Published online Aug 19, 2025. doi: 10.5498/wjp.v15.i8.105770
Revised: May 20, 2025
Accepted: June 17, 2025
Published online: August 19, 2025
Processing time: 115 Days and 0.3 Hours
Evaluating the relationship between sleep quality and depressive symptoms after laparoscopic hysterectomy under general anesthesia can help improve posto
To evaluate the correlation between sleep quality and depression symptoms in patients after laparoscopic hysterectomy under general anesthesia and explore factors associated with postoperative sleep disturbances and depression.
This retrospective case-control study included 102 females who underwent laparoscopic hysterectomy under general anesthesia at our hospital between January 2022 and June 2024, excluding those with severe cardiovascular/cere
Mean age of participants was (52.30 ± 8.39) years, with a body mass index of (23.56 ± 2.79) kg/m². Preoperative comorbidities included hypertension (25.49%), diabetes (14.71%), and heart disease (9.80%). Patients with poor preoperative sleep quality (higher PSQI scores) exhibited significantly more severe depressive symptoms (P < 0.05). Postoperative PSQI scores improved at 1-week, 1-month, and 3-months compared to baseline (P < 0.05). HAMD scores decreased at 1-week and 1-month postoperatively but returned to near preoperative levels at 3-months. Physiological indicators remained within normal ranges, and the postoperative complication rate was < 5%. Logistic regression showed that poor postoperative sleep quality was an independent predictor of depressive symptoms (odds ratio = 1.64, 95%CI: 1.22-2.20, P < 0.05).
Sleep quality was significantly correlated with depressive symptoms after laparoscopic hysterectomy under general anesthesia. Patients with poor postoperative sleep quality were more prone to depression. Early interventions for sleep disturbances are potentially beneficial for mitigating depression and improve mental health.
Core Tip: This study found a significant correlation between sleep quality and depressive symptoms after laparoscopic hysterectomy under general anesthesia. Patients with poor preoperative sleep quality experienced more severe depressive symptoms, and although postoperative sleep quality improved over time, depressive symptoms were still present at 3 months. Poor postoperative sleep quality was identified as an independent predictor of depression. Early interventions aimed at improving sleep quality post-surgery could help alleviate depressive symptoms, contributing to better mental health outcomes and improving overall recovery in these patients.
- Citation: Jia XP, Lou QX, Chen XZ, Zhang YZ. Relationship between sleep disturbances and depressive symptoms in patients after general anesthesia: A retrospective case-control study. World J Psychiatry 2025; 15(8): 105770
- URL: https://www.wjgnet.com/2220-3206/full/v15/i8/105770.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i8.105770
Postoperative sleep disturbance (PSD) has become an increasing concern in clinical medicine[1]. With continuous improvements in surgical techniques and anesthesia methods, although most patients survive the postoperative recovery period without complications, a decline in sleep quality is common[2]. After surgery, patients often present with symptoms such as difficulty falling asleep, ease of waking, early awakening, or shortened sleep duration. These sleep disturbances not only affect the quality of postoperative recovery; however, are also closely related to a variety of psychological and physiological problems, especially depressive symptoms[3,4]. Therefore, exploring the relationship between sleep disturbances and depressive symptoms after anesthesia is of great clinical value. As a common complication of anesthesia, sleep disturbances are often affected by many factors, such as preoperative psychological state, operation type, anesthesia method, drug use, and postoperative pain management[5]. Clinical studies have shown that the incidence of PSD is relatively high, especially in elderly patients, patients taking drugs for a prolonged time, and patients with previous sleep problems[6]. Sleep disturbance has dual effects on the physical and mental health of patients. Poor long-term sleep may lead to poor immune function, delayed wound healing, and increased postoperative complications[7]. Simultaneously, the incidence of depressive symptoms is significantly increased after anesthesia. Postoperative depressive symptoms include depressed mood, loss of interest, loss of appetite, and energy shortage, which affect the recovery process of patients and may even lead to an increase in postoperative complications[8]. Studies have shown a significant association between sleep disturbance and depression. A decline in sleep quality may aggravate depressive symptoms, which may interfere with the normal maintenance of sleep[9,10]. This vicious cycle causes patients to face greater challenges during the postoperative recovery process. Recent research has expanded our understanding of the relationship between sleep disorders and depressive symptoms beyond the postoperative recovery stage. Multiple neurobiological studies have found that the two may overlap in pathological mechanisms, such as dysfunction of the hypothalamic-pituitary-adrenal (HPA) axis, abnormal release of inflammatory factors, and reduced melatonin secretion, all of which are considered to play key roles in the comorbidity of sleep disorders and depression. In addition, brain imaging techniques, such as functional magnetic resonance imaging (fMRI), have shown that patients with postoperative sleep quality decline often have functional abnormalities involving the limbic system and prefrontal cortex, which are also closely related to emotional regulation. Therefore, revealing the intrinsic connection between the two from a neurobiological perspective may help develop more targeted intervention measures and provide a scientific basis for postoperative mental health management. We aimed to explore the relationship between sleep disturbances and depressive symptoms in patients after anesthesia through a retrospective case-control study. By systematically evaluating sleep quality and depressive symptoms at different time points after surgery and in combination with factors such as the basic situation of patients, type of operation, anesthesia method, and postoperative pain management, we analyzed whether there was a significant correlation between sleep disturbances and the occurrence of depressive symptoms. This study reveals potential risk factors for sleep disturbances and depression symptoms in patients after anesthesia surgery and provides a more accurate postoperative management strategy for clinical practice, especially for high-risk patient groups who are prone to sleep disturbances and depression. Existing research has generally found a significant cor
This was a retrospective case-control study. The data of 102 patients who underwent laparoscopic hysterectomy under general anesthesia in our hospital from January 2022 to June 2024 were collected. All patients were female, aged 30-70 years (average = 52.30 ± 8.39 years). All patients underwent laparoscopic hysterectomy under general anesthesia for benign gynecological diseases (such as uterine fibroids and adenomyosis). No cases of severe cardiovascular or ce
Inclusion criteria: Aged 30-70 years, laparoscopic hysterectomy performed for benign gynecological diseases (such as hysteromyoma and adenomyosis), smooth operation, and no serious complications after surgery. Patients were required to comply with follow-up requirements and cooperate with relevant assessments after surgery, including regular assessment of postoperative sleep quality and depression symptoms, with no severe psychiatric or other underlying conditions affecting their sleep and mood before surgery.
Exclusion criteria: Patients who had severe mental and psychological disorders (such as depression and anxiety) before surgery and had not received effective treatment; serious postoperative complications (such as cardiovascular and cerebrovascular events, infections) leading to a hospital stay of > 30 days; patients who were pregnant or lactating; patients who failed to have at least one follow-up visit after surgery; and severe sleep disturbances (such as insomnia and sleep apnea syndrome); patients with preoperative Pittsburgh Sleep Quality Index (PSQI) > 5 (indicating poor sleep) were excluded to minimize confounding of postoperative sleep assessments.
All patients were administered general anesthesia. Patients fasted for 8-10 hours before surgery, a vein was set-up after entering the operating room, and all vital signs were monitored. After sufficient oxygen and nitrogen removal, midazolam 0.03 mg/kg, fentanyl citrate 0.4-0.5 μg/kg, cis-atracurium 0.2 mg/kg and propofol (2-3 mg/kg) were infused intravenously. After anesthesia induction, tracheal intubation was performed using a visual laryngoscope to ensure airway patency and a ventilator was connected for the operation. Intraoperative anesthesia maintenance drugs: Intravenous micropump propofol 4-12 mg/kg/minute, remifentanil 0.1-0.3 μg/kg/minute, sevoflurane 1%-3%, intermittent intravenous injection cis-atracurium to maintain muscle relaxation. After the operation, all anesthetic drugs were discontinued, the patients entered a postanesthesia care unit to continue breathing support, and the tracheal catheter was removed after meeting extubation criteria.
Demographic characteristics: Patient age, weight, height, body mass index (BMI), marital status, occupation, and residential environment were obtained through patient self-reports and medical record queries.
Health status: Basic diseases before surgery, including hypertension, diabetes, heart disease, and liver and kidney dysfunction, were recorded, along with a history of drug use. Health status data were obtained through detailed medical history questions and physical examinations.
Sleep status: Preoperative sleep quality was assessed using the PSQI, a simple sleep assessment scale completed and explained by a professional.
Psychological state: Depressive mood was assessed preoperatively using the Hamilton Depression Scale (HAMD), a standardized depression scale. Trained clinical psychologists performed psychological status assessments.
Lifestyle information: including smoking, drinking habits, amount of exercise, and eating habits, was collected through patient self-reports and lifestyle questionnaires.
Laboratory examination data before surgery: Relevant examination data, including routine blood tests, liver and kidney function, blood glucose, and blood lipid levels were obtained from routine physical examination of the patient before the operation.
Assessment of sleep quality: Sleep quality was assessed using the PSQI, a commonly used self-rating scale that comprehensively assesses sleep quality over the previous month. The PSQI included following seven dimensions: Subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbance, hypnotic drug use, and daytime dys
Assessment of depression symptoms: The HAMD was used to assess depression symptoms, which is one of the most commonly used scales for the assessment of depression and can effectively quantify depressive symptoms in patients. The scale consists of 17 items and covers many symptoms such as emotion, insomnia, appetite, and loss of interest. The scoring criteria were 0-4 points, with 0 indicating no symptoms, and 4 indicating severe symptoms. Total HAMD scores range 0-52, with higher scores indicating more severe depressive symptoms. Specific evaluation criteria were as follows: 0-7 points: No depression symptoms, 8-16 points: Mild depression symptoms, 17-23 points: Moderate depression symptoms, 24 points or above: Severe depression symptoms. Patients were evaluated preoperatively, 1-week, 1-month, and 3-months after surgery and were completed by professionally trained anesthesiologists. Based on HAMD scores, changes in depressive symptoms at different time points post-surgery were analyzed, and the relationship between depression symptoms and sleep disturbance after surgery was further explored.
Monitoring of physiological indexes: (1) Heart rate and blood pressure monitoring: The resting heart rate and blood pressure of patients were measured preoperatively and at 1-week, 1-month, and 3-months postoperatively using a standardized sphygmomanometer. Measurements were taken after the patient sat in a quiet environment for 5 minutes, data were recorded three times for each measurement, with the average value calculated. The device had high accuracy and met clinical measurement standards. The normal heart rate range was 60-100 beats per minute; > 100 beats per minute or < 60 beats per minute required further assessment of the patient's cardiovascular health. Normal adult blood pressure should be maintained at approximately 120/80 mmHg. Postoperative blood pressure fluctuations greater or lower than 90/60 mmHg might indicate hypotension or cardiovascular problems that require timely intervention; and (2) Monitoring of blood oxygen saturation During the recovery period after surgery, patients were required to have their blood oxygen saturation (SpO2) monitored. A fingertip pulse oximeter was used for real-time monitoring. Each patient underwent measurements twice daily for 3 minute each, and the data were recorded. The SpO2 of patients after surgery should be maintained at 95% or above; a value < 90% might indicate respiratory dysfunction or complications requiring timely oxygen therapy or further examination. Normal body temperature was 36.5-37.5 °C. A temperature > 38 °C suggests a possible infection requiring further investigation and antibiotic therapy.
Laboratory examinations: (1) Routine and biochemical tests: Peripheral blood samples were collected pre-operatively and at 1-week, 1-month, and 3-months after surgery and sent to the clinical laboratory of the relevant hospital for routine blood tests, including white blood cell count, red blood cell count, and hemoglobin concentration. Blood samples were also used to test liver and kidney function, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine (Cr), and blood urea nitrogen (BUN) levels and other biochemical indicators; and (2) Determination of inflammatory factors: In order to further explore the biological mechanisms involving PSD and depressive symptoms, we measured levels of inflammatory factors including CRP and TNF-α at different time points before and after surgery. The ARCHITECT i2000SR immune analyzer (Abbott, United States) and related kits were used for quantitative detection to ensure high accuracy and reliability of the data. Evaluation criteria of the results were as follows: The normal level of CRP is < 15 mg/L, and if the CRP value after surgery was higher than this, it indicated the presence of inflammatory reactions or infection; Normal TNF-α level: < 10 pg/mL. Elevated TNF-α levels after surgery suggested the presence of systemic inflammatory responses.
Recovery post-surgery: (1) Hospital stay: The duration of hospital stay after surgery is an important indicator of patient recovery. Shorter hospital stays indicate better patient recovery. Postoperative hospitalization typically ranged 3-7 days, and postoperative complications or poor recovery were considered; (2) Complication rate: Postoperative infection, hemorrhage, and deep vein thrombosis: Patients with postoperative complications required prolonged hospitalization, which had an impact on postoperative rehabilitation. The criteria for the incidence of complications were that an incidence rate of < 5% was ideal, and that an incidence rate of > 10% indicated a need to strengthen postoperative management and intervention; and (3) Physical recovery: Whether the patient could move normally and recover the ability to take care of themself in daily life within 2-weeks after the operation. Two weeks after surgery, the patient's recovery was considered good if they could walk independently, eat food, and receive personal care, and poor if there was significant dysfunction.
Drug intervention and dosage: In this study, for sleep disturbances and depression symptoms in patients after surgery, some were administered drugs to intervene according to their needs. Commonly used drugs included the following: (1) Hypnotic drugs: For patients with significant sleep disturbances, zopiclone was administered orally at a commonly used dose of 7.5 mg per night for no more than 4 weeks. Zopiclone is a commonly used short-term hypnotic that improves sleep quality; and (2) Antidepressants: For patients presenting with mild-to-moderate depressive symptoms, sertraline was administered orally at a dose of 50 mg, starting in the morning, with the initial dose gradually increasing to 100 mg/day according to patient tolerance. Sertraline, a selective serotonin reuptake inhibitor, is widely used to treat depression. Drug use was strictly based on specific symptoms and treatment responses of patients. Drug dose and frequency were adjusted according to individual patient differences. Adverse drug reactions were monitored throughout the study period.
Analysis of intermediary effect: To further explore the possible mediating role of inflammatory markers in the relationship between sleep quality (PSQI) and depressive symptoms (HAMD), this study used Hayes' PROCESS macro program (version 4.0) to run Model 4 in SPSS 25.0 for mediation analysis. The independent variable is the total score of PSQI, the dependent variable is the total score of HAMD, and the mediating variable is CRP level. Due to the deviation of CRP data from normal distribution, logarithmic transformation was first performed to improve the skewness characteristics, thereby enhancing the stability and interpretability of the regression model. The mediation analysis used the Bootstrap method with non parametric bias correction (repeated sampling set at 5000 times) to generate a 95%CI to test the significance of indirect effects. If the Bootstrap confidence interval does not include 0, the mediating effect is considered significant.
Statistical analysis was performed using SPSS 25.0 statistical software. Measurement data are expressed as means ± SD, and inter-group comparisons were performed using independent sample t-tests. Enumeration data are expressed as frequency and percentage, and intra-group comparisons were performed using analysis of variance and χ2 tests. All data were subjected to normality tests and data conforming to a normal distribution were subjected to parametric tests. For non normally distributed data, Mann Whitney U test replaces t-test for inter group comparison; Spearman correlation replaced Pearson correlation. A post-hoc power analysis was performed using G*Power 3.1. For detecting a correlation coefficient of r = 0.62 (PSQI-HAMD association) with α = 0.05 and n = 102, the achieved power was 98%, confirming adequate sample size for primary analyses. The Shapiro-Wilk test was used to analyze normality of each variable, and the results showed that the PSQI total score (P = 0.107) and HAMD total score (P = 0.083) followed a normal distribution, which could be used for parameter testing. The Shapiro-Wilk test P values for CRP and TNF-α were 0.018 and 0.012, respectively, indicating a deviation from normal distribution. Nonparametric testing was used for the analysis. For the correlation between PSQI and HAMD, Pearson correlation analysis was used as the data followed a normal distribution. The skewed distribution data for CRP, TNF-α, etc., were compared using Mann-Whitney U-tests after grouping, and logarithmic transformation was performed during logistic regression analysis to improve the skewed distribution characteristics, thereby enhancing the stability and interpretability of the analysis results. Pearson’s correlation analysis was used to explore the correlation between the PSQI and HAMD scores. The distributions of the PSQI and HAMD scores were first examined for normality. Pearson’s correlation analysis was performed if the data were normally distributed. If the data were not normally distributed, the Spearman rank correlation analysis was used. Assuming that the data were normally distributed, the linear correlation coefficient (r value) between the two was directly calculated using Pearson’s correlation analysis. R values close to 1 indicate a strong positive correlation between the two; the poorer the sleep quality, the more serious the depressive symptoms. R values close to -1 indicated a strong negative correlation between the two, the better sleep quality, the milder depression symptoms are. R values close to 0 indicate no linear correlation between the two. Logistic regression analysis was also performed. Since “presence/absence of depression” is often used as the dichotomized result for depression symptoms. Logistic regression analysis can effectively assess the relationship between sleep disturbances and depressive symptoms. Depression group (1) was defined as having HAMD score greater than or equal to 17 points, and the nondepression group (0) was defined as having HAMD scores < 17 points. Logistic regression analysis was performed based on independent variables, such as total PSQI score, and miscellaneous variables (such as age, sex, and underlying disease) were controlled to analyze the independent predictive effect of sleep disturbance on depressive symptoms. Logistic regression analysis was performed using the odds ratios (OR). ORs > 1 indicate a positive correlation between poor sleep quality (high PSQI score) and depressive symptoms (high HAMD score). OR < 1 indicates a negative correlation, and OR close to 1 indicates no significant correlation. P < 0.05 indicates that the difference was statistically significant.
No notable differences were observed in age, weight, height, and BMI (P > 0.05). Some differences were observed in marital status, occupation, and living environment among the different groups, but none reached statistical significance (P > 0.05). In terms of health status, the prevalence rate of hypertension was 25.49%, which showed no statistically significant difference compared with other basic diseases (diabetes, heart disease, etc.) (P > 0.05). With regard to sleep status, the PSQI score was strongly correlated with preoperative health status. The level of depressive mood was significantly higher in patients with poor sleep quality (P > 0.05). HAMD scores showed that patients with severe depression before surgery had significantly different sleep quality (P > 0.05). No significant differences were found in lifestyle habits (smoking, drinking, and exercise) (P > 0.05; Table 1).
| Factor | Subitem | Group | n = 102 | mean ± SD | χ2/t | P value |
| Demographic characteristics | Age (years) | 102 | 44.36 ± 6.58 | - | - | |
| Body weight (kg) | 102 | 60.42 ± 7.83 | - | - | ||
| Height (cm) | 102 | 160.38 ± 5.62 | - | - | ||
| BMI (kg/m²) | 102 | 23.56 ± 2.79 | - | - | ||
| Marital status | Married/unmarried | 76/26 | - | 0.965 | 0.327 | |
| Occupation | Civil service/others | 58/44 | - | 1.2 | 0.274 | |
| Living condition | City/country | 59/43 | - | 0.585 | 0.444 | |
| Health condition | Hypertension | 26 | 0.2549 | 3.667 | 0.056 | |
| Diabetes | 15 | 0.1471 | 1.987 | 0.158 | ||
| Heart disease | 10 | 0.098 | 2.426 | 0.12 | ||
| Liver and kidney dysfunction | 8 | 0.0784 | 1.678 | 0.195 | ||
| Sleep status | PSQI score (score) | 0-5/6-10/11-15/16-21 | 52/36/10/4 | - | - | - |
| 3.56 ± 1.29 | 3.567 | 0.010a | ||||
| Psychology | HAMD score (score) | 0-7/8-14/15-21/22 and above | 55/32/10/5 | - | - | - |
| 6.20 ± 4.21 | 4.265 | 0.021b | ||||
| Life style | Smoking habits | Smoking/no smoking | 20/82 | - | 1.225 | 0.268 |
| Drinking habits | Drinking/not drinking | 18/84 | - | 0.982 | 0.321 | |
| Exercise habit | Yes/no | 42/60 | - | 1.836 | 0.176 | |
| Eating habits | Good/bad | 64/38 | - | 2.107 | 0.147 | |
| Preoperative examination data | Routine blood test | White blood cell count (× 109/L) | - | 5.56 ± 1.21 | - | - |
| Red blood cell count (× 109/L) | - | 4.35 ± 0.30 | - | - | ||
| Blood glucose (mmol/L) | 102 | 5.08 ± 1.05 | - | - | ||
| Blood lipid (mmol/L) | 102 | 4.12 ± 0.91 | - | - |
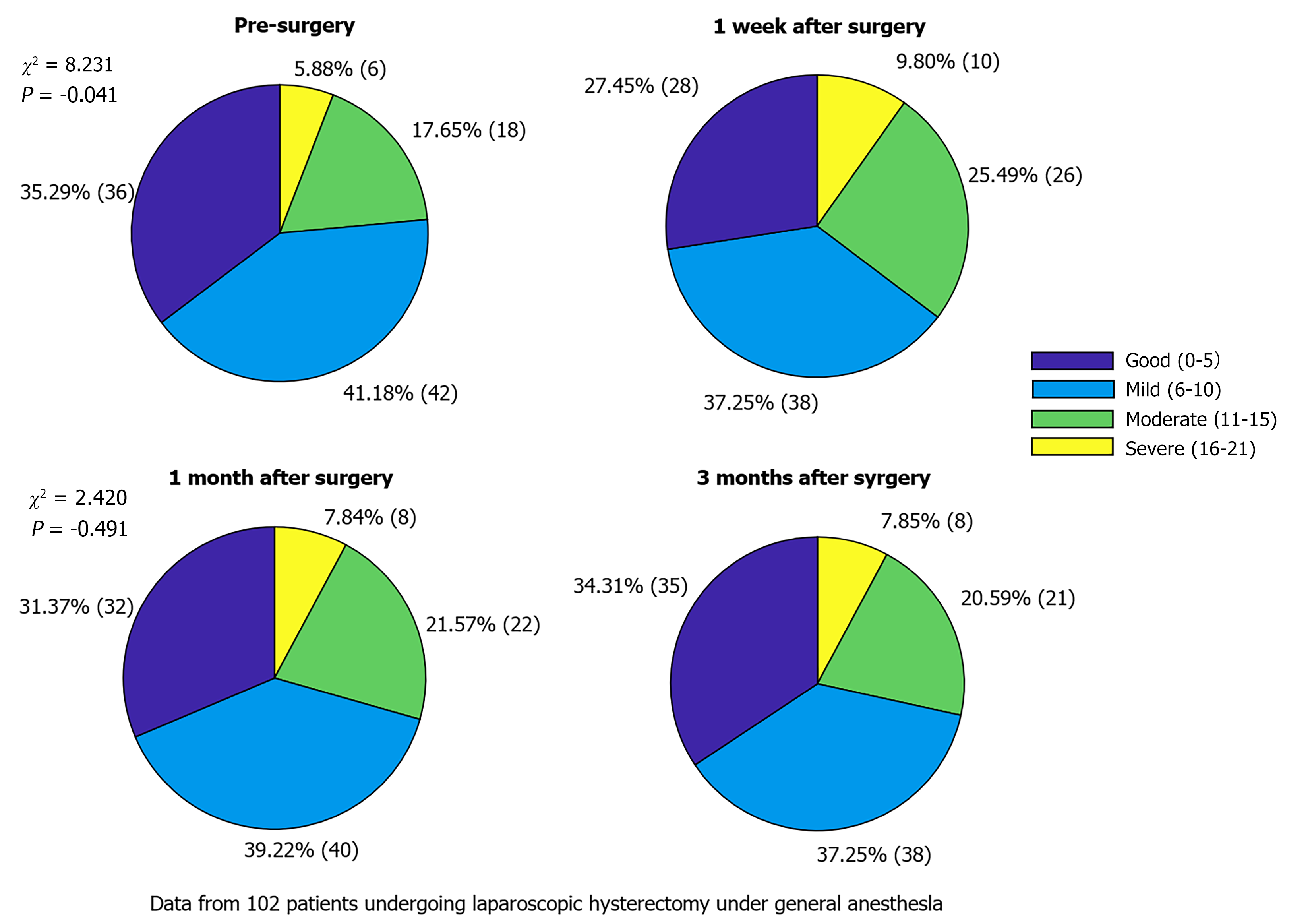
All patients did not experience any loss of contact or unexpected circumstances during the follow-up period, and there were no cases of withdrawal or exclusion during the follow-up period. Sleep quality of the patients significantly decreased 1 week after surgery (P < 0.05), whereas with prolongation of recovery time, patient sleep quality was improved but did not fully return to preoperative levels (P > 0.05; Table 2, Figure 1).
| Point of time | Good sleep quality (0-5) | Mild sleep disturbance (6-10) | Moderate sleep disturbance (11-15) | Severe sleep disturbance (16-21) | χ2 | P value |
| Pre-surgery | 36 (35.29) | 42 (41.18) | 18 (17.65) | 6 (5.88) | - | - |
| 1 week after surgery | 28 (27.45) | 38 (37.25) | 26 (25.49) | 10 (9.80) | 8.231 | 0.041a |
| 1 month after surgery | 32 (31.37) | 40 (39.22) | 22 (21.57) | 8 (7.84) | 3.223 | 0.358 |
| 3 months after surgery | 35 (34.31) | 38 (37.25) | 21 (20.59) | 8 (7.84) | 2.42 | 0.491 |
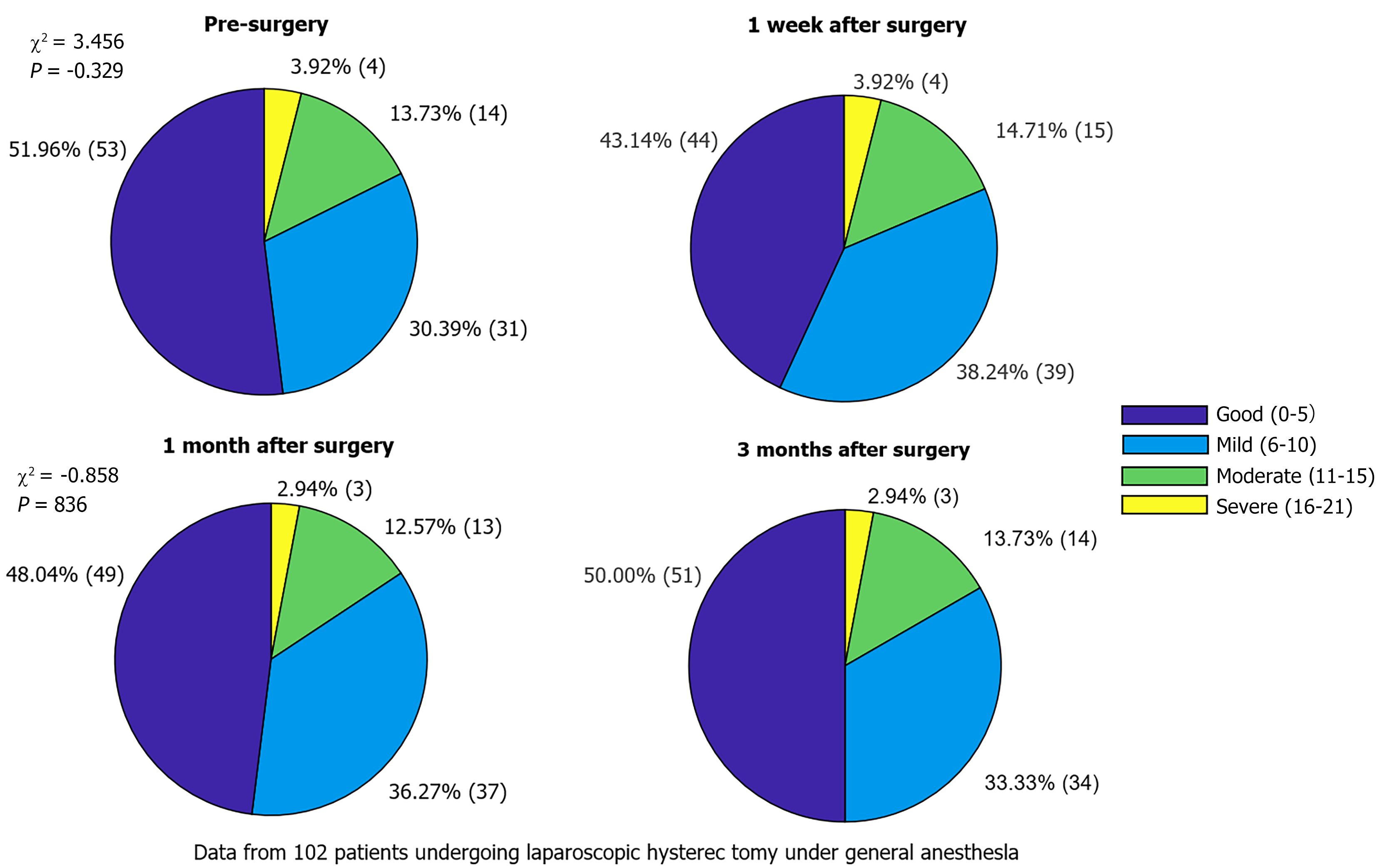
One week after surgery, the incidence of depressive symptoms was increased, and the proportion of patients with mild and moderate depression increased compared to that before the operation. However, at 1- and 3-months postoperatively, depression symptoms did not significantly worsen (P > 0.05) and gradually returned to preoperative levels (Table 3, Figure 2).
| Point of time | No depression symptoms (0-7) | Mild depression symptoms (8-16) | Moderate depression symptoms (17-23) | Severe depression symptoms (24 and above) | χ2 | P value |
| Pre-surgery | 53 (51.96) | 31 (30.39) | 14 (13.73) | 4 (3.92) | - | - |
| 1 week after surgery | 44 (43.14) | 39 (38.24) | 15 (14.71) | 4 (3.92) | 3.456 | 0.329 |
| 1 month after surgery | 49 (48.04) | 37 (36.27) | 13 (12.75) | 3 (2.94) | 1.268 | 0.738 |
| 3 months after surgery | 51 (50.00) | 34 (33.33) | 14 (13.73) | 3 (2.94) | 0.858 | 0.836 |
Most patients had a short postoperative hospital stay and a low incidence of complications (P > 0.05). Of these patients, 70.59% recovered well within 2 weeks after surgery and had good physical recovery (Table 4).
| Recovery indicators | Postoperative recovery | Frequency (n) | Proportion (%) | χ2 | P value |
| Hospital stay, days | |||||
| 3-5 | Good | 62 | 60.78 | 3.528 | 0.171 |
| 6-7 | Average | 35 | 34.31 | ||
| > 7 | Poor | 5 | 4.90 | ||
| Incidence of complications | |||||
| Postoperative infection | Low | 3 | 2.94 | 6.118 | 0.103 |
| Postoperative hemorrhage | Low | 2 | 1.96 | ||
| Deep vein thrombosis | Low | 2 | 1.96 | ||
| No complications | Ideal | 96 | 93.14 | ||
| Physical recovery | |||||
| Daily activities were resumed within 2 weeks after surgery | Good | 72 | 70.59 | 1.734 | 0.419 |
| Limited recovery within 2 weeks after surgery | Average | 24 | 23.53 | ||
| A significant dysfunction | Poor | 6 | 5.88 |
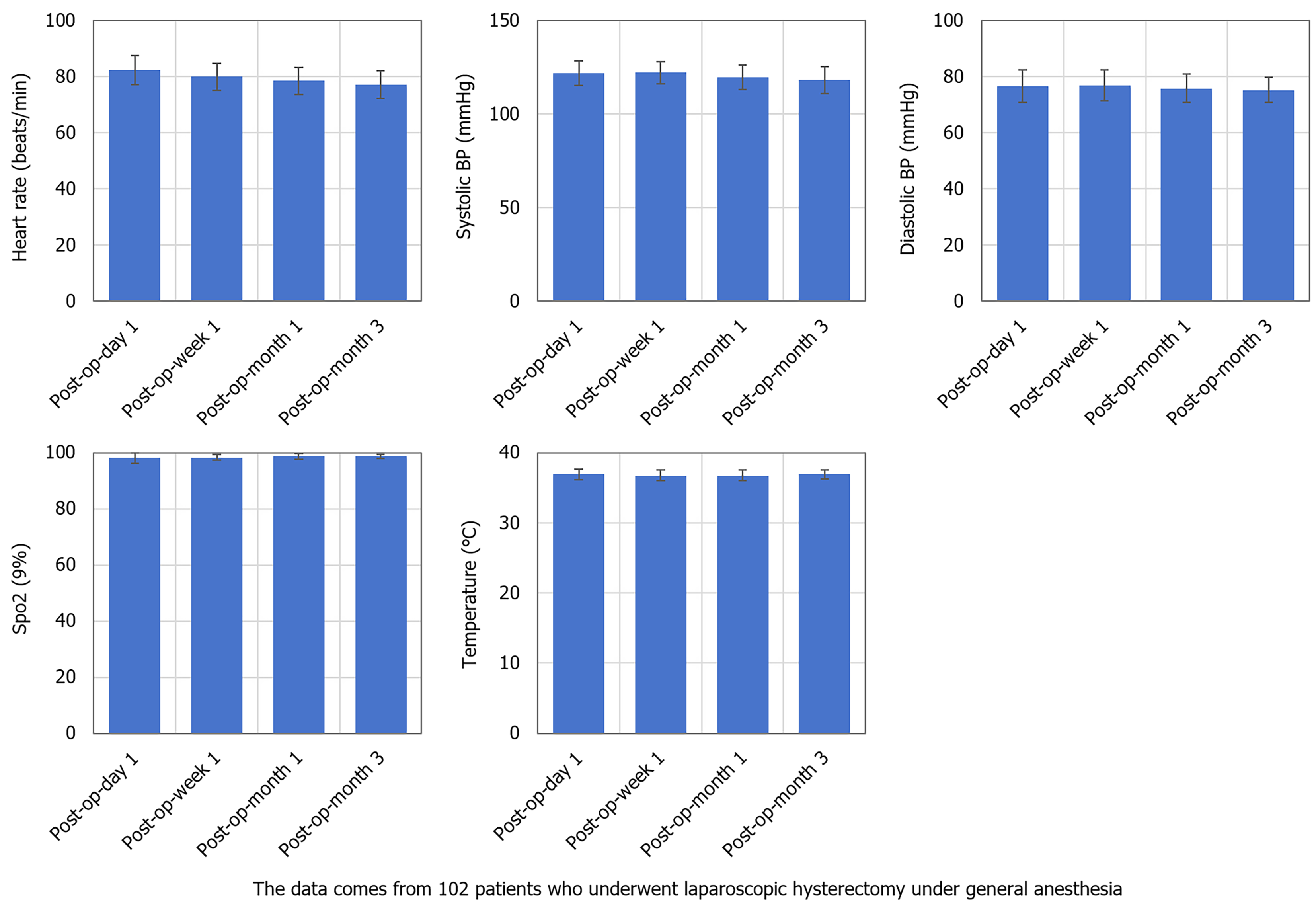
No obvious abnormalities were found in heart rate, blood pressure, SpO2, or body temperature after surgery. Monitoring data for all patients were within normal limits, and no episodes of excessive or slow heart rate, abnormal blood pressure, hypoxemia, or hyperthermia were observed (Table 5, Figure 3).
| Point of time | Heart rate (beats/minute) | Systolic blood pressure (mmHg) | Diastolic blood pressure (mmHg) | Oxygen saturation (%) | Temperature (°C) |
| 1 day after surgery | 82.45 ± 5.36 | 121.65 ± 6.88 | 76.90 ± 5.21 | 98.22 ± 1.25 | 37.01 ± 0.56 |
| 1 week after surgery | 80.30 ± 4.29 | 122.15 ± 5.74 | 77.12 ± 5.05 | 98.56 ± 1.12 | 36.87 ± 0.48 |
| 1 month after surgery | 78.90 ± 4.61 | 119.43 ± 7.11 | 75.87 ± 4.83 | 98.74 ± 1.08 | 36.86 ± 0.49 |
| 3 months after surgery | 77.56 ± 5.14 | 118.72 ± 6.98 | 75.26 ± 4.44 | 98.63 ± 1.15 | 36.83 ± 0.51 |
| F | 2.971 | 3.234 | 3.115 | 0.325 | 0.542 |
| P value | 0.035a | 0.022b | 0.027c | 0.808 | 0.662 |
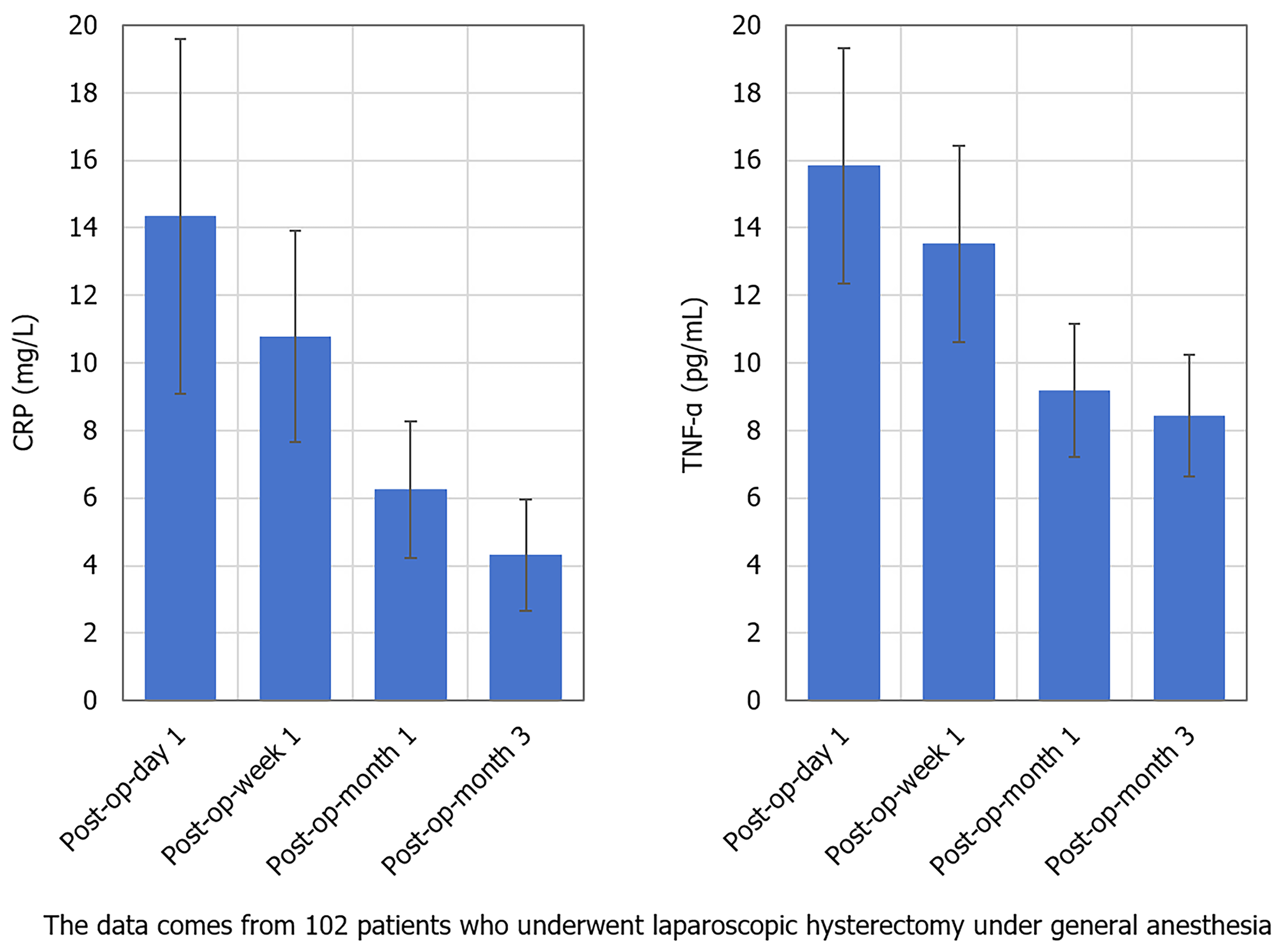
CRP levels were significantly changed at different time points after surgery (P < 0.05). CRP levels on the day after surgery were significantly higher than those at 1-week, 1-month, and 3-months after surgery, and gradually returned to the normal range over time. The change in TNF-α levels were also significantly different (P < 0.05). TNF-α values on the day after surgery were significantly higher than those at 1-week, 1-month, and 3-months after surgery, and inflammatory factor levels gradually decreased with the recovery process (Table 6, Figure 4).
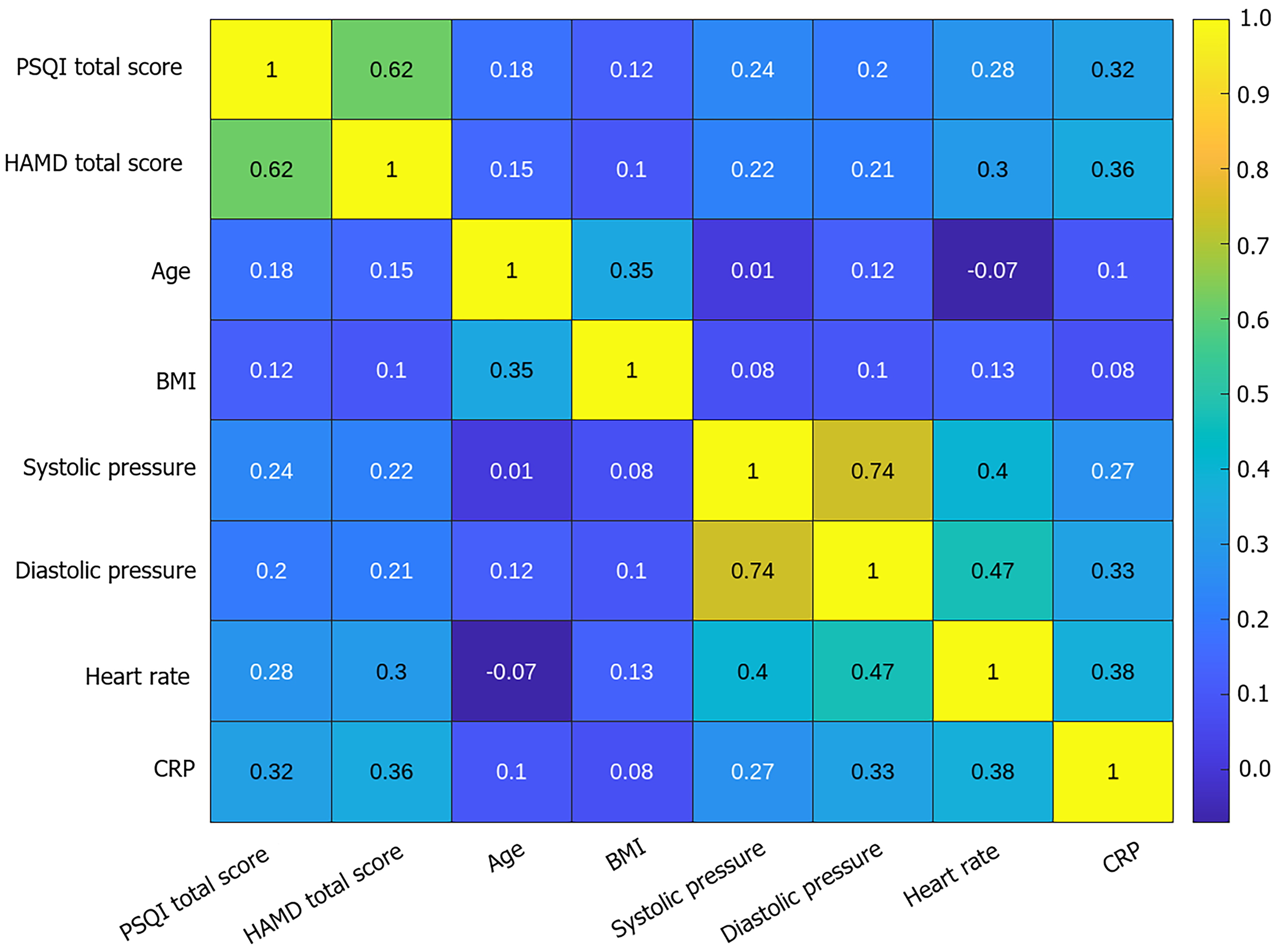
A significant positive correlation was noted between sleep disturbance and depressive symptoms. Physiological indicators, such as inflammatory factors (CRP), heart rate, and blood pressure, also had an effect on sleep quality (Table 7, Figure 5).
| Variable | PSQI score | HAMD score | Age | BMI | Systolic blood pressure (mmHg) | Diastolic blood pressure (mmHg) | Heart rate (beats/minute) | CRP (mg/L) |
| PSQI score | 1 | |||||||
| HAMD score | 0.62a | 1 | ||||||
| Age | 0.18 | 0.15 | 1 | |||||
| BMI | 0.12 | 0.1 | 0.35h | 1 | ||||
| Systolic blood pressure | 0.24b | 0.22b | 0.01 | 0.08 | 1 | |||
| Diastolic blood pressure | 0.2 | 0.21e | 0.12 | 0.1 | 0.74i | 1 | ||
| Heart rate | 0.28c | 0.30f | -0.07 | 0.13 | 0.40j | 0.47l | 1 | |
| CRP | 0.32d | 0.36g | 0.1 | 0.08 | 0.27k | 0.33m | 0.38n | 1 |
Sleep disturbances (PSQI scores) played a very significant role in predicting depression symptoms by logistic regression analysis. For every 1-point increase in the PSQI score, the risk of depression increased by 36.6%. Age and hypertension are also important factors that affect depression symptoms. Although diabetes and BMI contributed to depressive symptoms, the differences were not statistically significant (Table 8).
| Factor | β | SE | Wald χ2 | P value | OR | 95%CI |
| Constant term | -2.214 | 0.51 | 18.325 | < 0.001a | 0.11 | 0.049-0.246 |
| PSQI score | 0.312 | 0.074 | 17.045 | < 0.001b | 1.366 | 1.185-1.577 |
| Age | 0.015 | 0.008 | 4.232 | 0.04c | 1.016 | 1.000-1.033 |
| Gender (male = 1) | 0.292 | 0.271 | 1.157 | 0.282 | 1.339 | 0.801-2.239 |
| BMI | -0.084 | 0.054 | 2.481 | 0.115 | 0.92 | 0.817-1.036 |
| Hypertension (Yes = 1) | 0.745 | 0.298 | 6.264 | 0.012 | 2.106 | 1.161-3.821 |
| Diabetes (Yes = 1) | 0.567 | 0.322 | 3.07 | 0.08 | 1.763 | 0.927-3.341 |
Mediation analysis via PROCESS macro (Model 4) confirmed CRP partially mediated the PSQI-HAMD association (indirect effect = 0.18, 95%CI: 0.05-0.30, P < 0.001; Table 9).
| Path/effect type | Coefficient (β) | SE | t | P value | 95%CI |
| PSQI → CRP (a path) | 0.32 | 0.09 | 3.56 | < 0.001a | (0.14, 0.50) |
| CRP → HAMD (b path) | 0.56 | 0.15 | 3.73 | < 0.001a | (0.26, 0.85) |
| PSQI → HAMD (total effect, c) | 0.49 | 0.08 | 6.13 | < 0.001a | (0.33, 0.64) |
| PSQI → HAMD (direct effect, c′) | 0.31 | 0.09 | 3.44 | < 0.001a | (0.13, 0.49) |
| Indirect effect (a × b) | 0.18 | 0.07 (Boot SE) | - | < 0.001a | (0.05, 0.30) (Bootstrap 95%CI) |
Previous studies have shown a two-way relationship between sleep disturbances and depressive symptoms. Patients with depression usually suffer poor sleep quality and sleep disturbances may aggravate the severity of depressive symptoms[11]. This phenomenon has been widely confirmed in both clinical and experimental studies. For example, a retrospective study pointed out that patients with depression are more prone to sleep disturbances, especially difficulty falling asleep and early awakening[12]. Insufficient sleep or poor sleep quality is considered an important cause of depression, and depression itself can affect sleep structure and depth, forming a vicious circle[13]. It is often accompanied by changes in the structure of effective and rational sleep. In general, patients with depression have a long period of rapid eye movement (REM) sleep and a low proportion of non-REM sleep (NREM), which are closely related to symptoms such as depressed mood and energy deficiency during the day[14]. Sleep disturbances aggravate the occurrence and persistence of depressive mood by affecting neurobiological mechanisms of the brain[15]. For example, insufficient sleep leads to increased levels of stress hormones, such as cortisol, which in turn affect the emotional regulation system in the brain (such as the amygdala and prefrontal cortex)[16]. These neurochemical and physiological changes may be the core mechanism underlying the interaction between depression and sleep disturbances. This study also covered postoperative changes involving inflammatory factors such as CRP and TNF-α levels. Several studies have shown that inflammatory factors play important roles in sleep disturbance and depression. Some researchers have found that an increase in inflammatory factors significantly correlates with depression[17]. CRP and TNF-α are important inflammatory markers, and the increase in their levels is closely related to decline in sleep quality and the aggravation of depressive mood. In the postoperative recovery period, due to surgical trauma, immune responses, and physiological changes during the recovery process, the levels of inflammatory factors may temporarily increase, which not only reflects the body's immune response to trauma, but may also aggravate depressive symptoms and sleep disturbances through nerve-immune interactions[18]. Therefore, changes in inflammatory factors may provide a biological basis for understanding the relationship between PSD and depression. For example, inflammatory factors such as TNF-α may alter the regulation of emotional and sleep states by affecting neurotransmitter systems in specific areas of the brain (such as the serotonin and norepinephrine systems)[19]. Increased levels of inflammatory factors may further aggravate the comorbidity of depression and sleep disturbance by affecting cerebral blood flow and neural network function. Laparoscopic hysterectomy, a common gynecological surgery, is minimally invasive but still brings some physiological and psychological pressure to patients. Previous studies have shown that any form of surgery may affect the sleep quality of patients, particularly during the postoperative recovery period[20]. Postoperative pain, changes in the inpatient environment, residual anesthetics, and possible postoperative complications (such as infection and thrombosis) may lead to a decrease in sleep quality[21]. According to previous studies, short-term sleep disturbance after surgery is often related to improper pain control, psychological pressure, difficulty in postoperative physical recovery, and uncomfortable hospital environments, all of which may lead to decreased sleep efficiency and poor sleep quality[22]. Our findings implicate HPA axis dysregulation and neuroinflammation (e.g., TNF-α crossing blood-brain barrier) as shared pathways for PSD and depression. This supports combined interventions targeting sleep hygiene and inflammation (e.g., perioperative melatonin, CBT-I). Optimal timing may be within 48 hours post-surgery when inflammatory peaks occur (Figure 4). Bundled intervention includes: (1) Cognitive behavioral therapy for insomnia (CBT-I) to improve sleep onset and maintenance; (2) Omega-3 supplementation to reduce inflammation markers such as CRP; and (3) Symptom-triggered pharmacotherapy with individualized use of antidepressants or anxiolytics based on symptom severity. Moreover, the influence of postoperative recovery on depressive symptoms should not be ignored. Studies have shown that patients with poor postoperative recovery, especially those with complications or those requiring a longer hospital stay, often have more severe depressive symptoms. Such patients may suffer from long-term depressed mood and aggravation of depressive symptoms due to their continuous deterioration in physical health, pain, treatment delay, and inability to return to normal life. Conversely, patients with good postoperative recovery achieved rapid improvement in physical recovery and self-care ability and generally had better depressive mood and sleep quality. It is worth noting that there are still some noteworthy points regarding the clinical application of the conclusions drawn from this study. Clinical implementation faces multiple challenges, including low patient compliance and difficulties in follow-up. Mobile health applications can be used to improve patient engagement and self-management abilities; In addition, in resource scarce areas where medical equipment and personnel are limited, it is necessary to optimize and simplify the screening process, develop low-cost and highly operable screening protocols to improve accessibility and efficiency.
Results of this study reveal a close bidirectional relationship between postoperative sleep disorders and depressive symptoms, and emphasize the potential mediating role of inflammatory factors such as CRP and TNF-α. These findings provide important evidence for developing novel clinical interventions. Based on our research results, carrying out a joint intervention plan in the early postoperative recovery stage (e.g., within 48 hours post-surgery), including psychological support, pain relief optimization, sleep promotion measures (such as cognitive-behavioral therapy with CBT-i or short-acting sleep-aid drugs), and inflammation control strategies (such as nutritional support or anti-inflammatory treatment) are recommended. Simultaneously, high-risk individuals (such as those with sleeping or emotional problems before surgery) should be screened for personalized intervention management. However, there are also challenges in its clinical application, such as poor patient compliance, heavy burden on medical staff, and insufficient medical resources. Therefore, using electronic follow-up systems or mobile apps for remote monitoring and psychological intervention to increase patient participation, simultaneously training nurses and grassroots doctors, carrying out simplified sleep/emotion assessment processes, and improve intervention efficiency are recommended. In resource-limited areas, collective education, health lectures, and other forms of communication can be used as alternatives to individualized interventions to cover more patients. The optimization of intervention timing, delivery methods, and service models is expected to enhance the practicality and promotional value of postoperative mental health management. As a retrospective study, potential biases including selection bias and unmeasured confounders (e.g., preoperative psychological status, socioeconomic factors) exist. Retrospective data were utilized due to feasibility constraints during the coronavirus disease 2019 pandemic period (2022-2024).
In summary, the results of this study indicate that there exists a significant correlation between sleep quality and depressive symptoms in patients after laparoscopic hysterectomy under general anesthesia and that sleep disturbances may associated with the occurrence of depressive mood, and vice versa. These findings are not only consistent with previous related studies, but also provide new theoretical support for further exploration of the interaction between sleep and emotion in the process of postoperative recovery. Therefore, changes in the levels of inflammatory factors may play an important role in this process. This study had a small sample size, was limited to a specific postoperative population and lacked long-term follow-up data. Future research should further explore ways to alleviate depressive symptoms by improving postoperative sleep quality and exploring more personalized treatment strategies.
| 1. | Qiu D, Wang XM, Yang JJ, Chen S, Yue CB, Hashimoto K, Yang JJ. Effect of Intraoperative Esketamine Infusion on Postoperative Sleep Disturbance After Gynecological Laparoscopy: A Randomized Clinical Trial. JAMA Netw Open. 2022;5:e2244514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 80] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 2. | Wen Y, Xu J, Shen J, Tang Z, Li S, Zhang Q, Li J, Sun J. Esketamine Prevents Postoperative Emotional and Cognitive Dysfunction by Suppressing Microglial M1 Polarization and Regulating the BDNF-TrkB Pathway in Ageing Rats with Preoperative Sleep Disturbance. Mol Neurobiol. 2024;61:5680-5698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 3. | Wei W, Zhang A, Liu L, Zheng X, Tang C, Zhou M, Gu Y, Yao Y. Effects of subanaesthetic S-ketamine on postoperative delirium and cognitive function in elderly patients undergoing non-cardiac thoracic surgery: a protocol for a randomised, double-blinded, placebo-controlled and positive-controlled, non-inferiority trial (SKED trial). BMJ Open. 2022;12:e061535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Xu W, Zheng Y, Suo Z, Yang Y, Yang J, Wang Q, Zhou B, Ni C. Potential vicious cycle between postoperative pain and sleep disorders: A bibliometric analysis. Heliyon. 2024;10:e35185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Gu X, Zhu J. Roles of Exosomes and Exosomal MicroRNAs in Postoperative Sleep Disturbance. Nat Sci Sleep. 2021;13:1363-1375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Ceban F, Yan E, Pivetta B, Saripella A, Englesakis M, Gan TJ, Joshi GP, Chung F. Perioperative adverse events in adult patients with obstructive sleep apnea undergoing ambulatory surgery: An updated systematic review and meta-analysis. J Clin Anesth. 2024;96:111464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Wang Y, Wang T, Feng S, Li N, Zhang Y, Cheng Y, Wu H, Zhan S. An in-depth analysis of postoperative insomnia in elderly patients and its implications on rehabilitation. Sleep Breath. 2024;28:2187-2195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Yao ZW, Zhao BC, Yang X, Lei SH, Jiang YM, Liu KX. Relationships of sleep disturbance, intestinal microbiota, and postoperative pain in breast cancer patients: a prospective observational study. Sleep Breath. 2021;25:1655-1664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Pappu A, Singh M. Best perioperative practices in the management of obstructive sleep apnea patients undergoing ambulatory surgery. Curr Opin Anaesthesiol. 2024;37:644-650. [PubMed] [DOI] [Full Text] |
| 10. | Chen J, Mei X. Sensitivity to remifentanil and its predictive factor in male patients with moderate to severe obstructive sleep apnea syndrome. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2023;48:347-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Xue D, Guo X, Li Y, Sheng Z, Wang L, Liu L, Cao J, Liu Y, Lou J, Li H, Hao X, Zhou Z, Fu Q. Risk Factor Analysis and a Predictive Model of Postoperative Depressive Symptoms in Elderly Patients Undergoing Video-Assisted Thoracoscopic Surgery. Brain Sci. 2023;13:646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 12. | Coronado RA, Pennings JS, Master H, Brintz CE, Cole KR, Helmy J, Oleisky ER, Davidson C, Abtahi AM, Stephens BF, Archer KR. The Combined Influence of Sleep Disturbance and Depression on 12-month Outcomes After Lumbar Spine Surgery. Spine (Phila Pa 1976). 2024;49:1339-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Kaynar AM, Lin C, Sanchez AG, Lavage DR, Monroe A, Zharichenko N, Strassburger M, Saucier K, Groff YJ, Klatt BA, O'Malley MJ, Szigethy E, Wasan AD, Chelly JE. SuRxgWell: study protocol for a randomized controlled trial of telemedicine-based digital cognitive behavioral intervention for high anxiety and depression among patients undergoing elective hip and knee arthroplasty surgery. Trials. 2023;24:715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 14. | Wu H, Yang F, Zhang R, Xue H, Yang Y, Liao R, Li M, Wu X, Chen D, Chen G, Gong Y, Hou L. Study protocol for a randomised controlled clinical trial comparing desflurane-based versus propofol-based anaesthesia on postanaesthesia respiratory depression in patients with obstructive sleep apnoea after major abdominal surgery. BMJ Open. 2021;11:e051892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Liu H, Wang Q, Xu Z, Zhang L, Liu Y, Zhao L. Effects of oral pregabalin on postoperative sleep of patients after video-assisted thoracoscopic surgery: a randomized double-blind controlled trial. Minerva Anestesiol. 2024;90:872-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Wilson JM, Colebaugh CA, Flowers KM, Edwards RR, Partridge AH, Dominici LS, Schreiber KL. Early postoperative psychological distress as a mediator of subsequent persistent postsurgical pain outcomes among younger breast cancer patients. Breast Cancer Res Treat. 2022;196:363-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Fan X, Xu F, Ren H, Lu Z, Bu H, Ma L, Kong C, Wang T. The Analysis of Percutaneous Balloon Compression on Efficacy and Negative Emotion in the Treatment of Recurrent Trigeminal Neuralgia After Surgical Procedures. Pain Physician. 2021;24:E1255-E1262. [PubMed] |
| 18. | Azizoddin DR, Soens MA, Beck MR, Flowers KM, Edwards RR, Schreiber KL. Perioperative Sleep Disturbance Following Mastectomy: A Longitudinal Investigation of the Relationship to Pain, Opioid Use, Treatment, and Psychosocial Symptoms. Clin J Pain. 2023;39:76-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Chae D, Kim HC, Song Y, Choi YS, Han DW. Pharmacodynamic analysis of intravenous bolus remimazolam for loss of consciousness in patients undergoing general anaesthesia: a randomised, prospective, double-blind study. Br J Anaesth. 2022;129:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 71] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 20. | Leung KYQ, Cartoon J, Hammond NE. Depression screening in patients with aneurysmal subarachnoid haemorrhage and their caregivers: A systematic review. Aust Crit Care. 2023;36:1138-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Kim H, Kim Y, Bae J, Yoo S, Lim YJ, Kim JT. Comparison of remimazolam and dexmedetomidine for intraoperative sedation in patients undergoing lower extremity surgery under spinal anesthesia: a randomized clinical trial. Reg Anesth Pain Med. 2024;49:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 22. | Yurashevich M, Cooter Wright M, Sims SC, Tan HS, Berger M, Ji RR, Habib AS. Inflammatory changes in the plasma and cerebrospinal fluid of patients with persistent pain and postpartum depression after elective Cesarean delivery: an exploratory prospective cohort study. Can J Anaesth. 2023;70:1917-1927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |