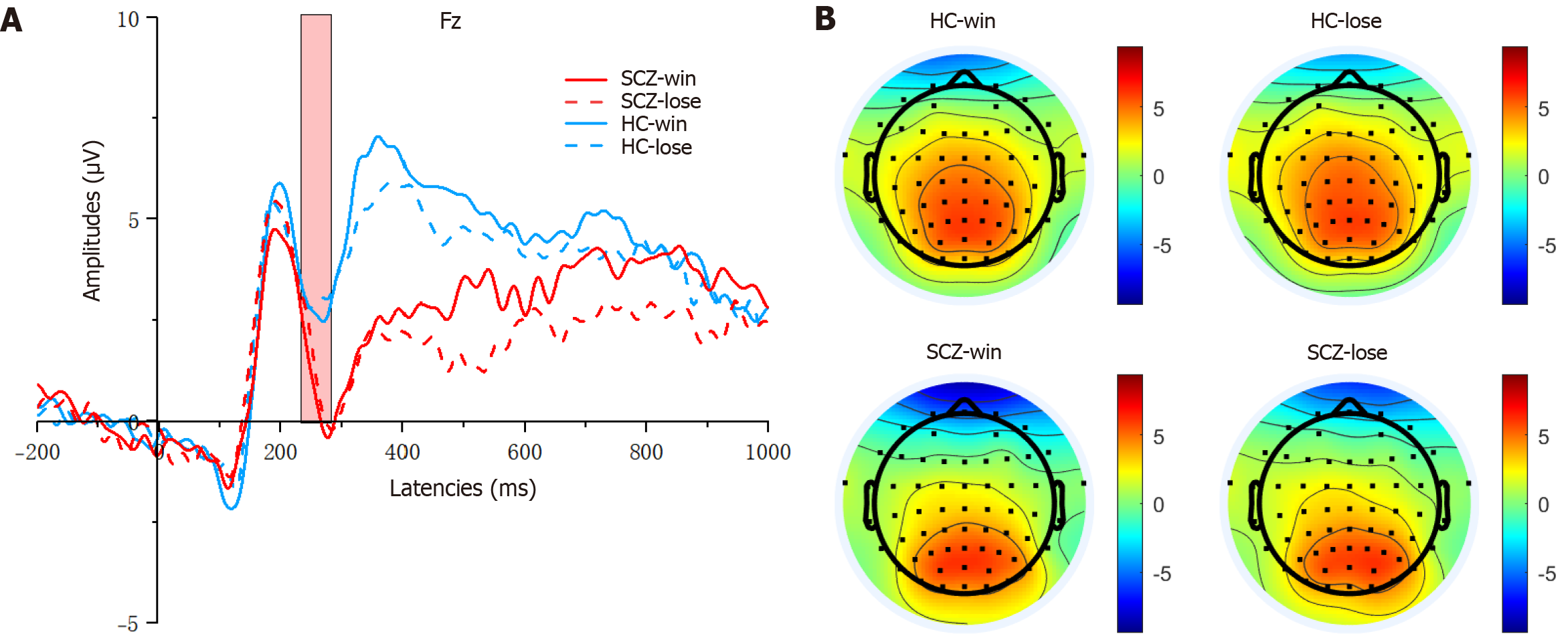
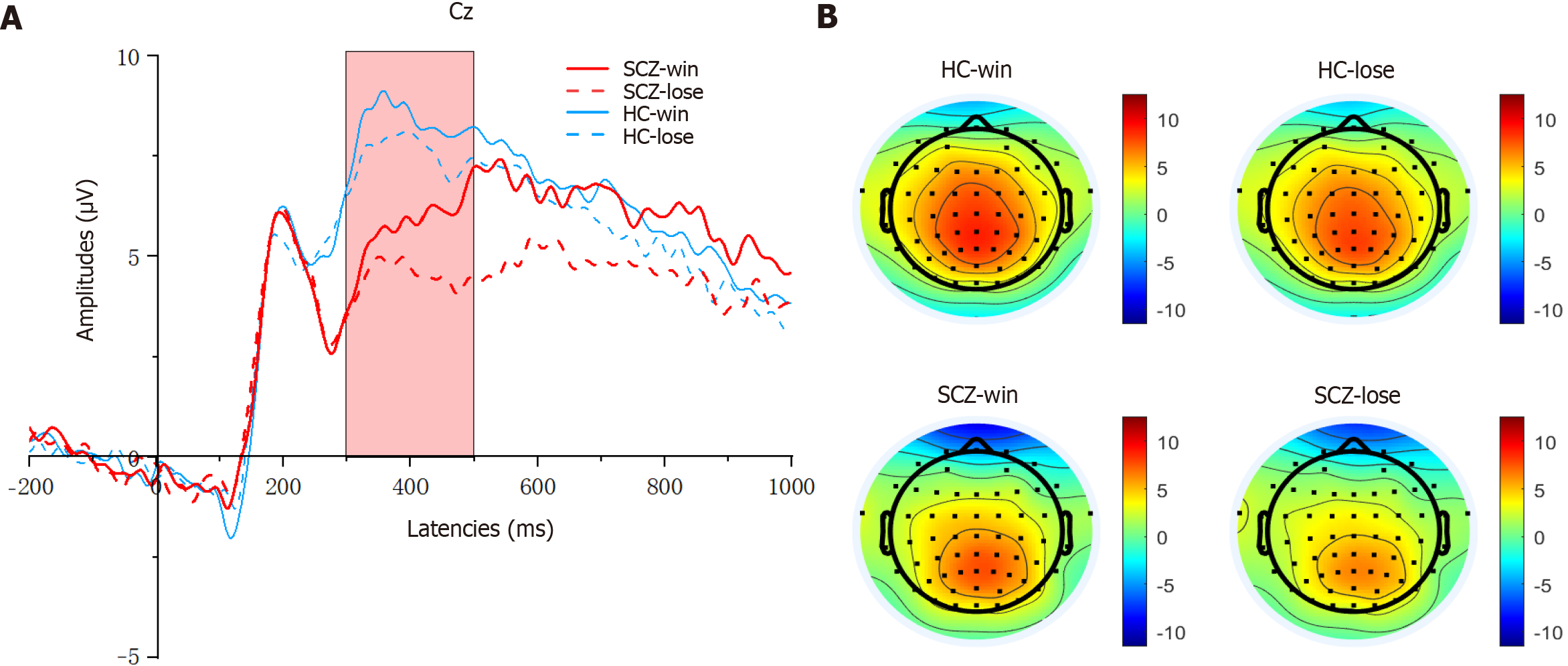
According to the grand averaged ERP waveforms and the results of previous literature[29,31,42-44], in the CRTT decision phase, mean amplitudes were quantified on three electrodes (F3, F4, and Fz) in a time window of 150-250 milliseconds for the P2, and in a time window of 200-300 milliseconds for N2. In the CRTT outcome phase, the activity was recorded at six electrodes (F3, F4, C3, C4, Fz, and Pz) during the time window of 300-500 milliseconds for the P3, and during the time window of 250-280 milliseconds following stimulus onset was used to calculate the FRN.
Statistical analyses were performed using SPSS 22.0 (IBM Corp, Armonk, NY, United States). Normality assumptions were verified through the Kolmogorov-Smirnov test, confirming normal distribution for all continuous variables. For continuous variables, independent sample t-tests were used to compare the differences between the SCZ group and HC group, whereas the χ2 test was employed for categorical variables. Two-way mixed analysis of variance (ANOVA) on the ERP components (N2, P2, and P3) and FRN was used to compare the differences between the SCZ group and the HC group. Pearson correlation analysis was employed to investigate the relationships between the ERP components and psychopathology parameters. The degrees of freedom for the F ratio were adjusted using the Greenhouse Geisser correction. Additionally, effect sizes were calculated using the partial eta-squared (η2). The threshold for significance was set to P < 0.05 (two-tailed). In cases of significant findings, post hoc Bonferroni corrections were conducted.
Table 1 presents the demographic characteristics of the participants. There were no significant differences between the SCZ and HC groups in terms of age, sex, education, or handedness. Forty patients were treated with antipsychotics: Six were treated with risperidone (mean dose 4.0 ± 1.3 mg/day), ten treated with olanzapine (mean dose 12.75 ± 2.15 mg/day), nine with quetiapine (mean dose 500.0 ± 100.65 mg/day), six with aripiprazole (mean dose 15.0 ± 9.37 mg/day), six with amisulpride (mean dose 250.50 ± 26.25 mg/day), and four with paliperidone (mean dose 7.50 ± 2.20 mg/day). For SCZ patients, the mean chlorpromazine-equivalent dose was 250.50 ± 10.66 mg/day, as calculated according to the previous report[35].
| Variable | HCs (n = 42) | SCZ (n = 40) | t/χ2 | P value |
| Age, years | 34.5 ± 8.0 | 35.8 ± 9.6 | 1.373 | 0.245 |
| Education, years | 13.4 ± 2.4 | 12.4 ± 2.5 | 1.735 | 0.087 |
| Handedness, right/middle/left | 17/14/11 | 16/14/10 | 0.029 | 0.986 |
| Sex, male/female | 15/27 | 16/24 | 0.160 | 0.689 |
| BIS-11 score | 65.93 ± 8.23 | 84.45 ± 11.02 | -8.650 | < 0.001 |
| RPQ proactive score | 0.83 ± 0.82 | 2.48 ± 2.40 | -4.108 | < 0.001 |
| RPQ reactive score | 3.67 ± 1.92 | 7.60 ± 2.34 | -8.366 | < 0.001 |
| RPQ total score | 4.57 ± 2.17 | 10.07 ± 3.83 | -8.050 | < 0.001 |
| PANSS total score | NA | 87.35 ± 12.22 | NA | NA |
| PANSS positive score | NA | 29.87 ± 5.15 | NA | NA |
| PANSS negative score | NA | 22.35 ± 6.44 | NA | NA |
| PANSS general score | NA | 35.17 ± 5.75 | NA | NA |
| MOAS score | NA | 7.89 ± 1.77 | NA | NA |
| BPAQ score | 34.76 ± 7.89 | NA | NA | NA |
The factor-analytically derived scoring method was used to compute the total score[33]. The first seven trials of punishment responses have been used to reflect unprovoked aggression in previous research[43,44].
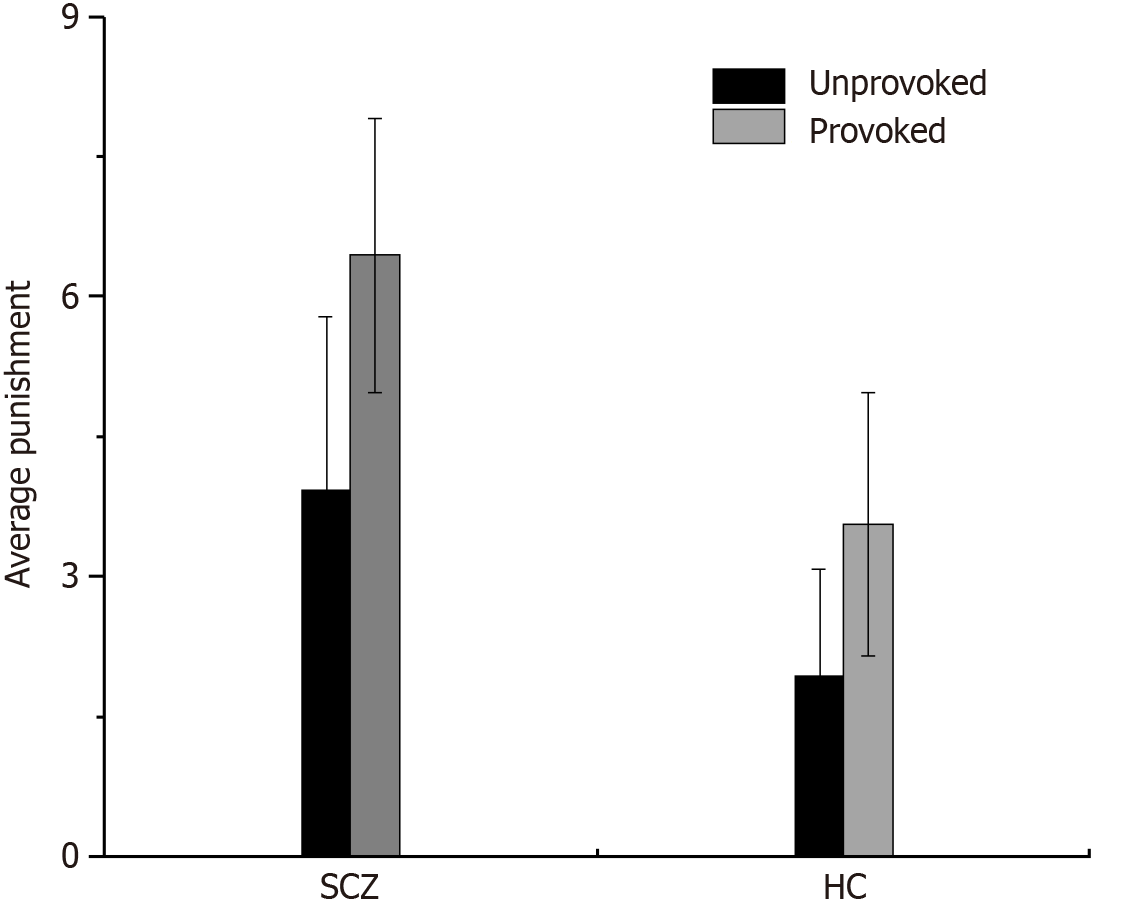
An ANOVA utilizing provocation (unprovoked vs provoked) as a within-subjects factor and group (SCZ vs HC) as a between-subjects factor for punishment selection revealed a significant main effect of provocation (F(1, 80) = 72.850, P = 0.000, η2 = 0.477), indicating greater reactive aggression in participants in the provoked condition than in those in the unprovoked condition. The main effect of the group was significant (F(1, 80) = 119.579, P = 0.000, η2 = 0.599), thus indicating that the SCZ patients exhibited greater reactive aggression compared with the HCs. However, the interaction effect between provocation and group was not significant (F(1, 80) = 3.285, P = 0.074, η2 = 0.039; Figure 2).
N2 and P2 components: Two-way mixed ANOVA was performed on the N2 and P2 components with the provocation level (high vs low) being used as the within-subjects factor and group (HC vs SCZ) as the between-subjects factor.
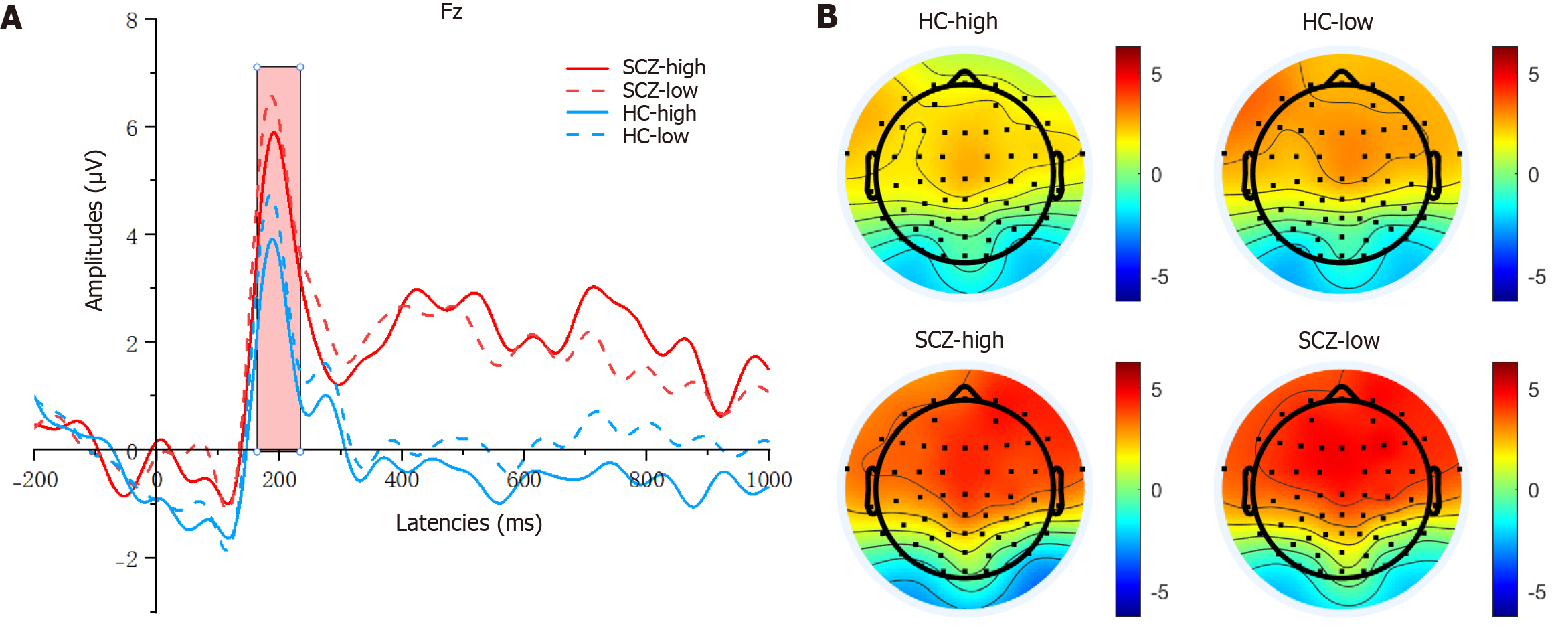
As shown in Figure 3, for the P2 amplitudes, the main effect of provocation was significant (F(1, 80) = 5.820, P = 0.018, η2 = 0.068), and the main effect of group was also significant (F(1, 80) = 7.164, P = 0.009, η2 = 0.082). Compared with the HC group, the SCZ group exhibited markedly higher (more positive) P2 amplitudes. Additionally, the P2 amplitudes following the high-provocation condition were notably lower than those following the low-provocation condition. How
As shown in Figure 4, for the N2 amplitudes, the main effect of provocation was significant (F(1, 80) = 4.760, P = 0.032, η2 = 0.056), and the main effect of group was significant (F(1, 80) = 5.288, P = 0.024, η2 = 0.062). The SCZ group exhibited markedly higher (more negative) N2 amplitudes compared with the HC group. Additionally, the N2 amplitudes fol
FRN and P3 components: Two-way mixed ANOVA was conducted on the FRN and P3 components, with outcome (win vs lose) being used as the within-subjects factor and group (HC vs SCZ) as the between-subjects factor.
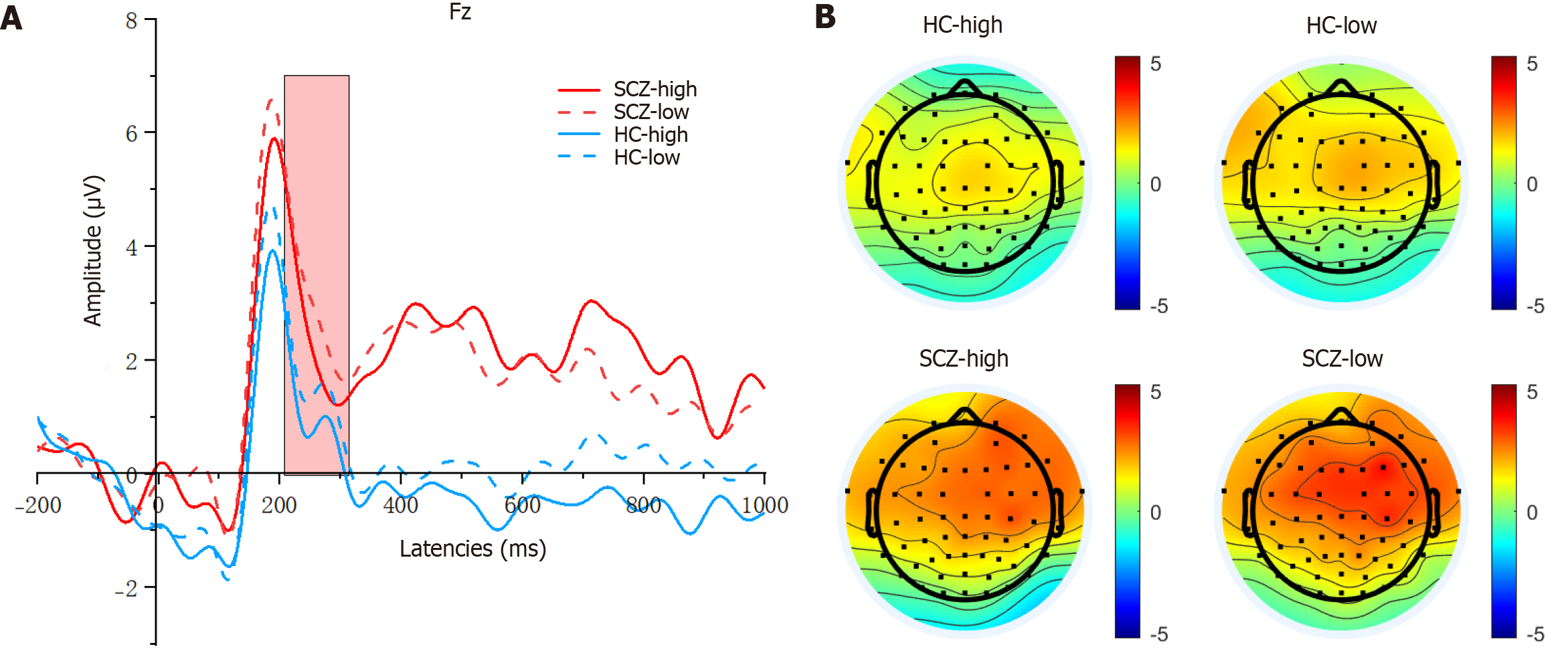
As shown in Figure 5, for the FRN amplitudes, the main effect of the group was significant (F(1, 80) = 4.619, P = 0.035, η2 = 0.055), and the FRN amplitudes were lower (more negative) in the SCZ group than in the HC group. However, the difference between the outcome and group was not significant (F(1, 80) = 0.000, P = 0.987, η2 = 0.000). Additionally, the main effect of outcome was not significant (F(1, 80) = 1.096, P = 0.298, η2 = 0.014). The FRN latency demonstrated no significant main effect for either the outcome (F(1, 80) = 0.313, P = 0.577, η2 = 0.004) or the group × outcome interaction (F(1, 80) = 0.429, P = 0.514, η2 = 0.005). However, the main effect was significant for the group (F(1, 80) = 15.296, P = 0.000, η2 = 0.161). The FRN latency in the HC group was significantly longer than that in the SCZ group.
As shown in Figure 6, for the P3 amplitudes, the main effect of outcome was significant (F(1, 80) = 6.057, P = 0.016, η2 = 0.700), and the P3 amplitudes were larger (more positive) in the win trials compared with the lose trials. The main effect of the group was significant, and the P3 amplitudes were also larger for the HC group than for the SCZ group (F(1, 80) = 4.651, P = 0.034, η2 = 0.055). Furthermore, the group × outcome interaction was not significant (F(1, 80) = 0.163, P = 0.688, η2 = 0.002). For the P3 latencies, neither significant main effects nor interactions were observed.
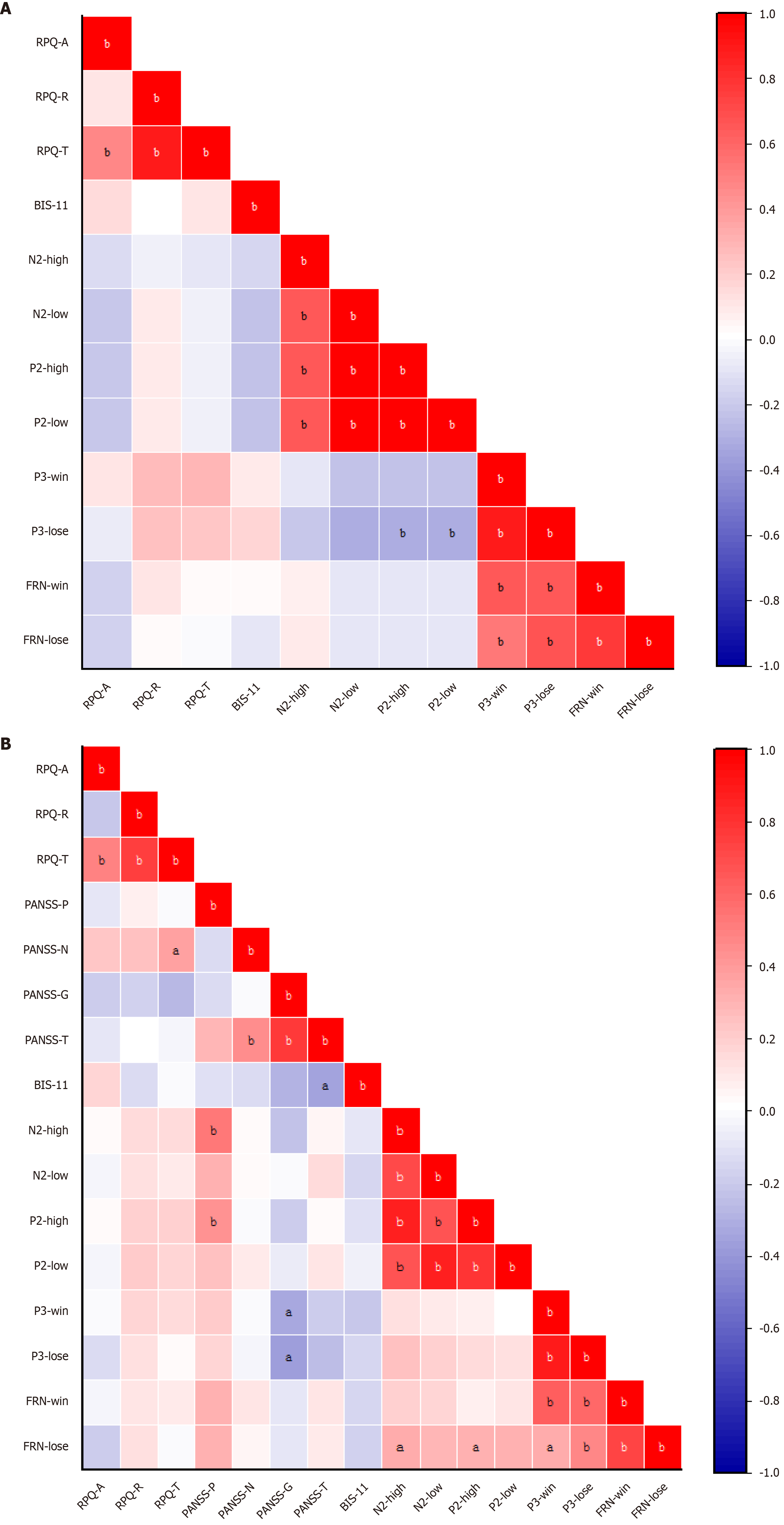
For the HC group, no significant correlations across any domains were observed (all P > 0.05). As shown in Figure 7, in the SCZ group, the N2 amplitudes evoked by high-intensity provocation was positively related to PANSS-P scores (r = 0.524, P = 0.000) and the P2 amplitudes evoked by high-intensity provocation was positively related to PANSS-G scores (r = 0.428, P = 0.006), whereas the P3 amplitudes evoked in the winning trials showed a negative correlation with the PANSS-G scores (r = -0.325, P = 0.041) and the P3 amplitudes evoked in the losing trials showed a negative correlation with the PANSS-G scores (r = -0.367, P = 0.020).
This is the first study to investigate the ERP characteristics of aggression with the CRTT in SCZ, which is also the first to clarify the neuroelectrophysiological mechanisms of aggression for SCZ patients. This study found that compared with that in HCs, the average punishment intensity of aggression in schizophrenic patients was higher during the CRTT. SCZ patients exhibited higher N2 amplitudes and P2 amplitudes during the decision phase of the CRTT. However, SCZ patients exhibited lower FRN amplitudes and lower P3 amplitudes during the outcome phase of the CRTT.
The CRTT is a widely used experimental paradigm designed to assess aggressive behavior in a controlled laboratory setting[41]. In the task, the intensity and duration of the selected punishment are commonly interpreted as behavioral indices of reactive aggression. In our study, SCZ patients have been found to exhibit significantly higher average punishment intensity. This elevated level of aggressive responding is typically interpreted as being an indicator of increased reactive aggression, particularly in response to perceived provocation during the task.
The heightened punishment intensity may reflect several underlying psychopathological and neurocognitive mechanisms. First, paranoid ideation and threat misattribution, which are prevalent in SCZ, may lead individuals to perceive the task environment as being more hostile or adversarial, thereby amplifying retaliatory behaviors. Second, impairments in impulse control and emotion regulation, which are often linked to dysfunction in prefrontal-limbic circuits[45-47], may hinder the modulation of aggressive impulses. Finally, cognitive deficits, such as impaired theory of mind or reduced social cue processing, could further exacerbate inappropriate or exaggerated aggressive responses, as SCZ patients may misinterpret the intentions or actions of their (fictitious) opponents[48,49]. Our findings underscore the complex interplay between psychotic symptomatology, social cognitive dysfunction, and affective dysregulation in contributing to aggression in SCZ.
In this study, ERP findings obtained from SCZ patients performing the CRTT revealed a distinct pattern of altered neural processing across task phases, reflecting abnormalities in conflict monitoring, salience detection, and feedback evaluation.
During the decision phase of the CRTT (i.e., when participants select the intensity of the punitive stimulus), SCZ patients exhibited increased N2 and P2 amplitudes compared with HCs. The N2 component, which is typically associated with conflict detection and cognitive control[50-52], suggests that patients may experience heightened neural conflict or increased effort in response selection. Moreover, the increased P2 amplitude, which is linked to early attentional allocation and salience detection[53,54], may indicate hyperresponsivity to socially provocative stimuli or aberrant attentional engagement during punishment decisions, thus possibly reflecting altered threat perception or exaggerated evaluation of social interactions.
In contrast, during the outcome phase (i.e., when the participants received feedback on the competition), SCZ patients demonstrated reduced FRN and lower P3 amplitudes. The FRN, which is typically elicited by negative or unexpected feedback, reflects early performance monitoring and reward prediction error processing[55,56]. The attenuation in SCZ may reflect blunted sensitivity to outcome valence, which is consistent with prior evidence of dysfunctional reward processing in this population[57,58]. Similarly, the P3 component, which is associated with the conscious evaluation of feedback and attentional resource allocation[59,60], was also diminished, suggesting deficits in integrating motivationally salient outcomes into goal-directed behavior.
Collectively, this ERP pattern indicates a dissociation between heightened early attentional or conflict-related processing and impaired feedback evaluation in SCZ patients during socially competitive interactions. These findings support the notion of dysregulated fronto-striatal and cingulo-parietal circuitries, and provide evidence of neurophysiological mechanisms for aggression in SCZ.
In summary, our study indicated that SCZ patients exhibited abnormal ERP characteristics evoked by the CRTT, which suggested the neural correlates of aggressive behavior in SCZ. These findings provide valuable insights into the under
There are some limitations in this study. First, due to the small sample size, this study is preliminary in nature. In the future, large samples with the same parameters are needed to replicate these findings. Second, the current study involved only a cross-sectional design, thus making it difficult to distinguish causal mechanisms. Third, due to the low spatial resolution of the ERP equipment, future studies should combine high-spatial-resolution fMRI further to clarify the neurophysiological mechanism of aggression in SCZ. Moreover, the investigation of the specific impacts of different treatment modalities on ERP characteristics could yield a more comprehensive understanding of the neural mechanisms involved in this phenomenon.
In conclusion, our study demonstrated that patients with SCZ exhibited abnormal ERP characteristics evoked by the CRTT. This ERP pattern reflects a dissociation between heightened early attentional or conflict-related processing and impaired feedback evaluation in SCZ patients. These findings uncover the distinct neurodynamic characteristics of aggressive behavior in SCZ, offering a novel perspective for understanding its pathological mechanism and guiding the development of personalized therapeutic strategies. To elucidate the causal mechanisms, we recommend that future research adopt a longitudinal design and incorporate more comprehensive evidence regarding structural and functional aspects.
We thank the subjects and their families who participated in this study and we would like to acknowledge everyone who helped us in this project.
| 1. | Jauhar S, Johnstone M, McKenna PJ. Schizophrenia. Lancet. 2022;399:473-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 506] [Article Influence: 168.7] [Reference Citation Analysis (0)] |
| 2. | Ogonah MGT, Seyedsalehi A, Whiting D, Fazel S. Violence risk assessment instruments in forensic psychiatric populations: a systematic review and meta-analysis. Lancet Psychiatry. 2023;10:780-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 3. | Kersten R, Greitemeyer T. Human aggression in everyday life: An empirical test of the general aggression model. Br J Soc Psychol. 2024;63:1091-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 4. | Li W, Yang Y, Hong L, An FR, Ungvari GS, Ng CH, Xiang YT. Prevalence of aggression in patients with schizophrenia: A systematic review and meta-analysis of observational studies. Asian J Psychiatr. 2020;47:101846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 5. | Whiting D, Gulati G, Geddes JR, Fazel S. Association of Schizophrenia Spectrum Disorders and Violence Perpetration in Adults and Adolescents From 15 Countries: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2022;79:120-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 79] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 6. | Chai Y, Tang JY, Ma DCF, Luo H, Chan SKW. Self-Harm and Suicide Rates Before and After an Early Intervention Program for Patients With First-Episode Schizophrenia. JAMA Netw Open. 2024;7:e2426795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Solmi M, Seitidis G, Mavridis D, Correll CU, Dragioti E, Guimond S, Tuominen L, Dargél A, Carvalho AF, Fornaro M, Maes M, Monaco F, Song M, Il Shin J, Cortese S. Incidence, prevalence, and global burden of schizophrenia - data, with critical appraisal, from the Global Burden of Disease (GBD) 2019. Mol Psychiatry. 2023;28:5319-5327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 155] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 8. | Ostuzzi G, Vita G, Bertolini F, Tedeschi F, De Luca B, Gastaldon C, Nosé M, Papola D, Purgato M, Del Giovane C, Correll CU, Barbui C. Continuing, reducing, switching, or stopping antipsychotics in individuals with schizophrenia-spectrum disorders who are clinically stable: a systematic review and network meta-analysis. Lancet Psychiatry. 2022;9:614-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 9. | Romero-Martínez Á, Sarrate-Costa C, Moya-Albiol L. Reactive vs proactive aggression: A differential psychobiological profile? Conclusions derived from a systematic review. Neurosci Biobehav Rev. 2022;136:104626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 10. | Potegal M, Nordman JC. Non-angry aggressive arousal and angriffsberietschaft: A narrative review of the phenomenology and physiology of proactive/offensive aggression motivation and escalation in people and other animals. Neurosci Biobehav Rev. 2023;147:105110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 11. | Krakowski MI, Tural U, Czobor P. Separate pathways to violent behavior in schizophrenia and in the general population. J Psychiatr Res. 2022;151:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Whiting D, Lichtenstein P, Fazel S. Violence and mental disorders: a structured review of associations by individual diagnoses, risk factors, and risk assessment. Lancet Psychiatry. 2021;8:150-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 130] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 13. | Brugman S, Lobbestael J, Sack AT, Cima MJ, Schuhmann T, Emmerling F, Arntz A. Cognitive predictors of reactive and proactive aggression in a forensic sample: A comparison with a non-clinical sample. Psychiatry Res. 2018;269:610-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Ahmed AO, Richardson J, Buckner A, Romanoff S, Feder M, Oragunye N, Ilnicki A, Bhat I, Hoptman MJ, Lindenmayer JP. Do cognitive deficits predict negative emotionality and aggression in schizophrenia? Psychiatry Res. 2018;259:350-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Vaskinn A, Rokicki J, Bell C, Tesli N, Bang N, Hjell G, Fischer-Vieler T, Haukvik UK, Friestad C. Violent Offending in Males With or Without Schizophrenia: A Role for Social Cognition? Schizophr Bull. 2024;50:663-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Gebreegziabhere Y, Habatmu K, Mihretu A, Cella M, Alem A. Cognitive impairment in people with schizophrenia: an umbrella review. Eur Arch Psychiatry Clin Neurosci. 2022;272:1139-1155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 127] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 17. | Hostetler N, Tavares TP, Ritchie MB, Oliver LD, Chen VV, Greening S, Finger EC, Mitchell DGV. Prefrontal cortex structural and developmental associations with callous-unemotional traits and aggression. Sci Rep. 2024;14:4087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Park K, Chung C. Differential Alterations in Cortico-Amygdala Circuitry in Mice with Impaired Fear Extinction. Mol Neurobiol. 2020;57:710-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Nikolic M, Pezzoli P, Jaworska N, Seto MC. Brain responses in aggression-prone individuals: A systematic review and meta-analysis of functional magnetic resonance imaging (fMRI) studies of anger- and aggression-eliciting tasks. Prog Neuropsychopharmacol Biol Psychiatry. 2022;119:110596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Haukvik UK, Wolfers T, Tesli N, Bell C, Hjell G, Fischer-Vieler T, Bang N, Melle I, Andreassen OA, Rasmussen K, Agartz I, Westlye LT, Friestad C, Rokicki J. Individual-level deviations from normative brain morphology in violence, psychosis, and psychopathy. Transl Psychiatry. 2025;15:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Stauffer EM, Bethlehem RAI, Dorfschmidt L, Won H, Warrier V, Bullmore ET. The genetic relationships between brain structure and schizophrenia. Nat Commun. 2023;14:7820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 22. | Williams JA, Burgess S, Suckling J, Lalousis PA, Batool F, Griffiths SL, Palmer E, Karwath A, Barsky A, Gkoutos GV, Wood S, Barnes NM, David AS, Donohoe G, Neill JC, Deakin B, Khandaker GM, Upthegrove R; PIMS Collaboration. Inflammation and Brain Structure in Schizophrenia and Other Neuropsychiatric Disorders: A Mendelian Randomization Study. JAMA Psychiatry. 2022;79:498-507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 171] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 23. | Donoghue T, Voytek B. Automated meta-analysis of the event-related potential (ERP) literature. Sci Rep. 2022;12:1867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 24. | Krakowski MI, De Sanctis P, Foxe JJ, Hoptman MJ, Nolan K, Kamiel S, Czobor P. Disturbances in Response Inhibition and Emotional Processing as Potential Pathways to Violence in Schizophrenia: A High-Density Event-Related Potential Study. Schizophr Bull. 2016;42:963-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Patel PK, Green MF, Barch D, Wynn JK. Mechanisms and correlates of incentivized response inhibition in schizophrenia and bipolar disorder. J Psychiatr Res. 2025;183:282-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Hoptman MJ. Impulsivity and aggression in schizophrenia: a neural circuitry perspective with implications for treatment. CNS Spectr. 2015;20:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 27. | Timmins MA, Fanning JR, McCloskey MS, Berman ME, Coccaro EF. Laboratory assessment of aggression: The Taylor Aggression Paradigm in adults with and without a disorder of impulsive aggression. J Psychiatr Res. 2023;163:413-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 28. | Wang Y, Yang Q, Zhu B, Ye S, Tian X, Krueger F. High levels of psychopathic traits increase the risk of transferring reactive aggression to innocent people after provocation: Evidence from an ERP study. Biol Psychol. 2020;153:107891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Wiswede D, Taubner S, Münte TF, Roth G, Strüber D, Wahl K, Krämer UM. Neurophysiological correlates of laboratory-induced aggression in young men with and without a history of violence. PLoS One. 2011;6:e22599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Bertsch K, Böhnke R, Kruk MR, Naumann E. Influence of aggression on information processing in the emotional stroop task--an event-related potential study. Front Behav Neurosci. 2009;3:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Chen CY, Chiou CR, Ko CH. Juveniles with a history of violent behavior show cognitive performance and electrophysiology consistent with inhibitory control and emotional feedback processing problems. Aggress Behav. 2019;45:6-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Bernat EM, Ellis JS, Bachman MD, Hicks BM. P3 amplitude reductions are associated with shared variance between internalizing and externalizing psychopathology. Psychophysiology. 2020;57:e13618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Lobbestael J, Emmerling F, Brugman S, Broers N, Sack AT, Schuhmann T, Bonnemayer C, Benning R, Arntz A. Toward a More Valid Assessment of Behavioral Aggression: An Open Source Platform and an Empirically Derived Scoring Method for Using the Competitive Reaction Time Task (CRTT). Assessment. 2021;28:1065-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Giancola PR, Parrott DJ. Further evidence for the validity of the Taylor Aggression Paradigm. Aggress Behav. 2008;34:214-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 135] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 35. | Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1567] [Cited by in RCA: 1718] [Article Influence: 78.1] [Reference Citation Analysis (0)] |
| 36. | Dinić BM, Raine A, Vujić A, van Dongen JDM. Cross-cultural Validity of the Reactive-Proactive Aggression Questionnaire Among Adults Across Five Countries. J Interpers Violence. 2022;37:NP6261-NP6283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Meule A. Sorting the confusion about the numerous versions of the Barratt Impulsiveness Scale. Neurol Sci. 2023;44:3721-3722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Knoedler DW. The Modified Overt Aggression Scale. Am J Psychiatry. 1989;146:1081-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Lim K, Peh OH, Yang Z, Rekhi G, Rapisarda A, See YM, Rashid NAA, Ang MS, Lee SA, Sim K, Huang H, Lencz T, Lee J, Lam M. Large-scale evaluation of the Positive and Negative Syndrome Scale (PANSS) symptom architecture in schizophrenia. Asian J Psychiatr. 2021;62:102732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 40. | Tsuang HC, Chen WJ, Kuo SY, Hsiao PC. Handedness and schizotypy: The potential effect of changing the writing-hand. Psychiatry Res. 2016;242:198-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 41. | Warburton WA, Bushman BJ. The competitive reaction time task: The development and scientific utility of a flexible laboratory aggression paradigm. Aggress Behav. 2019;45:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 42. | Krämer UM, Büttner S, Roth G, Münte TF. Trait aggressiveness modulates neurophysiological correlates of laboratory-induced reactive aggression in humans. J Cogn Neurosci. 2008;20:1464-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Pincham HL, Bryce D, Fonagy P, Fearon RMP. Psychosocial intervention in at-risk adolescents: using event-related potentials to assess changes in decision making and feedback processing. Eur Child Adolesc Psychiatry. 2019;28:223-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Dambacher F, Schuhmann T, Lobbestael J, Arntz A, Brugman S, Sack AT. No Effects of Bilateral tDCS over Inferior Frontal Gyrus on Response Inhibition and Aggression. PLoS One. 2015;10:e0132170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 45. | Leclerc MP, Regenbogen C, Hamilton RH, Habel U. Some neuroanatomical insights to impulsive aggression in schizophrenia. Schizophr Res. 2018;201:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 46. | Kaldewaij R, Koch SBJ, Hashemi MM, Zhang W, Klumpers F, Roelofs K. Anterior prefrontal brain activity during emotion control predicts resilience to post-traumatic stress symptoms. Nat Hum Behav. 2021;5:1055-1064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 47. | Benzait A, Krenz V, Wegrzyn M, Doll A, Woermann F, Labudda K, Bien CG, Kissler J. Hemodynamic correlates of emotion regulation in frontal lobe epilepsy patients and healthy participants. Hum Brain Mapp. 2023;44:1456-1475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 48. | Lam BY, Raine A, Lee TM. Effect of theory of mind and peer victimization on the schizotypy-aggression relationship. NPJ Schizophr. 2016;2:16001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | McGuire J, Brüne M, Langdon R. Judgment of moral and social transgression in schizophrenia. Compr Psychiatry. 2017;76:160-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | Larson MJ, Clayson PE, Clawson A. Making sense of all the conflict: a theoretical review and critique of conflict-related ERPs. Int J Psychophysiol. 2014;93:283-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 316] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 51. | Jost K, Wendt M, Luna-Rodriguez A, Jacobsen T. Electrophysiological correlates of proportion congruency manipulation in a temporal flanker task. Psychophysiology. 2022;59:e14092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 52. | Coffman BA, Haigh SM, Murphy TK, Leiter-Mcbeth J, Salisbury DF. Reduced auditory segmentation potentials in first-episode schizophrenia. Schizophr Res. 2018;195:421-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 53. | Favero JD, Luck C, Lipp OV, Nguyen AT, Marinovic W. N1-P2 event-related potentials and perceived intensity are associated: The effects of a weak pre-stimulus and attentional load on processing of a subsequent intense stimulus. Biol Psychol. 2023;184:108711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 54. | Chen Z, Qin Y, Peng M, Zhao W, Shi X, Lai D, Yin E, Yan Y, Yao D, Liu T. Event-related potential patterns of selective attention modulated by perceptual load. Brain Behav. 2023;13:e2907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 55. | Weismüller B, Bellebaum C. Expectancy affects the feedback-related negativity (FRN) for delayed feedback in probabilistic learning. Psychophysiology. 2016;53:1739-1750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 56. | Walentowska W, Severo MC, Moors A, Pourtois G. When the outcome is different than expected: Subjective expectancy shapes reward prediction error at the FRN level. Psychophysiology. 2019;56:e13456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 57. | Abram SV, Roach BJ, Holroyd CB, Paulus MP, Ford JM, Mathalon DH, Fryer SL. Reward processing electrophysiology in schizophrenia: Effects of age and illness phase. Neuroimage Clin. 2020;28:102492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 58. | Clayson PE, Wynn JK, Infantolino ZP, Hajcak G, Green MF, Horan WP. Reward processing in certain versus uncertain contexts in schizophrenia: An event-related potential (ERP) study. J Abnorm Psychol. 2019;128:867-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 59. | Weber J, Abeln V, Steichele K, Foitschik T, Stuckenschneider T. Inefficient resource allocation is associated with reduced alpha activity in parietal regions in individuals with Parkinson's disease. Eur J Neurosci. 2021;53:1225-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 60. | Kranczioch C, Bryant D. Attentional awakening, resource allocation and the focus of temporal attention. Neuroreport. 2011;22:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |