Published online Aug 19, 2025. doi: 10.5498/wjp.v15.i8.107696
Revised: April 19, 2025
Accepted: June 23, 2025
Published online: August 19, 2025
Processing time: 133 Days and 21.1 Hours
Schizophrenia is characterized by psychotic symptoms, negative symptoms, and cognitive deficits, profoundly affecting individuals and their families. The eti
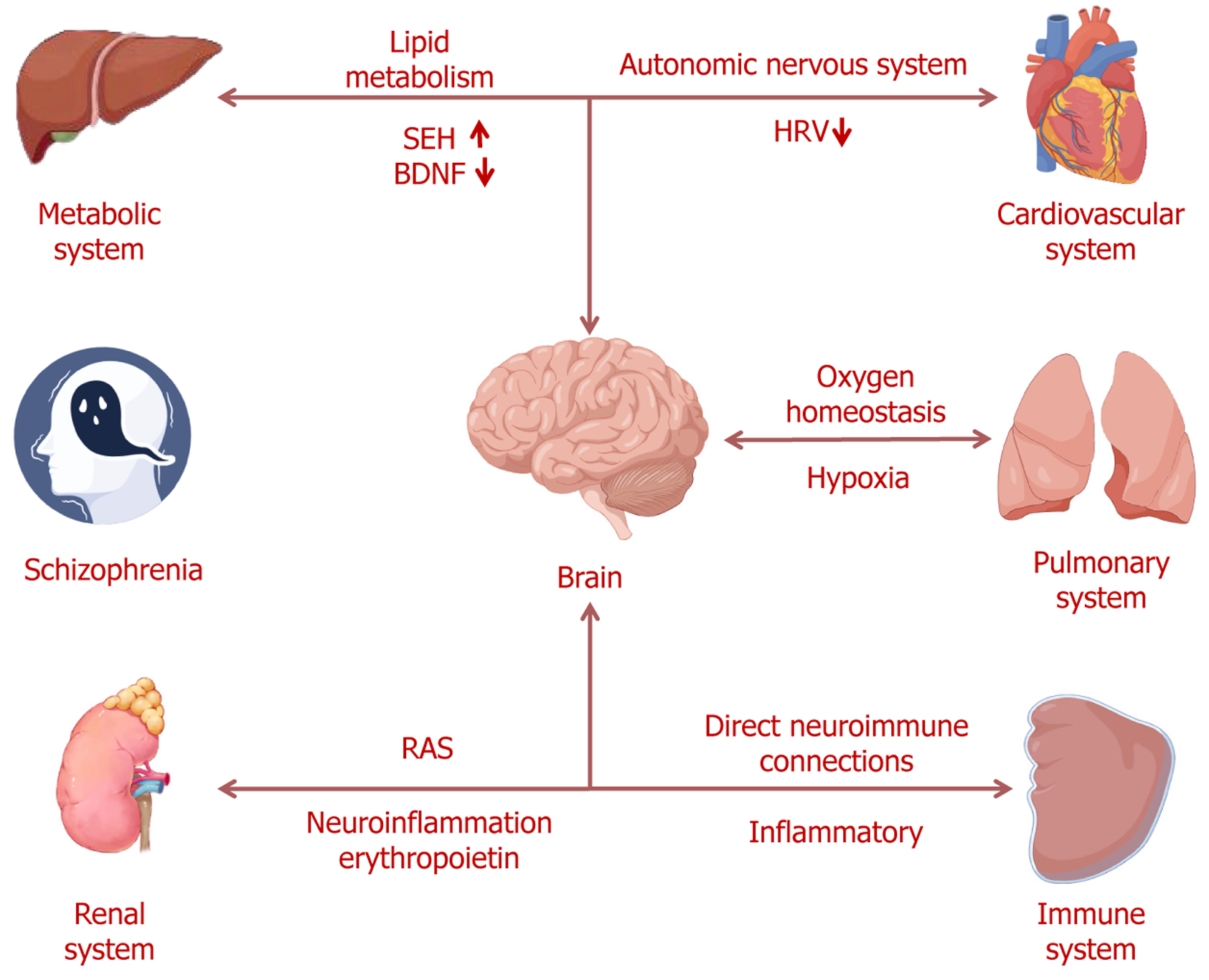
Core Tip: While existing reviews of schizophrenia focus primarily on central nervous system pathophysiology, this minireview uniquely highlights the growing evidence of interactions between the brain and peripheral organ systems, including the cardiovascular, pulmonary, hepatic, immune, and renal systems. It underscores the critical role of brain-organ interaction via the autonomic nervous system, endocrine system, and immune system in the pathophysiology of schizophrenia, providing new insights into its underlying mechanisms and potential therapeutic targets.
- Citation: Lin J, Feng ST, Wu ZY, Dong LR, Yin DQ, Zhu H, Jia HX, Ning YZ. Interaction between the brain and multiple organ systems in schizophrenia. World J Psychiatry 2025; 15(8): 107696
- URL: https://www.wjgnet.com/2220-3206/full/v15/i8/107696.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i8.107696
Schizophrenia, as a noteworthy public health concern, has a world lifetime prevalence of around 1%[1]. Epidemiological studies show that patients with schizophrenia have a mortality rate that is two to three times greater than the general population, associated with the alterations in non-central nervous systems[2]. Despite schizophrenia is characterized by a multitude of brain abnormalities, several structural and functional imbalances in schizophrenia are related to the organic causes[3,4]. Recent researches have demonstrated that schizophrenia is often accompanied by a range of dysfunctions in the specific organ systems, including cardiovascular, hepatic and metabolic, immune, pulmonary, and renal systems[5-9]. These body systems are interconnected in varying degrees in the pathology of schizophrenia, indicating schizophrenia engage multiple systems[10]. For instance, patients with schizophrenia typically manifests in early adulthood, whereas heart failure and coronary artery disease emerge later in life[5]. Previous meta-analyses have further indicated that a significantly higher risk of schizophrenia in those with other diseases of organ systems, such as the digestive system and immune system[11,12]. These organic systems are not only affected by schizophrenia, but also through the biological pathways on brain function, thereby influencing the onset and progression of the schizophrenia[13,14].
Given the intricate connections between the brain and the organ systems, the integration of evidence on brain-organ crosstalk in schizophrenia is required. In this narrative review, we summarize the evidence of the interactions between the brain and the heart, liver, spleen, lungs, and kidneys in schizophrenia. Besides, we will review the association between the brain and multiple organic systems via the autonomic nervous system, endocrine system, and immune system. The purpose of this review is to provide new perspectives on the pathophysiology of schizophrenia.
The interaction between the brain and the cardiovascular system is primarily mediated by the autonomic nervous system[15]. By modulating both sympathetic and parasympathetic nervous systems, the autonomic nervous system controls heart rate, rhythm, as well as the contraction and relaxation of blood vessels, ensuring the proper functioning of the cardiovascular system. Heart rate variability (HRV), as a key physiological indicator, not only reflects the dynamic regu
The interaction between the brain and the pulmonary system is primarily mediated by oxygen homeostasis, with hypoxia influencing neurodevelopment, gene expression, and structural integrity, thereby contributing to the pathophysiology of schizophrenia[26]. The main functions of the pulmonary system are to maintain oxygen supply and facilitate the expulsion of carbon dioxide through gas exchange, and adequate oxygenation is crucial for normal brain function[27]. Recent studies have found that patients with schizophrenia exhibit histogenous hypoxia and acid retention, with reduced venous oxygen pressure emerging as a characteristic variable of schizophrenia[28]. Hypoxia not only alters gene expression but may also disrupt critical neurodevelopmental signaling pathways[29]. Prior studies have indicated that hypoxia not only impacts gene expression but may also interfere with the expression of many candidate genes related to schizophrenia, thereby disrupting key neurodevelopmental signaling pathways[30]. These changes may impair neuronal differentiation, migration, and synapse formation, ultimately leading to abnormalities in brain structure and function, and thus contributing to the onset of schizophrenia[31]. Autopsy-based studies have further demonstrated that the ex
Immune system dysregulation is increasingly recognized as a key component in the pathophysiology of schizophrenia[35]. The spleen, as a vital immune organ, plays a central role in host defense against bacteria, viruses, and other path
The liver plays a pivotal role in lipid metabolism, maintaining systemic lipid homeostasis[44]. Increasing evidence indicates that lipid metabolism abnormalities in patients with schizophrenia, which are closely associated with clinical symptoms[45,46]. These lipid metabolism disorders not only alter the overall physiological state but may also impact brain function via the brain-liver axis. Specifically, reduced lipid content in the dorsolateral prefrontal cortex of schizophrenia patients is closely related to cognitive dysfunction[47]. Furthermore, postmortem studies have demonstrated a synchronized increase in the expression levels of soluble epoxide hydrolase in both the liver and the brain in schizophrenia[48]. Moreover, a prior study has identified a negative correlation between brain-derived neurotrophic factor (BDNF) levels in the parietal cortex and those in the liver of patients with schizophrenia[49]. BDNF plays a crucial role in neuroprotection and neuroplasticity, and its dysregulation in both organs may impact the pathophysiology of schizophrenia via the brain-liver axis[50]. These cross-organ metabolic and neuroregulatory abnormalities further highlight the importance of brain-liver interactions in schizophrenia. Thus, lipid metabolism abnormalities in the liver and brain, as well as BDNF expression dysregulation, may jointly influence the pathophysiological processes of schizophrenia through the interaction of the brain-liver axis.
The interaction between the brain and the renal system is primarily mediated by the renin-angiotensin system (RAS). RAS plays a pivotal role in blood pressure regulation and is also implicated in various biological processes, including neuroinflammation, oxidative stress, and neurodevelopment[51]. In schizophrenia, aberrant activation of the RAS has been linked to neurotransmitter imbalances, heightened inflammatory responses, and neuronal damage[52]. Increasing evidence suggests that the increased RAS activity may exacerbate neuroinflammation, thereby influencing the clinical presentation of schizophrenia, particularly in terms of affective disturbances and cognitive impairments[53,54]. Fur
The multiple organic systems involvement highlights that the pathophysiological mechanisms of schizophrenia extend well beyond the brain itself[62]. Bidirectional communication between the brain and peripheral organs is mediated through several complex pathways, most notably the autonomic nervous system, the endocrine system, and the immune system[63-65]. Autonomic dysfunction has been found to be correlated with multiple aspects of schizophrenia patho
In summary, the pathogenesis of schizophrenia involves not only the central nervous system but also the dynamic interactions between the brain and other organs (Figure 1). The brain-organs crosstalk is mediated through various biological pathways, affecting the function of peripheral organs and, in turn, regulating brain function via feedback mechanisms in schizophrenia. Although significant progress has been made in elucidating these interactions, current research remains fragmented, and many critical questions remain unanswered. To achieve a more comprehensive understanding of inter-organ dynamics and their contributions to schizophrenia, the application of multi-omics approaches is particularly valuable. Multi-omics technologies enable simultaneous detection of changes across multiple biological levels, thereby uncovering shared molecular mechanisms and dynamic fluctuations of signaling molecules among different organs. For instance, transcriptomic analysis can identify genes that are co-expressed or differentially expressed across multiple organs, revealing potential molecular pathways. Metabolomic profiling can detect alterations in metabolites within blood or tissues, providing insights into metabolic links between peripheral dysfunction and central nervous system pathology. We propose the construction of a “brain-organ network” model as a future research direction. This model would integrate transcriptomic, proteomic, and metabolomic data from multiple organs, and apply network-based computational approaches to identify key nodes and pathways involved in inflammation, oxidative stress, and neurotransmission. Such a systems-level strategy could help uncover novel biomarkers and therapeutic targets for schizophrenia, particularly those that address multi-organ dysregulation. Furthermore, future studies should incorporate animal models and longitudinal interventional designs to validate these interactions and dissect causal mechanisms underlying brain-organ communication. These efforts will be essential for advancing our understanding of schizophrenia as a systemic disorder and for the development of more effective, multi-target treatment strategies.
| 1. | Li X, Wei N, Song J, Liu J, Yuan J, Song R, Liu L, Mei L, Yan S, Wu Y, Pan R, Yi W, Jin X, Li Y, Liang Y, Sun X, Cheng J, Su H. The global burden of schizophrenia and the impact of urbanization during 1990-2019: An analysis of the global burden of disease study 2019. Environ Res. 2023;232:116305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 2. | Correll CU, Solmi M, Veronese N, Bortolato B, Rosson S, Santonastaso P, Thapa-Chhetri N, Fornaro M, Gallicchio D, Collantoni E, Pigato G, Favaro A, Monaco F, Kohler C, Vancampfort D, Ward PB, Gaughran F, Carvalho AF, Stubbs B. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry. 2017;16:163-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 966] [Cited by in RCA: 1151] [Article Influence: 143.9] [Reference Citation Analysis (0)] |
| 3. | Karlsgodt KH, Sun D, Cannon TD. Structural and Functional Brain Abnormalities in Schizophrenia. Curr Dir Psychol Sci. 2010;19:226-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | Năstase MG, Vlaicu I, Trifu SC. Genetic polymorphism and neuroanatomical changes in schizophrenia. Rom J Morphol Embryol. 2022;63:307-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Veeneman RR, Vermeulen JM, Abdellaoui A, Sanderson E, Wootton RE, Tadros R, Bezzina CR, Denys D, Munafò MR, Verweij KJH, Treur JL. Exploring the Relationship Between Schizophrenia and Cardiovascular Disease: A Genetic Correlation and Multivariable Mendelian Randomization Study. Schizophr Bull. 2022;48:463-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 6. | Grant RK, Brindle WM, Donnelly MC, McConville PM, Stroud TG, Bandieri L, Plevris JN. Gastrointestinal and liver disease in patients with schizophrenia: A narrative review. World J Gastroenterol. 2022;28:5515-5529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Ermakov EA, Melamud MM, Buneva VN, Ivanova SA. Immune System Abnormalities in Schizophrenia: An Integrative View and Translational Perspectives. Front Psychiatry. 2022;13:880568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 82] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 8. | Li C, Chen W, Lin F, Li W, Wang P, Liao G, Zhang L. Functional Two-Way Crosstalk Between Brain and Lung: The Brain-Lung Axis. Cell Mol Neurobiol. 2023;43:991-1003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 49] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 9. | Carswell C, Cogley C, Bramham K, Chilcot J, Noble H, Siddiqi N. Chronic kidney disease and severe mental illness: a scoping review. J Nephrol. 2023;36:1519-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Valença AGF, Smith BJ. Schizophrenia Outside the Brain. Adv Exp Med Biol. 2022;1400:53-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | Wijarnpreecha K, Jaruvongvanich V, Cheungpasitporn W, Ungprasert P. Association between celiac disease and schizophrenia: a meta-analysis. Eur J Gastroenterol Hepatol. 2018;30:442-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Mongan D, Sabherwal S, Susai SR, Föcking M, Cannon M, Cotter DR. Peripheral complement proteins in schizophrenia: A systematic review and meta-analysis of serological studies. Schizophr Res. 2020;222:58-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 13. | Hutchinson MR, Hill-Yardin EL. Moving from brain-organ axes to mind-body unity: Convergence research by neuroscientists with multi-pronged expertise. Neuroscience. 2024;559:123-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Schmitt A, Falkai P, Papiol S. Neurodevelopmental disturbances in schizophrenia: evidence from genetic and environmental factors. J Neural Transm (Vienna). 2023;130:195-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 15. | Wehrwein EA, Orer HS, Barman SM. Overview of the Anatomy, Physiology, and Pharmacology of the Autonomic Nervous System. Compr Physiol. 2016;6:1239-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 276] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 16. | Grégoire JM, Gilon C, Carlier S, Bersini H. Autonomic nervous system assessment using heart rate variability. Acta Cardiol. 2023;78:648-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 17. | Bär KJ, Letzsch A, Jochum T, Wagner G, Greiner W, Sauer H. Loss of efferent vagal activity in acute schizophrenia. J Psychiatr Res. 2005;39:519-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 138] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Chang JS, Yoo CS, Yi SH, Hong KH, Oh HS, Hwang JY, Kim SG, Ahn YM, Kim YS. Differential pattern of heart rate variability in patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:991-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Valenza G, Sclocco R, Duggento A, Passamonti L, Napadow V, Barbieri R, Toschi N. The central autonomic network at rest: Uncovering functional MRI correlates of time-varying autonomic outflow. Neuroimage. 2019;197:383-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 20. | Thayer JF, Sternberg E. Beyond heart rate variability: vagal regulation of allostatic systems. Ann N Y Acad Sci. 2006;1088:361-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 479] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 21. | Clamor A, Lincoln TM, Thayer JF, Koenig J. Resting vagal activity in schizophrenia: meta-analysis of heart rate variability as a potential endophenotype. Br J Psychiatry. 2016;208:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 124] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 22. | Hempel RJ, Tulen JH, van Beveren NJ, Röder CH, Hengeveld MW. Cardiovascular variability during treatment with haloperidol, olanzapine or risperidone in recent-onset schizophrenia. J Psychopharmacol. 2009;23:697-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Kim JH, Ann JH, Lee J. Relationship between heart rate variability and the severity of psychotic symptoms in schizophrenia. Acta Neuropsychiatr. 2011;23:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Malaspina D, Dalack G, Leitman D, Corcoran C, Amador XF, Yale S, Glassman A, Gorman JM. Low heart rate variability is not caused by typical neuroleptics in schizophrenia patients. CNS Spectr. 2002;7:53-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Clamor A, Koenig J, Thayer JF, Lincoln TM. A randomized-controlled trial of heart rate variability biofeedback for psychotic symptoms. Behav Res Ther. 2016;87:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Klauser P, Baker ST, Cropley VL, Bousman C, Fornito A, Cocchi L, Fullerton JM, Rasser P, Schall U, Henskens F, Michie PT, Loughland C, Catts SV, Mowry B, Weickert TW, Shannon Weickert C, Carr V, Lenroot R, Pantelis C, Zalesky A. White Matter Disruptions in Schizophrenia Are Spatially Widespread and Topologically Converge on Brain Network Hubs. Schizophr Bull. 2017;43:425-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 27. | Powers KA, Dhamoon AS. Physiology, Pulmonary Ventilation and Perfusion. 2023 Jan 23. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025. [PubMed] |
| 28. | Huang X, Lu QL, Zhu XM, Zeng YB, Liu Y, Hu HY. Histogenous Hypoxia and Acid Retention in Schizophrenia: Changes in Venous Blood Gas Analysis and SOD in Acute and Stable Schizophrenia Patients. Front Psychiatry. 2021;12:792560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Bonkowsky JL, Son JH. Hypoxia and connectivity in the developing vertebrate nervous system. Dis Model Mech. 2018;11:dmm037127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Schmidt-Kastner R, Guloksuz S, Kietzmann T, van Os J, Rutten BPF. Analysis of GWAS-Derived Schizophrenia Genes for Links to Ischemia-Hypoxia Response of the Brain. Front Psychiatry. 2020;11:393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Schmidt-Kastner R, van Os J, W M Steinbusch H, Schmitz C. Gene regulation by hypoxia and the neurodevelopmental origin of schizophrenia. Schizophr Res. 2006;84:253-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | Prabakaran S, Swatton JE, Ryan MM, Huffaker SJ, Huang JT, Griffin JL, Wayland M, Freeman T, Dudbridge F, Lilley KS, Karp NA, Hester S, Tkachev D, Mimmack ML, Yolken RH, Webster MJ, Torrey EF, Bahn S. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Mol Psychiatry. 2004;9:684-697, 643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 594] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 33. | Cui C, Jiang X, Wang Y, Li C, Lin Z, Wei Y, Ni Q. Cerebral Hypoxia-Induced Molecular Alterations and Their Impact on the Physiology of Neurons and Dendritic Spines: A Comprehensive Review. Cell Mol Neurobiol. 2024;44:58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 34. | Singh DK, Ling EA, Kaur C. Hypoxia and myelination deficits in the developing brain. Int J Dev Neurosci. 2018;70:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Gangadin SS, Enthoven AD, van Beveren NJM, Laman JD, Sommer IEC. Immune Dysfunction in Schizophrenia Spectrum Disorders. Annu Rev Clin Psychol. 2024;20:229-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 36. | Noble BT, Brennan FH, Popovich PG. The spleen as a neuroimmune interface after spinal cord injury. J Neuroimmunol. 2018;321:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 37. | Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1342] [Cited by in RCA: 1506] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 38. | Cantone AF, Burgaletto C, Di Benedetto G, Gaudio G, Giallongo C, Caltabiano R, Broggi G, Bellanca CM, Cantarella G, Bernardini R. Rebalancing Immune Interactions within the Brain-Spleen Axis Mitigates Neuroinflammation in an Aging Mouse Model of Alzheimer's Disease. J Neuroimmune Pharmacol. 2025;20:15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 39. | Zhang X, Lei B, Yuan Y, Zhang L, Hu L, Jin S, Kang B, Liao X, Sun W, Xu F, Zhong Y, Hu J, Qi H. Brain control of humoral immune responses amenable to behavioural modulation. Nature. 2020;581:204-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 220] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 40. | Khandaker GM, Cousins L, Deakin J, Lennox BR, Yolken R, Jones PB. Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry. 2015;2:258-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 649] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 41. | Zhang J, Chang L, Pu Y, Hashimoto K. Abnormal expression of colony stimulating factor 1 receptor (CSF1R) and transcription factor PU.1 (SPI1) in the spleen from patients with major psychiatric disorders: A role of brain-spleen axis. J Affect Disord. 2020;272:110-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 42. | Larson KC, Gergits FW, Renoux AJ, Weisman EJ, Dejanovic B, Huang L, Pandya B, McLaren DG, Lynch BA, Fisher R, Thackaberry E, Gray D, Gaudreault F, Mirescu C. Rescue of in vitro models of CSF1R-related adult-onset leukodystrophy by iluzanebart: mechanisms and therapeutic implications of TREM2 agonism. J Neuroinflammation. 2025;22:26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | Yan L, Li Y, Fan F, Gou M, Xuan F, Feng W, Chithanathan K, Li W, Huang J, Li H, Chen W, Tian B, Wang Z, Tan S, Zharkovsky A, Hong LE, Tan Y, Tian L. CSF1R regulates schizophrenia-related stress response and vascular association of microglia/macrophages. BMC Med. 2023;21:286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Jones JG. Hepatic glucose and lipid metabolism. Diabetologia. 2016;59:1098-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 192] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 45. | Solberg DK, Bentsen H, Refsum H, Andreassen OA. Association between serum lipids and membrane fatty acids and clinical characteristics in patients with schizophrenia. Acta Psychiatr Scand. 2015;132:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 46. | Wu X, Huang Z, Wu R, Zhong Z, Wei Q, Wang H, Diao F, Wang J, Zheng L, Zhao J, Zhang J. The comparison of glycometabolism parameters and lipid profiles between drug-naïve, first-episode schizophrenia patients and healthy controls. Schizophr Res. 2013;150:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 47. | Maas DA, Martens MB, Priovoulos N, Zuure WA, Homberg JR, Nait-Oumesmar B, Martens GJM. Key role for lipids in cognitive symptoms of schizophrenia. Transl Psychiatry. 2020;10:399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 48. | Zhang J, Tan Y, Chang L, Hammock BD, Hashimoto K. Increased expression of soluble epoxide hydrolase in the brain and liver from patients with major psychiatric disorders: A role of brain - liver axis. J Affect Disord. 2020;270:131-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 49. | Yang B, Ren Q, Zhang JC, Chen QX, Hashimoto K. Altered expression of BDNF, BDNF pro-peptide and their precursor proBDNF in brain and liver tissues from psychiatric disorders: rethinking the brain-liver axis. Transl Psychiatry. 2017;7:e1128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 137] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 50. | Lin CC, Huang TL. Brain-derived neurotrophic factor and mental disorders. Biomed J. 2020;43:134-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 51. | Xue B, Zhang Y, Johnson AK. Interactions of the Brain Renin-Angiotensin-System (RAS) and Inflammation in the Sensitization of Hypertension. Front Neurosci. 2020;14:650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 52. | Gadelha A, Vendramini AM, Yonamine CM, Nering M, Berberian A, Suiama MA, Oliveira V, Lima-Landman MT, Breen G, Bressan RA, Abílio V, Hayashi MA. Convergent evidences from human and animal studies implicate angiotensin I-converting enzyme activity in cognitive performance in schizophrenia. Transl Psychiatry. 2015;5:e691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 53. | Sirota P, Meiman M, Herschko R, Bessler H. Effect of neuroleptic administration on serum levels of soluble IL-2 receptor-alpha and IL-1 receptor antagonist in schizophrenic patients. Psychiatry Res. 2005;134:151-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 54. | Naudin J, Capo C, Giusano B, Mège JL, Azorin JM. A differential role for interleukin-6 and tumor necrosis factor-alpha in schizophrenia? Schizophr Res. 1997;26:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 108] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 55. | Rodriguez-Pallares J, Rey P, Parga JA, Muñoz A, Guerra MJ, Labandeira-Garcia JL. Brain angiotensin enhances dopaminergic cell death via microglial activation and NADPH-derived ROS. Neurobiol Dis. 2008;31:58-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 169] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 56. | Sánchez-Lemus E, Honda M, Saavedra JM. Angiotensin II AT1 receptor blocker candesartan prevents the fast up-regulation of cerebrocortical benzodiazepine-1 receptors induced by acute inflammatory and restraint stress. Behav Brain Res. 2012;232:84-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 57. | Corsi-Zuelli FMDG, Brognara F, Quirino GFDS, Hiroki CH, Fais RS, Del-Ben CM, Ulloa L, Salgado HC, Kanashiro A, Loureiro CM. Neuroimmune Interactions in Schizophrenia: Focus on Vagus Nerve Stimulation and Activation of the Alpha-7 Nicotinic Acetylcholine Receptor. Front Immunol. 2017;8:618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 58. | Noguchi CT, Asavaritikrai P, Teng R, Jia Y. Role of erythropoietin in the brain. Crit Rev Oncol Hematol. 2007;64:159-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 182] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 59. | Peng B, Kong G, Yang C, Ming Y. Erythropoietin and its derivatives: from tissue protection to immune regulation. Cell Death Dis. 2020;11:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 60. | Wüstenberg T, Begemann M, Bartels C, Gefeller O, Stawicki S, Hinze-Selch D, Mohr A, Falkai P, Aldenhoff JB, Knauth M, Nave KA, Ehrenreich H. Recombinant human erythropoietin delays loss of gray matter in chronic schizophrenia. Mol Psychiatry. 2011;16:26-36, 1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 61. | Ehrenreich H, Degner D, Meller J, Brines M, Béhé M, Hasselblatt M, Woldt H, Falkai P, Knerlich F, Jacob S, von Ahsen N, Maier W, Brück W, Rüther E, Cerami A, Becker W, Sirén AL. Erythropoietin: a candidate compound for neuroprotection in schizophrenia. Mol Psychiatry. 2004;9:42-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 139] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 62. | Tian YE, Cropley V, Maier AB, Lautenschlager NT, Breakspear M, Zalesky A. Heterogeneous aging across multiple organ systems and prediction of chronic disease and mortality. Nat Med. 2023;29:1221-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 218] [Article Influence: 109.0] [Reference Citation Analysis (0)] |
| 63. | Sylvia KE, Demas GE. A gut feeling: Microbiome-brain-immune interactions modulate social and affective behaviors. Horm Behav. 2018;99:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 64. | Stogios N, Gdanski A, Gerretsen P, Chintoh AF, Graff-Guerrero A, Rajji TK, Remington G, Hahn MK, Agarwal SM. Autonomic nervous system dysfunction in schizophrenia: impact on cognitive and metabolic health. NPJ Schizophr. 2021;7:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 65. | Bucolo C, Leggio GM, Drago F, Salomone S. Dopamine outside the brain: The eye, cardiovascular system and endocrine pancreas. Pharmacol Ther. 2019;203:107392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 66. | Kowalchuk C, Castellani LN, Chintoh A, Remington G, Giacca A, Hahn MK. Antipsychotics and glucose metabolism: how brain and body collide. Am J Physiol Endocrinol Metab. 2019;316:E1-E15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 67. | Juruena MF, Eror F, Cleare AJ, Young AH. The Role of Early Life Stress in HPA Axis and Anxiety. Adv Exp Med Biol. 2020;1191:141-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 213] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 68. | Stárka L. The origin of 7alpha-hydroxy-dehydroepiandrosterone and its physiological role: a history of discoveries. Physiol Res. 2017;66:S285-S294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 69. | Harris LW, Pietsch S, Cheng TM, Schwarz E, Guest PC, Bahn S. Comparison of peripheral and central schizophrenia biomarker profiles. PLoS One. 2012;7:e46368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |