Published online Jun 19, 2025. doi: 10.5498/wjp.v15.i6.106262
Revised: March 28, 2025
Accepted: April 3, 2025
Published online: June 19, 2025
Processing time: 98 Days and 8.5 Hours
Hypertension, a prevalent condition among older adults, has been identified as a risk factor for cognitive decline. Nutritional status is a pivotal factor in preserving cognitive function in hypertensive older adults. Nutritional psychiatry under
Core Tip: In hypertensive older adults, body mass index (BMI), serum hemoglobin, and serum albumin levels have been identified as independent protective factors for cognitive function. Conversely, alkaline phosphatase (ALP) has been associated with cognitive decline. BMI, hemoglobin, and albumin are commonly used as indicators of nutritional status, and poor nutritional status has been linked to poorer cognitive function. While ALP itself does not directly impact cognitive function, it serves as a marker of overall status and can predict cognitive decline.
- Citation: Nagamine T. Nutritional psychiatry for hypertensive older adults. World J Psychiatry 2025; 15(6): 106262
- URL: https://www.wjgnet.com/2220-3206/full/v15/i6/106262.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i6.106262
Hypertension, a prevalent condition in older adults, has been linked to a decline in cognitive function[1]. Older adults are susceptible to a deterioration in nutritional status due to their physical and social environment. Nutritional status is a pivotal factor in preserving cognitive function in hypertensive older adults[2]. Nutritional psychiatry is an emerging field that explores the relationship between diet, nutrition, and mental health. It investigates how specific nutrients, dietary patterns, and gut microbiota influence brain function and mental well-being[3]. However, the intricate relationship between nutritional factors and cognitive decline remains to be fully elucidated. This article aims to explore the mechanisms by which nutritional factors influence cognitive function in hypertensive older adults from the perspective of nutritional psychiatry.
Hypertension can impair brain function by altering cerebral blood flow and metabolism. Hypertension exerts a variety of effects on the blood vessels of the brain, leading to a decline in cognitive function[1]. Hypertension can damage the blood vessels in the brain, causing them to become stiff and reducing their capacity to deliver oxygen and nutrients to brain cells. Over time, this can result in cognitive decline and dementia[4]. Hypertension is a significant risk factor for stroke, which can cause substantial brain damage and cognitive impairment. Hypertension has been linked to the development of white matter lesions, which are areas of damage in the white matter. White matter plays a crucial role in facilitating communication between different brain regions, and damage to this part of the brain can disrupt cognitive processing[5]. Hypertension can trigger an inflammatory response throughout the body, including the brain. Prolonged inflammation can lead to damage to brain cells and contribute to cognitive decline[6]. Hypertension has been demonstrated to disrupt the equilibrium of neurotransmitters, which are chemical messengers in the brain that are imperative for cognitive functions such as memory and learning.
Hypertension has been demonstrated to damage the blood–brain barrier; a protective layer that regulates the passage of substances into the brain, potentially allowing harmful substances to enter and contributing to cognitive decline. Hypertension may be associated with hormonal changes that can affect cognitive function, positioning hypertensive older adults as a group with risk factors for cognitive decline[7]. When discussing hypertension and hormonal changes affecting cognitive function, several hormones are implicated. These include cortisol, aldosterone, thyroid hormones, and atrial natriuretic peptide[8]. A study that explored differences in clinical data and cognitive function in hypertensive older adults with different nutritional statuses and analyzed the relationship between nutritional status and cognitive impairment found that a decline in nutritional status predicted a decline in cognitive function. Among the nutritional and hematological factors, normal body mass index (BMI), serum hemoglobin, and serum albumin were independent factors for protecting cognitive function, and increased serum alkaline phosphatase (ALP) was an independent factor for declining cognitive function[9]. Normal BMI may reflect overall metabolic health, which is crucial for brain function. Adequate hemoglobin ensures sufficient oxygen delivery to the brain. Serum albumin reflects nutritional status and overall health, both of which are important for cognitive health. Elevated ALP can indicate liver or bone issues, which may indirectly affect brain function. ALP is less widely known than BMI and hemoglobin. What ALP is and what high ALP levels mean will be discussed in the next chapter.
High BMI is often associated with cognitive decline, but low BMI in older adults can also be a risk factor[10]. Older adults with low BMI may be malnourished, lacking essential nutrients that support brain health. Nutrient deficiencies can impair cognitive function and increase the risk of dementia. Nutrient deficiencies refer to a state in which the body lacks adequate levels of essential nutrients, such as vitamins, minerals, and fatty acids, required for optimal physiological function. Low BMI can be associated with muscle loss (sarcopenia), which can lead to weakness, frailty, and reduced physical activity. Reduced physical activity can have a detrimental effect on brain health and cognitive function. Low BMI can be an indication of underlying health conditions, such as cancer, infections, or gastrointestinal disorders, which can contribute to cognitive decline. While high BMI is associated with chronic inflammation, some studies suggest that low BMI in older adults may also be linked to increased inflammation, which can damage brain cells and impair cognitive function. Low BMI has been demonstrated to disrupt the balance of certain hormones, such as leptin and ghrelin, which play a role in appetite regulation and energy balance[10]. These hormonal changes may also affect brain function and contribute to cognitive decline.
Low hemoglobin levels, often indicative of anemia, can be associated with cognitive decline through several interconnected mechanisms[10]. The primary function of hemoglobin is to carry oxygen throughout the body. When hemoglobin levels are low, the brain receives less oxygen, leading to hypoxia. Chronic hypoxia can damage brain cells, impair neuronal function, and contribute to cognitive decline. Anemia can affect blood viscosity and potentially reduce blood flow to the brain, leading to ischemia and contributing to cognitive impairment. Iron, a vital element in hemoglobin production, also plays a role in brain function. Iron deficiency, a common cause of anemia, can independently impair cognitive abilities, affecting attention, memory, and executive functions[11]. Anemia can sometimes be associated with deficiencies in other nutrients, such as B vitamins (especially B12 and folate), which are also crucial for brain health and cognitive function.
Low hemoglobin can be indicative of various chronic diseases, including kidney disease, heart failure, and chronic inflammation. These conditions have the potential to contribute to cognitive decline through several pathways, such as inflammation, reduced blood flow to the brain, and metabolic disturbances. Anemia, particularly anemia of chronic disease, has been associated with chronic inflammation. Inflammation can result in damage to brain tissue, impairment of neuronal function, and contribute to neurodegenerative processes, which can increase the risk of cognitive decline. Low hemoglobin level is frequently observed in frail older adults; a state of decreased physiological reserve that renders individuals more vulnerable to stressors and can increase the risk of cognitive decline. Frailty can also limit an individual's ability to obtain and prepare nutritious food, further contributing to anemia and cognitive issues.
Nonetheless, reverse causality may also be posited to explain the relationship between cognitive decline and anemia, such that cognitive impairment, particularly in the context of dementia, may precipitate alterations in dietary habits, diminished food intake, and subsequent nutritional deficiencies, including iron deficiency, which can contribute to anemia. Individuals with dementia may experience forgetfulness regarding meals, difficulty in meal preparation, or a diminution in interest in food.
In the context of aging, decreased albumin levels frequently coincide with cognitive decline, although the underlying relationship is multifaceted and not invariably direct. Low albumin can be indicative of various chronic diseases, including liver disease, kidney disease, heart failure, and malnutrition. These conditions can contribute to cognitive decline through several mechanisms, such as inflammation, reduced blood flow to the brain, and metabolic disturbances. Albumin, a pivotal protein in the blood, is a crucial indicator of protein malnutrition. Malnutrition, particularly in older adults, can result in deficiencies in essential nutrients that are crucial for brain health, such as B vitamins, -3 fatty acids, and antioxidants, thereby directly impacting cognitive function. Low albumin can be a marker of malnutrition, and deficiencies in essential nutrients can directly impact cognitive function[12]. These deficiencies can affect neurotransmitter production, neuronal signaling, and overall brain health. Low albumin is frequently observed in frail older adults. Frailty is defined as a state of decreased physiological reserve, which renders individuals more vulnerable to stressors and increases the risk of cognitive decline. Frailty can also limit an individual's ability to obtain and prepare nutritious food, thereby contributing to malnutrition and cognitive issues.
Inflammation has the potential to damage brain tissue, impair neuronal function, and contribute to neurodegenerative processes. Low albumin may be a consequence of the inflammatory state, or it could exacerbate it. Low albumin may contribute to increased oxidative stress, which can damage brain cells and impair cognitive function. Oxidative stress is implicated in various neurodegenerative diseases. Albumin plays a role in maintaining blood volume and pressure. Consequently, low albumin levels can potentially result in diminished blood flow to the brain, consequently depriving it of oxygen and nutrients that are indispensable for maintaining optimal cognitive function.
Nonetheless, reverse causality may also be posited in that cognitive decline may give rise to malnutrition. In certain instances, cognitive impairment per se, particularly in the case of dementia, has been demonstrated to result in alterations in eating habits, diminished food intake, and subsequent malnutrition and low albumin levels. Individuals suffering from dementia may neglect to eat, encounter difficulties in the preparation of meals, or exhibit a waning interest in food.
The relationship between elevated ALP levels and cognitive decline in hypertensive older adults is complex and not fully understood. While ALP itself is not directly causative of cognitive decline, it often acts as an indicator for other underlying health issues that do contribute to cognitive impairment[13]. ALP is produced in the liver, and elevated levels can indicate liver damage or disease (e.g., cirrhosis, hepatitis, or biliary obstruction). Liver dysfunction can lead to the accumulation of toxins in the blood, which can cross the blood–brain barrier and negatively impact cognitive function, as well as disrupt the metabolism of substances crucial for brain health. ALP is also produced in bones, and elevated levels can be seen in conditions such as osteoporosis. While these bone disorders themselves might not directly cause cognitive decline, they can be associated with other health problems (e.g., chronic pain or reduced mobility) that can indirectly affect cognitive function. In some cases, medications used to treat these conditions may have cognitive side effects. Kidney disease can also cause elevated ALP. Kidney dysfunction can lead to the buildup of toxins in the blood and disrupt electrolyte balance, both of which can negatively affect brain function and cognition. Some studies have shown a link between elevated ALP and heart failure. Heart failure can reduce blood flow to the brain, leading to ischemia and contributing to cognitive decline. While not a direct cause, some studies have shown a possible connection between diabetes, elevated ALP, and cognitive decline. Diabetes itself is a well-established risk factor for cognitive impairment, and some research suggests that elevated ALP may be involved in inflammatory processes. Chronic inflammation is increasingly recognized as a contributor to cognitive decline and neurodegenerative diseases. It is conceivable that the conditions that result in elevated ALP may also promote inflammation, which subsequently affects the brain. Research studies have indicated that elevated ALP may contribute to vascular dysfunction, including damage to blood vessels in the brain. This can result in reduced blood flow, ischemia, and an increased risk of stroke; all of which can impair cognitive function. Although not a direct mechanism, some conditions that cause elevated ALP can also lead to deficiencies in nutrients essential for brain health, indirectly affecting cognitive function.
However, it has been posited that ALP may directly impair vascular endothelial cells and contribute to cognitive decline. Vascular endothelial cells, which line the interior of blood vessels, fulfill numerous critical functions, including regulating blood coagulation, controlling inflammatory responses, and coordinating vasodilation and vasoconstriction. A decline in vascular endothelial function has been associated with the progression of arteriosclerosis and an elevated risk of developing cerebrovascular disease, which is often accompanied by impaired cognitive function. Elevated ALP levels have been shown to be associated with diminished vascular endothelial function[14]. The precise mechanism by which elevated ALP leads to a reduction in vascular endothelial function remains to be fully elucidated, although several potential mechanisms have been proposed. ALP affects sphingosine-1-phosphate (S1P) signaling. S1P is a bioactive lipid that plays a vital role in regulating vascular tone, endothelial barrier function, angiogenesis, and inflammation[15]. ALP modulates S1P signaling, contributing to increased vascular permeability and vascular calcification. ALP has been shown to activate substances that promote inflammatory responses, such as the dephosphorylation of proinflammatory molecules. This can lead to increased levels of cytokines and chemokines, which, in turn, induce endothelial dysfunction. Exposure of vascular endothelial cells to chronic inflammation disrupts the delicate balance of signaling pathways, impairing nitric oxide (NO) production and increasing endothelial permeability. ALP has been reported to increase the production of reactive oxygen species (ROS), potentially through the hydrolysis of phosphate-containing molecules that contribute to oxidative stress[16]. ROS, such as superoxide and hydrogen peroxide, can induce oxidative stress in vascular endothelial cells, leading to damage of cellular components, including lipids, proteins, and DNA. This oxidative stress compromises the functionality of the endothelium by reducing NO bioavailability and promoting endothelial cell apoptosis.
Nutritional psychiatry acknowledges the significance of micronutrients in maintaining brain health, underscoring the potential for identifying and correcting deficiencies in hypertensive older adults as a pivotal strategy for preserving cognitive function. A balanced diet is imperative for ensuring adequate nutrient intake, and hypertensive older adults may require specific dietary guidance or supplementation to address potential deficiencies. Nutrient deficiencies, including B vitamins, vitamin D, and magnesium, are more prevalent in older adults and can be exacerbated by certain medications used to treat hypertension. These deficiencies have the potential to adversely affect cognitive function[17].
Nutritional psychiatry underscores the significance of anti-inflammatory diets in promoting mental and cognitive health. The identification of specific dietary patterns capable of reducing inflammatory markers could prove pivotal in averting cognitive decline in this demographic. A diet abundant in processed foods, saturated fats, and added sugars has the potential to intensify inflammatory responses. Conversely, a diet replete with fruits, vegetables, whole grains, and -3 fatty acids can elicit anti-inflammatory effects. In hypertensive older adults, the management of inflammation through dietary interventions is imperative for regulation of blood pressure and preservation of cognitive health. Nutritional psychiatry explores the potential of dietary antioxidants in safeguarding against oxidative damage in the brain[18]; a matter of particular significance in hypertensive individuals where oxidative stress is already a prevalent concern. A diet deficient in antioxidants, found in colorful fruits and vegetables, can exacerbate oxidative stress. Hypertensive older adults stand to benefit most from diets abundant in vitamins C and E, selenium, and other antioxidants. There are several key dietary patterns that prevent cognitive decline in hypertensive older adults. The dietary approaches to stop hypertension (DASH) diet is a healthy eating plan designed to lower blood pressure. It emphasizes fruits, vegetables, whole grains, and lean protein, while limiting sodium, saturated fats, and added sugars[19]. Think of it as a balanced approach to eating, focusing on whole, unprocessed foods for better heart health. By effectively reducing blood pressure, the DASH diet indirectly supports cognitive health by minimizing vascular damage in the brain. The Mediterranean diet is a way of eating based on the traditional foods of countries bordering the Mediterranean Sea[20]. It emphasizes fruits, vegetables, whole grains, healthy fats like olive oil, and fish, while limiting red meat. It is associated with reduced risk of cardiovascular disease, which is closely linked to cognitive decline. The Mediterranean–DASH Intervention for Neurodegenerative Delay diet combines the Mediterranean and DASH diets, focusing on foods that specifically benefit brain health[21]. It prioritizes leafy greens, berries, nuts, and fish, aiming to slow cognitive decline and reduce the risk of Alzheimer's disease. Essentially, it is a brain-boosting diet that emphasizes nutrient-rich foods known to protect cognitive function.
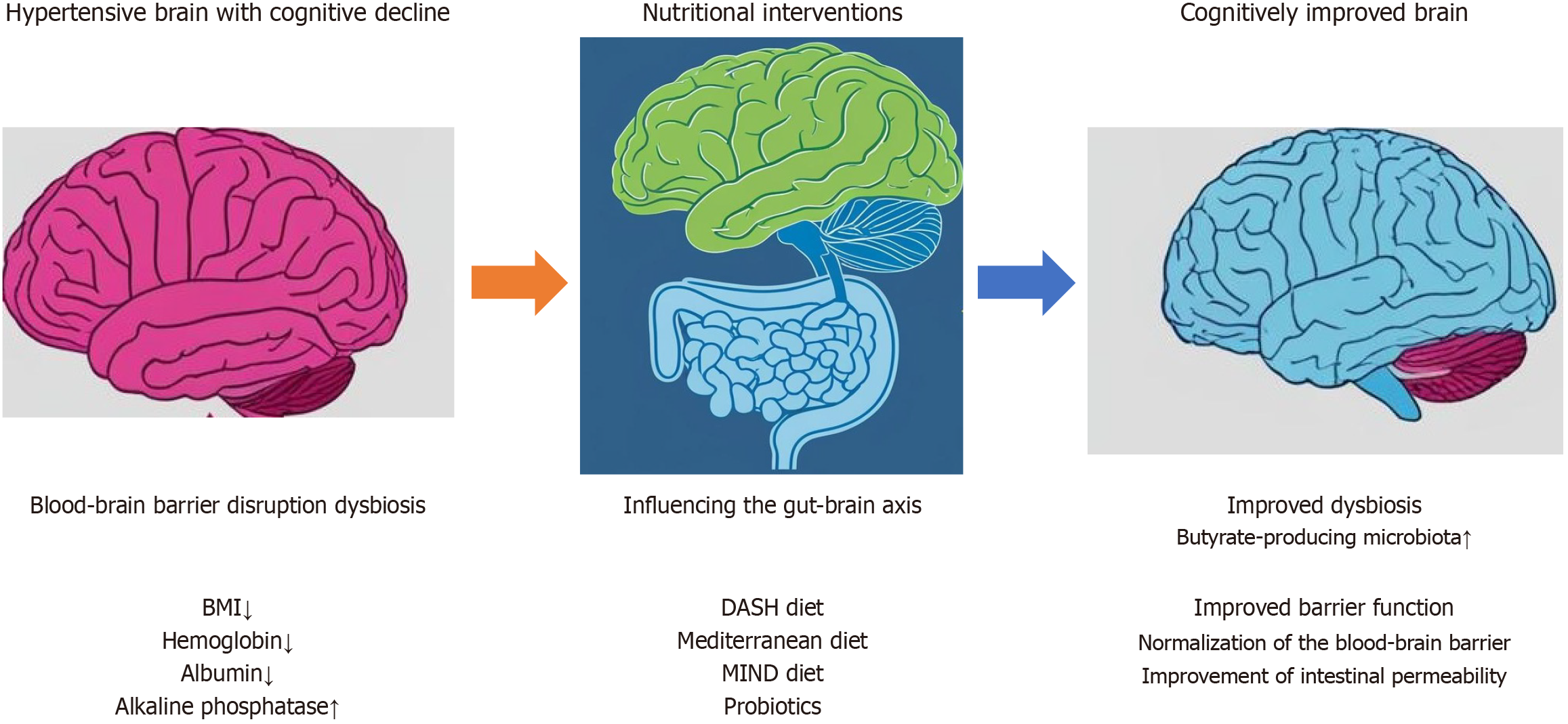
Nutritional psychiatry is a field that emphasizes the critical link between diet and mental health, as illustrated by the concept of the gut–brain axis. In hypertensive older adults, targeting the gut microbiome through dietary interventions has emerged as a promising avenue for supporting cognitive function[22]. Diet plays a major role in shaping the gut microbiome. A diet rich in fiber, prebiotics, and probiotics can promote a healthy gut microbiome, which can have positive effects on both blood pressure and cognitive function. Recent research has indicated a potential link between hypertension and alterations in the gut microbiome. An imbalance in the gut microbiome can lead to inflammation and affect neurotransmitter production, thereby impacting both blood pressure and cognitive function. Probiotics intervention has been shown to enhance cognitive function in hypertensive older adults by improving blood flow and oxygen supply to the brain. This improvement in brain function can be attributed to enhanced gut health, which allows for more efficient nutrient absorption from food. These nutrients can then be used by the brain to function properly (Figure 1). Some specific probiotics that are important for cognitive function include Lactobacillus rhamnosus, Bifidobacterium longum, Lactobacillus casei, Lactobacillus acidophilus, and butyrate-producing microbiota[23].
The limitation of this paper is that it does not include the results of randomized controlled trials (RCTs) and effective longitudinal studies to support the clinical relevance of the findings. There are many confounding factors in the field of nutritional psychiatry, and effective RCTs have not been conducted[24]. In the future, it will be necessary to build evidence by examining nutritional factors that affect cognitive function in hypertension not only through RCTs, but also through Mendelian randomization trials and epidemiological studies in cohorts. Nevertheless, the substantiation for nutritional psychiatry remains unconfirmed by RCTs. This is due to the fact that nutrients do not exert a precise effect in a brief period, akin to psychotropic drugs, but rather, they act on diverse components of the body and function as an entity. Consequently, the implementation of nutritional psychiatry in clinical practice necessitates an individualized approach that considers the background of each patient.
Nutritional factors have been demonstrated to exert a substantial influence on cognitive function in hypertensive older adults. By addressing inflammation, oxidative stress, the gut–brain axis, nutrient deficiencies, and insulin resistance through dietary interventions, nutritional psychiatry emerges as a promising approach to supporting cognitive health in this vulnerable population. Further research is necessary to fully elucidate these mechanisms and develop targeted nutritional strategies for the prevention of cognitive decline in hypertensive older adults.
| 1. | Canavan M, O'Donnell MJ. Hypertension and Cognitive Impairment: A Review of Mechanisms and Key Concepts. Front Neurol. 2022;13:821135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 74] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 2. | Bhagwasia M, Rao AR, Banerjee J, Bajpai S, Raman AV, Talukdar A, Jain A, Rajguru C, Sankhe L, Goswami D, Shanthi GS, Kumar G, Varghese M, Dhar M, Gupta M, A-Koul P, Mohanty RR, Chakrabarti SS, Yadati SR, Dey S, Dey AB. Association Between Cognitive Performance and Nutritional Status: Analysis From LASI-DAD. Gerontol Geriatr Med. 2023;9:23337214231194965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 3. | Logan AC, Jacka FN. Nutritional psychiatry research: an emerging discipline and its intersection with global urbanization, environmental challenges and the evolutionary mismatch. J Physiol Anthropol. 2014;33:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 4. | Ungvari Z, Toth P, Tarantini S, Prodan CI, Sorond F, Merkely B, Csiszar A. Hypertension-induced cognitive impairment: from pathophysiology to public health. Nat Rev Nephrol. 2021;17:639-654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 299] [Article Influence: 74.8] [Reference Citation Analysis (0)] |
| 5. | Chang YL, Chao RY, Hsu YC, Chen TF, Tseng WI. White matter network disruption and cognitive correlates underlying impaired memory awareness in mild cognitive impairment. Neuroimage Clin. 2021;30:102626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Pacholko A, Iadecola C. Hypertension, Neurodegeneration, and Cognitive Decline. Hypertension. 2024;81:991-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 7. | Yao J, Wang S, Li M, Song B, Lan C, Jia J, Yang Y. Correlation between blood pressure control status and cognitive impairment in older adults: A national cross-sectional study. PLoS One. 2025;20:e0317861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Hodes A, Lichtstein D. Natriuretic hormones in brain function. Front Endocrinol (Lausanne). 2014;5:201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Xu Q, Lu S, Shi Z, Yang Y, Yu J, Wang Z, Zhang B, Hong K. Nutritional status of elderly hypertensive patients and its relation to the occurrence of cognitive impairment. World J Psychiatry. 2025;15:103092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Klok MD, Jakobsdottir S, Drent ML. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes Rev. 2007;8:21-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 810] [Cited by in RCA: 869] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 11. | Yavuz BB, Cankurtaran M, Haznedaroglu IC, Halil M, Ulger Z, Altun B, Ariogul S. Iron deficiency can cause cognitive impairment in geriatric patients. J Nutr Health Aging. 2012;16:220-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Min JY, Ha SW, Yang SH, Kang MJ, Jeong DE, Min KB, Kim B. Chronic Status of Serum Albumin and Cognitive Function: A Retrospective Cohort Study. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 13. | Zhang YL, Jia SY, Yang B, Miao J, Su C, Cui ZG, Yang LM, Guo JH. Non-linear association of liver enzymes with cognitive performance in the elderly: A cross-sectional study. PLoS One. 2024;19:e0306839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 14. | Jackson EK, Cheng D, Ritov VB, Mi Z. Alkaline Phosphatase Activity Is a Key Determinant of Vascular Responsiveness to Norepinephrine. Hypertension. 2020;76:1308-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Lucaciu A, Brunkhorst R, Pfeilschifter JM, Pfeilschifter W, Subburayalu J. The S1P-S1PR Axis in Neurological Disorders-Insights into Current and Future Therapeutic Perspectives. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 16. | López-Posadas R, González R, Ballester I, Martínez-Moya P, Romero-Calvo I, Suárez MD, Zarzuelo A, Martínez-Augustin O, Sánchez de Medina F. Tissue-nonspecific alkaline phosphatase is activated in enterocytes by oxidative stress via changes in glycosylation. Inflamm Bowel Dis. 2011;17:543-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Kaur D, Rasane P, Singh J, Kaur S, Kumar V, Mahato DK, Dey A, Dhawan K, Kumar S. Nutritional Interventions for Elderly and Considerations for the Development of Geriatric Foods. Curr Aging Sci. 2019;12:15-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 18. | van Zonneveld SM, van den Oever EJ, Haarman BCM, Grandjean EL, Nuninga JO, van de Rest O, Sommer IEC. An Anti-Inflammatory Diet and Its Potential Benefit for Individuals with Mental Disorders and Neurodegenerative Diseases-A Narrative Review. Nutrients. 2024;16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 19. | Filippou CD, Tsioufis CP, Thomopoulos CG, Mihas CC, Dimitriadis KS, Sotiropoulou LI, Chrysochoou CA, Nihoyannopoulos PI, Tousoulis DM. Dietary Approaches to Stop Hypertension (DASH) Diet and Blood Pressure Reduction in Adults with and without Hypertension: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv Nutr. 2020;11:1150-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 282] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 20. | Widmer RJ, Flammer AJ, Lerman LO, Lerman A. The Mediterranean diet, its components, and cardiovascular disease. Am J Med. 2015;128:229-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 569] [Cited by in RCA: 572] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 21. | van den Brink AC, Brouwer-Brolsma EM, Berendsen AAM, van de Rest O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diets Are Associated with Less Cognitive Decline and a Lower Risk of Alzheimer's Disease-A Review. Adv Nutr. 2019;10:1040-1065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 344] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 22. | Grosso G. Nutritional Psychiatry: How Diet Affects Brain through Gut Microbiota. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 23. | Salami M. Interplay of Good Bacteria and Central Nervous System: Cognitive Aspects and Mechanistic Considerations. Front Neurosci. 2021;15:613120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 24. | Carnegie R, Zheng J, Sallis HM, Jones HJ, Wade KH, Evans J, Zammit S, Munafò MR, Martin RM. Mendelian randomisation for nutritional psychiatry. Lancet Psychiatry. 2020;7:208-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |