Published online May 19, 2025. doi: 10.5498/wjp.v15.i5.102953
Revised: February 13, 2025
Accepted: February 24, 2025
Published online: May 19, 2025
Processing time: 178 Days and 5.1 Hours
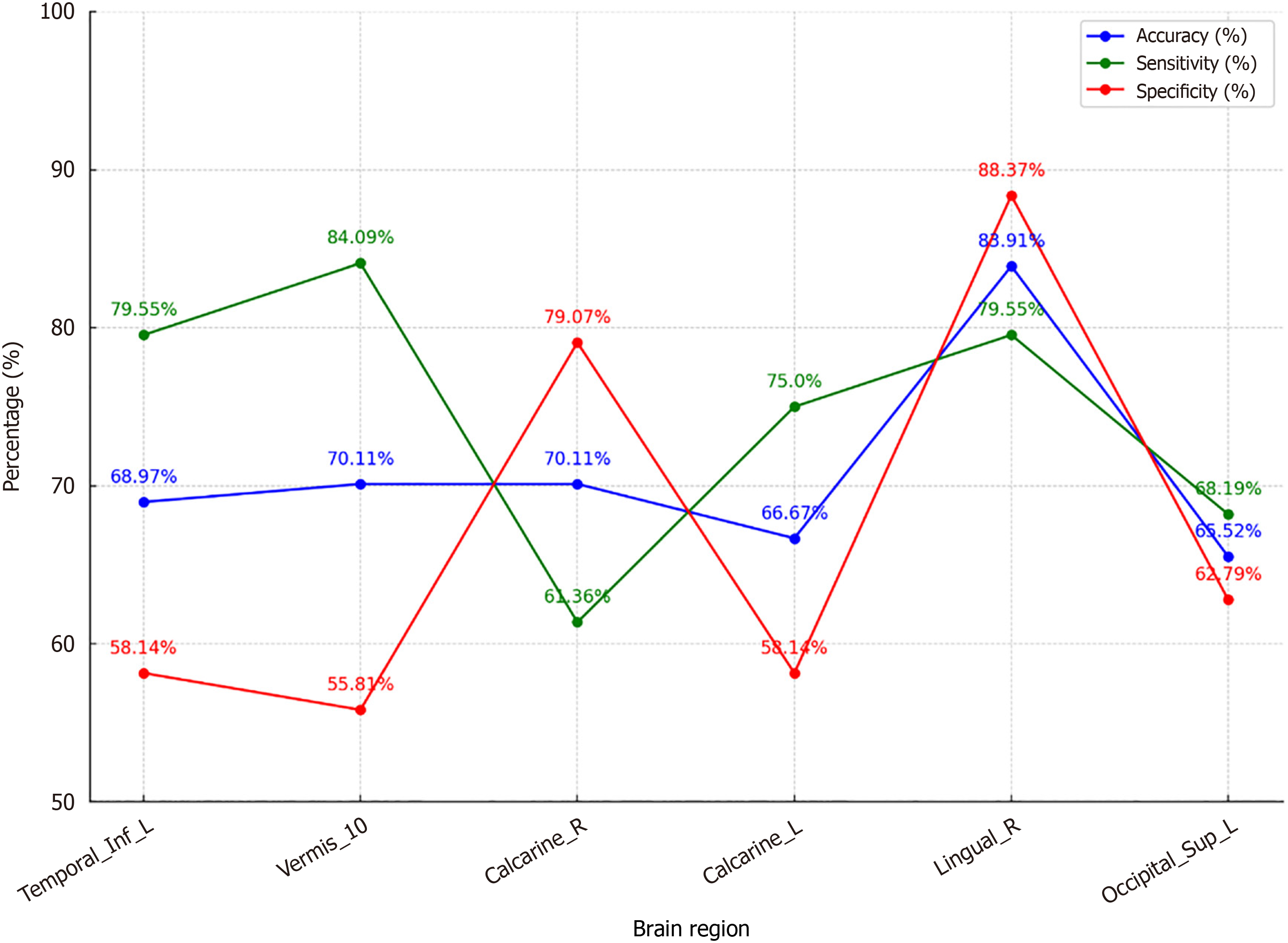
Yu et al's study has advanced the understanding of the neural mechanisms underlying major depressive disorder (MDD) in adolescents, emphasizing the significant role of the amygdala. While traditional diagnostic methods have limitations in objectivity and accuracy, this research demonstrates a notable advancement through the integration of machine learning techniques with neuroimaging data. Utilizing resting-state functional magnetic resonance imaging (fMRI), the study investigated functional connectivity (FC) in adolescents with MDD, identifying notable reductions in regions such as the left inferior temporal gyrus and right lingual gyrus, alongside increased connectivity in Vermis-10. The application of support vector machines (SVM) to resting-state fMRI (rs-fMRI) data achieved an accuracy of 83.91%, sensitivity of 79.55%, and specificity of 88.37%, with an area under the curve of 0.6765. These results demonstrate how SVM analysis of rs-fMRI data represents a significant improvement in diagnostic precision, with reduced FC in the right lingual gyrus emerging as a particularly critical marker. These findings underscore the critical role of the amygdala in MDD pathophysiology and highlight the potential of rs-fMRI and SVM as tools for identifying reliable neuroimaging biomarkers.
Core Tip: Yu et al's research sheds light on adolescent major depressive disorder (MDD), focusing on the amygdala. The study explored neuroimaging biomarkers for diagnostics using resting-state functional magnetic resonance imaging to analyze functional connectivity (FC) in adolescents with MDD. It found reduced FC in the left inferior temporal gyrus and right lingual gyrus, alongside increased connectivity in Vermis-10. Support vector machines effectively distinguished MDD patients from healthy controls, highlighting reduced FC in the right lingual gyrus as a key marker. These findings suggest FC changes as reliable biomarkers, offering a non-invasive, accurate diagnostic approach for adolescent MDD.
- Citation: Byeon H. Unveiling the invisible: How cutting-edge neuroimaging transforms adolescent depression diagnosis. World J Psychiatry 2025; 15(5): 102953
- URL: https://www.wjgnet.com/2220-3206/full/v15/i5/102953.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i5.102953
Adolescent major depressive disorder (MDD) is a significant mental health challenge, impacting approximately 10%-20% of adolescents globally, with prevalence rates continuing to rise in recent years due to various social, environmental, and biological factors[1]. This disorder is characterized by persistent low mood and loss of interest in activities, and can severely affect adolescents' developmental trajectories. Prompt and accurate diagnosis is crucial, as early intervention can dramatically improve long-term outcomes.
Traditional methods for diagnosing adolescent MDD rely heavily on clinical interviews and self-report measures, which often suffer from subjectivity and can lead to misdiagnosis. Adolescents may have difficulty articulating their emotional states due to developmental factors, such as limited emotional awareness or social stigma, complicating the diagnostic process. Furthermore, cultural differences in symptom expression and clinician bias can introduce additional variability. These challenges highlight the need for more objective biomarkers that can complement traditional diagnostic approaches. For instance, subjective assessments may fail to capture subtle neurobiological changes that precede overt symptoms, leading to delays in diagnosis and treatment.
Advancements in neuroimaging, particularly resting-state functional magnetic resonance imaging (rs-fMRI), offer new possibilities for understanding the neurobiological foundations of psychiatric disorders like MDD. Rs-fMRI enables the examination of functional connectivity (FC) between brain regions, providing insights into the brain's communication networks and how they may be disrupted in mental health disorders. In adolescent MDD, FC alterations have been identified as key indicators of disease pathology. For example, reduced FC in regions such as the amygdala, prefrontal cortex (PFC), and default mode network has been consistently associated with emotional dysregulation and cognitive deficits in MDD patients[2]. These findings suggest that FC not only reflects underlying neural dysfunction but also serves as a potential biomarker for early detection and monitoring of treatment response.
The PFC plays a critical role in regulating emotions, decision-making, and executive functions, all of which are impaired in individuals with MDD[3]. Studies have shown that hypoactivity in the PFC, particularly in the dorsolateral PFC, is linked to deficits in cognitive control and emotional regulation, hallmark features of depression[4]. Additionally, altered connectivity between the PFC and subcortical regions, such as the amygdala, has been implicated in the patho
In adolescent MDD, the amygdala has been identified as a key region involved in emotion regulation, with studies showing altered connectivity patterns in individuals with MDD. Using the amygdala as a focal point, rs-fMRI can help identify changes in FC that may serve as potential biomarkers for MDD. The integration of machine learning, particularly support vector machines (SVM), with neuroimaging data has enhanced the potential for developing precise diagnostic tools. SVM can efficiently handle complex data, identifying patterns that distinguish between healthy and clinical populations. This capability is instrumental in creating predictive models based on neuroimaging biomarkers for adolescent MDD.
This editorial explores these innovative approaches, discussing how rs-fMRI and SVM can be used to identify reliable biomarkers for adolescent MDD. By advancing diagnostic precision, this paper aims to facilitate early intervention and improve treatment outcomes, offering a new perspective on managing adolescent depression and contributing to a deeper understanding of its neurobiological underpinnings, as demonstrated in the study by Yu et al[6].
The exploration of neuroimaging techniques and machine learning in the context of psychiatric disorders, particularly MDD, has been an area of significant research interest. Early studies[7,8] on neuroimaging in depression primarily used structural MRI to investigate morphological changes in the brain, such as alterations in gray matter volume. These studies provided foundational insights but were limited in their ability to capture dynamic brain processes. The advent of rs-fMRI marked a pivotal shift, allowing researchers to assess FC between brain regions[9]. This approach has been particularly illuminating in understanding the default mode network and its disruptions in depressive disorders.
In the context of adolescent MDD, several studies illuminate the critical role of the amygdala and its connectivity with other brain regions, fundamentally altering our understanding of emotional and cognitive processing in this population. The amygdala's FC, particularly with regions such as the hippocampus and brainstem, tends to be lower in individuals with MDD, correlating inversely with the severity of depression symptoms. This underpins its potential influence on processes integral to memory and emotional response in adolescents, thereby contributing to heightened susceptibility to depressive episodes[10].
Moreover, genetic variations such as the BDNF Val66Met polymorphism have been found to modulate amygdala-cortical connectivity, significantly affecting depression risk in adolescents. This genetic factor appears to enhance connectivity within amygdala-cortical networks, implying a potential risk factor for developing depression, especially among female adolescents[11]. The interplay between genetic predispositions and FC highlights the intricate neurobiological mechanisms underpinning MDD.
Additionally, the structural and functional dynamics between the amygdala and PFC are pivotal in understanding mood regulation. Aberrant connectivity between these regions is implicated in the onset of depressive states during adolescence, which could explain the variability in mood and affective responses observed in clinically depressed individuals[12]. Furthermore, studies reveal that treatment responses, particularly to antidepressants like fluoxetine, can alter amygdala-prefrontal connectivity, providing insights into the neurophysiological biomarkers of treatment efficacy[13]. These findings underscore the potential of FC as a biomarker for MDD, offering a non-invasive means to identify at-risk individuals. These findings underscore the potential of FC as a biomarker for MDD, offering a non-invasive means to identify at-risk individuals.
The integration of machine learning with neuroimaging data has further propelled the field forward, particularly through the application of SVM. SVM is favored for their robustness in managing high-dimensional data, making them a popular choice for classifying individuals based on neuroimaging features. Numerous studies have employed SVM to distinguish between depressed and non-depressed adolescents, achieving notable accuracy and specificity. For example, research employing SVM on resting-state fMRI data demonstrated good performance, underscoring the potential utility of these models in clinical evaluations of adolescent depression[14].
However, the predictive performance of SVM in depression studies varies across different research initiatives. Key factors contributing to these discrepancies include variations in sample sizes, imaging protocols, and feature selection methods. The differences in neuroimaging methodologies and the heterogeneity of clinical presentations among adolescents contribute to the challenges in achieving consistent predictive accuracy[15]. Additionally, the complexity of depression as a disorder, which involves diverse neural circuits and variable symptomatology, necessitates more nuanced approaches in data analysis and interpretation.
Further complications arise from the inherent variability in neuroimaging studies, such as differing scanner types and imaging settings, which can influence the quality and comparability of the data used for model training and validation[16]. To address these issues, it is essential for future studies to standardize protocols, adopt harmonization techniques, and explore sophisticated models like multi-component classifiers that can accommodate data variability and improve the robustness of predictions[17].
Beyond SVM, recent research has delved into the application of advanced machine learning techniques, including deep learning and ensemble methods, to enhance the analysis of neuroimaging data. These approaches are particularly promising for capturing the complex patterns inherent in neuroimaging data that often challenge traditional algorithms. Convolutional neural networks (CNN), for instance, have shown considerable potential in extracting subtle connectivity alterations in rs-fMRI data. By refining network parameters, CNN offer improved pattern recognition capabilities, which proves beneficial in distinguishing intricate neurobiological changes associated with various mental health conditions[18].
Furthermore, ensemble methods like Random Forests and Gradient Boosting Machines have emerged as powerful tools through their ability to aggregate multiple models to boost predictive performance. These techniques leverage the diversity of model predictions to reduce variance and enhance accuracy, enabling more robust classification and regression tasks in neuroimaging studies. Ensemble methods thus provide a comprehensive framework for addressing the limitations of single-model approaches, offering heightened predictive power that is particularly advantageous in handling the high-dimensional and often noisy datasets characteristic of neuroimaging[19].
In addition, the development and utilization of specialized machine learning toolboxes for neuroimaging, such as those integrated within the scikit-learn library, facilitate the application of both supervised and unsupervised learning approaches in this field. These toolboxes are designed to handle the complexities of neuroimaging datasets, supporting researchers in implementing a range of machine learning methodologies beyond SVM to advance diagnostic processes[20]. Collectively, these advancements underscore the critical role of sophisticated machine learning systems in expanding the capabilities of neuroimaging analyses, paving the way for improved diagnostic accuracy and insight into cognitive and psychiatric disorders.
Despite these advancements, challenges remain in translating neuroimaging findings into clinical practice. The variability in imaging methodologies and the need for large, diverse datasets are critical issues that future studies must address. Additionally, integrating neuroimaging biomarkers with clinical and genetic data could provide a more comprehensive understanding of MDD, paving the way for personalized medicine approaches.
In summary, the study by Yu et al[6] underscores the transformative potential of combining neuroimaging and ma
| Neuroimaging technique | Machine learning approaches | Deep learning approaches | Findings/applications | Ref. |
| fMRI | SVM, XGBoost | CNN | Identification of functional connectivity alterations, prediction of treatment response, generalizable markers across imaging sites | Anderson et al[21], Shi et al[22], Yamashita et al[23] |
| sMRI | PCA, ICA | Autoencoders | Detection of gray matter volume reductions, feature extraction, and identification of structural biomarkers | Zhang et al[24], Ho et al[25] |
| PET | Feature selection | Evaluation of altered glucose metabolism, identification of treatment-specific biomarkers | McGrath et al[26], Kang and Cho[27] | |
| EEG | Time-series analysis | CNN, RNN, LSTM | Analysis of altered neural oscillations, connectivity patterns, and prediction models using EEG-based approaches | Uyulan et al[28], Yang et al[29] |
In this study, a total of 44 adolescents diagnosed with MDD and 43 healthy controls (HC) were recruited (Table 2). The inclusion criteria for the MDD group required participants to meet the DSM-IV criteria for a depressive episode, as confirmed by the Mini International Neuropsychiatric Interview for Children and Adolescents and a Hamilton Depression Rating Scale score ≥ 17. HC were required to have no history of psychiatric disorders, neurological conditions, or significant medical illnesses, as verified through clinical interviews. Exclusion criteria for both groups included the presence of comorbid psychiatric disorders, substance abuse, or any contraindications for MRI scanning, such as metallic implants or claustrophobia. The adolescents with MDD included 10 males and 34 females with an average age of 15 ± 2 years. All participants underwent rs-fMRI to assess FC in the brain. Patients were selected from the psychiatric outpatient department of the First Affiliated Hospital of Chongqing Medical University, China. Demographic and clinical data were analyzed using t-tests and χ2 tests to ensure comparability between groups. Table 1 shows that there were no significant differences in age, sex, or education level between patients and controls, confirming the validity of the sample matching (Table 2).
| Variable | MDD group (n = 44) | Control group (n = 43) | P value |
| Age (years) | 15 ± 2 | 15 ± 2 | > 0.05 |
| Sex (male/female) | 10/34 | 11/32 | > 0.05 |
| Education (years) | 10 ± 3 | 10 ± 3 | > 0.05 |
| HAMD-17 Score | 21 ± 5 | 5 ± 3 | < 0.05 |
The rs-fMRI data were preprocessed using a standardized pipeline to ensure data quality and consistency. Preprocessing steps included motion correction to minimize artifacts caused by head movement, spatial normalization to align individual brain images to a standard template, and temporal filtering to remove low-frequency drifts and high-frequency noise. Additionally, nuisance regression was performed to account for non-neuronal signals, including those from white matter, cerebrospinal fluid, and global signal fluctuations. These steps are critical for reducing noise and enhancing the reliability of FC analysis.
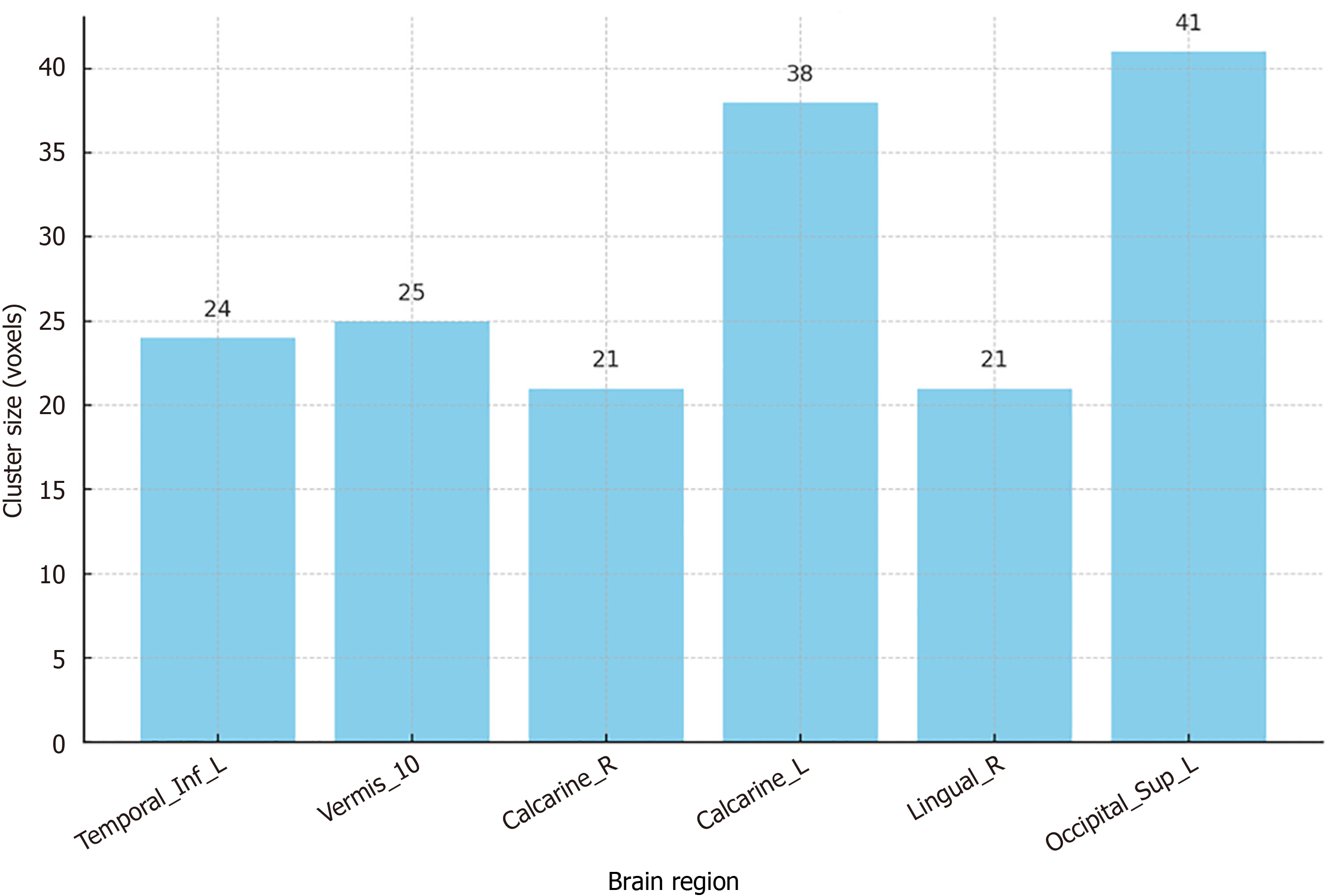
The study used the REST plus software to calculate FC between the amygdala and other brain regions (Figure 1). The rationale for selecting the bilateral amygdala as the seed region was based on its well-documented role in emotion regulation and its consistent involvement in MDD pathophysiology. By focusing on amygdala-based FC, the study aimed to identify specific connectivity patterns that could serve as biomarkers for adolescent MDD. Fisher's transformation was applied to achieve a normal distribution of z-scores for statistical analysis. Significant differences in FC were observed in several brain regions, including the left inferior temporal gyrus, bilateral calcarine, right lingual gyrus, and left superior occipital gyrus, with increased connectivity in Vermis-10.
A SVM was employed to classify FC patterns between patients and controls. Although the SVM model demonstrated an accuracy of 83.91%, sensitivity of 79.55%, and specificity of 88.37%, the area under the receiver operating characteristic (ROC) curve (AUC) was 0.6765, indicating moderate predictive performance (Figure 2). The AUC value suggests that while the model is capable of distinguishing between groups, its discriminative ability is not yet optimal. This may be attributed to the heterogeneity of MDD symptoms and the relatively small sample size, which can limit the generalizability of the findings. Future studies should explore larger datasets and more advanced machine learning algorithms to improve predictive accuracy.
Notably, the study observed a higher proportion of females in the MDD group compared to males, reflecting the known sex differences in MDD prevalence during adolescence. While this aligns with epidemiological trends, it also raises questions about potential sex-specific differences in FC patterns. Further subgroup analyses stratified by sex could provide insights into whether these differences influence diagnostic accuracy or treatment response, offering a more nuanced understanding of adolescent MDD.
The ROC analysis of the right lingual gyrus's FC value confirmed its potential as a biomarker for distinguishing between MDD patients and HC. However, the moderate AUC highlights the need for further refinement of the model to improve its diagnostic accuracy. These findings suggest that while rs-fMRI and SVM can effectively identify neuroi
The findings from the study by Yu et al[6] have several important clinical implications for the diagnosis and management of adolescent MDD, offering a pathway to more effective and personalized treatment strategies. First, the identification of FC alterations in specific brain regions, such as the right lingual gyrus and the left inferior temporal gyrus, provides clinicians with potential neuroimaging biomarkers that could enhance the accuracy of MDD diagnosis in adolescents. The ability to use rs-fMRI as a non-invasive diagnostic tool represents a significant advancement over traditional methods, which often rely on subjective symptom reporting and clinical interviews. By integrating these biomarkers into clinical practice, healthcare providers can achieve earlier and more reliable diagnoses, potentially leading to timely interventions that could mitigate the long-term effects of MDD.
Second, the use of SVM in analyzing neuroimaging data demonstrates the potential of machine learning algorithms to improve diagnostic processes. The moderate predictive performance observed in this study indicates that while current models are promising, there is scope for further refinement and optimization. By continuing to develop and validate these models, clinicians could have access to more robust diagnostic tools that not only differentiate between healthy and MDD-affected individuals but also provide insights into the severity and progression of the disorder. This could facilitate more tailored treatment plans, aligning therapeutic approaches with the specific neurobiological profiles of patients.
Third, the study underscores the importance of a multidisciplinary approach in managing adolescent MDD. The integration of neuroimaging findings with clinical assessments could lead to a more comprehensive understanding of the disorder, enabling healthcare teams to address both the psychological and biological aspects of depression. This holistic approach could improve patient outcomes by ensuring that treatment plans are informed by a complete picture of the patient's mental health, encompassing both symptomatic and underlying neurological factors.
Fourth, these findings highlight the potential for neuroimaging biomarkers to inform treatment decisions beyond diagnosis. By understanding the specific brain connectivity patterns associated with MDD, clinicians could identify which patients are more likely to respond to certain interventions, such as cognitive-behavioral therapy or pharmacotherapy. This precision medicine approach could enhance treatment efficacy, reduce the trial-and-error nature of current practices, and ultimately improve patient adherence and satisfaction.
Lastly, the study encourages the exploration of neuroimaging as a tool for monitoring treatment efficacy and disease progression. Regular neuroimaging assessments could provide objective measures of treatment response, allowing clinicians to adjust therapeutic strategies in real time based on observed changes in brain connectivity. This dynamic approach to treatment management could improve long-term outcomes by ensuring that interventions remain aligned with the evolving needs of the patient.
In summary, the integration of neuroimaging biomarkers into clinical practice has the potential to revolutionize the diagnosis and treatment of adolescent MDD, offering a more objective, personalized, and effective approach to managing this challenging disorder. By leveraging these advancements, healthcare providers can enhance the quality of care for adolescents with MDD, improving both short-term and long-term mental health outcomes.
Despite the promising findings, this study is subject to several limitations that must be addressed in future research to enhance the applicability and robustness of the results. These limitations also point towards important future research directions. First, the study's relatively small sample size limits the generalizability of the findings across broader populations. A larger and more diverse cohort would provide a more reliable basis for assessing the efficacy of the identified neuroimaging biomarkers in diagnosing adolescent MDD. Future studies should aim to include participants from various demographic backgrounds to ensure that the findings are representative of different population segments.
Second, the moderate AUC value observed in the SVM analysis indicates room for improvement in predictive accuracy. While SVM are effective in handling complex datasets, future research should explore the application of advanced ensemble machine learning algorithms, such as Random Forests or Gradient Boosting Machines, which may offer enhanced predictive performance by leveraging multiple models to improve classification accuracy. These algorithms can capture intricate patterns in neuroimaging data, potentially leading to more precise and reliable diagnostic tools. Furthermore, future work should investigate the potential of combining neuroimaging data with other readily accessible biomarkers, such as genetic, proteomic, or clinical measures. Integrating these diverse data sources could significantly improve diagnostic accuracy and allow for a more personalized approach to treatment.
Third, the study focused exclusively on rs-fMRI to assess FC. While rs-fMRI provides valuable insights into brain network dynamics, incorporating additional imaging modalities, such as structural MRI or diffusion tensor imaging (DTI), could offer a more comprehensive view of the neurobiological changes associated with MDD. Multimodal imaging approaches, which combine rs-fMRI with structural MRI and DTI, could enrich our understanding of the disorder by revealing both structural and functional brain alterations, thereby improving diagnostic capabilities. This multimodal approach will be crucial in disentangling the complex interplay between structural and functional changes in the brain associated with MDD.
Fourth, the study's cross-sectional design limits the ability to infer causal relationships between observed FC alterations and MDD. Longitudinal studies are needed to explore the temporal dynamics of these neuroimaging biomarkers and their relationship with the onset and progression of MDD symptoms. Such studies could elucidate whether FC changes precede clinical manifestations, thereby serving as predictive markers for future depressive episodes.
Fifth, the study's reliance on a single neuroimaging session for each participant may not capture the variability in brain connectivity over time. Future research should incorporate repeated imaging sessions to assess the stability and reliability of the identified biomarkers. This approach would provide insights into the consistency of these neuroimaging patterns and their potential as long-term diagnostic tools.
Sixth, the current study did not account for potential confounding factors, such as medication usage or comorbid psychiatric conditions, which may influence brain connectivity patterns. Future studies should control for these variables to isolate the specific effects of MDD on neuroimaging findings. Additionally, exploring the interaction between these factors and FC alterations could provide a deeper understanding of the complex interplay between neurobiology and mental health.
Lastly, the study highlights the need for ongoing refinement of predictive models to enhance their clinical utility. Future research should focus on developing and validating robust predictive models that integrate neuroimaging data with clinical and demographic variables, offering a comprehensive assessment tool for adolescent MDD. By addressing these limitations and pursuing these research directions, we can advance our understanding of MDD and improve diagnostic and therapeutic strategies for adolescents facing this challenging disorder.
Yu et al's study advances our understanding of managing adolescent MDD by demonstrating the potential of neuro
| 1. | Shorey S, Ng ED, Wong CHJ. Global prevalence of depression and elevated depressive symptoms among adolescents: A systematic review and meta-analysis. Br J Clin Psychol. 2022;61:287-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 572] [Article Influence: 143.0] [Reference Citation Analysis (0)] |
| 2. | Rice F, Riglin L, Thapar AK, Heron J, Anney R, O'Donovan MC, Thapar A. Characterizing Developmental Trajectories and the Role of Neuropsychiatric Genetic Risk Variants in Early-Onset Depression. JAMA Psychiatry. 2019;76:306-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 3. | Smith KM, Renshaw PF, Bilello J. The diagnosis of depression: current and emerging methods. Compr Psychiatry. 2013;54:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Chen X, Lu B, Li HX, Li XY, Wang YW, Castellanos FX, Cao LP, Chen NX, Chen W, Cheng YQ, Cui SX, Deng ZY, Fang YR, Gong QY, Guo WB, Hu ZJ, Kuang L, Li BJ, Li L, Li T, Lian T, Liao YF, Liu YS, Liu ZN, Lu JP, Luo QH, Meng HQ, Peng DH, Qiu J, Shen YD, Si TM, Tang YQ, Wang CY, Wang F, Wang HN, Wang K, Wang X, Wang Y, Wang ZH, Wu XP, Xie CM, Xie GR, Xie P, Xu XF, Yang H, Yang J, Yao SQ, Yu YQ, Yuan YG, Zhang KR, Zhang W, Zhang ZJ, Zhu JJ, Zuo XN, Zhao JP, Zang YF; DIRECT consortium, Yan CG. The DIRECT consortium and the REST-meta-MDD project: towards neuroimaging biomarkers of major depressive disorder. Psychoradiology. 2022;2:32-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 5. | Woodward ND, Cascio CJ. Resting-State Functional Connectivity in Psychiatric Disorders. JAMA Psychiatry. 2015;72:743-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 150] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 6. | Yu ZH, Yu RQ, Wang XY, Ren WY, Zhang XQ, Wu W, Li X, Dai LQ, Lv YL. Resting-state functional magnetic resonance imaging and support vector machines for the diagnosis of major depressive disorder in adolescents. World J Psychiatry. 2024;14:1696-1707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 7. | Bouckaert F, De Winter FL, Emsell L, Dols A, Rhebergen D, Wampers M, Sunaert S, Stek M, Sienaert P, Vandenbulcke M. Grey matter volume increase following electroconvulsive therapy in patients with late life depression: a longitudinal MRI study. J Psychiatry Neurosci. 2016;41:105-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 8. | Sacher J, Neumann J, Fünfstück T, Soliman A, Villringer A, Schroeter ML. Mapping the depressed brain: a meta-analysis of structural and functional alterations in major depressive disorder. J Affect Disord. 2012;140:142-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 250] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 9. | Mulders PC, van Eijndhoven PF, Schene AH, Beckmann CF, Tendolkar I. Resting-state functional connectivity in major depressive disorder: A review. Neurosci Biobehav Rev. 2015;56:330-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 594] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 10. | Cullen KR, Westlund MK, Klimes-Dougan B, Mueller BA, Houri A, Eberly LE, Lim KO. Abnormal amygdala resting-state functional connectivity in adolescent depression. JAMA Psychiatry. 2014;71:1138-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 243] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 11. | Wheeler AL, Felsky D, Viviano JD, Stojanovski S, Ameis SH, Szatmari P, Lerch JP, Chakravarty MM, Voineskos AN. BDNF-Dependent Effects on Amygdala-Cortical Circuitry and Depression Risk in Children and Youth. Cereb Cortex. 2018;28:1760-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Chang KD. Amygdalar-Prefrontal Connectivity Changes During Adolescence: Implications for Development of Mood Disorders. Biol Psychiatry. 2017;82:458-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Zhang A, Yang C, Li G, Wang Y, Liu P, Liu Z, Sun N, Zhang K. Functional connectivity of the prefrontal cortex and amygdala is related to depression status in major depressive disorder. J Affect Disord. 2020;274:897-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 14. | Mao N, Che K, Chu T, Li Y, Wang Q, Liu M, Ma H, Wang Z, Lin F, Wang B, Ji H. Aberrant Resting-State Brain Function in Adolescent Depression. Front Psychol. 2020;11:1784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 15. | Shad MU, Bidesi AP, Chen LA, Ernst M, Rao U. Neurobiology of decision making in depressed adolescents: a functional magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry. 2011;50:612-621.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Gallo S, El-Gazzar A, Zhutovsky P, Thomas RM, Javaheripour N, Li M, Bartova L, Bathula D, Dannlowski U, Davey C, Frodl T, Gotlib I, Grimm S, Grotegerd D, Hahn T, Hamilton PJ, Harrison BJ, Jansen A, Kircher T, Meyer B, Nenadić I, Olbrich S, Paul E, Pezawas L, Sacchet MD, Sämann P, Wagner G, Walter H, Walter M; PsyMRI, van Wingen G. Functional connectivity signatures of major depressive disorder: machine learning analysis of two multicenter neuroimaging studies. Mol Psychiatry. 2023;28:3013-3022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 45] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 17. | Bajaj S, Blair KS, Dobbertin M, Patil KR, Tyler PM, Ringle JL, Bashford-Largo J, Mathur A, Elowsky J, Dominguez A, Schmaal L, Blair RJR. Machine learning based identification of structural brain alterations underlying suicide risk in adolescents. Discov Ment Health. 2023;3:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Shen YF, Liu YZ, Ye WH, Zhang YM, Qian JC, Wang YQ. Research on Discharge Sound Recognition Based on Machine Learning and Convolutional Neural Network Training Algorithm. 2021 IEEE International Conference on Advances in Electrical Engineering and Computer Applications (AEECA), Dalian, China, 2021; 441-446. [DOI] [Full Text] |
| 19. | Lad R, Metkewar PS. Review of Machine Learning Classifier Toolbox of Neuroimaging Data. 2020 IEEE Pune Section International Conference (PuneCon), Pune, India, 2020; 175-179. [DOI] [Full Text] |
| 20. | Abraham A, Pedregosa F, Eickenberg M, Gervais P, Mueller A, Kossaifi J, Gramfort A, Thirion B, Varoquaux G. Machine learning for neuroimaging with scikit-learn. Front Neuroinform. 2014;8:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 761] [Cited by in RCA: 1152] [Article Influence: 104.7] [Reference Citation Analysis (0)] |
| 21. | Anderson KM, Collins MA, Kong R, Fang K, Li J, He T, Chekroud AM, Yeo BTT, Holmes AJ. Convergent molecular, cellular, and cortical neuroimaging signatures of major depressive disorder. Proc Natl Acad Sci U S A. 2020;117:25138-25149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 22. | Shi Y, Zhang L, Wang Z, Lu X, Wang T, Zhou D, Zhang Z. Multivariate Machine Learning Analyses in Identification of Major Depressive Disorder Using Resting-State Functional Connectivity: A Multicentral Study. ACS Chem Neurosci. 2021;12:2878-2886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 23. | Yamashita A, Sakai Y, Yamada T, Yahata N, Kunimatsu A, Okada N, Itahashi T, Hashimoto R, Mizuta H, Ichikawa N, Takamura M, Okada G, Yamagata H, Harada K, Matsuo K, Tanaka SC, Kawato M, Kasai K, Kato N, Takahashi H, Okamoto Y, Yamashita O, Imamizu H. Generalizable brain network markers of major depressive disorder across multiple imaging sites. PLoS Biol. 2020;18:e3000966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (6)] |
| 24. | Zhang L, Wang M, Liu M, Zhang D. A Survey on Deep Learning for Neuroimaging-Based Brain Disorder Analysis. Front Neurosci. 2020;14:779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 25. | Ho TC, Gutman B, Pozzi E, Grabe HJ, Hosten N, Wittfeld K, Völzke H, Baune B, Dannlowski U, Förster K, Grotegerd D, Redlich R, Jansen A, Kircher T, Krug A, Meinert S, Nenadic I, Opel N, Dinga R, Veltman DJ, Schnell K, Veer I, Walter H, Gotlib IH, Sacchet MD, Aleman A, Groenewold NA, Stein DJ, Li M, Walter M, Ching CRK, Jahanshad N, Ragothaman A, Isaev D, Zavaliangos-Petropulu A, Thompson PM, Sämann PG, Schmaal L. Subcortical shape alterations in major depressive disorder: Findings from the ENIGMA major depressive disorder working group. Hum Brain Mapp. 2022;43:341-351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 26. | McGrath CL, Kelley ME, Holtzheimer PE, Dunlop BW, Craighead WE, Franco AR, Craddock RC, Mayberg HS. Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA Psychiatry. 2013;70:821-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 347] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 27. | Kang SG, Cho SE. Neuroimaging Biomarkers for Predicting Treatment Response and Recurrence of Major Depressive Disorder. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 28. | Uyulan C, Ergüzel TT, Unubol H, Cebi M, Sayar GH, Nezhad Asad M, Tarhan N. Major Depressive Disorder Classification Based on Different Convolutional Neural Network Models: Deep Learning Approach. Clin EEG Neurosci. 2021;52:38-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 29. | Yang Y, Zhu DM, Zhang C, Zhang Y, Wang C, Zhang B, Zhao W, Zhu J, Yu Y. Brain Structural and Functional Alterations Specific to Low Sleep Efficiency in Major Depressive Disorder. Front Neurosci. 2020;14:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |